A New [PMo12O40]3−-Based NiII Compound: Electrochemical and Photocatalytic Properties for Water Pollutant Removal
Abstract
1. Introduction
2. Results and Discussion
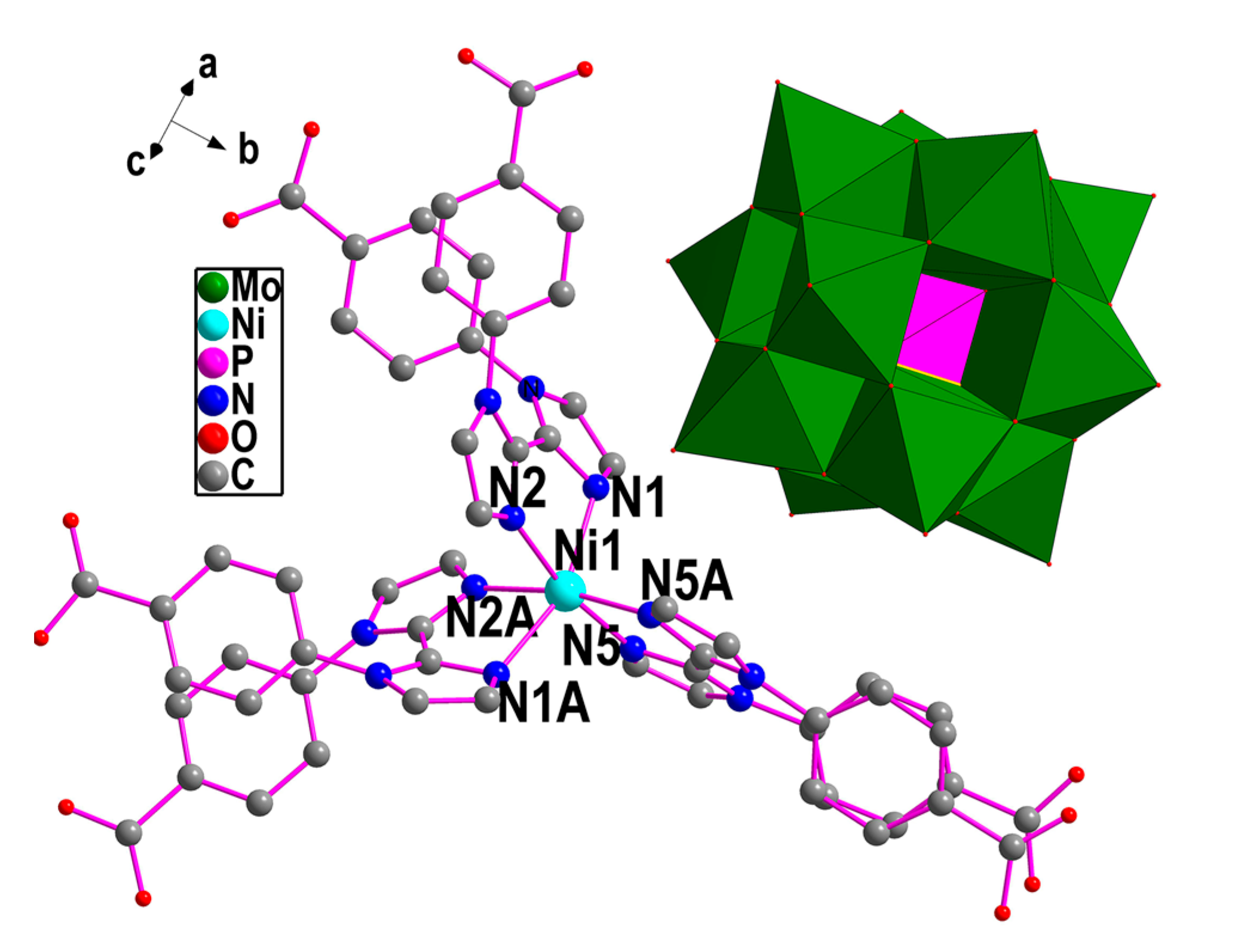
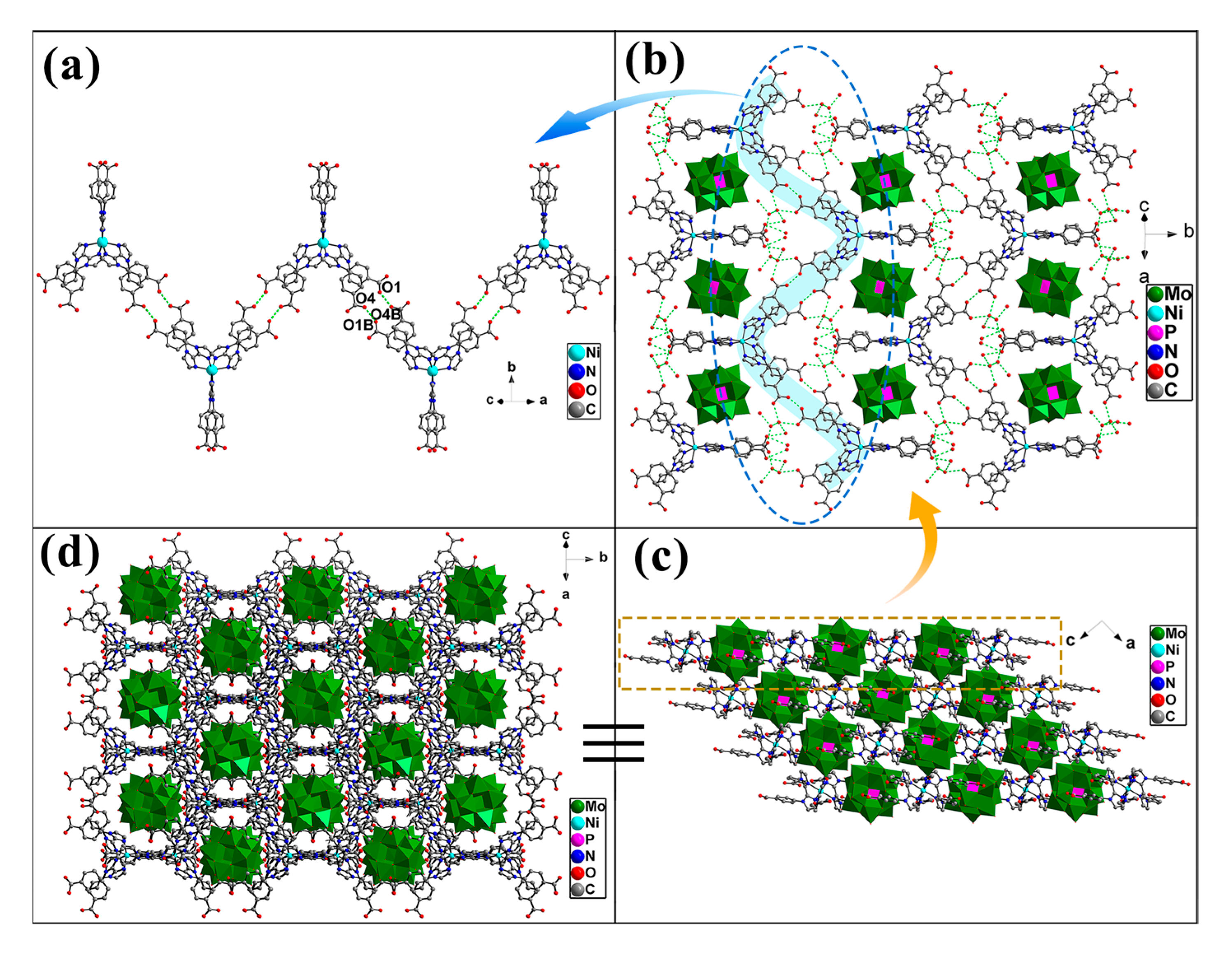
2.1. Crystal Structure of Compound 1
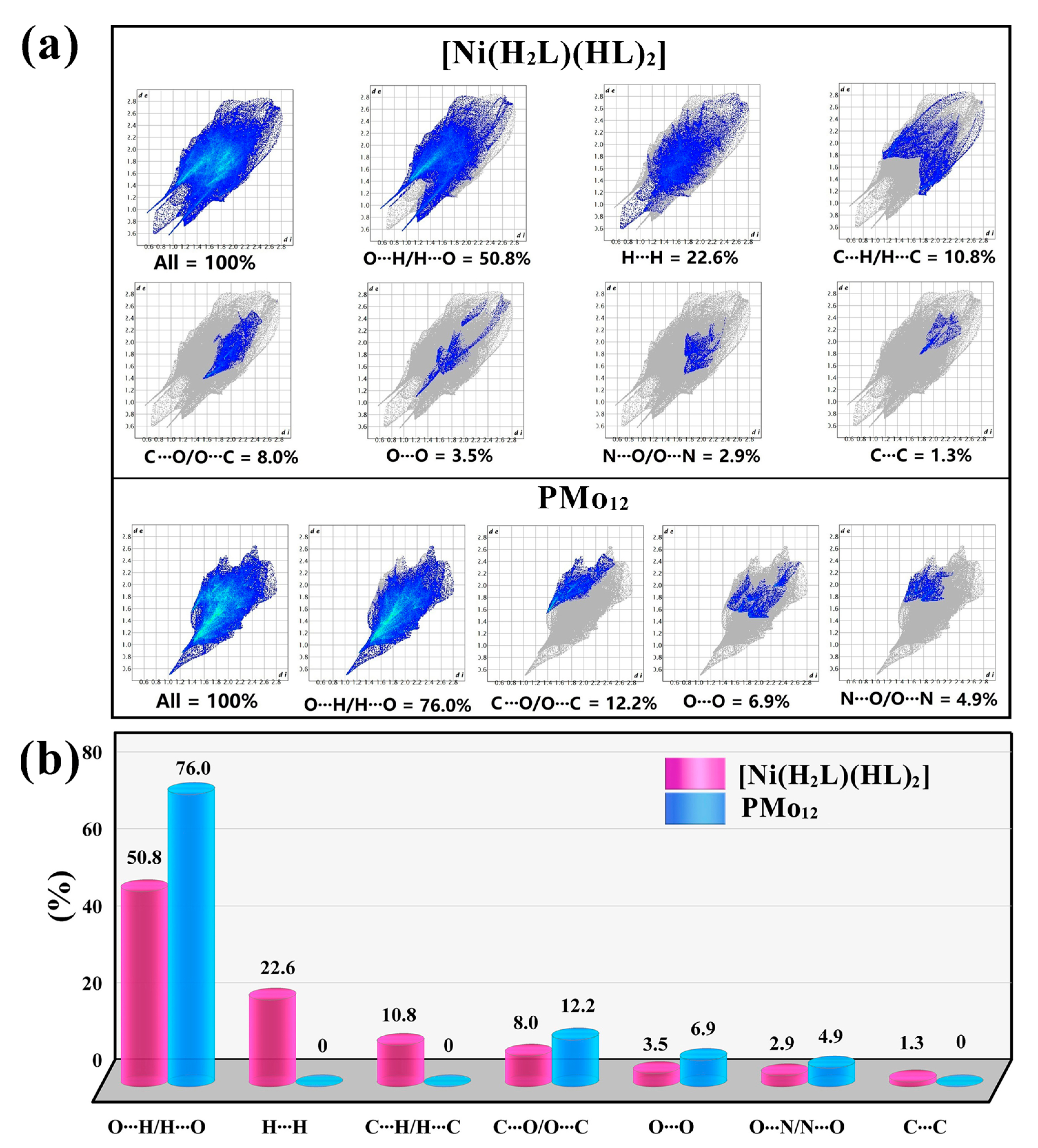
2.2. Hirshfeld Surface Analysis
2.3. Powder X-Ray Diffraction and FT-IR Spectra
2.4. TG Analyses
2.5. Electrochemical Properties
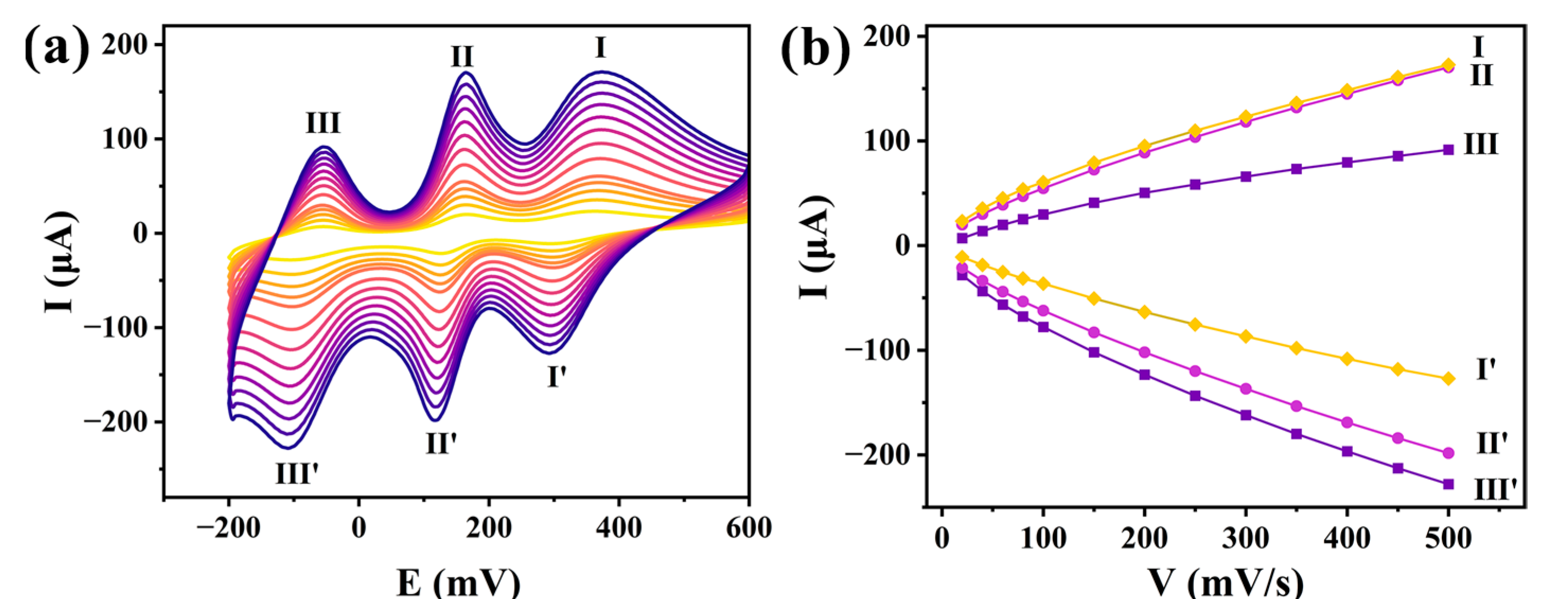
2.5.1. Cyclic Voltammetric Behaviors
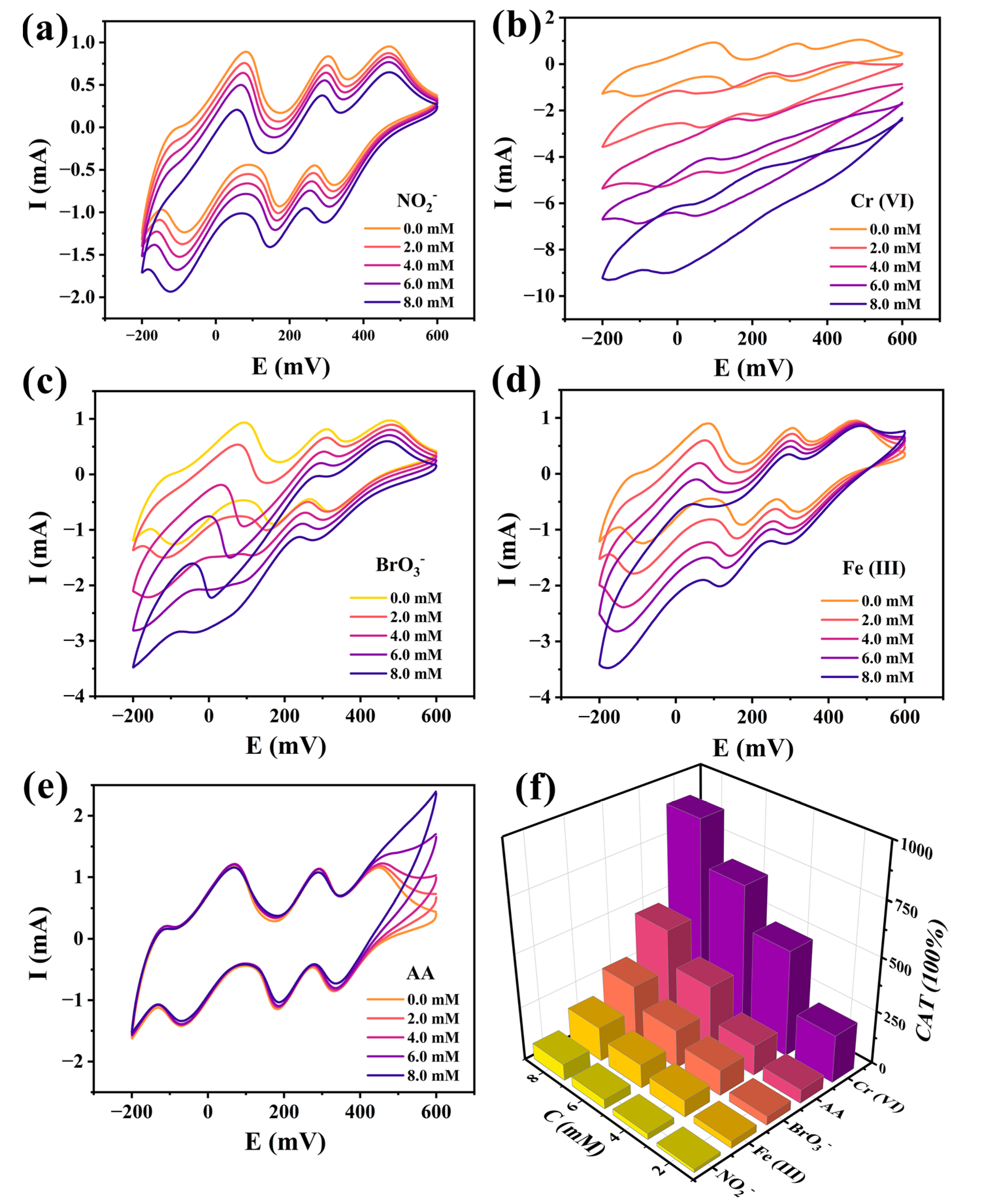
2.5.2. Electrocatalytic Properties
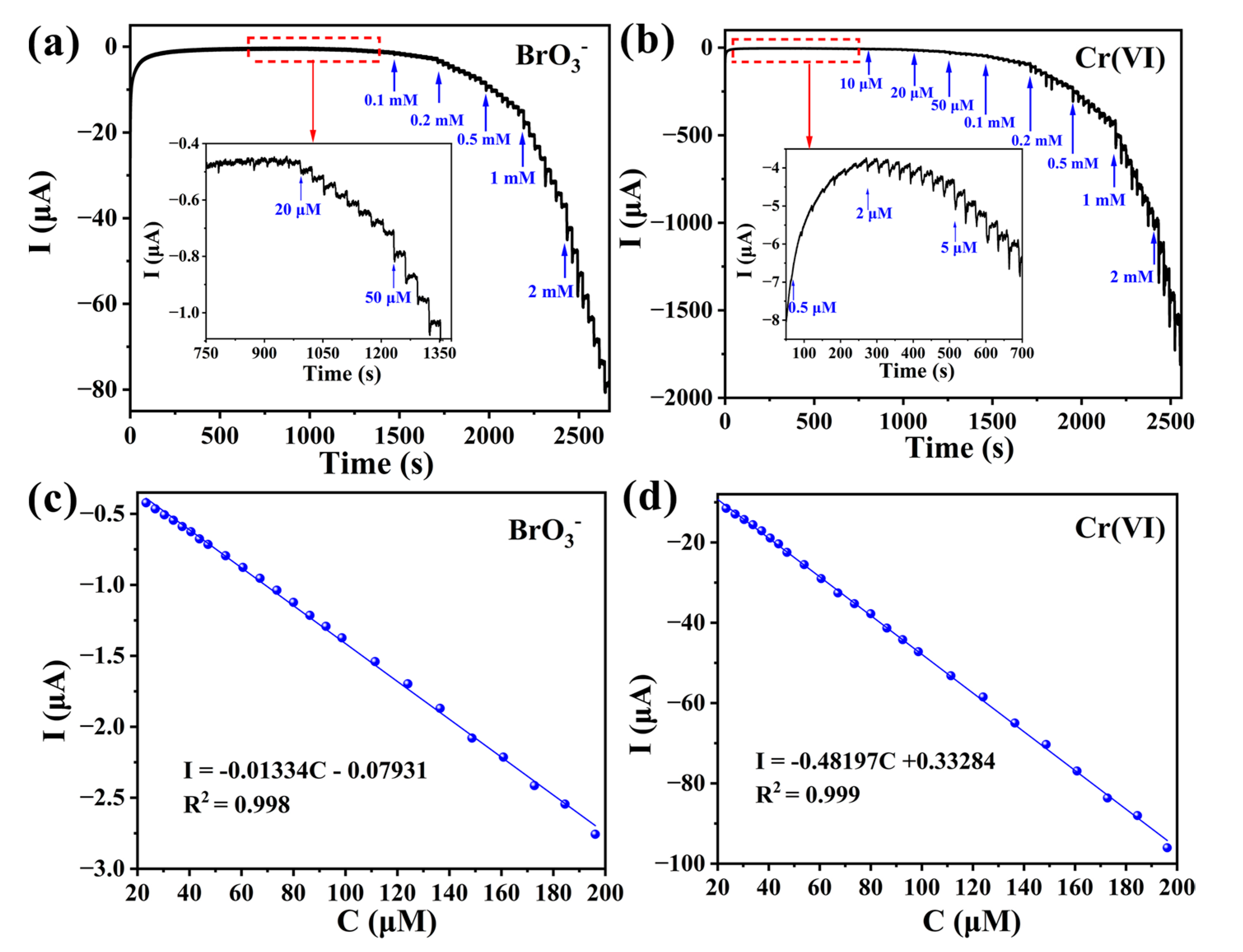
2.5.3. Electrochemical Sensing Activities
2.6. Photocatalytic Performance
2.6.1. Optical Band Gap
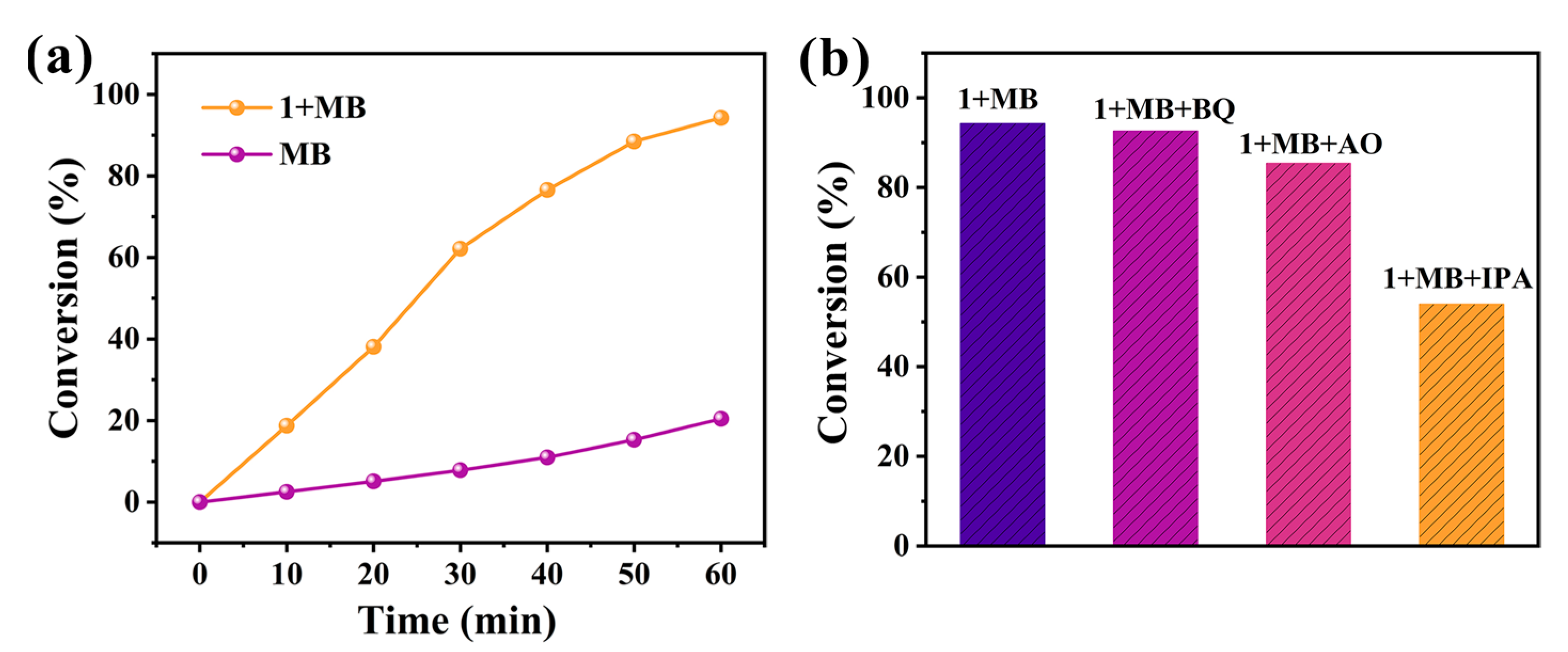
2.6.2. Photocatalytic Degradation of MB
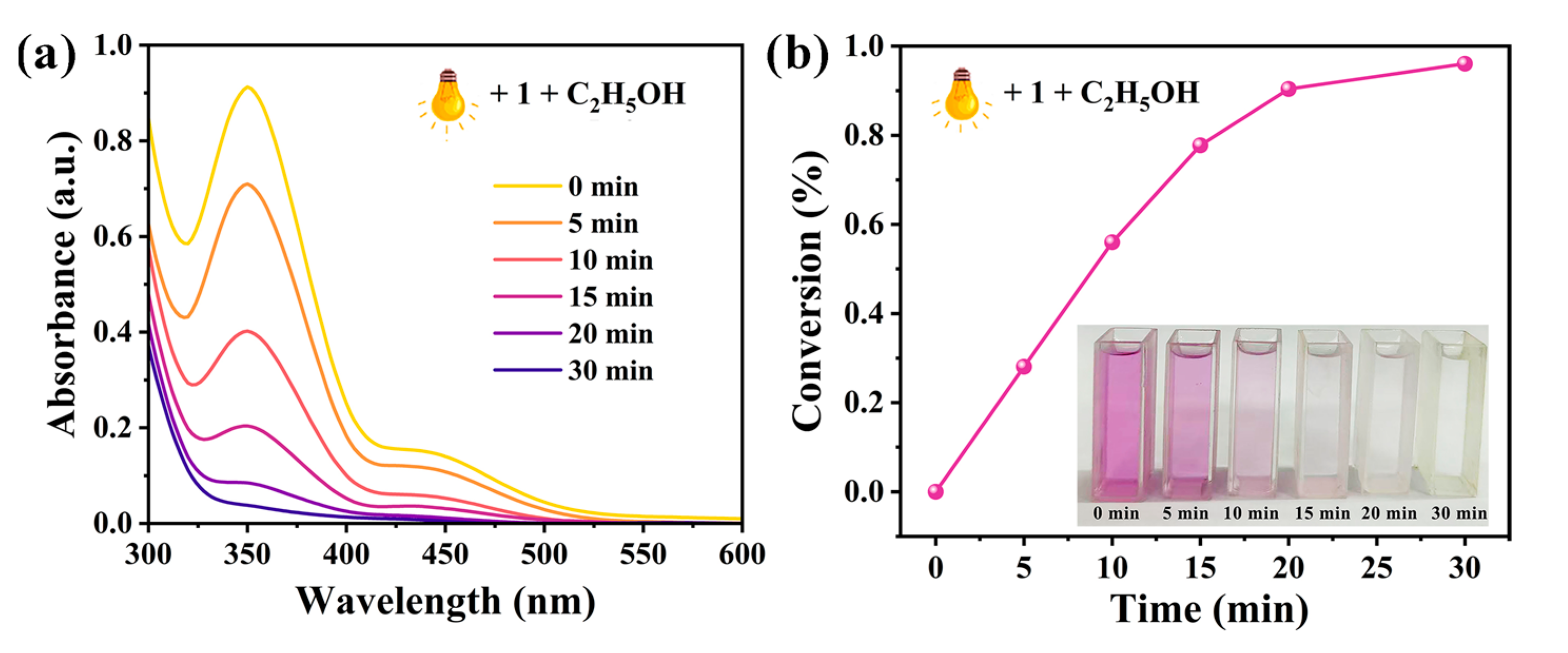
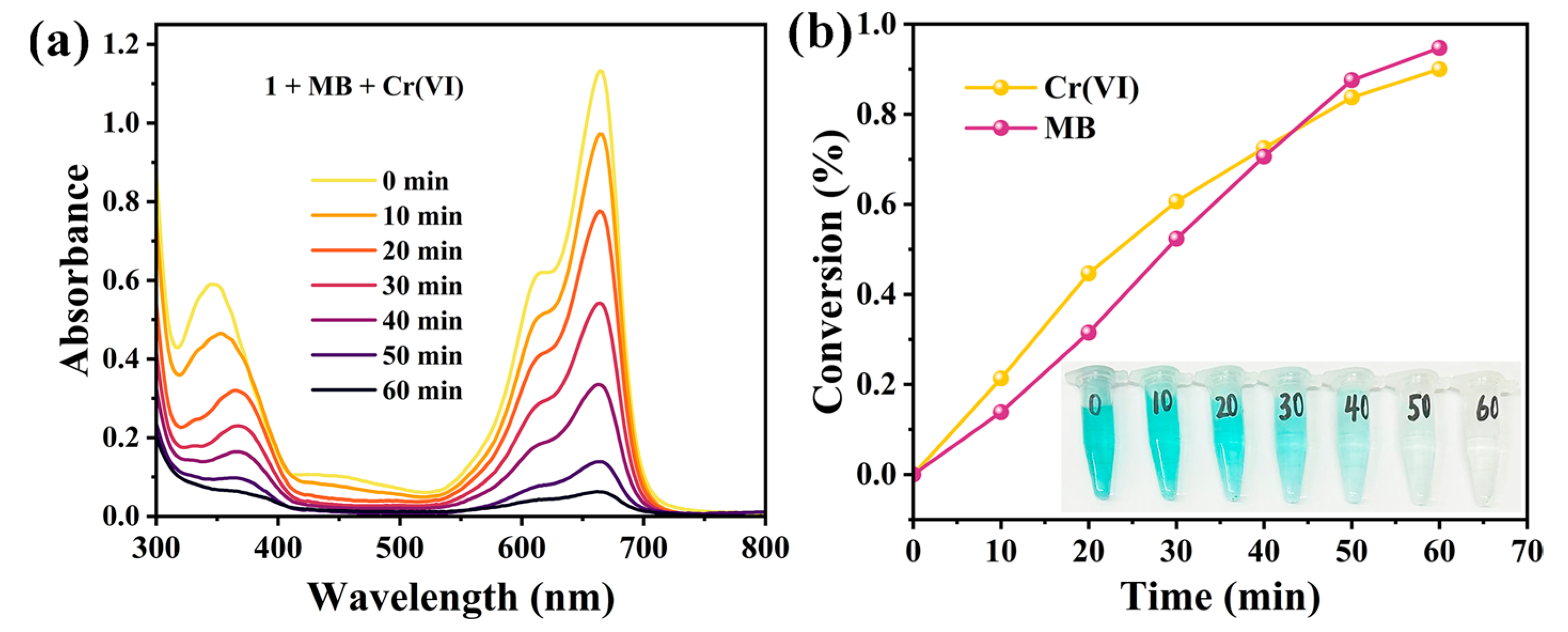
2.6.3. Photocatalytic Reduction of Cr(VI)
3. Experimental Section
3.1. Materials and Methods
3.2. Syntheses
Synthesis of [Ni(H2L)(HL)2](PMo12O40)·3H3O·4H2O (1)
3.3. X-Ray Crystallography
3.4. Preparation of Carbon Paste Electrode
3.5. Procedures of Photocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murtaza, G.; Ahmed, M.I.; Yu, K.; An, X.; Shah, S.S.A.; Sohail, M. Challenges and outlooks for the polyoxometalates, metal-organic frameworks (POMs-MOFs) hybrid materials in water treatment technologies. Environ. Res. 2025, 272, 121156. [Google Scholar] [CrossRef]
- Jiao, X.; Jia, K.; Yu, Y.; Liu, D.; Zhang, J.; Zhang, K.; Zheng, H.; Sun, X.; Tong, Y.; Wei, Q.; et al. Nanocellulose-based functional materials towards water treatment. Carbohydr. Polym. 2025, 350, 122977. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, H.H.; Rehman, S.U.; Khurshid, A.; Tahir, M.; Ajmal, S.; Tabish, M.; Alam, M.M.; Arif, M.; Al-Sehemi, A.G.; et al. Recent trends in metal-organic framework-based photocatalysts for Cr(VI) reduction: Confronts and prospects. J. Alloys Compd. 2024, 997, 174785. [Google Scholar] [CrossRef]
