AMPEC4: Naja ashei Venom-Derived Peptide as a Stimulator of Fibroblast Migration with Antibacterial Activity
Abstract
1. Introduction
2. Results
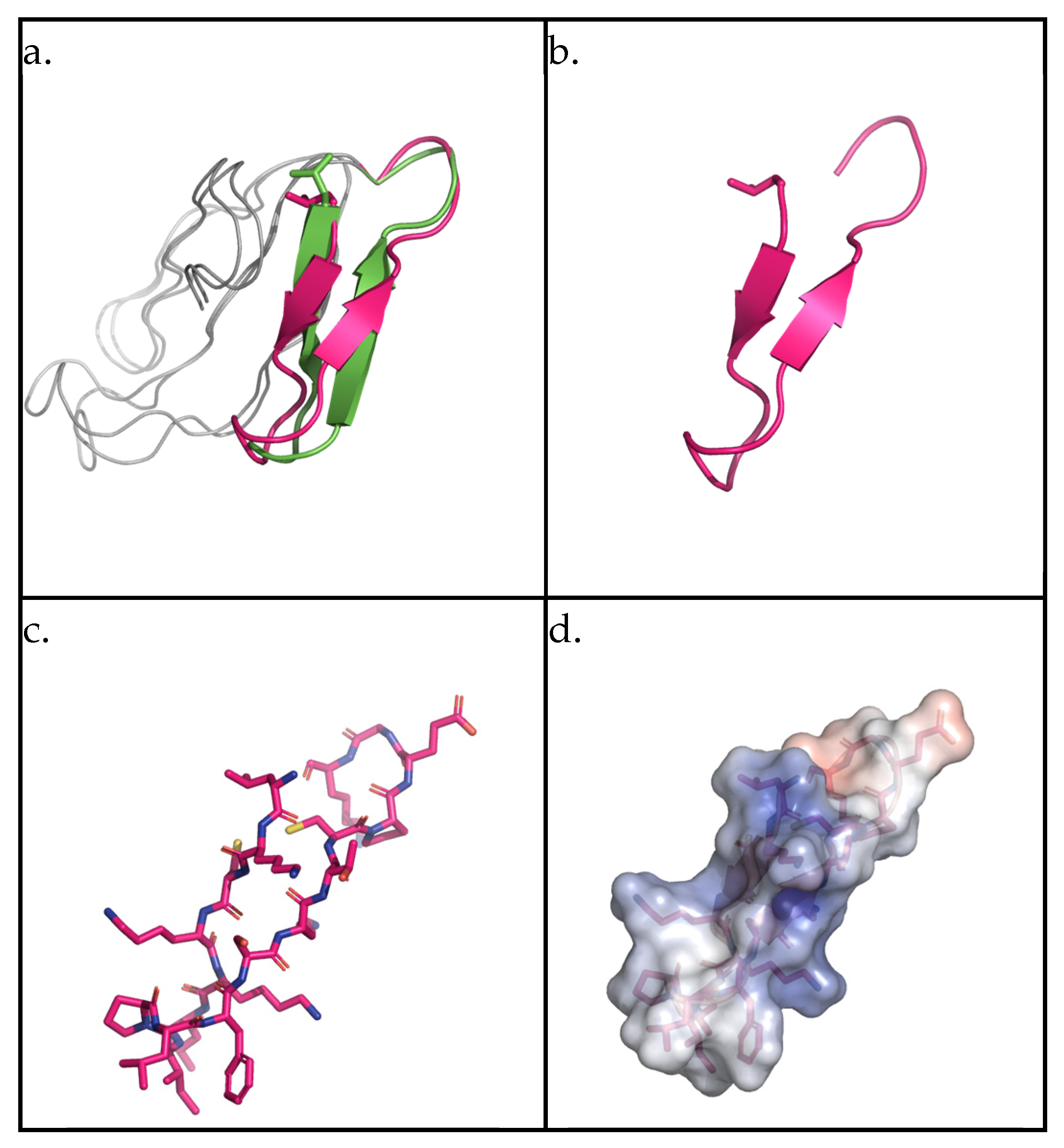
2.1. AMPEC4 Structural Characterization
2.2. AMPEC4 with Anti-Escherichia coli Activity
2.2.1. Minimum Inhibitory Concentration (MIC)
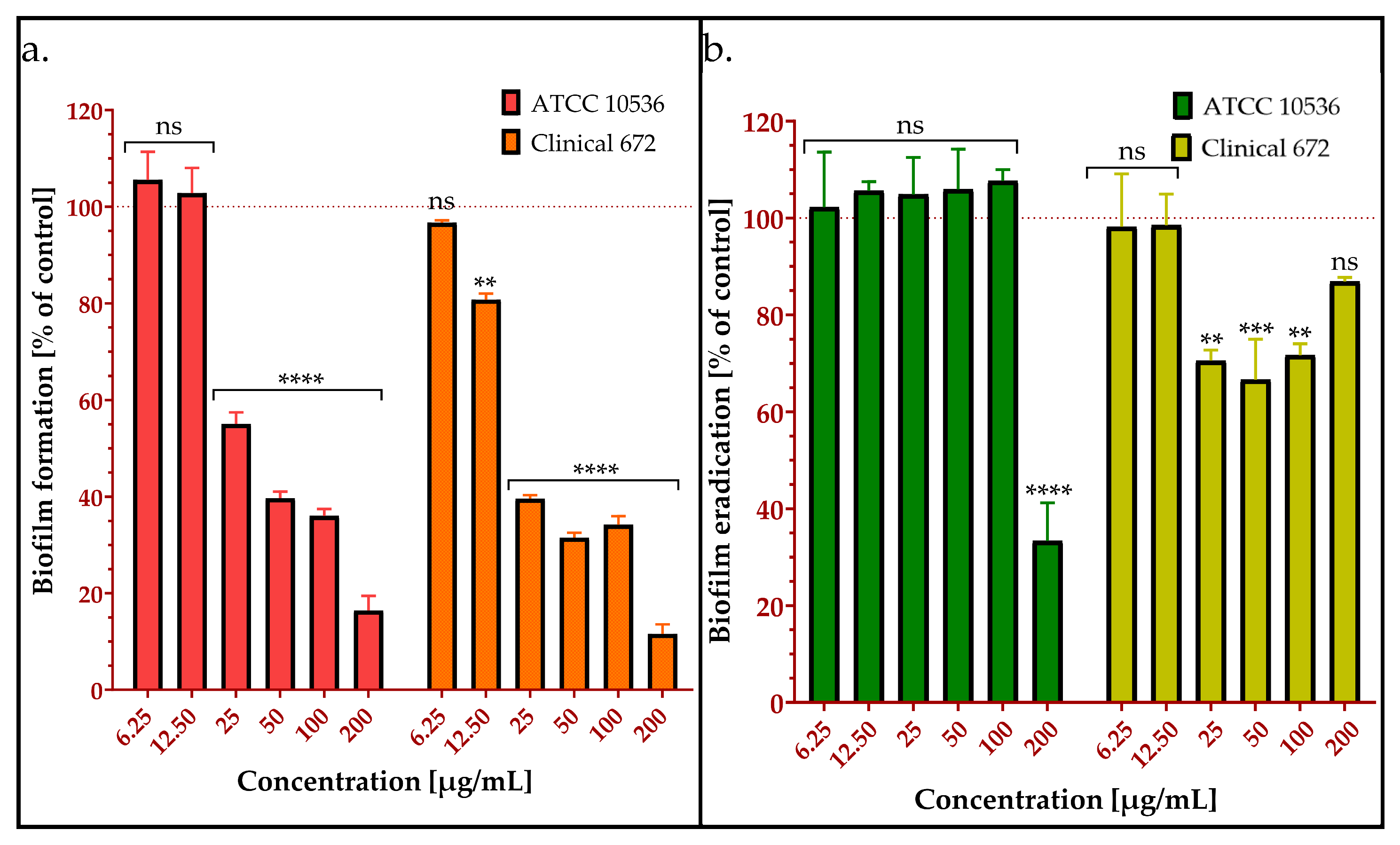
2.2.2. Inhibition of Biofilm Formation by AMPEC4
2.2.3. Biofilm Eradication by AMPEC4
2.2.4. AMPEC4 Increase E. coli Membrane Permeability
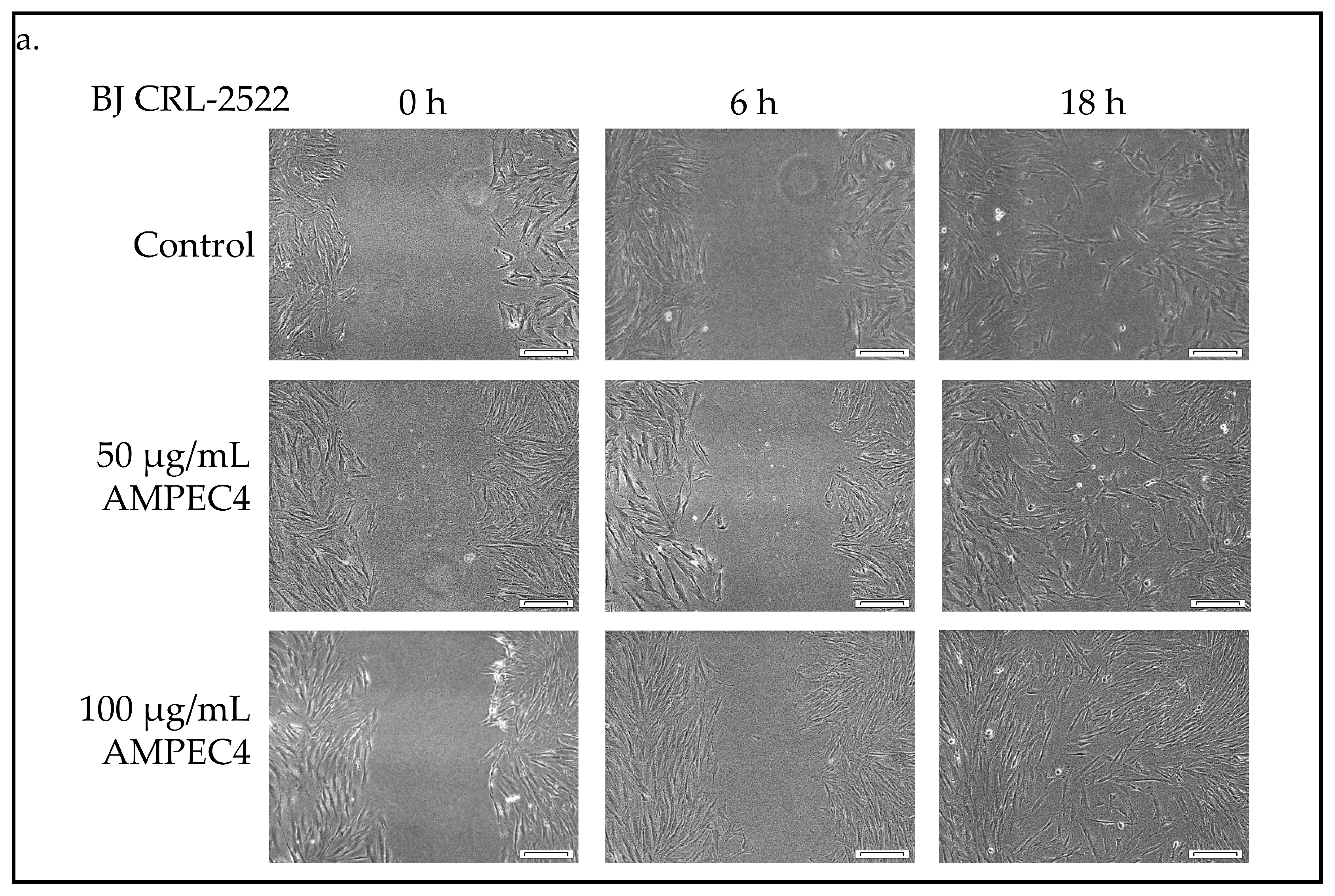
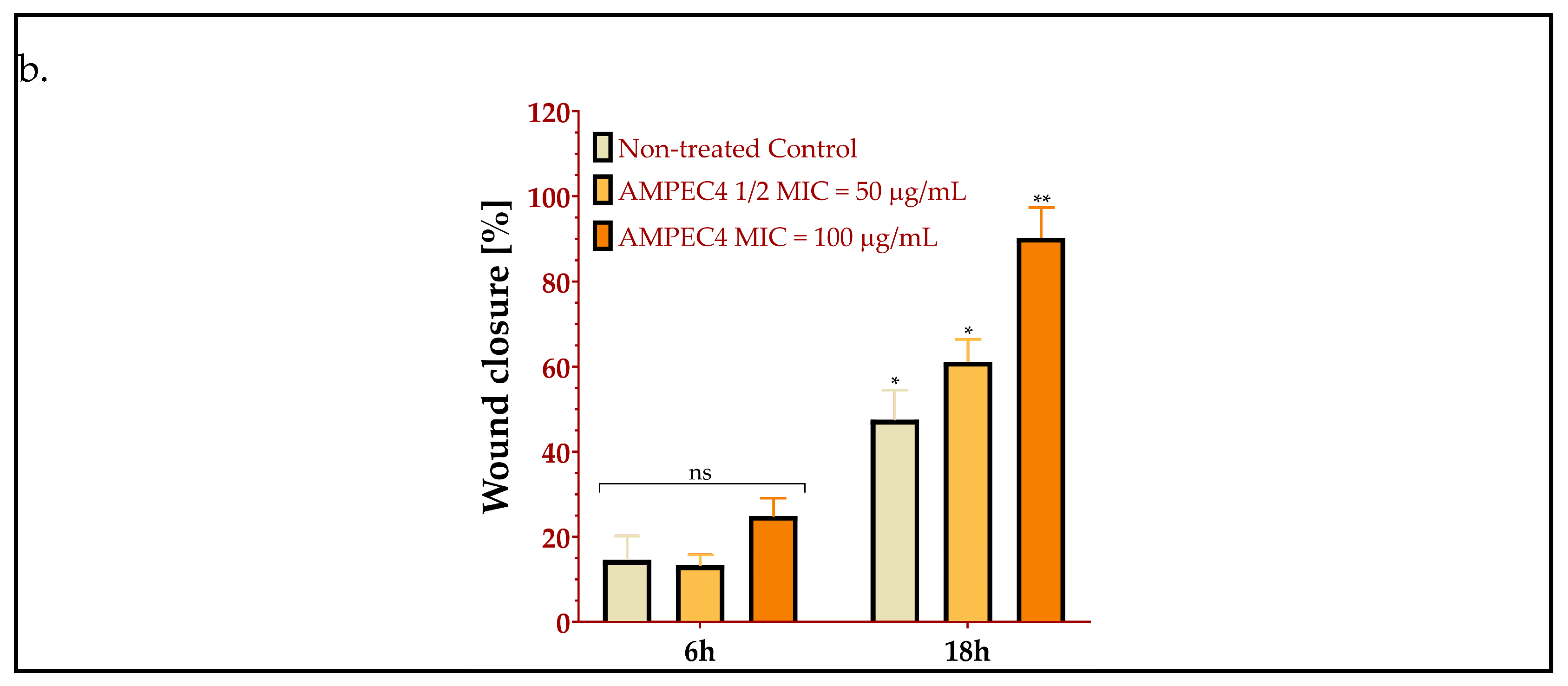
2.3. AMPEC4 Promotes the Migration of Fibroblasts
2.4. Biocompatibility of AMPEC4
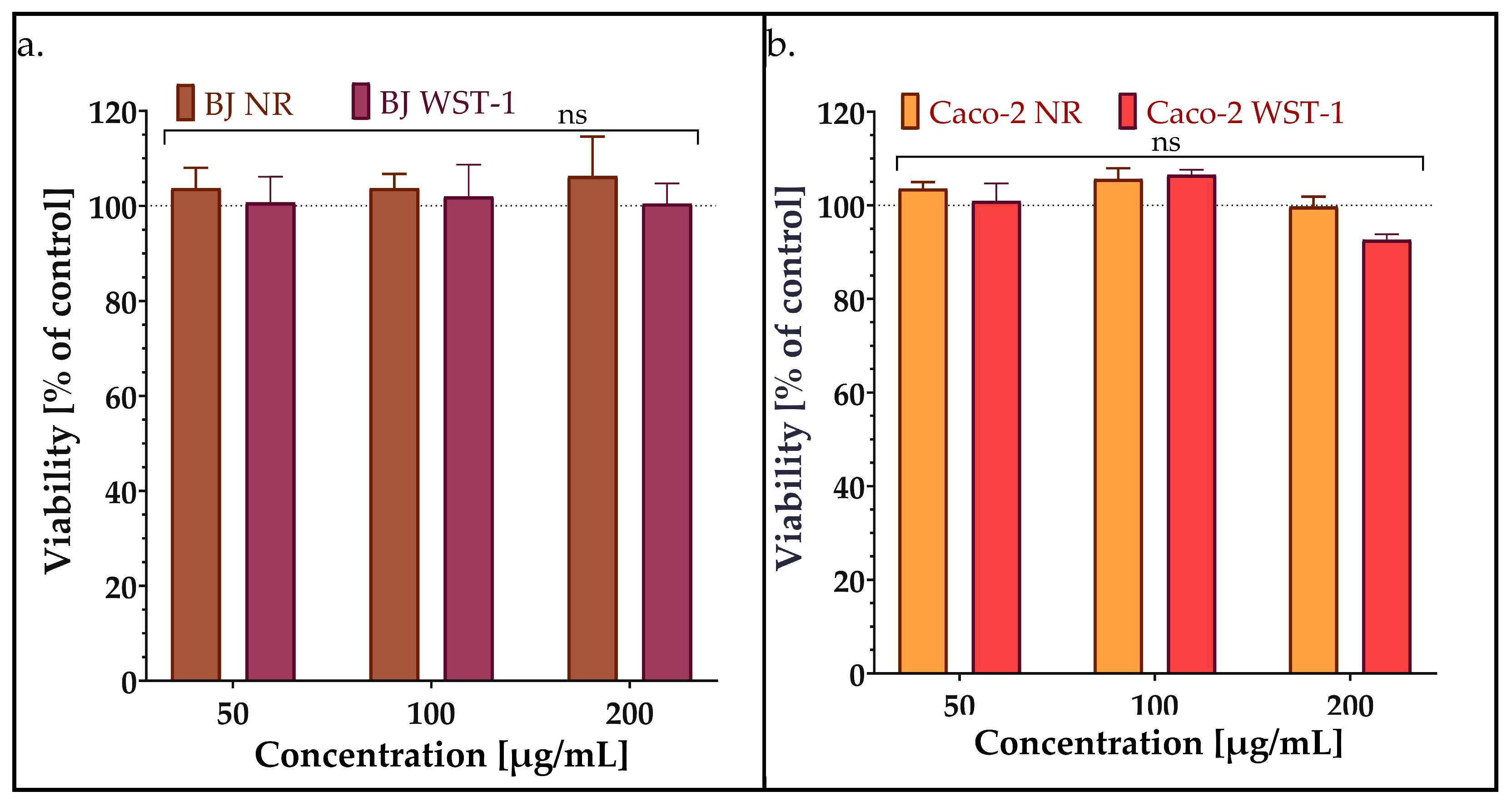
2.4.1. Viability/Cytotoxicity
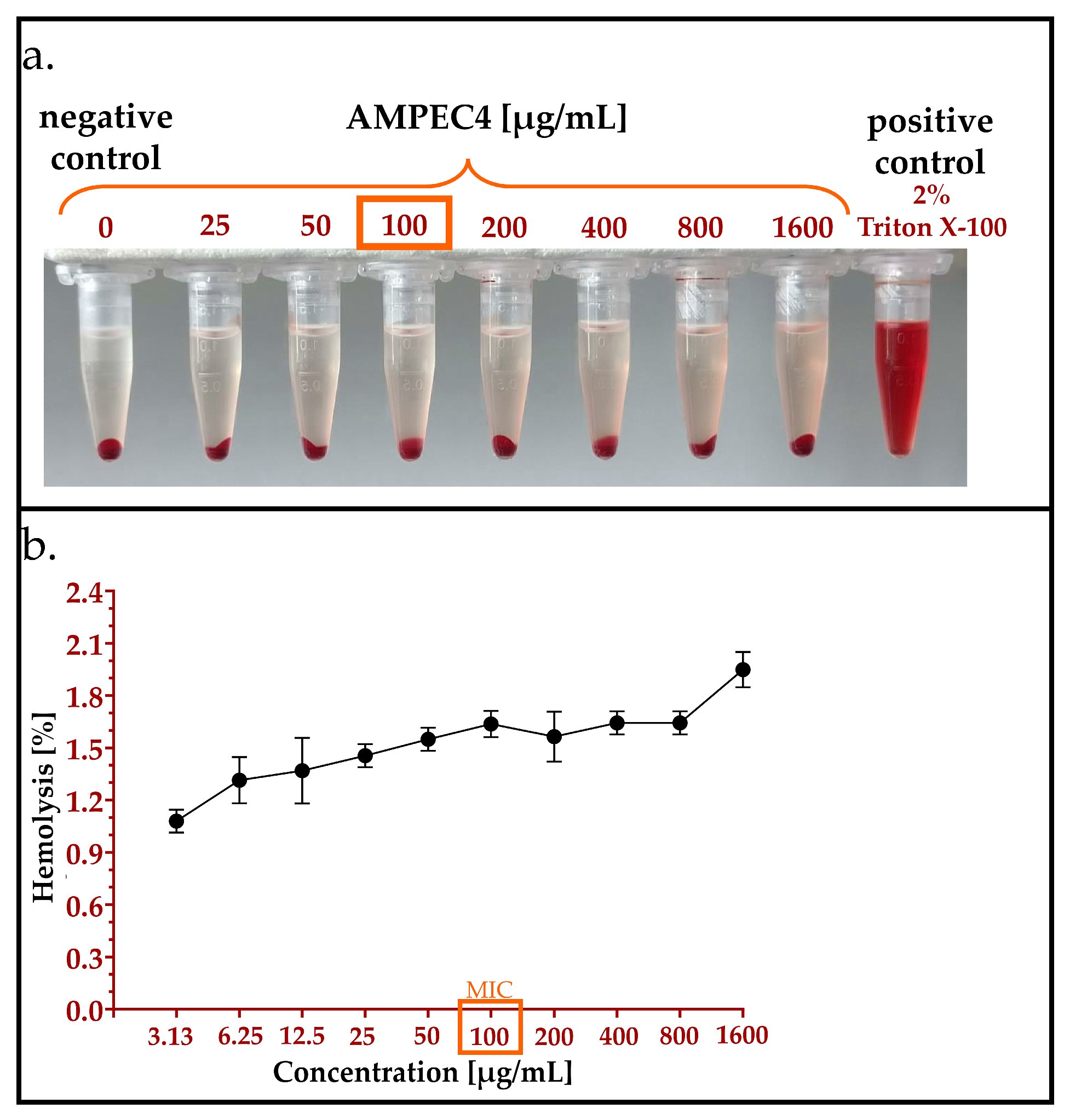
2.4.2. Hemolysis Assay
2.4.3. Genotoxicity Assay
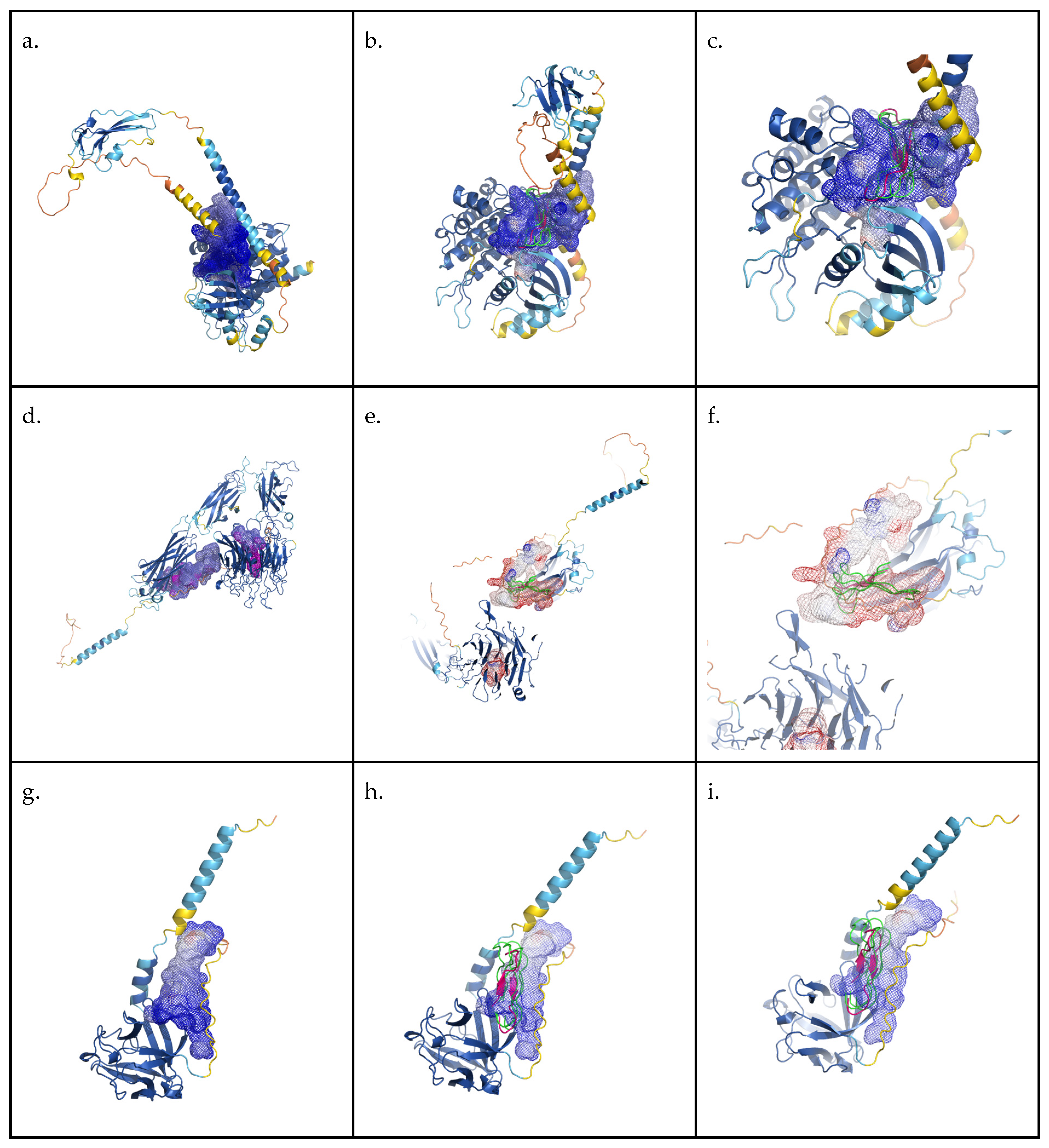
2.4.4. In Silico Binding Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. AMPEC4 Peptide Design
4.3. Structure Analysis and Visualization
4.4. AMPEC4 Peptide Synthesis
4.5. Antimicrobial Activity Testing
4.5.1. Determination of Minimum Inhibitory Concentration (MIC)
4.5.2. Inhibition of Biofilm Formation
4.5.3. Biofilm Eradication
4.5.4. Bacterial Membrane Permeabilization Assay
4.6. Cell Line Studies
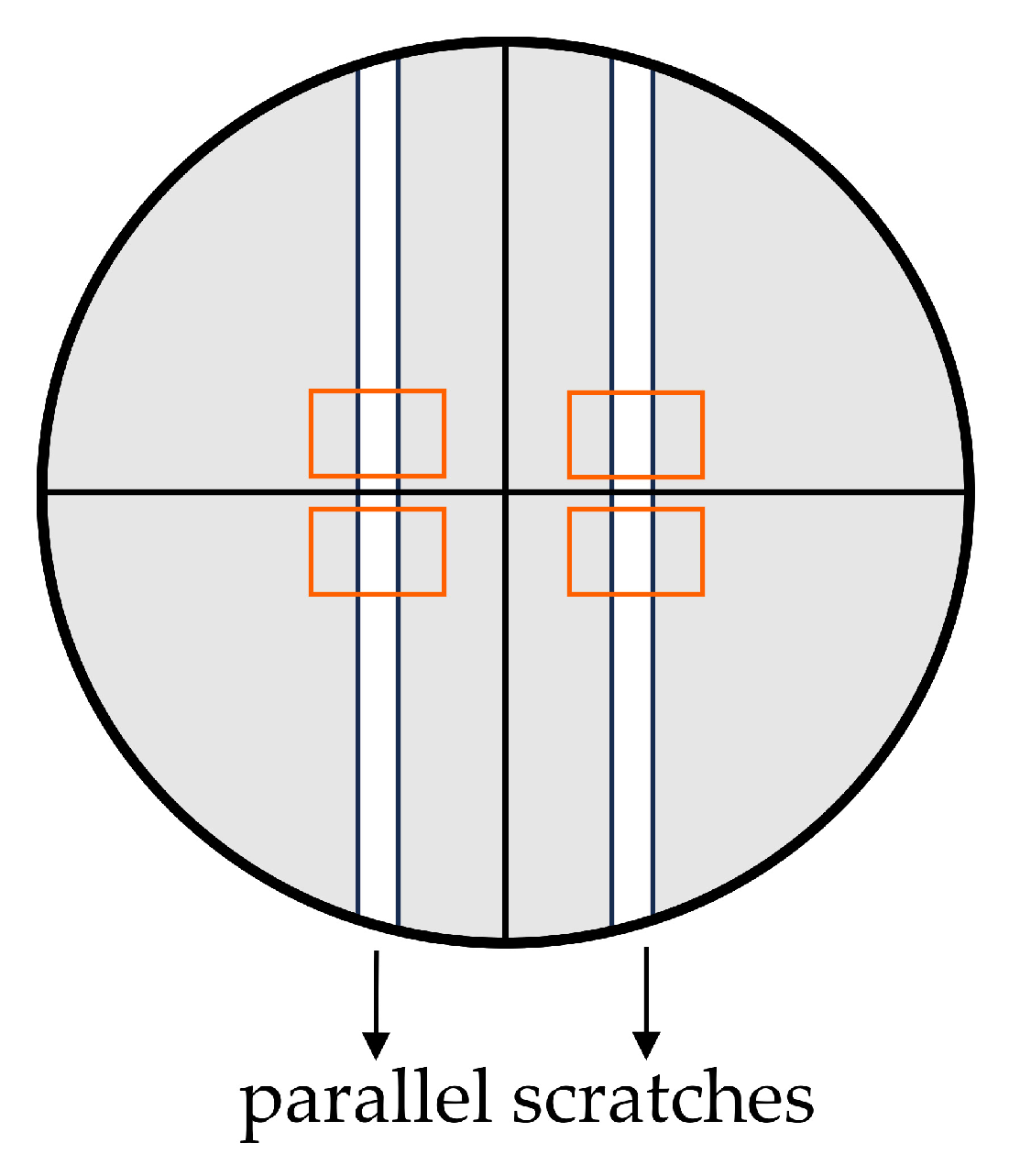
4.6.1. Scratch Assay
4.6.2. Biocompatibility Assay
Viability Assay
Hemolysis Assay
