Bio-Compounds, Antioxidant Activity, and Phenolic Content of Broccoli After Impregnation with Beetroot Juice
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Compounds of Broccoli Extracts
2.2. Total Phenolic Content (TPC) and Antioxidant Capacity (AC)
2.3. Vacuum Impregnation
2.4. Moisture Ratio Curves and Water Content Curves
2.5. Modeling of Broccoli Drying Process
2.6. Dry Matter (DM), Water Activity (AW), and Density (ρb)
2.7. Color
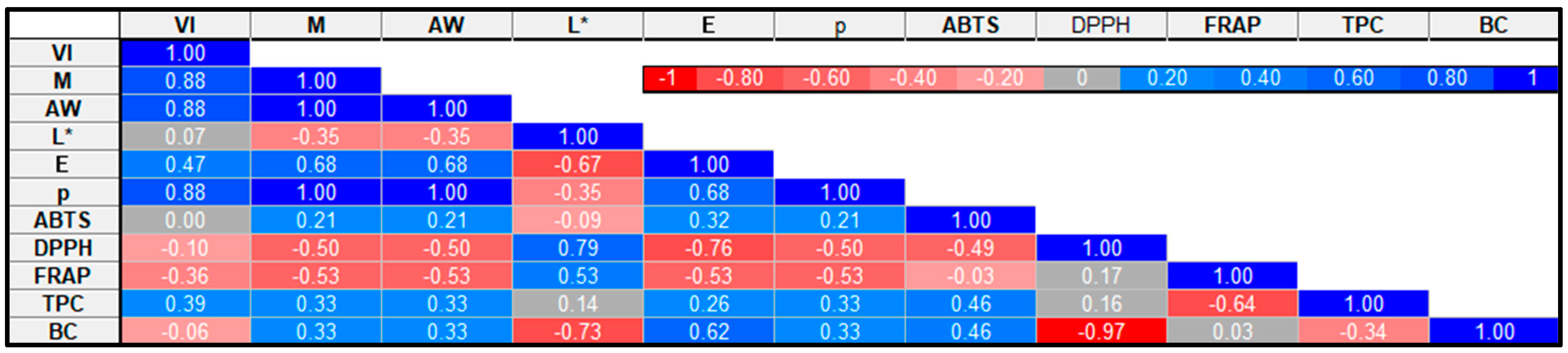
2.8. Pearson Correlation of Drying Kinetics and Selected Broccoli Quality Attributes
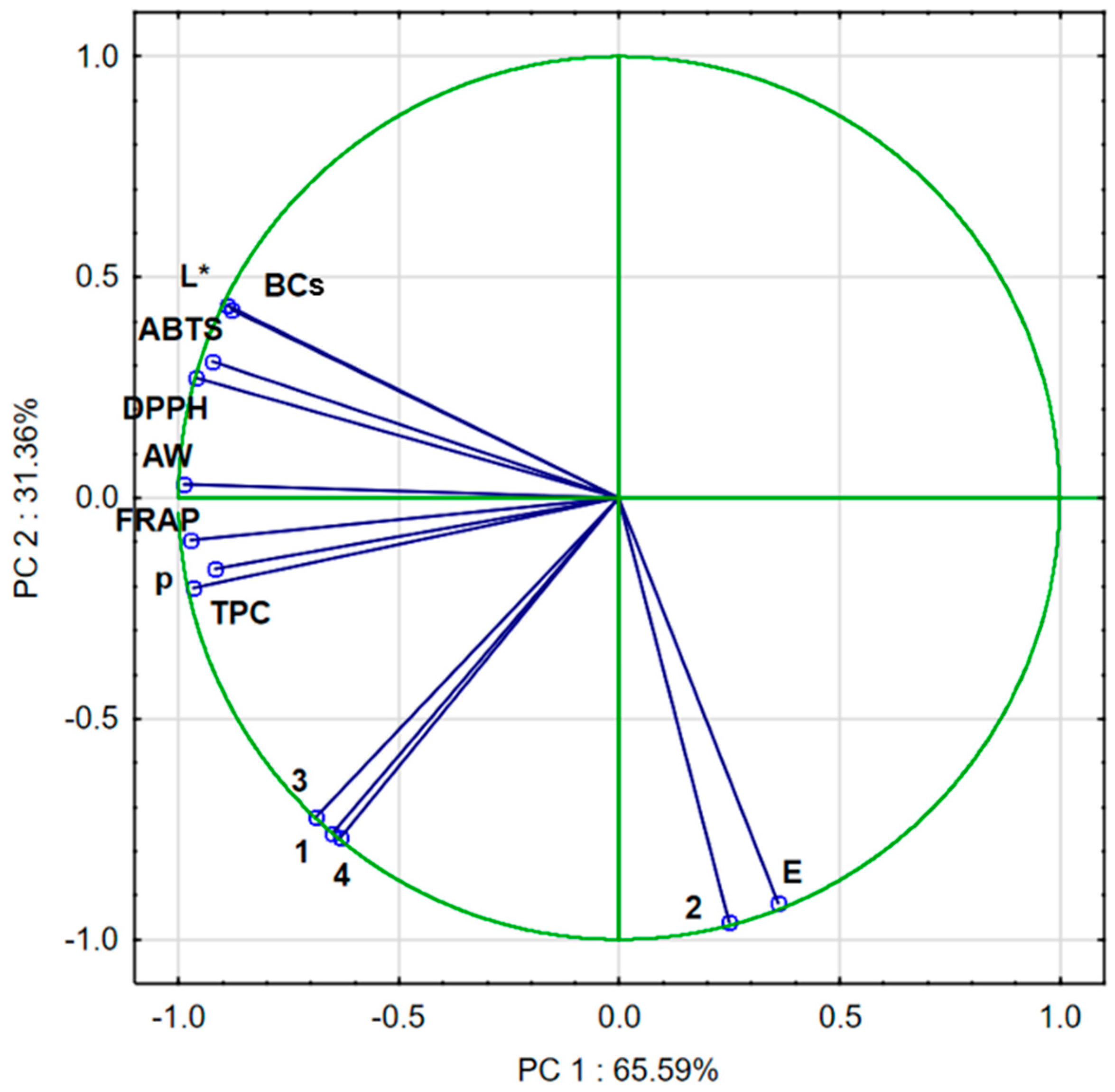
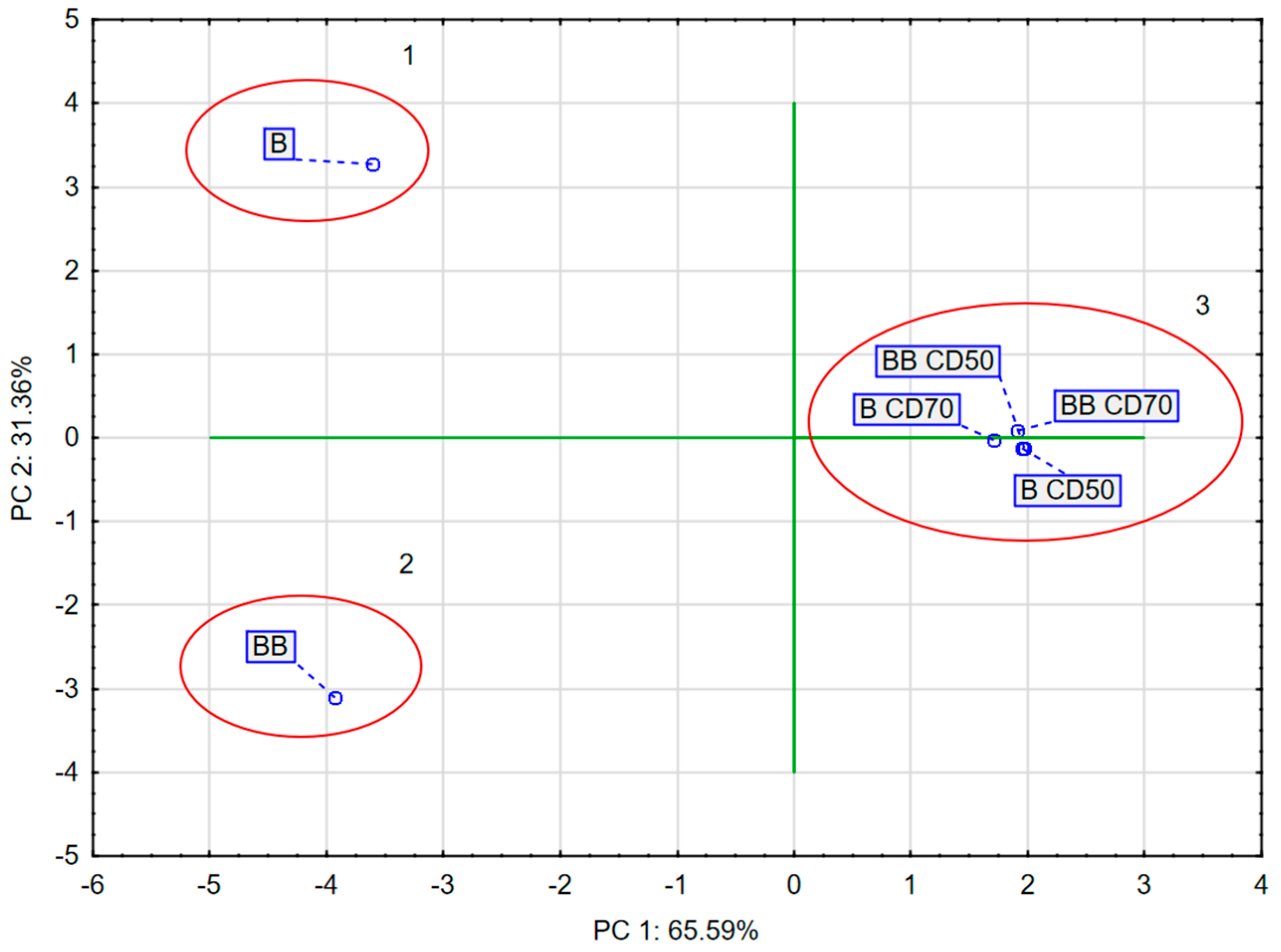
2.9. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Plants
3.2. Impregnation Solution
3.3. Pre-Treatment Before Drying Process
- m—mass of the impregnated sample;
- m0—initial mass.
