Physical Properties and Volatile Profile Changes of Cauliflower Treated with Onion and Beetroot Juices Using Vacuum Impregnation Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Volatile Organic Compound (VOC) Profiles
2.2. Vacuum Impregnation
2.3. Dry Matter (DM), Water Activity (AW), and Density
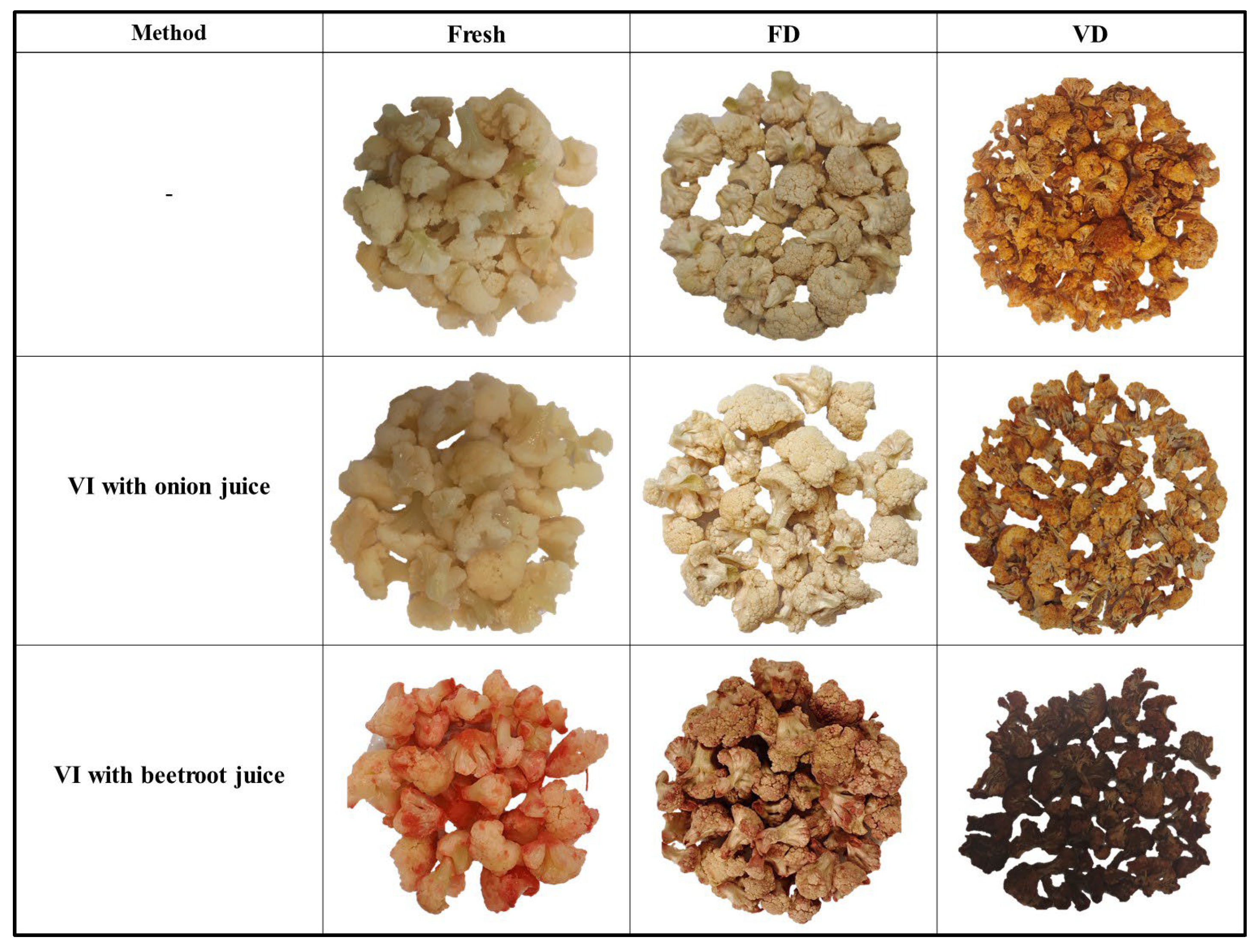
2.4. Color
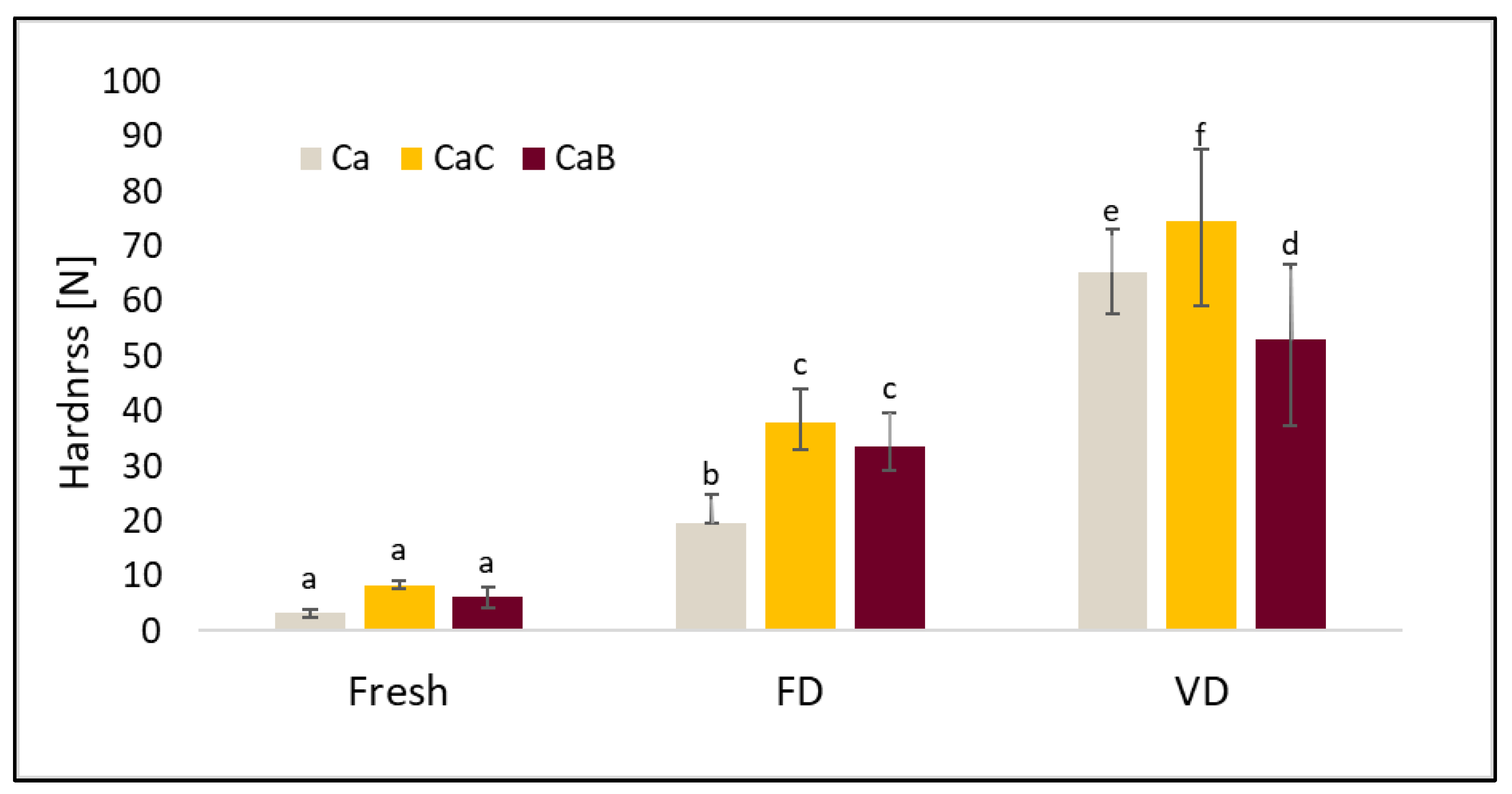
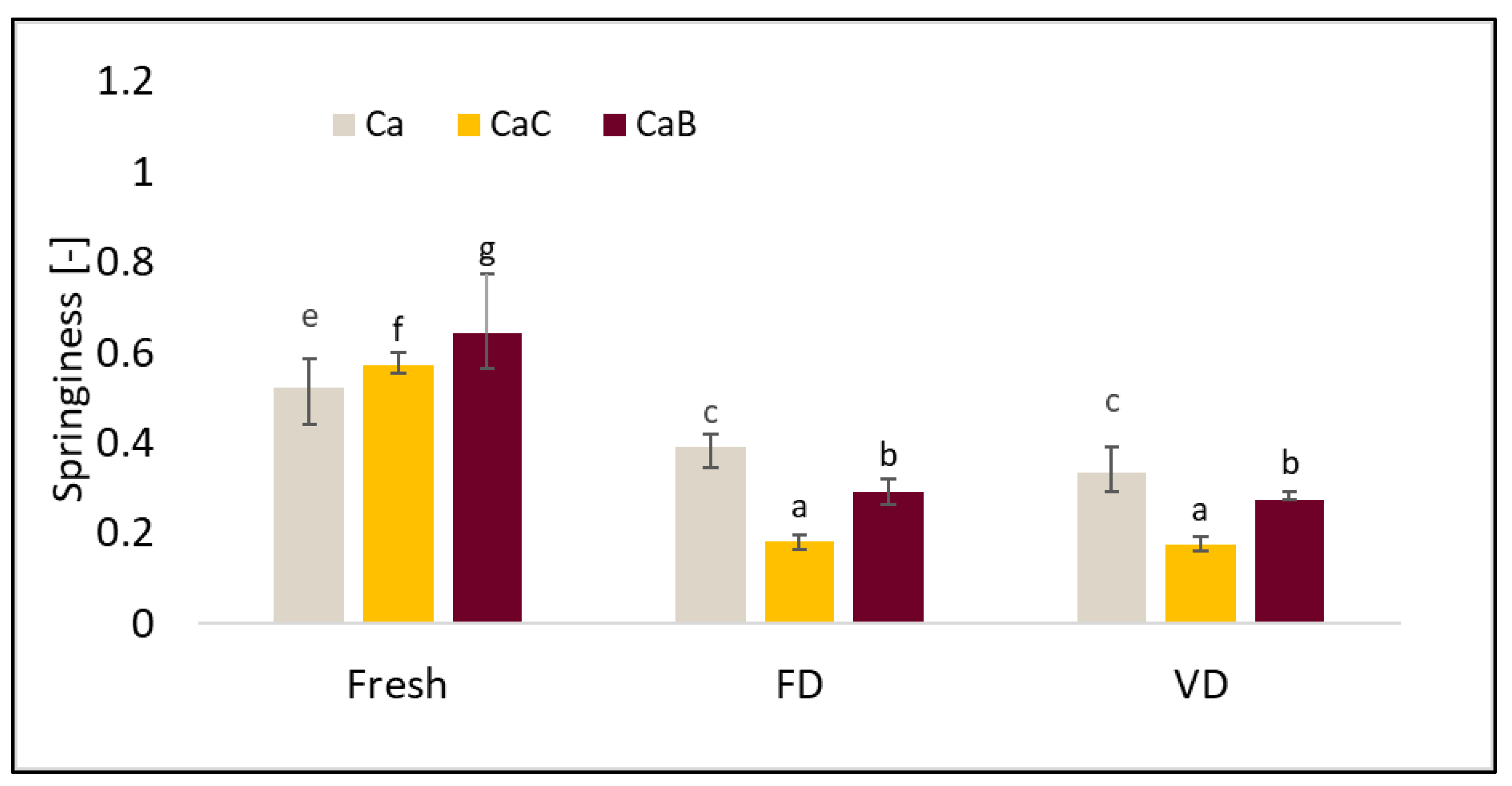
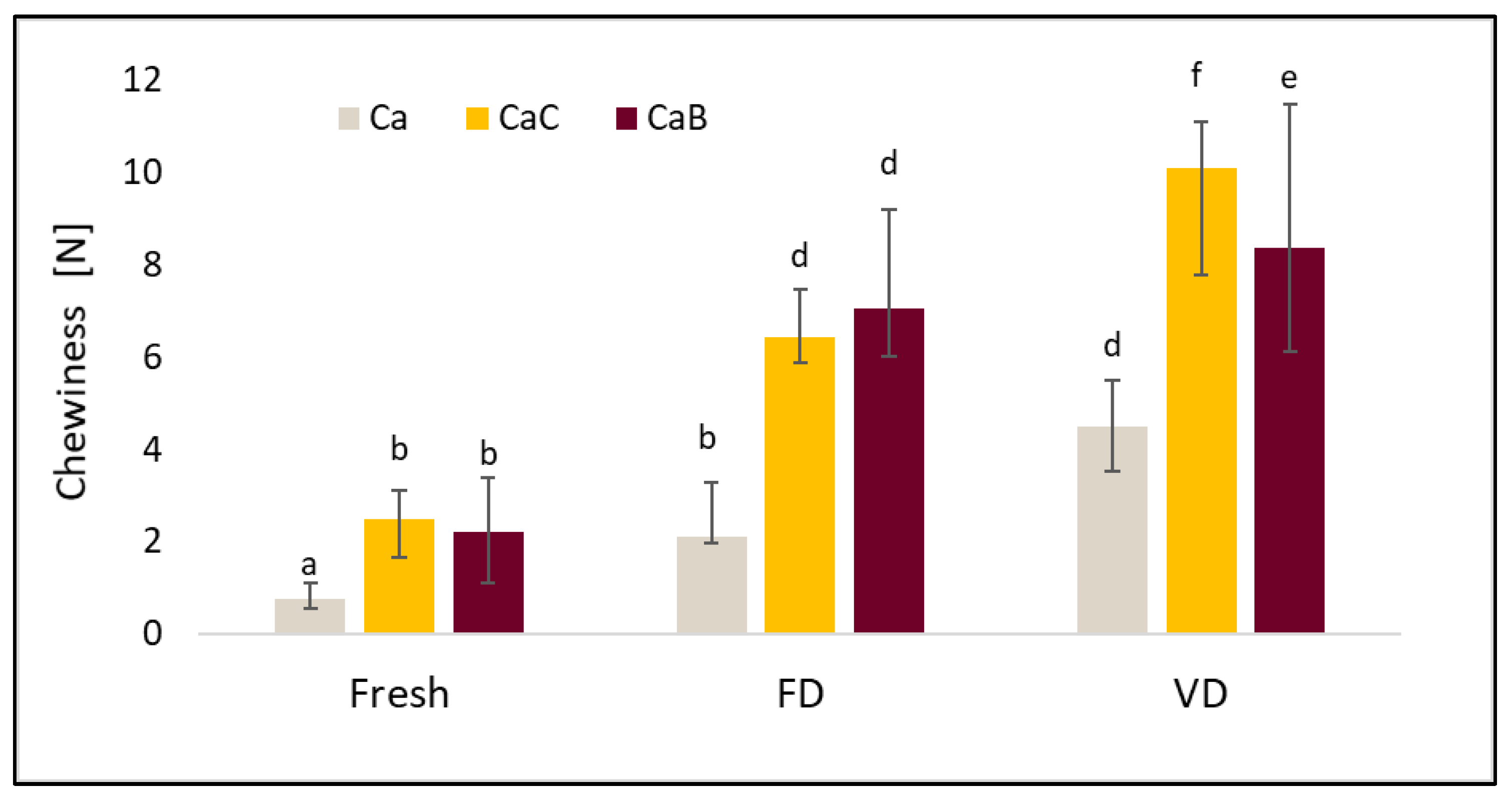
2.5. Texture Profile Analysis (TPA)
3. Materials and Methods
3.1. Preparation of Sample
3.2. Pretreatment Before Drying Process
3.3. Drying
3.4. VOC Extraction and Analysis
3.4.1. Methods
3.4.2. Identification
3.5. Physical Properties
3.6. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowacka, M.; Mannozzi, C.; Dalla Rosa, M.; Tylewicz, U. Sustainable Approach for Development Dried Snack Based on Actinidia deliciosa Kiwifruit. Appl. Sci. 2023, 13, 2189. [Google Scholar] [CrossRef]
- Mitrus, M.; Tydman, K.; Milanowski, M.; Soja, J.; Lewko, P.; Kupryaniuk, K.; Wójtowicz, A. Influence of the forming die design on processing and physical properties of gluten-free crisps. Agric. Eng. 2024, 28, 87–96. [Google Scholar] [CrossRef]
- Vaughan, J.; Geissler, C. The New Oxford Book of Food Plants; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Cieslik, E.; Cieslik, I.; Borowski, M. Characteristics of the health-promoting properties of glucosinolates (PL). ZPPNR 2017, 588, 3–14. [Google Scholar] [CrossRef]
- Korzeniowska-Ginter, R.; Grużyńska, M. Culinary use of cruciferous vegetables in terms of their health-promoting properties (PL). Probl. Hig. Epidemiol. 2013, 94, 639–641. [Google Scholar]