Genotoxicity
4.7. Docking Simulations
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The Chemistry of Snake Venom and Its Medicinal Potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake Venom Peptides: Tools of Biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, L.; Torres, M.D.T.; de la Fuente-Nunez, C. Biologically Active Peptides from Venoms: Applications in Antibiotic Resistance, Cancer, and Beyond. Int. J. Mol. Sci. 2022, 23, 15437. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Mendes, B.; Lancellotti, M.; Franchi, G.C.; Passos, Ó.; Ramos, M.J.; Fernandes, P.A.; Alves, C.; Vale, N.; Gomes, P.; et al. Lessons from a Single Amino Acid Substitution: Anticancer and Antibacterial Properties of Two Phospholipase A2-Derived Peptides. Curr. Issues Mol. Biol. 2022, 44, 46–62. [Google Scholar] [CrossRef]
- Lee, A.C.L.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Peña-Carrillo, M.S.; Pinos-Tamayo, E.A.; Mendes, B.; Domínguez-Borbor, C.; Proaño-Bolaños, C.; Miguel, D.C.; Almeida, J.R. Dissection of Phospholipases A2 Reveals Multifaceted Peptides Targeting Cancer Cells, Leishmania and Bacteria. Bioorg. Chem. 2021, 114, 105041. [Google Scholar] [CrossRef]
- Ali, N.; Shamoon, A.; Yadav, N.; Sharma, T. Peptide Combination Generator: A Tool for Generating Peptide Combinations. ACS Omega 2020, 5, 5781–5783. [Google Scholar] [CrossRef]