- Kamalesh, R.; Saravanan, A.; Ragini, Y.P.; Vickram, A.S. A review on optimizing photocatalytic performance for effective removal of emerging pollutants: Approaches and applications. J. Water Process Eng. 2025, 69, 106883. [Google Scholar] [CrossRef]
- Bao, J.; Liu, M.; Yin, X.; Alimaje, K.; Ma, Y.; Han, Z. Polyoxotungstate-based supramolecular complexes as multifunctional electrocatalysts for sensing water contaminants. J. Solid State Chem. 2023, 317, 123717. [Google Scholar] [CrossRef]
- Murmu, G.; Panigrahi, T.H.; Saha, S. Recent advances in the development of polyoxometalates and their composites for the degradation of toxic chemical dyes. Prog. Solid State Chem. 2024, 76, 100489. [Google Scholar] [CrossRef]
- Shi, H.; Xu, M.; Wang, L.; Ma, Q.; Zhao, M.; Li, Q.; Xu, Z.; Ji, L.; Yu, F.; Ma, J. Application of capacitive deionization in heavy metal ions removal and recovery: A review. Sep. Purif. Technol. 2025, 364, 132027. [Google Scholar] [CrossRef]
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental persistence; bioaccumulation; and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Sun, C.; Xing, X.; Li, J.; Xiong, W.; Li, H. Recent advances in carbon-based catalysts for electrocatalytic nitrate reduction to ammonia. Carbon Lett. 2025, 35, 1–19. [Google Scholar] [CrossRef]
- Raza, H.; Yildiz, I.; Yasmeen, F.; Munawar, K.S.; Ashfaq, M.; Abbas, M.; Ahmed, M.; Younus, H.A.; Zhang, S.; Ahmad, N. Synthesis of a 2D copper(II)-carboxylate framework having ultrafast adsorption of organic dyes. J. Colloid Interface Sci. 2021, 602, 43–54. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Zhang, Z. Structure-induced selective adsorption of diverse dyes and Cr(VI) photoreduction using two new polyoxometalate-based metal-viologen complexes. CrystEngComm 2024, 26, 1363–1370. [Google Scholar] [CrossRef]