- Szwejda-Grzybowska, J. Anti-carcinogenic components of cruciferous vegetables and their importance in cancer prevention (PL). BiCT 2021, 4, 44. [Google Scholar]
- Kapusta-Duch, J.; Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kuboń, M.; Leszczyńska, T.; Borczak, B. Health-Promoting Properties of Fresh and Processed Purple Cauliflower. Sustainability 2019, 11, 4008. [Google Scholar] [CrossRef]
- Gębczyński, P.; Tabaszewska, M.; Kur, K.; Zbylut-Górska, M.; Słupski, J. Effect of the Drying Method and Storage Conditions on the Quality and Content of Selected Bioactive Compounds of Green Legume Vegetables. Molecules 2024, 29, 1732. [Google Scholar] [CrossRef]
- Sroy, S.; Miller, F.A.; Fundo, J.F.; Silva, C.L.M.; Brandão, T.R.S. Freeze-Drying Processes Applied to Melon Peel: Assessment of Physicochemical Attributes and Intrinsic Microflora Survival during Storage. Foods 2022, 11, 1499. [Google Scholar] [CrossRef]
- Krzykowski, A.; Rudy, S.; Polak, R.; Biernacka, B.; Krajewska, A.; Janiszewska-Turak, E.; Kowalska, I.; Żuchowski, J.; Skalski, B.; Dziki, D. Drying of Red Chili Pepper (Capsicum annuum L.): Process Kinetics, Color Changes, Carotenoid Content and Phenolic Profile. Molecules 2024, 29, 5164. [Google Scholar] [CrossRef]
- Sujinda, N.; Saengsuwan, T.; Chaichana, N. A study on drying characteristics, color, and vitamin C preservation of green banana slices using a vacuum heat pump system. Agric. Eng. 2024, 28, 176–184. [Google Scholar] [CrossRef]