3.4. Convective Drying (CD)
3.5. Bioactive Compounds, Antioxidant Capacity, and Phenols
3.5.1. Extraction Procedure
3.5.2. Identification of Bioactive Compounds by UPLC-PDA–MS Method
3.5.3. Antioxidant Capacity (AC) and Total Phenolic Content (TPC)
3.6. Mathematical Modeling
3.7. Physical Properties
3.7.1. Dry Weight
3.7.2. Water Activity
3.7.3. Bulk Density
3.7.4. Color
3.8. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manríquez-Zúñiga, A.N.; de la Torre, A.R.; Valdés-Santiago, L.; Hernández-Bustos, D.A.; Cuéllar-Sojo, S.; Hernández-Rayas, A.; Perez-Vega, S.; Molina-Guerrero, C.E. Broccoli Leaves (Brassica oleracea var. italica) as a Source of Bioactive Compounds and Chemical Building Blocks: Optimal Extraction Using Dynamic Maceration and Life Cycle Assessment. Sustainability 2023, 15, 16616. [Google Scholar] [CrossRef]
- Fanesi, B.; Ismaiel, L.; Nartea, A.; Orhotohwo, O.L.; Kuhalskaya, A.; Pacetti, D.; Lucci, P.; Falcone, P.M. Bioactives and Technological Quality of Functional Biscuits Containing Flour and Liquid Extracts from Broccoli By-Products. Antioxidants 2023, 12, 2115. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Toxqui-Terán, A.; Espinosa-Solis, V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var Italica) Stalk and Floret Juice Powders. Molecules 2021, 26, 1973. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Chróst, M.; Starowicz, M. Effect of Sous-Vide Processing Time on Chemical and Sensory Properties of Broccoli, Green Beans and Beetroots. Appl. Sci. 2023, 13, 4086. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Sujinda, N.; Saengsuwan, T.; Chaichana, N. A study on drying characteristics, color, and vitamin C preservation of green banana slices using a vacuum heat pump system. Agric. Eng. 2024, 28, 176–184. [Google Scholar] [CrossRef]
- Malkina, V.; Kiurchev, S.; Hutsol, T.; Verkholantseva, V.; Kiurcheva, L.; Miroshnichenko, M.; Biliuk, M.; Pidlisnyj, V.; Gurgulu, H.; Kowalczyk, Z. Optimization of parameters of a vibroconveyor system for infrared drying of soy. Agric. Eng. 2022, 26, 157–166. [Google Scholar] [CrossRef]
- Gavahian, M.; Nayi, P.; Masztalerz, K.; Szumny, A.; Figiel, A. Cold plasma as an emerging energy-saving pretreatment to enhance food drying: Recent advances, mechanisms involved, and considerations for industrial applications. Trends Food Sci. Technol. 2024, 143, 104210. [Google Scholar] [CrossRef]
- Menon, A.; Stojceska, V.; Tassou, S.A. A systematic review on the recent advances of the energy efficiency improvements in non-conventional food drying technologies. Trends Food Sci. Technol. 2020, 100, 67–76. [Google Scholar] [CrossRef]
- Kroehnke, J.; Szadzińska, J.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Musielak, G.; Mierzwa, D. Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa)—The influence of ultrasound on process kinetics and product quality. Ultrason. Sonochemistry 2021, 71, 105377. [Google Scholar] [CrossRef]
- Szychowski, P.J.; Lech, K.; Sendra-Nadal, E.; Hernández, F.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, A.A. Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying method. Food Chem. 2018, 255, 157–164. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Iwiński, H.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Coriandrum sativum L.—Effect of Multiple Drying Techniques on Volatile and Sensory Profile. Foods 2021, 10, 403. [Google Scholar] [CrossRef]