- Huang, K.; Huang, L.; Shen, Y.; Hua, Y.; Song, R.; Li, Z.; Zhang, H. Two novel 3D polyoxometalate-based metal-organic frameworks for structure-directed selective adsorption and photodegradation of organic dyes. Inorg. Chem. Commun. 2024, 170, 113350. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, G.; Li, W.B.; Zhong, X.F.; Zhang, Y.Y.; Ye, J.W.; Yang, T.; Mo, Z.W.; Chen, X.M. Boosting the degradation of antibiotics via peroxymonosulfate activation with a Cu-based metal-organic framework. Chem. Sci. 2024, 15, 9733–9741. [Google Scholar] [CrossRef]
- Wang, H.; Zou, P.; Xu, L.; Jiang, R.; Shi, H.; Tang, B.Z.; Zhao, Z. Molecular engineering towards efficient aggregation induced delayed fluorescence luminogens as emitters and sensitizers for high-performance organic light-emitting diodes. Chem. Asian J. 2024, 19, e202400827. [Google Scholar] [CrossRef]
- Mal, S.S.; Banerjee, A.; Kortz, U. The cyclic 48-tungsto-8-phosphate [H7P8W48O184]33− Contant-Tézé polyanion and its derivatives [H6P4W24O94]18− and [H2P2W12O48]12−: Structural aspects and reactivity. Dalton Trans. 2025, 54, 5208–5233. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.X.; Guo, M.S.; Du, J.; Ma, Y.Y.; Han, Z.G. Polyoxometalate chemistry of {M[P4Mo6]2}: From structure assembly to functional application. Coord. Chem. Rev. 2024, 518, 216092. [Google Scholar] [CrossRef]
- Chen, S.J.; Ge, R.; Hong, L.H.; Sun, Y.Q.; Cai, P.W.; Sun, C.; Zeng, Q.X.; Li, X.X.; Zheng, S.T. Assembly of four ternary polyoxometalate aggregates by integrating presynthesized {XW9Nb3O40} (X = Si/Ge) units using molybdenum-oxo clusters. Inorg. Chem. 2025, 64, 142–150. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Y.; Huang, B.; Xiao, Z.; Wu, P. Application of polyoxometalates in photocatalytic degradation of organic pollutants. Nanoscale Adv. 2021, 3, 4646–4658. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, F.; Ning, S.; Cheng, P. Polyoxometalate-based metal-organic frameworks for heterogeneous catalysis. Inorg. Chem. Front. 2021, 8, 1865–1899. [Google Scholar] [CrossRef]