- Rudy, S.; Dziki, D.; Biernacka, B.; Polak, R.; Krzykowski, A.; Krajewska, A.; Stanisławczyk, R.; Rudy, M.; Żurek, J.; Rudzki, G. Impact of Drying Process on Grindability and Physicochemical Properties of Celery. Foods 2024, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, M.; Stępień, B.; Łyczko, J.; Kamiński, P. The Influence of the Vacuum Impregnation, Beetroot Juice, and Various Drying Methods on Selected Properties of Courgette and Broccoli Snacks. Foods 2023, 12, 4294. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules 2021, 26, 4597. [Google Scholar] [CrossRef]
- Kręcisz, M.; Kolniak-Ostek, J.; Stępień, B.; Łyczko, J.; Pasławska, M.; Musiałowska, J. Influence of Drying Methods and Vacuum Impregnation on Selected Quality Factors of Dried Sweet Potato. Agriculture 2021, 11, 858. [Google Scholar] [CrossRef]
- Kręcisz, M.; Klemens, M.; Latański, A.; Stępień, B. The Use of Beetroot Juice as an Impregnating Solution to Change Volatile Compounds, Physical Properties and Influence the Kinetics of the Celery Drying Process. Molecules 2024, 29, 4050. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum Impregnation of Fish Gelatin Combined with Grape Seed Extract Inhibits Protein Oxidation and Degradation of Chilled Tilapia Fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef]
- Demir, H.; Çelik, S.; Sezer, Y.Ç. Effect of Ultrasonication and Vacuum Impregnation Pretreatments on the Quality of Beef Marinated in Onion Juice a Natural Meat Tenderizer. Food Sci. Technol. Int. Cienc. Tecnol. Los Aliment. Int. 2022, 28, 340–352. [Google Scholar] [CrossRef]
- Hofmeister, L.C.; Souza, J.A.R.; Laurindo, J.B.Z. Use of Dyed Solutions to Visualize Different Aspects of Vacuum Impregnation of Minas Cheese. LWT Food Sci. Technol. 2005, 38, 379–386. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Rodríguez-Lafitte, E.; Villagrán, Z.; Aurora-Vigo, E.F.; Ruvalcaba-Gómez, J.M.; Símpalo-López, W.B.; Martínez-Esquivias, F.; Sarango-Córdova, C.H. Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters. Appl. Sci. 2024, 14, 10850. [Google Scholar] [CrossRef]