- Torrent, M.; Victoria Nogues, M.; Boix, E. Discovering New In Silico Tools for Antimicrobial Peptide Prediction. Curr. Drug Targets 2012, 13, 1148–1157. [Google Scholar] [CrossRef]
- Santamaría, C.; Larios, S.; Angulo, Y.; Pizarro-Cerda, J.; Gorvel, J.P.; Moreno, E.; Lomonte, B. Antimicrobial Activity of Myotoxic Phospholipases A2 from Crotalid Snake Venoms and Synthetic Peptide Variants Derived from Their C-Terminal Region. Toxicon 2005, 45, 807–815. [Google Scholar] [CrossRef]
- Bicho, G.F.H.; Nunes, L.O.C.; Fiametti, L.O.; Argentin, M.N.; Candido, V.T.; Camargo, I.L.B.C.; Cilli, E.M.; Santos-Filho, N.A. Synthesis, Characterization, and Study of the Antimicrobial Potential of Dimeric Peptides Derived from the C-Terminal Region of Lys49 Phospholipase A2 Homologs. Toxins 2024, 16, 308. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Tu, Q.; Zhang, Z.; Chen, M.; Mwangi, J.; Li, Y.; Jin, Y.; Zhao, X.; Lai, R. Targeting Surface Nucleolin Induces Autophagy-Dependent Cell Death in Pancreatic Cancer via AMPK Activation. Oncogene 2019, 38, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Bougis, P.; Tessier, M.; Van Rietschoten, J.; Rochat, H.; Faucon, J.F.; Dufourcq, J. Are Interactions with Phospholipids Responsible for Pharmacological Activities of Cardiotoxins? Mol. Cell Biochem. 