- Li, K.; Liu, T.; Ying, J.; Tian, A.; Wang, X. Recent research progress on polyoxometalate-based electrocatalysts in energy generation. J. Mater. Chem. A 2024, 12, 13576–13604. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal-organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, C.; Cheng, M.; Chen, M.; Chen, S.; Lei, L.; Chen, Y.; Yi, H.; Fu, Y.; Li, L. Polyoxometalate@metal-organic framework composites as effective photocatalysts. ACS Catal. 2021, 11, 13374–13396. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, E.; Zhou, S.; Wu, Z.; Zhang, W.; Zhao, M.; Dong, G. A new two-fold interpenetrating metal-organic framework based on polyoxometalate: Synthesis, structure, efficient hydrogen evolution and dye degradation. J. Solid State Chem. 2023, 323, 124066. [Google Scholar] [CrossRef]
- Li, M.T.; Zhou, X.H.; Liang, Q.M.; Chen, J.; Sun, J.W.; Yu, Y.; Wang, L.Y. POMs-based metal-organic frameworks with interpenetrating structures and their carbon nanotube-coated materials for lithium-ion anode applications. J. Solid State Chem. 2024, 337, 124787. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Hui, K.; Ying, J.; Tian, A. The applications of Keggin-based metal-organic compounds in sensing and catalysis. Dalton Trans. 2024, 53, 15412–15420. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chishti, A.N.; Chen, P.; Lv, Z.; Tan, Y.; Zhang, H.; Zha, J.; Ma, Z.; Ni, L.; Zhang, L.N.; et al. A Keggin-type polyoxomolybdate-based crystalline material formed by hydrothermal transformation: Photo/electro-catalytic properties and mechanism study. CrystEngComm 2022, 24, 8134–8140. [Google Scholar] [CrossRef]