- Derossi, A.; De Pilli, T.; Severini, C. Application of Vacuum Impregnation Techniques to Improve the pH Reduction of Vegetables: Study on Carrots and Eggplants. Food Bioprocess Technol. 2013, 6, 3217–3226. [Google Scholar] [CrossRef]
- Gras, M.; Vidal-Brotóns, N.; Betoret, A.; Chiralt; Fito, P. The Response of Some Vegetables to Vacuum Impregnation. Innov. Food Sci. Emerg. Technol. 2002, 3, 263–269. [Google Scholar] [CrossRef]
- Kręcisz, M.; Kolniak-Ostek, J.; Łyczko, J.; Stępień, B. Evaluation of bioactive compounds, volatile compounds, drying process kinetics and selected physical properties of vacuum impregnation celery dried by different methods. Food Chem. 2023, 413, 135490. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhou, S. Phenolic Components and Health Beneficial Properties of Onions. Agriculture 2021, 11, 872. [Google Scholar] [CrossRef]
- Yuasa, M.; Ueno, M.; Kawabeta, K.; Morikawa, M.; Uemura, M.; Matsuzawa, T.; Tominaga, M. Taste characteristics, volatile components, sensory properties, and antioxidant activity of fresh onion (Allium cepa L.) leaves. Bull Natl. Res. Cent. 2022, 46, 270. [Google Scholar] [CrossRef]
- The Good Scents Company. Available online: https://www.thegoodscentscompany.com/data/rw1008101.html?utm_sourc (accessed on 28 April 2025).
- Smolecule. Available online: https://www.smolecule.com/products/s1895504?utm_source=chatgpt.com (accessed on 28 April 2025).