- Matys, A.; Nowacka, M.; Witrowa-Rajchert, D.; Wiktor, A. Chemical and Thermal Characteristics of PEF-Pretreated Strawberries Dried by Various Methods. Molecules 2024, 29, 3924. [Google Scholar] [CrossRef]
- Mieszczakowska-Frąc, M.; Dyki, B.; Konopacka, D. Effects of Ultrasound on Polyphenol Retention in Apples After the Application of Predrying Treatments in Liquid Medium. Food Bioprocess Technol. 2016, 9, 543–552. [Google Scholar] [CrossRef]
- Panayampadan, A.S.; Alam, M.S.; Aslam, R.; Kaur, J. Vacuum Impregnation Process and Its Potential in Modifying Sensory, Physicochemical and Nutritive Characteristics of Food Products. Food Eng. Rev. 2022, 14, 229–256. [Google Scholar] [CrossRef]
- Aguirre-García, M.; Hernández-Carranza, P.; Cortés-Zavaleta, O.; Ruiz-Espinosa, H.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Mass transfer analysis of bioactive compounds in apple wedges impregnated with beetroot juice: A 3D modelling approach. J. Food Eng. 2020, 282, 110003. [Google Scholar] [CrossRef]
- Tappi, S.; Velickova, E.; Mannozzi, C.; Tylewicz, U.; Laghi, L.; Rocculi, P. Multi-Analytical Approach to Study Fresh-Cut Apples Vacuum Impregnated with Different Solutions. Foods 2022, 11, 488. [Google Scholar] [CrossRef]
- Nuñez, H.; Jaques, A.; Belmonte, K.; Elitin, J.; Valdenegro, M.; Ramírez, C.; Córdova, A. Development of an Apple Snack Enriched with Probiotic Lacticaseibacillus rhamnosus: Evaluation of the Refractance Window Drying Process on Cell Viability. Foods 2024, 13, 1756. [Google Scholar] [CrossRef]
- Trusińska, M.; Drudi, F.; Rybak, K.; Tylewicz, U.; Nowacka, M. Effect of the Pulsed Electric Field Treatment on Physical, Chemical and Structural Changes of Vacuum Impregnated Apple Tissue in Aloe Vera Juices. Foods 2023, 12, 3957. [Google Scholar] [CrossRef]
- Assis, F.R.; Rodrigues, L.G.G.; Tribuzi, G.; de Souza, P.G.; Carciofi, B.A.M.; Laurindo, J.B. Fortified apple (Malus spp., var. Fuji) snacks by vacuum impregnation of calcium lactate and convective drying. LWT 2019, 113, 108298. [Google Scholar] [CrossRef]
- Chong, J.X.; Lai, S.; Yang, H. Chitosan combined with calcium chloride impacts fresh-cut honeydew melon by stabilising nanostructures of sodium-carbonate-soluble pectin. Food Control 2015, 53, 195–205. [Google Scholar] [CrossRef]
- González-Pérez, J.E.; Jiménez-González, O.; Ramírez-Corona, N.; Guerrero-Beltrán, J.A.; López-Malo, A. Vacuum Impregnation on Apples with Grape Juice Concentrate: Effects of Pressure, Processing Time, and Juice Concentration. Innov. Food Sci. Emerg. Technol. 2022, 77, 102981. [Google Scholar] [CrossRef]
- Demir, H.; Çelik, S.; Sezer, Y.Ç. Effect of ultrasonication and vacuum impregnation pretreatments on the quality of beef marinated in onion juice a natural meat tenderizer. Food Sci. Technol. Int. 2021, 28, 340–352. [Google Scholar] [CrossRef]
- Arnal, M.; Gallego, M.; Mora, L.; Talens, P. Vacuum impregnation as a sustainable technology to obtain iron-fortified broad bean (Vicia faba) flours. Food Funct. 2023, 14, 5429–5441. [Google Scholar] [CrossRef]
- de Oliveira, P.M.; Ramos, A.M.; Martins, E.M.F.; Vieira, É.N.R.; de Souza Soares, A.; de Noronha, M.C. Comparison of vacuum impregnation and soaking techniques for addition of the probiotic Lactobacillus acidophilus to minimally processed melon. Int. J. Food Sci. Technol. 2017, 52, 2547–2554. [Google Scholar] [CrossRef]