- Yu, X.Y.; Lv, Y.; Weng, X.; Geng, J.; Yang, Y.; Jin, H.; Cui, H.; Fu, B.; Qu, X.; Shi, H.; et al. Five new multifunctional inorganic-organic hybrids based on Keggin-type polyoxometalates and a 3;5-di(1H-imidazol-1-yl)benzoic acid ligand. CrystEngComm 2023, 25, 2185–2195. [Google Scholar] [CrossRef]
- Tong, N.; Wang, X.; Zhao, X.Y.; Zhang, W.; Zhu, Y.S. Construction of anderson-type polyoxometalate-based complexes involving in situ ligand transformation with capacitive and electrochemical sensing performances. Inorg. Chim. Acta 2024, 562, 121901. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y.; Zheng, Y.; Guo, Q.; Yi, W.; Lian, Q.; Guo, Q.; Yu, X.Y.; Yu, X. A three-dimensional octamolybdate-modified silver crystalline material with catalytic and antibacterial activities. Inorg. Chem. Commun. 2024, 170, 113143. [Google Scholar] [CrossRef]
- Geng, J.Q.; Lu, Y.; Zhang, R.; Lv, Y.X.; Huang, S.T.; Yang, Y.Y.; Qu, X.; Shi, H.F.; Jin, H.; Yu, X.Y. Two new inorganic-organic hybrids constructed by Keggin-type polyoxometalates sandwiched between lanthanide-organic layers: Synthesis, structure and properties. J. Mol. Struct. 2024, 1302, 137502. [Google Scholar] [CrossRef]
- Cui, Z.W.; Lu, J.J.; Lin, H.Y.; Luan, J.; Chang, Z.H.; Li, X.H.; Wang, X.L. Four Keggin-type polyoxometalate-based complexes derived from bis(pyrazine)-bis(amide) ligands for electrochemical sensing of multiple analytes and adsorbing dye molecules. CrystEngComm 2022, 24, 828–836. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Sun, C.; Ying, J.; Tian, A. Five compounds based on [Mo12O40]8− and [β-Mo8O26]4− anions: Electrochemical sensing, photocatalytic and supercapacitor properties. Inorg. Chim. Acta 2022, 541, 121088. [Google Scholar] [CrossRef]