1983, 55, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.Y.; Chiang, C.M.; Hseu, Y.C.; Vyas, A.A.; Rules, G.S.; Wu, W.G. Two Distinct Types of Cardiotoxin as Revealed by the Structure and Activity Relationship of Their Interaction with Zwitterionic Phospholipid Dispersions. J. Biol. Chem. 1994, 269, 14473–14483. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Kao, P.H.; Fu, Y.S.; Hu, W.P.; Chang, L. Sen Bactericidal Effect of Naja Nigricollis Toxin γ Is Related to Its Membrane-Damaging Activity. Peptides 2011, 32, 1755–1763. [Google Scholar] [CrossRef]
- Chen, L.W.; Kao, P.H.; Fu, Y.S.; Lin, S.R.; Chang, L. Sen Membrane-Damaging Activity of Taiwan Cobra Cardiotoxin 3 Is Responsible for Its Bactericidal Activity. Toxicon 2011, 58, 46–53. [Google Scholar] [CrossRef]
- Sanders, D.J.; Inniss, S.; Sebepos-Rogers, G.; Rahman, F.Z.; Smith, A.M. The Role of the Microbiome in Gastrointestinal Inflammation. Biosci. Rep. 2021, 41, BSR20203850. [Google Scholar] [CrossRef]
- Shogan, B.D.; Chen, J.; Duchalais, E.; Collins, D.; Chang, M.; Krull, K.; Krezalek, M.A.; Larson, D.W.; Walther-Antonio, M.R.; Chia, N.; et al. Alterations of the Rectal Microbiome Are Associated with the Development of Postoperative Ileus in Patients Undergoing Colorectal Surgery. J. Gastrointest. Surg. 2020, 24, 1663–1672. [Google Scholar] [CrossRef]
- Jaiswal, P.; Sharma, S.; Pratap, A.; Ansari, M.; Shukla, V.K.; Basu, S.; Banerjee, T. Significant Presence of Biofilm-Producing Gut-Derived Bacteria in Anal Fistula of Chronic Duration. Int. Wound J. 2021, 18, 519–524. [Google Scholar] [CrossRef]