- Maruyama, F.T. Identification ff Dimethyl Trisulfide as a Major Aroma Component of Cooked Brassicaceous Vegetables. J. Food Sci. 1970, 35, 540–543. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wang, D.; He, Z.; Gong, J.; Tan, C. Effects of Different Probiotics on the Volatile Components of Fermented Coffee Were Analyzed Based on Headspace-Gas Chromatography-Ion Mobility Spectrometry. Foods 2023, 12, 2015. [Google Scholar] [CrossRef]
- Tian, M.; Lin, K.; Yang, L.; Jiang, B.; Zhang, B.; Zhu, X.; Ren, D.; Yu, H. Characterization of key aroma compounds in gray sufu fermented using Leuconostoc mesenteroides subsp. Mesenteroides F24 as a starter culture. Food Chem. X 2023, 20, 100881. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Liu, S.; Hong, P.; Zhou, C.; Zhong, S. Characterization of the Effect of Different Cooking Methods on Volatile Compounds in Fish Cakes Using a Combination of GC–MS and GC-IMS. Food Chem. X 2024, 22, 101291. [Google Scholar] [CrossRef]
- Kurek, M.A.; Finnseth, C.; Skipnes, D.; Rode, T.M. Impact of High-Pressure Processing (HPP) on Selected Quality and Nutritional Parameters of Cauliflower (Brassica oleracea var. Botrytis). Appl. Sci. 2022, 12, 6013. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Socha, R.; Filipiak-Florkiewicz, A.; Topolska, K. Sous-vide Technique as an Alternative to Traditional Cooking Methods in the Context of Antioxidant Properties of Brassica Vegetables. J. Sci. Food Agric. 2019, 99, 173–182. [Google Scholar] [CrossRef]
- Cebula, S.; Kunicki, E.; Kalisz, A. Quality Changes in Curds of White, Green and Romanesco Cauliflower during Storage. Pol. J. Food Nutr. Sci. 2006, 15, 155. [Google Scholar]
- Gębczyński, P.; Kmiecik, W. Effects of traditional and modified technology, in the production of frozen cauliflower, on the contents of selected antioxidative compounds. Food Chem. 2007, 1, 229–235. [Google Scholar] [CrossRef]
- Kręcisz, M.; Stępień, B.; Pikor, K. Effect of Tomato Juice and Different Drying Methods on Selected Properties of Courgette. Appl. Sci. 2024, 14, 7105. [Google Scholar] [CrossRef]
- Xu, H.; Lei, M.; Li, J.; Zou, S.; Yin, W.; Jiang, W.; Xianyu, D.; Li, D.; Zhao, C.; Yu, L. Effects of different drying methods on the physicochemical and functional properties of Pyracantha fortuneana (Maxim.) Li fruit. LWT 2023, 187, 115383. [Google Scholar] [CrossRef]
- Pasten, A.; Vega-Galvez, A.; Uribe, E.; Carvajal, M.; Mejías, N.; Araya, M.; Goñi, M.G. A Comparison of the Effects of Low-Temperature Vacuum Drying and Other Methods on Cauliflower’s Nutritional–Functional Properties. Processes 2024, 12, 1629. [Google Scholar] [CrossRef]
- Tappi, S.; Velickova, E.; Mannozzi, C.; Tylewicz, U.; Laghi, L.; Rocculi, P. Multi-Analytical Approach to Study Fresh-Cut Apples Vacuum Impregnated with Different Solutions. Foods 2022, 11, 488. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Professor at the Biology Department; Baylor University: Waco, TX, USA, 2017. [Google Scholar]
- Apaliya, M.T.; Kwaw, E.; Osae, R.; Alolga, R.N.; Aidoo, P.; Mensah, L.A.; Sackey, A.S.; Wilson, C.L. Effect of different drying methods on the rehydration kinetics, physiochemical and functional properties of unripe plantain (Musa parasidiaca) flour. Food Chem. Adv. 2024, 4, 100610. [Google Scholar] [CrossRef]
- Figiel, A.; Tajner-Czopek, A. The effect of Candy moisture on texture. J. Food Serv. 2006, 7, 189–195. [Google Scholar] [CrossRef]