- Harchani, A.; Sutuła, S.; Trzybiński, D.; Woźniak, K.; Haddad, A. New series of hybrid organic-inorganic polyoxoselenomolybdate compounds: Crystal structure, Hirshfeld Surface analysis. Inorg. Chim. Acta 2020, 501, 119271. [Google Scholar] [CrossRef]
- Ammari, Y.; Abid, S.; Naifer, K.H. Synthesis, crystal structure and optical properties of Anderson-type heteropolyanion with cobalt cations. Appl. Phys. A 2020, 126, 101. [Google Scholar] [CrossRef]
- Skorepova, E.; Harchani, A.; Ftini, M.M.; Dušek, M.; Haddad, A. Crystal structure of a new polyoxometalate (POM) compound with a high level of crystallographic disorder. Acta Cryst. 2020, C76, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, H.; Wang, P.; Wang, P.; Zha, J.; Liu, Y.; Gautam, J.; Zhang, L.N.; Wang, Y.; Xie, J.; et al. Inorganic-organic hybrid supramolecular architectures based on Keggin polyoxometalates and crown ether: Synthesis, crystal structure and electrochemical properties. CrystEngComm 2021, 23, 8482–8489. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Xu, N.; Chang, Z.; Liu, G.; Lin, H.; Wang, X. Four octamolybdate complexes constructed from a quinoline-imidazole-monoamide ligand: Structures and electrochemical, photocatalytic and magnetic properties. CrystEngComm 2020, 22, 8322–8329. [Google Scholar] [CrossRef]
- Geng, J.Q.; Lu, Y.; Yang, L.; Jiang, X.; Huang, L.K.; Qu, X.S.; Yang, Y.Y.; Jin, H.; Li, X.M.; Yu, X.Y. A new [SiMo12O40]4−-based metal organic framework: Synthesis, Structure, photo-/electro-catalytic and absorption properties. Transit. Metal Chem. 2024, 49, 319–329. [Google Scholar] [CrossRef]
- Xu, C. Cu/Cu2O/NH2-MIL-88B (Fe) heterojunction as the photocatalyst to remove hexavalent chromium heavy metal ions in water. RSC Adv. 2025, 15, 2462–2469. [Google Scholar] [CrossRef]