- Kręcisz, M.; Stępień, B.; Łyczko, J.; Kamiński, P. The Influence of the Vacuum Impregnation, Beetroot Juice, and Various Drying Methods on Selected Properties of Courgette and Broccoli Snacks. Foods 2023, 12, 4294. [Google Scholar] [CrossRef]
- Alzamora, S.M.; Salvatori, D.; Tapia, M.S.; López-Malo, A.; Welti-Chanes, J.; Fito, P. Novel functional foods from vegetable matrices impregnated with biologically active compounds. J. Food Eng. 2005, 67, 205–214. [Google Scholar] [CrossRef]
- Vinod, B.R.; Asrey, R.; Sethi, S.; Menaka, M.; Meena, N.K.; Shivaswamy, G. Recent advances in vacuum impregnation of fruits and vegetables processing: A concise review. Heliyon 2024, 10, e28023. [Google Scholar] [CrossRef]
- Fernandez-Leon, M.F.; Fernandez-Leon, A.M.; Lozano, M.; Ayuso, M.C.; Gonzalez-Gómez, D. Identification, quantification and comparison of the principal bioactive compounds and external quality parameters of two broccoli cultivars. J. Funct. Foods 2012, 4, 465–473. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Perez-Vicente, A.; García-Viguera, C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem. 2024, 52, 135–138. [Google Scholar] [CrossRef]
- Li, S.; Lin, J.; Wei, J.; Zhou, L.; Wang, P.; Qu, S. Sinigrin Impedes the Breast Cancer Cell Growth through the Inhibition of PI3K/AKT/MTOR Phosphorylation-Mediated Cell Cycle Arrest. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 33–43. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T. Targeting cancer stem cells with sulforaphane, a dietary component from broccoli and broccoli sprouts. Future Oncol. 2013, 9, 1097–1103. [Google Scholar] [CrossRef]
- Gudino, I.; Martin, A.; Casquete, R.; Prieto, M.H.; Ayuso, M.C.; Córdoba, M.G. Evaluation of broccoli (Brassica oleracea var. italica) crop by-products as sources of bioactive compounds. Sci. Hortic. 2022, 304, 111284. [Google Scholar] [CrossRef]
- Sallas-Millan, J.A.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional food obtained from fermentation of broccoli by-products (stalk): Metagenomics profile and glucosinolate and phenolic compounds characterization by LC-ESI-QqQ-MS/MS. LWT-Food Sci. Technol. 2022, 169, 113915. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihjala, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Kidoń, M. Zastosowanie impregnacji próżniowej do modyfikacji właściwości fizykochemicznych, sensorycznych i odżywczych produktów pochodzenia roślinnego—Przegląd. Int. J. Mol. Sci. 2014, 15, 16577. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Rodríguez-Lafitte, E.; Villagrán, Z.; Aurora-Vigo, E.F.; Ruvalcaba-Gómez, J.M.; Símpalo-López, W.B.; Martínez-Esquivias, F.; Sarango-Córdova, C.H. Optimization of Vacuum Impregnation with Aqueous Extract from Hibiscus sabdariffa Calyces in Apple Slices by Response Surface Methodology: Effect on Soluble Phenols, Flavonoids, Antioxidant Activity, and Physicochemical Parameters. Appl. Sci. 2024, 14, 10850. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Pasławska, M.; Stępień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of Vacuum Impregnation with Apple-Pear Juice on Content of Bioactive Compounds and Antioxidant Activity of Dried Chokeberry Fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef]
- Kręcisz, M.; Kolniak-Ostek, J.; Stępień, B.; Łyczko, J.; Pasławska, M.; Musiałowska, J. Influence of Drying Methods and Vacuum Impregnation on Selected Quality Factors of Dried Sweet Potato. Agriculture 2021, 11, 858. [Google Scholar] [CrossRef]