- Kibret, A.A.; Oumer, M.; Moges, A.M. Prevalence and Associated Factors of Hemorrhoids among Adult Patients Visiting the Surgical Outpatient Department in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 2021, 16, e0249736. [Google Scholar] [CrossRef]
- Bender, F.; Eckerth, L.; Fritzenwanker, M.; Liese, J.; Askevold, I.; Imirzalioglu, C.; Padberg, W.; Hecker, A.; Reichert, M. Drug Resistant Bacteria in Perianal Abscesses Are Frequent and Relevant. Sci. Rep. 2022, 12, 14866. [Google Scholar] [CrossRef]
- Liang, Y.; Ren, T.; Li, R.; Yu, Z.; Wang, Y.; Zhang, X.; Qin, Z.; Li, J.; Hu, J.; Luo, C. Natural Products with Potential Effects on Hemorrhoids: A Review. Molecules 2024, 29, 12673. [Google Scholar] [CrossRef] [PubMed]
- Dowling Enez, V.E.; Izarra Henriquez, C.V. Anal Abscess Microbiology as an Anal Fistula Predictor. J. Coloproctology 2020, 40, 129–134. [Google Scholar] [CrossRef]
- Lessa, F.C.; Sievert, D.M. Antibiotic Resistance: A Global Problem and the Need to Do More. Clin. Infect. Dis. 2023, 77, S1–S3. [Google Scholar] [CrossRef]
- Dżugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Geevarghese, A.; Herman, I.M. Bioactive Peptides Derived from Vascular Endothelial Cell Extracellular Matrices Promote Microvascular Morphogenesis and Wound Healing in Vitro. Wound Repair Regen. 