- Jiang, X.; Weng, X.; Liang, Z.; Yang, L.; Deng, S.; Yu, L.; Wei, Q.; Yu, F.; Jin, H.; Yu, X.Y. Two new multifunctional coordination polymers based on N-heterocyclic carboxylic acid ligand: Syntheses, structures and properties. J. Mol. Struct. 2025, 1328, 141336. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Zhu, H.; Zhang, L.; Sang, X.; Zhu, Z.; You, W.; Zhang, F. Development of polyoxometalate-based Ag-H2biim inorganic-organic hybrid compounds functionalized for the acid electrocatalytic hydrogen evolution reaction. Dalton Trans. 2023, 52, 15725–15733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, J.; Lin, H.; Wang, X.; Chang, Z.; Chen, Y.; Zhang, Y. Polyoxometalate-based metal-organic complexes constructed from a new bis-pyrimidine-amide ligand with high capacitance performance and selectivity for the detection of Cr(VI). Chin. Chem. Lett. 2022, 33, 4389–4394. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Gong, L.; Dong, L.; Gu, Y.; Wang, M.; Yang, B. Pyridine polymer tubular structures connected with polyoxometalates as bifunctional electrocatalysts for water splitting. Dalton Trans. 2025, 54, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Cui, L.P.; Yu, K.; Lv, J.H.; Ma, Y.J.; Zhao, T.T.; Zhou, B.B. Supramolecular host-guest assembly based on phosphotungstate nanostructures for pseudocapacitive and electrochemical sensing applications. ACS Appl. Nano Mater. 2022, 5, 10452–10461. [Google Scholar] [CrossRef]
- Lv, Y.; Lu, Y.; Yu, X.; Yu, L.; Qu, X.; Yang, Y.; Jin, H.; Wei, Q.; Li, X.; Yu, X.Y. Three new multifunctional supramolecular compounds based on Keggin-type polyoxoanions and 3;5-di(1H-imidazol-1-yl)benzoic acid: Syntheses, structures, and properties. Molecules 2025, 30, 580. [Google Scholar] [CrossRef]
- Liu, X.; Liu, T.; Ying, J.; Yang, M.; Zhang, Y.; Tian, A. Two novel viologen/polyoxometalate-based compounds for the efficient photocatalytic reduction of Cr(VI) under visible light irradiation. Appl. Organomet. Chem. 2025, 39, e7929. [Google Scholar] [CrossRef]