- Kręcisz, M.; Kolniak-Ostek, J.; Łyczko, J.; Stępień, B. Evaluation of bioactive compounds, volatile compounds, drying process kinetics and selected physical properties of vacuum impregnation celery dried by different methods. Food Chem. 2023, 413, 135490. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska-Kubzdela, E.; Szadzińska, J.; Biegańska-Marecik, R.; Spiżewski, T.; Mierzwa, D. Effect of ultrasound on mass transfer during vacuum impregnation and selected quality parameters of products: A case study of carrots. Ultrason. Sonochemistry 2023, 99, 106592. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochemistry 2019, 58, 104643. [Google Scholar] [CrossRef]
- Md Salim, N.S.; Gariépy, Y.; Raghavan, V. Suszenie gorącym powietrzem i suszenie gorącym powietrzem wspomagane mikrofalami plastrów łodyg brokułów (Brassica oleracea L. var. Italica). J. Food Process Preserv. 2016, 41, e12905. [Google Scholar] [CrossRef]
- Zielinska, M.; Markowski, M. Air drying characteristics and moisture diffusivity of carrots. Chem. Eng. Process. Process Intensif. 2010, 49, 212–218. [Google Scholar] [CrossRef]
- Kręcisz, M.; Klemens, M.; Latański, A.; Stępień, B. The Use of Beetroot Juice as an Impregnating Solution to Change Volatile Compounds, Physical Properties and Influence the Kinetics of the Celery Drying Process. Molecules 2024, 29, 4050. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules 2021, 26, 4597. [Google Scholar] [CrossRef]
- Kręcisz, M.; Stępień, B.; Pikor, K. Effect of Tomato Juice and Different Drying Methods on Selected Properties of Courgette. Appl. Sci. 2024, 14, 7105. [Google Scholar] [CrossRef]
- Liu, K.; Hao, Y.; Chen, Y.; Gao, Q. Effects of dry heat treatment on the structure and physicochemical properties of waxy potato starch. Int. J. Biol. Macromol. 2019, 132, 1044–1050. [Google Scholar] [CrossRef]
- Xu, H.; Wu, M.; Zhang, X.; Wang, B.; Wang, S.; Zheng, Z.; Li, D.; Wang, F. Application of blanching pretreatment in herbaceous peony (Paeonia lactiflora Pall.) flower processing: Improved drying efficiency, enriched volatile profile and increased phytochemical content. Ind. Crops Prod. 2022, 188, 115663. [Google Scholar] [CrossRef]
- Mowafy, S.; Guo, J.; Lei, D.; Liu, Y. Application of novel blanching and drying technologies improves the potato drying kinetics and maintains its physicochemical attributes and flour functional properties. Innov. Food Sci. Emerg. Technol. 2024, 94, 103648. [Google Scholar] [CrossRef]
- Arslan, D.; Musa Özcan, M.M.; Mengeş, H.O. Evaluation of drying methods with respect to drying parameters, some nutritional and colour characteristics of peppermint (Mentha x piperita L.). Energy Convers. Manag. 2010, 51, 2769–2775. [Google Scholar] [CrossRef]
- Jałoszyński, K.; Szarycz, M.; Jarosz, B. The Effect of Convective and Microwave-Vacuum Drying on the Preservation of Aromatic Compounds in Parsley (PL). Agric. Eng. 2006, 12, 209–215. [Google Scholar]
- Kolniak-Ostek, J.; Oszmianski, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of Sea Buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Kaushal, P.; Sharma, H.K. Osmo-convective dehydration kinetics of jackfruit (Artocarpus heterophyllus). J. Saudi Soc. Agric. Sci. 2016, 15, 118–126. [Google Scholar] [CrossRef]