2011, 19, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, A.; Sakamoto, H.; Kuroda, J.; Kondo, Y.; Kamei, Y.; Nonaka, S.; Furukawa, S.; Yamamoto, S.; Satoh, A. Keratinocyte-Driven Dermal Collagen Formation in the Axolotl Skin. Nat. Commun. 2025, 16, 1757. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Mu, L.; Zhou, Y.; Chen, Y.; Wu, J.; Wang, Y.; Yang, H.; Li, M.; Xu, W.; et al. A Frog-Derived Immunomodulatory Peptide Promotes Cutaneous Wound Healing by Regulating Cellular Response. Front. Immunol. 2019, 10, 2421. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kim, H.; Kwak, G.; Yoon, H.Y.; Jo, S.D.; Lee, J.E.; Cho, D.; Kwon, I.C.; Kim, S.H. Development of Biocompatible HA Hydrogels Embedded with a New Synthetic Peptide Promoting Cellular Migration for Advanced Wound Care Management. Adv. Sci. 2018, 5, 1800852. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Z.; Zhao, Y.; Zou, Z.; Lu, S.; Zhang, B.; Li, S. Sponges of Carboxymethyl Chitosan Grafted with Collagen Peptides for Wound Healing. Int. J. Mol. Sci. 2019, 20, 3890. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legáth, Ľ.; Legáth, J. Antimicrobial Activity of Protein Fraction from Naja Ashei Venom against Staphylococcus Epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef]
- Bachem. Available online: https://www.bachem.com/knowledge-center/peptide-calculator/ (accessed on 18 March 2025).