| Compounds | LRI Exp 1 | LRI Lit 2 | Match 3 | Ca% | Ca SD 4 | CaB% | CaB SD | CaC% | CaC SD | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | 781 | nd 5 | nd | nd | nd | 11.33 | 1.95 | ||
| 2 | Heptene <2-methyl-1-> | 784 | 784 | 89 | 63.54 | 6.06 | 72.86 | 7.86 | 61.55 | 1.18 |
| 3 | Pentanethiol <4-methyl-2-> | 804 | 793 | 89 | nd | nd | 1.11 | 0.68 | nd | nd |
| 4 | Hexanal | 791 | 801 | 85 | 3.39 | 1.29 | nd | nd | nd | nd |
| 5 | Ethylcyclobutanol<2-> | 831 | 828 | 90 | 7.56 | 4.01 | 4.86 | 3.62 | 4.62 | 1.77 |
| 6 | Hexenal-<2E> | 864 | 855 | 95 | 1.17 | 0.56 | 0.76 | 0.21 | 0.88 | 0.04 |
| 7 | Hexanol <1> | 875 | 867 | 93 | nd | nd | nd | nd | 0.16 | 0.01 |
| 8 | Heptanal | 901 | 906 | 90 | 0.20 | 0.11 | 0.14 | 0.10 | 0.09 | 0.04 |
| 9 | Sorbaldehyde | 909 | 914 | 94 | nd | nd | nd | nd | 0.17 | 0.08 |
| 10 | Pinene <alpha-> | 932 | 933 | 96 | 0.55 | 0.51 | 0.64 | 0.50 | 0.27 | 0.08 |
| 11 | Camphene | 948 | 943 | 95 | 0.17 | 0.17 | 0.20 | 0.18 | 0.08 | 0.03 |
| 12 | Heptenal <2E-> | 953 | 956 | 90 | 0.13 | 0.09 | nd | nd | 0.08 | 0.02 |
| 13 | Benzaldehyde | 958 | 960 | 98 | 1.46 | 0.83 | 0.72 | 0.50 | 0.83 | 0.26 |
| 14 | Dimethyl trisulfide | 966 | 964 | 90 | nd | nd | nd | nd | 0.67 | 0.65 |
| 15 | Hexanoic acid | 969 | 967 | 90 | nd | nd | 0.10 | 0.07 | nd | nd |
| 16 | Sabinene | 971 | 972 | 86 | 0.07 | 0.07 | nd | nd | nd | nd |
| 17 | Pinene <beta-> | 977 | 978 | 90 | 0.18 | 0.19 | 0.23 | 0.21 | nd | nd |
| 18 | Octene <2-methyl-6-methylene-2-> | 978 | 968 | 90 | nd | nd | nd | nd | 0.62 | 0.34 |
| 19 | Myrcene | 988 | 991 | 95 | 0.33 | 0.36 | 0.90 | 1.07 | 0.09 | 0.04 |
| 20 | Pentyl furan <2-> | 994 | 988 | 91 | nd | nd | 0.24 | 0.07 | nd | nd |
| 21 | Mentha-1(7),8-diene <meta-> | 1009 | 1001 | 85 | nd | nd | 0.71 | 0.30 | nd | nd |
| 22 | Heptadienal <2,4-trans,trans-> | 1009 | 1013 | 88 | 1.71 | 0.80 | nd | nd | 0.80 | 0.16 |
| 23 | Cymene <ortho-> | 1022 | 1024 | 92 | 0.18 | 0.15 | 0.21 | 0.20 | nd | nd |
| 24 | Hexanol <2-ethyl-> | 1026 | 1029 | 92 | nd | nd | nd | nd | 0.24 | 0.01 |
| 25 | Limonene | 1028 | 1030 | 93 | 0.42 | 0.34 | 0.56 | 0.54 | 0.14 | 0.03 |
| 26 | Eucalyptol | 1031 | 1032 | 95 | 0.41 | 0.43 | 0.34 | 0.44 | nd | nd |
| 27 | Oct-3-en-2-one | 1035 | 1036 | 92 | nd | nd | 0.15 | 0.10 | 0.19 | 0.07 |
| 28 | Decane <2-methyl-> | 1053 | 1051 | 90 | nd | nd | 0.06 | 0.05 | nd | nd |
| 29 | Octenal <2-> | 1054 | 1059 | 93 | nd | nd | 0.07 | 0.04 | 0.06 | 0.02 |
| 30 | Nona-3,5-dien-2-one | 1065 | 1068 | 87 | 4.43 | 1.99 | 1.85 | 0.92 | 2.23 | 0.70 |
| 31 | Unknown (probably isomer of previous compound) | 1089 | 5.28 | 2.74 | 1.38 | 0.57 | 2.49 | 1.03 | ||
| 32 | Undecane <n-> | 1099 | 1100 | 95 | nd | nd | 0.07 | 0.04 | nd | nd |
| 33 | Nonanal | 1102 | 1104 | 92 | 0.22 | 0.12 | 0.18 | 0.05 | 0.12 | 0.02 |
| 34 | Thujone <beta-> | 1104 | 1118 | 90 | 0.08 | 0.09 | 0.09 | 0.09 | nd | nd |
| 35 | Unknown (probably fatty acid) | 1308 | 9.86 | 4.27 | 13.05 | 2.38 | 12.26 | 1.09 |
| Compounds | LRI Exp 1 | LRI Lit 2 | Match 3 | Ca FD% | CaB FD% | CaC FD% | Ca VD% | CaB VD% | CaC VD% | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Butenolide | 805 | 807 | 88 | 19.74 ± 2.62 | nd 4 | nd | 2.16 ± 0.92 | nd | nd |
| 2 | Formate <pentyl-> | 814 | 823 | 91 | 1.42 ± 0.44 | nd | nd | 3.55 ± 0.47 | nd | nd |
| 3 | Ethylcyclobutanol<2-> | 830 | 828 | 89 | 12.44 ± 1.58 | 3.43 ± 0.53 | 3.55 ± 0.27 | 12.94 ± 0.39 | 3.51 ± 0.86 | 11.00 ± 1.71 |
| 4 | Isovaleric acid | 850 | 842 | 86 | Nd | 0.58 ± 0.20 | 0.77 ± 0.16 | nd | 1.66 ± 0.66 | 5.22 ± 1.18 |
| 5 | Unknown | 856 | Nd | nd | 0.38 ± 0.09 | nd | nd ±0.04 | 2.32 ± 0.47 | ||