- Sarimeseli, A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Henderson, S.M.; Pabis, S. Grain Drying Theory II: Temperature Effect on Drying Coefficient. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Demir, V.; Gunhan, T.; Yagcioglu, A.K.; Degrmencioglu, A. Mathematical modeling and the determination of some quality parameters of air-dried bay leaves. Biosyst. Eng. 2004, 88, 325–335. [Google Scholar] [CrossRef]
- Soysal, Y.; Öztekin, S.; Eren, Ö. Microwave drying of parsley: Modeling, kinetics, and energy aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- ASAE S269.3; Wafers, Pellet, and Crumbles—Definitions and Methods for Determining Density, Durability and Moisture Content. ASAE Standard: Washington, DC, USA, 1989.


| Rt (min) | [M−H]− (m/z) a | MS/MS Fragments (m/z) a | Compound | B | B CD50 | B CD70 | BB | BB CD50 | BB CD70 |
|---|---|---|---|---|---|---|---|---|---|
| 2.56 | 341.0826 | 179.0250/161.0179 | Caffeoyl hexoside | 16.02 ± 0.25 e | 0.25 ± 0.04 b | 0.40 ± 0.12 c | 4.60 ± 0.62 d | 0.19 ± 0.05 a | 0.19 ± 0.06 a |
| 3.28 | 353.0844 | 191.0560/179.0348 | Caffeoylquinic acid | 87.02 ± 2.15 f | 38.34 ± 1.15 e | 33.39 ± 1.18 d | 23.89 ± 1.26 c | 13.57 ± 0.74 a | 15.91 ± 0.84 b |
| 3.35 | 353.0811 | 179.0290 | Caffeoylquinic acid | 0.49 ± 0.04 b | 0.29 ± 0.11 a | 0.66 ± 0.09 c | 7.26 ± 0.35 e | 0.94 ± 0.04 d | 0.92 ± 0.14 d |
| 3.40 | 447.0410 | 357.0850 | Orientin b | 0.80 ± 0.02 c | 0.56 ± 0.54 b | 0.28 ± 0.03 a | 7.01 ± 0.24 d | 0.20 ± 0.26 a | 0.19 ± 0.03 a |
| 3.85 | 609.1469 | 447.0531/285.0361 | Kaempferol diglucoside | 239.69 ± 4.13 e | 4.85 ± 0.16 a | 5.17 ± 0.12 b | 41.04 ± 1.32 d | 8.28 ± 0.86 c | 4.61 ± 0.24 a |
| 4.03 | 337.0337 | 191.0497/163.0337 | Coumaroyl-quinic acid | 1.94 ± 0.06 c | 1.18 ± 0.04 b | 1.27 ± 0.15 b | 9.61 ± 0.36 d | 0.79 ± 0.26 a | 0.89 ± 0.36 a |
| 4.20 | 353.0904 | 191.0596/ | Caffeoylquinic acid b | 21.95 ± 1.18 | 1.25 ± 0.14 | 1.95 ± 0.08 | 16.35 ± 1.65 | 1.78 ± 0.42 | 1.44 ± 0.05 |
| 4.22 | 1111.3350 | 949.2182/301.0816 | Quercetin-3-caffeoyltriglucoside-7-glucoside | 2.69 ± 0.08 c | 1.11 ± 0.06 b | 0.27 ± 0.06 a | 5.50 ± 0.54 d | 0.41 ± 0.32 a | 5.41 ± 0.23 d |
| 4.48 | 1095.0083 | 933.4816/787.2160 | Kempferol-3-caffeoyltriglucoside-7-glucoside | 6.31 ± 0.04 c | 12.09 ± 0.45 d | 11.24 ± 0.12 d | 1.33 ± 0.26 a | 5.69 ± 0.16 b | 13.77 ± 0.54 e |
| 4.83 | 1139.2931 | 977.2447/285.0431 | Kempferol-3-sinapoylsophorotrioside-7-glucosided | 2.03 ± 0.19 a | 2.28 ± 0.08 a | 2.64 ± 0.20 b | 3.84 ± 0.36 c | 4.39 ± 0.54 d | 3.00 ± 0.64 c |
| 4.94 | 385.1083 | 205.0451/189.0714 | Sinapoyl hexose | 56.49 ± 2.53 g | 7.63 ± 0.34 d | 4.10 ± 0.26 b | 10.60 ± 0.64 f | 5.99 ± 0.12 c | 1.21 ± 0.08 a |
| 5.80 | 609.1398 | 447.0898/285.0439 | Kaempferol diglucoside | 68.74 ± 2.64 d | 11.12 ± 0.04 a | 10.04 ± 0.42 a | 31.68 ± 0.64 c | 11.14 ± 0.24 a | 18.58 ± 01.24 b |
| 6.51 | 341.0989 | 12.58 ± 1.24 b | 18.13 ± 1.06 c | 12.20 ± 0.65 b | 31.01 ± 0.68 | 10.38 ± 0.46 a | 18.10 ± 0.67 c | ||
| 6.53 | 301.0023 | 723.1643/429.1104 | Quercetin b | 11.52 ± 1.36 a | 38.35 ± 1.74 c | 19.17 ± 0.34 b | 60.20 ± 1.12 d | 17.53 ± 0.84 b | 25.00 ± 1.29 b |
| 6.91 | 359.1560 | 197.0451/179.1125/161.0216 | Rosmarinic acid b | 1.62 ± 0.02 b | 2.32 ± 0.06 c | 2.23 ± 0.08 c | 0.90 ± 0.04 a | 2.78 ± 0.22 d | 4.23 ± 0.46 e |
| 7.15 | 947.2666 | 785.2501/293.0054 | Kempferol-3-feruloyldiglucoside-7-glucoside | 47.88 ± 2.54 e | 8.61 ± 0.13 c | 6.97 ± 0.64 b | 111.59 ± 2.65 f | 5.28 ± 0.46 a | 23.65 ± 0.58 d |