- Wu, L.; Qin, D.; Fang, F.; Wang, W.; Zhao, W. Development of efficient photocatalyst MIL-68(Ga)_NH2 metal-organic framework for the removal of Cr(VI) and Cr(VI)/RhB from wastewater under visible light. Materials 2022, 15, 3761. [Google Scholar] [CrossRef]


| 1 | |
|---|---|
| Formula | C60H57N12O59Mo12NiP |
| Mol. wt. | 3131.13 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a (Å) | 18.685 (2) |
| b (Å) | 22.837 (2) |
| c (Å) | 21.457 (2) |
| α (°) | 90 |
| β (°) | 94.624 (5) |
| γ (°) | 90 |
| V (Å3) | 9126.0 (16) |
| Z | 4 |
| Dc (g cm−3) | 2.279 |
| µ (mm−1) | 1.922 |
| F (000) | 6080.9 |
| Reflection collected | 93,335 |
| Rint | 0.1566 |
| GOF | 1.029 |
| Final R ab indices [I > 2σ(I)] | R1 = 0.0505; wR2 = 0.0986 |
| R indices (all data) | R1 = 0.0891; wR2 = 0.1131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Liu, S.; Shi, D.; Yang, Y.; Yu, F.; Lu, T.; Yu, X.-Y.; Zhao, Y. A New [PMo12O40]3−-Based NiII Compound: Electrochemical and Photocatalytic Properties for Water Pollutant Removal. Molecules 2025, 30, 2172. https://doi.org/10.3390/molecules30102172
Lin G, Liu S, Shi D, Yang Y, Yu F, Lu T, Yu X-Y, Zhao Y. A New [PMo12O40]3−-Based NiII Compound: Electrochemical and Photocatalytic Properties for Water Pollutant Removal. Molecules. 2025; 30(10):2172. https://doi.org/10.3390/molecules30102172
Chicago/Turabian StyleLin, Guoqing, Shufeng Liu, Dai Shi, Ying Yang, Fangle Yu, Tong Lu, Xiao-Yang Yu, and Yuguang Zhao. 2025. "A New [PMo12O40]3−-Based NiII Compound: Electrochemical and Photocatalytic Properties for Water Pollutant Removal" Molecules 30, no. 10: 2172. https://doi.org/10.3390/molecules30102172
APA StyleLin, G., Liu, S., Shi, D., Yang, Y., Yu, F., Lu, T., Yu, X.-Y., & Zhao, Y. (2025). A New [PMo12O40]3−-Based NiII Compound: Electrochemical and Photocatalytic Properties for Water Pollutant Removal. Molecules, 30(10), 2172. https://doi.org/10.3390/molecules30102172