| 6 | Hexenal <2E> | 863 | 850 | 95 | 1.16 ± 0.25 | 0.59 ± 0.04 | 0.58 ± 0.03 | 1.11 ± 0.16 | 0.29 ± 0.00 | 1.09 ± 0.04 |
| 7 | Isothiocyanate <2-propenyl-> | 883 | 880 | 93 | nd | 0.42 ± 0.08 | nd | nd | nd | nd |
| 8 | Heptanal | 906 | 900 | 95 | 0.36 ± 0.09 | 0.12 ± 0.01 | 0.12 ± 0.00 | 0.32 ± 0.07 | 0.16 ± 0.03 | 0.27 ± 0.03 |
| 9 | Pyrazine <2,5-dimethyl-> | 910 | 912 | 90 | 1.05 ± 0.38 | nd | 0.43 ± 0.02 | 0.82 ± 0.15 | nd | 1.13 ± 0.32 |
| 10 | Hexanoate <methyl-> | 920 | 922 | 94 | 0.33 ± 0.04 | nd | nd | 0.07 ± 0.01 | nd | nd |
| 11 | Pinene <alpha-> | 932 | 933 | 98 | nd | 0.79 ± 0.01 | 0.84 ± 0.06 | nd | 0.68 ± 0.13 | 0.38 ± 0.06 |
| 12 | Camphene | 948 | 953 | 97 | 0.37 ± 0.22 | 1.27 ± 0.02 | 1.28 ± 0.05 | 0.42 ± 0.22 | 1.06 ± 0.22 | 0.58 ± 0.08 |
| 13 | Heptenal <2E> | 952 | 956 | 91 | 0.19 ± 0.02 | nd | nd | 0.17 ± 0.02 | nd | nd |
| 14 | Benzaldehyde | 957 | 960 | 98 | 4.77 ± 1.29 | 1.13 ± 0.14 | 1.75 ± 0.20 | 17.38 ± 4.95 | 6.66 ± 1.14 | 9.11 ± 0.47 |
| 15 | Trisulfide <dimethyl-> | 965 | 969 | 85 | 0.83 ± 0.46 | nd | 0.49 ± 0.08 | 0.52 ± 0.04 | nd | 1.95 ± 0.62 |
| 16 | Hexanoic acid | 971 | 974 | 95 | nd | nd | 0.78 ± 0.15 | nd | nd | 1.30 ± 0.18 |
| 17 | Pinene <beta-> | 976 | 978 | 90 | nd | 0.33 ± 0.25 | 0.34 ± 0.02 | nd | 0.27 ± 0.10 | 0.14 ± 0.03 |
| 18 | Caproic acid | 971 | 979 | 92 | 4.04 ± 2.35 | 0.53 ± 0.00 | nd | 1.34 ± 0.40 | 0.23 ± 0.04 | nd |
| 19 | Oct-1-en-3-ol | 977 | 978 | 87 | 0.87 ± 0.13 | nd | nd | 1.05 ± 0.12 | nd | nd |
| 20 | Unknown | 982 | 1.19 ± 0.36 | nd | nd | 0.78 ± 0.15 | nd | nd | ||
| 21 | Hept-5-en-2-one <6-methyl-> | 981 | 986 | 85 | nd | 2.15 ± 0.13 | 1.86 ± 0.06 | nd | 1.91 ± 0.62 | 1.47 ± 0.26 |
| 22 | Myrcene | 988 | 991 | 98 | 15.24 ± 11.93 | 62.09 ± 0.59 | 62.10 ± 1.69 | 13.98 ± 8.30 | 54.61 ± 5.27 | 22.79 ± 3.79 |
| 23 | Pyrazine <2-ethyl-, 6-methyl-> | 995 | 994 | 90 | 1.06 ± 0.40 | 0.59 ± 0.12 | 0.54 ± 0.07 | 0.80 ± 0.15 | 1.96 ± 0.45 | 1.35 ± 0.31 |
| 24 | Decane | 1000 | 1000 | 90 | nd | 0.55 ± 0.04 | 0.69 ± 0.02 | nd | 0.10 ± 0.04 | 0.55 ± 0.06 |
| 25 | Mentha-1(7),8-diene <p-> | 1003 | 1004 | 97 | nd | 3.28 ± 0.08 | 3.29 ± 0.08 | nd | 2.72 ± 0.39 | 1.21 ± 0.19 |
| 26 | Heptadienal <2,4-trans,trans-> | 1009 | 1013 | 90 | 1.03 ± 0.21 | nd | 0.37 ± 0.07 | 1.67 ± 0.19 | nd | 1.67 ± 0.19 |
| 27 | Cymene <para-> | 1022 | 1025 | 96 | nd | 1.81 ± 0.10 | 1.70 ± 0.06 | nd | 1.73 ± 0.18 | 0.71 ± 0.11 |
| 28 | Hexanol <2-ethyl-> | 1026 | 1028 | 90 | 1.40 ± 0.37 | 0.49 ± 0.04 | 0.76 ± 0.02 | 0.26 ± 0.08 | 0.32 ± 0.04 | 0.39 ± 0.02 |
| 29 | Limonene | 1027 | 1030 | 90 | nd | 0.39 ± 0.02 | 0.37 ± 0.02 | nd | 0.35 ± 0.02 | 0.27 ± 0.00 |
| 30 | 3-Octen-2-one | 1034 | 1047 | 93 | 0.61 ± 0.12 | nd | nd | 0.51 ± 0.04 | nd | nd |
| 31 | Nona-3,5-dien-2-one | 1065 | 1068 | 86 | 5.24 ± 1.18 | 2.24 ± 0.08 | 2.11 ± 0.26 | 10.24 ± 0.80 | 0.53 ± 0.18 | 7.89 ± 0.76 |
| 32 | Unknown (probably isomer of previous compound) | 1090 | 6.62 ± 1.74 | 1.82 ± 0.01 | 2.07 ± 0.04 | 7.01 ± 0.69 | 0.13 ± 0.02 | 6.05 ± 0.88 | ||
| 33 | Nonanal | 1102 | 1104 | 93 | nd | nd | 0.22 ± 0.01 | nd | nd | 0.48 ± 0.01 |
| 34 | Geranial | 1160 | 1174 | 86 | nd | 0.19 ± 0.01 | 0.14 ± 0.01 | nd | 0.13 ± 0.05 | 0.06 ± 0.03 |
| 35 | Citral | 1183 | 1174 | 90 | nd | 0.19 ± 0.03 | 0.13 ± 0.01 | nd | 0.12 ± 0.03 | 0.03 ± 0.01 |
| 36 | Dodecane | 1199 | 1200 | 96 | 5.29 ± 2.28 | 3.33 ± 0.52 | 3.93 ± 0.29 | 1.48 ± 0.45 | 0.19 ± 0.04 | 1.09 ± 0.21 |
| 37 | Unknown (probably fatty acid) | 1308 | 12.40 ± 2.53 | 9.85 ± 0.64 | 6.45 ± 0.72 | 22.98 ± 2.27 | 20.79 ± 3.47 | 18.83 ± 2.26 | ||