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Zapała, L.; Ciszkowicz, E.; Kosińska-Pezda, M.; Maciołek, U.; Kozioł, A.E.; Miłoś, A.; Woźnicka, E.; Bocian, A.; Zapała, W.; Rydel-Ciszek, K.; et al. Novel Silver(I) Complexes with Fenamates: Insights into Synthesis, Spectral Characterization, and Bioactivity. J. Inorg. Biochem. 2025, 266, 112846. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining Conditions for Biofilm Inhibition and Eradication Assays for Gram-Positive Clinical Reference Strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium Iodide Staining Underestimates Viability of Adherent Bacterial Cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef]
- Bridges, D.F.; Lacombe, A.; Wu, V.C.H. Integrity of the Escherichia Coli O157:H7 Cell Wall and Membranes After Chlorine Dioxide Treatment. Front. Microbiol. 2020, 11, 888. [Google Scholar] [CrossRef]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. OpenComet: An Automated Tool for Comet Assay Image Analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The Comet Assay: A Method to Measure DNA Damage in Individual Cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res 2024, 52, D368–D375. [Google Scholar] [CrossRef]
- Steinkellner, G.; Rader, R.; Thallinger, G.G.; Kratky, C.; Gruber, K. VASCo: Computation and Visualization of Annotated Protein Surface Contacts. BMC Bioinform. 2009, 10, 32. [Google Scholar] [CrossRef]
- Hendlich, M.; Rippmann, F.; Barnickel, G. LIGSITE: Automatic and Efficient Detection of Potential Small Molecule-Binding Sites in Proteins. J. Mol. Graph. Model. 1997, 15, 359–363. [Google Scholar] [CrossRef]
- PyMOL. The PyMOL Molecular Graphics System; Version 3.0; Schrödinger, LLC.: New York, NY, USA, 2024. [Google Scholar]
- Hus, K.K.; Buczkowicz, J.; Petrilla, V.; Petrillová, M.; Łyskowski, A.; Legáth, J.; Bocian, A. First Look at the Venom of Naja Ashei. Molecules 2018, 23, 609. [Google Scholar] [CrossRef] [PubMed]
- Hus, K.K.; Marczak, Ł.; Petrilla, V.; Petrillová, M.; Legáth, J.; Bocian, A. Different Research Approaches in Unraveling the Venom Proteome of Naja Ashei. Biomolecules 2020, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.H.; Lin, S.R.; Chang, L. Sen Differential Binding to Phospholipid Bilayers Modulates Membrane-Damaging Activity of Naja Naja Atra Cardiotoxins. Toxicon 2009, 54, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Dyba, B.; Rudolphi-Szydło, E.; Barbasz, A.; Czyżowska, A.; Hus, K.K.; Petrilla, V.; Petrillová, M.; Legáth, J.; Bocian, A. Effects of 3ftx Protein Fraction from Naja Ashei Venom on the Model and Native Membranes: Recognition and Implications for the Mechanisms of Toxicity. Molecules 2021, 26, 2164. [Google Scholar] [CrossRef]
- Dyba, B.; Rudolphi-Szydło, E.; Kreczmer, B.; Barbasz, A.; Petrilla, V.; Petrillova, M.; Legáth, J.; Bocian, A.; Hus, K.K. Exploring the Effects of Three-Finger Toxins from Naja Ashei Venom on Neuronal and Immunological Cancer Cell Membranes. Sci. Rep. 2024, 14, 18570. [Google Scholar] [CrossRef]
- Fanelli, G.; Pasqua, M.; Prosseda, G.; Grossi, M.; Colonna, B. AcrAB Efflux Pump Impacts on the Survival of Adherent-Invasive Escherichia Coli Strain LF82 inside Macrophages. Sci. Rep. 2023, 13, 2692. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Stone, T.A.; Deber, C.M. Peptide-Based Efflux Pump Inhibitors of the Small Multidrug Resistance Protein from Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2019, 63, 1–10. [Google Scholar] [CrossRef]
- Sanchez-Carbonel, A.; Mondragón, B.; López-Chegne, N.; Peña-Tuesta, I.; Huayan-Dávila, G.; Blitchtein, D.; Carrillo-Ng, H.; Silva-Caso, W.; Aguilar-Luis, M.A.; del Valle-Mendoza, J. The Effect of the Efflux Pump Inhibitor Carbonyl Cyanide M-Chlorophenylhydrazone (CCCP) on the Susceptibility to Imipenem and Cefepime in Clinical Strains of Acinetobacter Baumannii. PLoS ONE 2021, 16, e0259915. [Google Scholar] [CrossRef]
- Huttunen, J.; Gynther, M.; Huttunen, K.M. Targeted Efflux Transporter Inhibitors—A Solution to Improve Poor Cellular Accumulation of Anti-Cancer Agents. Int. J. Pharm. 2018, 550, 278–289. [Google Scholar] [CrossRef]
- Fletcher, J.E.; Jiang, M.S. Possible Mechanisms of Action of Cobra Snake venom cardiotoxins and bee venom melittin. Toxicon 1993, 31, 669–695. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. The Myth of Cobra Venom Cytotoxin: More than Just Direct Cytolytic Actions. Toxicon X 2022, 14, 100123. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Bocian, A.; Petrilla, V.; Adamczyk-Grochala, J.; Szymura, K.; Hendzel, W.; Kaleniuk, E.; Hus, K.K.; Petrillova, M.; Wnuk, M. Snake Venoms Promote Stress-Induced Senescence in Human Fibroblasts. J. Cell Physiol. 2019, 234, 6147–6160. [Google Scholar] [CrossRef] [PubMed]
- Offor, B.C.; Piater, L.A. Snake Venom Toxins: Potential Anticancer Therapeutics. J. Appl. Toxicol. 2024, 44, 666–685. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nature 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Ngamwongsatit, P.; Banada, P.P.; Panbangred, W.; Bhunia, A.K. WST-1-Based Cell Cytotoxicity Assay as a Substitute for MTT-Based Assay for Rapid Detection of Toxigenic Bacillus Species Using CHO Cell Line. J. Microbiol. Methods 2008, 73, 211–215. [Google Scholar] [CrossRef]
- Nemeth, J.; Ledergerber, B.; Preiswerk, B.; Nobile, A.; Karrer, S.; Ruef, C.; Kuster, S.P. Multidrug-Resistant Bacteria in Travellers Hospitalized Abroad: Prevalence, Characteristics, and Influence on Clinical Outcome. J. Hosp. Infect. 2012, 82, 254–259. [Google Scholar] [CrossRef]