| 38 | Tetradecane <n-> | 1400 | 1400 | 95 | 3.54 ± 1.17 | 1.86 ± 0.11 | 1.97 ± 0.05 | 1.12 ± 0.22 | 0.15 ± 0.05 | 0.82 ± 0.08 |
| Material | WG | °Bx |
|---|---|---|
| CaC | 4.03 ± 1.43 | 11.5 ± 0.08 |
| CaB | 8.90 ± 1.11 | 7.5 ± 0.12 |
| Method | Water Activity [-] | Dry Mass [%] | Bulk Density [kg/m3] |
|---|---|---|---|
| Ca | 0.970 ± 0.006 d | 8.91 ± 0.24 | 275.44 ± 8.20 d |
| Ca_FD | 0.145 ± 0.011 a | 98.64 ± 0.89 | 30.71 ± 2.31 a |
| Ca_VD | 0.294 ± 0.016 b | 97.94 ± 0.57 | 122.14 ± 7.35 b |
| CaC | 0.978 ± 0.002 e | 9.22 ± 0.16 | 365.94 ± 14.49 d |
| CaC_FD | 0.173 ± 0.006 a | 96.09 ± 3.22 | 36.69 ± 4.32 a |
| CaC_VD | 0.369 ± 0.013 c | 95.39 ± 2.90 | 160.11 ± 1.74 c |
| CaB | 0.982 ± 0.001 d | 9.88 ± 0.15 | 368.23 ± 13.55 d |
| CaB_FD | 0.257 ± 0.016 b | 96.44 ± 0.70 | 35.78 ± 1.70 a |
| CaB_VD | 0.328 ± 0.034 c | 95.74 ± 0.72 | 160.99 ± 3.89 c |
| Method | L* | a* | b* | C* | BI | ∆E |
|---|---|---|---|---|---|---|
| Ca | 73.10 ± 3.20 c | −0.65 ± 0.19 f | 11.53 ± 1.79 e | 11.50 ± 1.81 | 16.27 ± 2.70 | - |
| Ca_FD | 81.45 ± 3.96 a | 1.80 ± 0.46 d | 16.55 ± 1.68 b | 16.60 ± 1.68 | 26.30 ± 3.28 | 9.87 |
| Ca_VD | 79.84 ± 6.25 b | 0.40 ± 0.21 e | 20.61 ± 1.93 a | 20.62 ± 1.93 | 29.77 ± 2.22 | 11.31 |
| CaC | 71.33 ± 4.39 c | −0.87 ± 0.45 f | 12.91 ± 2.73 d | 12.87 ± 2.75 | 18.84 ± 4.60 | 2.48 |
| CaC_FD | 72.54 ± 4.60 c | 2.12 ± 1.45 d | 20.46 ± 3.96 a | 20.54 ± 3.98 | 35.30 ± 9.52 | 9.18 |
| CaC_VD | 51.17 ± 3.70 e | 11.98 ± 1.14 b | 20.78 ± 2.02 a | 21.07 ± 1.99 | 68.96 ± 6.87 | 26.65 |
| CaB | 59.28 ± 2.52 e | 15.67 ± 2.52 a | 14.44 ± 1.85 c | 14.98 ± 1.81 | 46.83 ± 5.33 | 20.95 |
| CaB_FD | 62.48 ± 2.66 d | 15.44 ± 0.47 a | 19.01 ± 1.38 a | 19.41 ± 1.36 | 54.06 ± 3.80 | 20.03 |
| CaB_VD | 35.56 ± 3.67 f | 9.58 ± 1.80 c | 12.03 ± 1.36 d | 12.43 ± 1.27 | 61.70 ± 9.17 | 38.70 |
| Code | Material | Type of Drying |
|---|---|---|
| Ca | Cauliflower | - |
| Ca_FD | Cauliflower | freeze-drying |
| Ca_VD | Cauliflower | vacuum drying |
| CaC | Cauliflower with onion juice | - |
| CaC_FD | Cauliflower with onion juice | freeze-drying |
| CaC_VD | Cauliflower with onion juice | vacuum drying |
| CaB | Cauliflower with beetroot juice | - |
| CaB_FD | Cauliflower with beetroot juice | freeze-drying |
| CaB_VD | Cauliflower with beetroot juice | vacuum drying |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kręcisz, M.; Stępień, B.; Klemens, M.; Latański, A. Physical Properties and Volatile Profile Changes of Cauliflower Treated with Onion and Beetroot Juices Using Vacuum Impregnation Process. Molecules 2025, 30, 2147. https://doi.org/10.3390/molecules30102147
Kręcisz M, Stępień B, Klemens M, Latański A. Physical Properties and Volatile Profile Changes of Cauliflower Treated with Onion and Beetroot Juices Using Vacuum Impregnation Process. Molecules. 2025; 30(10):2147. https://doi.org/10.3390/molecules30102147
Chicago/Turabian StyleKręcisz, Magdalena, Bogdan Stępień, Marta Klemens, and Aleks Latański. 2025. "Physical Properties and Volatile Profile Changes of Cauliflower Treated with Onion and Beetroot Juices Using Vacuum Impregnation Process" Molecules 30, no. 10: 2147. https://doi.org/10.3390/molecules30102147
APA StyleKręcisz, M., Stępień, B., Klemens, M., & Latański, A. (2025). Physical Properties and Volatile Profile Changes of Cauliflower Treated with Onion and Beetroot Juices Using Vacuum Impregnation Process. Molecules, 30(10), 2147. https://doi.org/10.3390/molecules30102147