- Chen, C.R.; Makhatadze, G.I. ProteinVolume: Calculating Molecular van Der Waals and Void Volumes in Proteins. BMC Bioinform. 2015, 16, 101. [Google Scholar] [CrossRef]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Miłoś, A.; Jenkins, H.; Ślusarczyk, L.; Matwijczuk, A. Design, Spectroscopy, and Assessment of Cholinesterase Inhibition and Antimicrobial Activities of Novel Coumarin–Thiadiazole Hybrids. Int. J. Mol. Sci. 2022, 23, 6314. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Sidor, E.; Hęclik, J.; Lecka-Szlachta, K.; Dżugan, M. The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods. Antibiotics 2023, 12, 235. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-Szlachta, K.; Pasternakiewicz, A.; Dżugan, M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules 2022, 27, 725. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar] [CrossRef]
- Wendland, R.J.; Conway, M.T.; Worthington, K.S. Evaluating the Polymerization Effectiveness and Biocompatibility of Bio-Sourced, Visible Light-Based Photoinitiator Systems. J. Biomed. Mater. Res. A 2024, 112, 1662–1674. [Google Scholar] [CrossRef] [PubMed]
- Sroka, M.; Zaborniak, I.; Chmielarz, P.; Bała, J.; Wolski, K.; Ciszkowicz, E.; Awsiuk, K.; Raczkowska, J. Grafting of Multifunctional Polymer Brushes from a Glass Surface: Surface-Initiated Atom Transfer Radical Polymerization as a Versatile Tool for Biomedical Materials Engineering. Macromol. Chem. Phys. 2024, 225, 2300284. [Google Scholar] [CrossRef]
- Klamut, M.; Zaborniak, I.; Bałbustyn, J.; Niemiec, M.; Ciszkowicz, E.; Błoniarz, P.; Chmielarz, P. Precise Tailoring of Thermoresponsive Characteristics: Revealing ATRP Opportunities for Controlled Poly(Ethylene Glycol)-Based Monomers Composition in Cyclodextrin-Containing Polymers. Polymer 2024, 312, 127645. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Gołacki, K.; Kamysz, W. Ultrashort Cationic Lipopeptides—Effect of Antimicrobial Activity and Hemolysis. Molecules 2020, 25, 257. [Google Scholar] [CrossRef]
- Kamysz, E.; Sikorska, E.; Bauer, M.; Sikora, K.; Neubauer, D. Influence of Lipidation Pattern of the KR12 Fragment of Peptide LL-37 on Its Antibacterial and Hemolytic Activities. Int. J. Mol. Sci. 2023, 24, 5505. [Google Scholar] [CrossRef]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA Modifications with the Comet Assay: A Compendium of Protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef]
- Roy, I.; Nadar, P.; Khurana, S. Neutral Comet Assay to Detect and Quantitate DNA Double Strand Breaks in Hematopoietic Stem Cells. Bio Protoc. 2021, 11, e4130. [Google Scholar] [CrossRef]
- Ji, Y.; Karbaschi, M.; Abdulwahed, A.; Quinete, N.S.; Evans, M.D.; Cooke, M.S. A High-Throughput Comet Assay Approach for Assessing Cellular DNA Damage. J. Vis. Exp. 2022, 2022, 63559. [Google Scholar] [CrossRef]
- Costea, M.A.; Rosan, C.A.; Laslo, V.; Agud, E.; Purcarea, C.; Vicas, S.I. The Comet Assay as a Sustainable Method for Evaluating the Genotoxicity Caused by the Soluble Fraction Derived from Sewage Sludge on Diverse Cell Types, Including Lymphocytes, Coelomocytes and Allium Cepa L. Cells. Sustainability 2024, 16, 457. [Google Scholar] [CrossRef]
- Honorato, R.V.; Trellet, M.E.; Jiménez-García, B.; Schaarschmidt, J.J.; Giulini, M.; Reys, V.; Koukos, P.I.; Rodrigues, J.P.; Karaca, E.; van Zundert, G.C.; et al. The HADDOCK2.4 Web Server for Integrative Modeling of Biomolecular Complexes. Nat. Protoc. 2024, 19, 3219–3241. [Google Scholar] [CrossRef]


| Bacterial Strains | Reference | Clinical | ||
|---|---|---|---|---|
| ATCC 10536 | 325 | 665 | 672 | |
| AMPEC4 | 100.0 | 200.0 | 100.0 | 200.0 |
| Ampicillin | 31.3 | 62.5 | NAA 1 | NAA 1 |
| Ciprofloxacin | 0.5 | 0.25 | 0.25 | 0.5 |
| Chloramphenicol | 1.0 | 3.9 | 3.9 | 3.9 |
| Molecular Target | Cavity Volume (Å3) |
|---|---|
| P21802 # | 26,172 |
| P11362 # | 23,015 |
| P22455 # | 21,638 |
| P06756 * | 18,607 |
| P22607 # | 15,623 |
| P05106 * | 11,377 |
| P42702 # | 7466 |
| P22455 # | 7209 |
| P21802 # | 5469 |
| Q13873 # | 4800 |
| P22607 # | 4350 |
| P36894 # | 3773 |
| P06756 * | 3411 |
| Q13873 # | 2976 |
| Q13873 # | 2460 |
| Q13873 # | 2376 |
| O76093 $ | 2371 |
| Q13873 # | 2354 |
| P42702 # | 2194 |
| P22607 # | 2184 |
| Q13873 # | 2103 |
| UniProt ID | Cavity Volume (Å3) | HADDOCK Score | Z-Score |
|---|---|---|---|
| P36894 | 3773 | −51.2 ± 8.9 | −1.7 |
| P06756 | 3411 | −89.9 ± 11.0 | −1.7 |
| O76093 | 2371 | −95.7 ± 10.1 | −1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciszkowicz, E.; Miłoś, A.; Łyskowski, A.; Buczkowicz, J.; Nieczaj, A.; Lecka-Szlachta, K.; Hus, K.K.; Sikora, K.; Neubauer, D.; Bauer, M.; et al. AMPEC4: Naja ashei Venom-Derived Peptide as a Stimulator of Fibroblast Migration with Antibacterial Activity. Molecules 2025, 30, 2167. https://doi.org/10.3390/molecules30102167
Ciszkowicz E, Miłoś A, Łyskowski A, Buczkowicz J, Nieczaj A, Lecka-Szlachta K, Hus KK, Sikora K, Neubauer D, Bauer M, et al. AMPEC4: Naja ashei Venom-Derived Peptide as a Stimulator of Fibroblast Migration with Antibacterial Activity. Molecules. 2025; 30(10):2167. https://doi.org/10.3390/molecules30102167
Chicago/Turabian StyleCiszkowicz, Ewa, Anna Miłoś, Andrzej Łyskowski, Justyna Buczkowicz, Anna Nieczaj, Katarzyna Lecka-Szlachta, Konrad K. Hus, Karol Sikora, Damian Neubauer, Marta Bauer, and et al. 2025. "AMPEC4: Naja ashei Venom-Derived Peptide as a Stimulator of Fibroblast Migration with Antibacterial Activity" Molecules 30, no. 10: 2167. https://doi.org/10.3390/molecules30102167
APA StyleCiszkowicz, E., Miłoś, A., Łyskowski, A., Buczkowicz, J., Nieczaj, A., Lecka-Szlachta, K., Hus, K. K., Sikora, K., Neubauer, D., Bauer, M., Kamysz, W., & Bocian, A. (2025). AMPEC4: Naja ashei Venom-Derived Peptide as a Stimulator of Fibroblast Migration with Antibacterial Activity. Molecules, 30(10), 2167. https://doi.org/10.3390/molecules30102167