| 7.35 | 1095.2855 | 787.1799/285.1093 | Kempferol-3-caffeoyltriglucoside-7-glucoside | 5.90 ± 0.42 a | 24.87 ± 0.54 e | 15.52 ± 0.16 b | 17.72 ± 1.12 c | 22.98 ± 0.98 d | 17.24 ± 1.16 c |
| 7.58 | 577.1445 | 285.0321 | Kempferol-3,7-O-di-rhamnopyranoside | 15.28 ±0.65 f | 5.26 ± 0.32 d | 1.06 ± 0.08 a | 10.56 ± 1.24 e | 1.81 ± 0.21 c | 1.42 ± 0.28 b |
| 8.12 | 919.3005 | 723.1643/429.1104/301.0721 | Quercetin derivative | 10.12 ± 0.24 c | 1.62 ± 0.14 a | 1.90 ± 0.16 b | 9.28 ± 1.16 c | 2.13 ± 0.08 b | 2.02 ± 0.62 b |
| 8.31 | 753.2167 | 523.1585/223.0616/205.0515 | 1,2-Disinapoyl-gentobioside | 5.40 ± 0.16 a | 70.13 ± 2.25 d | 74.18 ± 2.14 d | 23.12 ± 1.54 b | 90.89 ± 2.54 e | 48.47 ± 2.12 c |
| 8.60 | 723.2103 | 499.1444/175.0398 | 1-sinapoyl-2-feruloyl-gentibiose | 21.13 ± 1.31 b | 93.64 ± 7.32 d | 104.85 ± 3.16 e | 3.94 ± 0.62 a | 122.49 ± 3.25 f | 73.89 ± 1.98 c |
| 8.81 | 1139.2924 | 977.4442/285.1507 | Kempferol-3-sinapoylsophorotrioside-7-glucoside | 3.90 ± 0.24 a | 20.55 ± 1.02 e | 19.85 ± 1.08 d | 14.22 ± 0.52 c | 11.99 ± 1.12 b | 10.20 ± 0.52 b |
| 8.93 | 693.2132 | 499.1516/259.5630 | 1,2-diferuoyl-diglucoside | 1.19 ± 0.14 c | 0.93 ± 0.24 c | 0.80 ± 0.08 b | 9.29 ± 0.32 d | 0.39 ± 0.36 a | 0.87 ± 0.26 b |
| 9.21 | 959.2661 | 735.3117/511.1374 | Trisinapoyl-gentionbiose | 2.72 ± 0.04 a | 62.78 ± 2.47 c | 62.23 ± 1.74 c | 85.71 ± 3.52 | 60.22 ± 0.76 b | 52.04 ± 1.14 d |
| 9.49 | 929.2539 | 723.2125 | Feruloyl-disinapoyl-gentionbiose | 1.99 ± 0.27 a | 18.96 ± 1.12 e | 16.63 ± 1.54 d | 5.85 ± 0.24 b | 22.22 ± 0.24 f | 11.90 ± 0.76 c |
| 9.81 | 1109.3099 | 947.0086/285.0297 | Kempferol-3-feruloylsophorotrioside | 5.32 ± 0.67 b | 10.00 ± 0.64 c | 9.86 ± 0.68 c | 2.61 ± 0.12 a | 9.77 ± 0.56 c | 6.86 ± 1.08 a |
| 10.07 | 929.2347 | 705.1979 | Feruloyl-disinapoyl-gentionbiose | 1.11 ± 0.17 b | 1.61 ± 0.18 b | 2.06 ± 0.16 c | 1.66 ± 0.32 b | 1.16 ± 0.06 b | 0.52 ± 0.36 a |
| 10.56 | 489.2639 | Kaempferol 3-O-(6″-acetyl-hexoside) | 9.01 ± 0.63 f | 2.11 ± 0.14 b | 1.66 ± 0.24 a | 6.90 ± 0.68 e | 4.28 ± 0.23 d | 3.62 ± 0.09 c | |
| 10.73 | 959.2738 | 735.1979/511.2109 | Trisinapoyl-gentionbiose | 446.37 ± 0.42 e | 3.69 ± 0.08 b | 2.37 ± 0.35 a | 15.50 ± 0.54 d | 5.13 ± 0.42 c | 3.28 ± 0.11 b |
| 10.85 | 625.4776 | /463.2062/301.0021 | Quercetin-3,4′-O-di-beta-glucoside | 0.81 ± 0.06 a | 2.43 ± 0.12 b | 2.86 ± 0.42 b | 166.88 ± 4.54 c | 2.21 ± 0.09 b | 2.44 ± 0.25 b |
| Total [mg/100g] | 1107.87 ± 33.79 | 466.92 ± 16.54 | 427.82 ± 13.12 | 739.64 ± 24.98 | 446.95 ± 11.86 | 371.85 ± 9.46 |
| Rt (min) | [M−H]− | MS/MS | Compound |
|---|---|---|---|
| (m/z) | Fragments (m/z) | ||
| 2.60 | 341.0860 | Vulgaxanthin II | |
| 2.63 | 356.0997 | Cyclodopa glucoside | |
| 2.93 | 385.1063 | N-Formylcyclodopa glucoside | |
| 5.29 | 551.1737 | 389.1350 | Betanin |
| 9.15 | 535.1428 | Mono-decarboxy betanin |
| Rt (min) | [M−H]− | MS/MS | Compound |
|---|---|---|---|
| (m/z) | Fragments (m/z) | ||
| 0.90 | 195.0461 | 177.0476/129.0088 | Gluconic acid |
| 1.87 | 436.0401 | 372.0553/178.0165 | Glucoraphanin |
| 3.19 | 447.0482 | 259.0295/134.0551 | Glucobrassicin |
| 3.76 | 447.0535 | 254.1377/139.0027 | Indolymethyl glucosinolate |
| 4.66 | 422.0568 | 358.1490/259.0676 | Glucoiberin |
| 4.97 | 477.0605 | 284.0360/154.0144 | Neoglucobrassicin |
| 6.16 | 477.0588 | 235.9673 | Methoxyglucobrassicin 1 |
| 6.32 | 477.0568 | 235.9234 | Methoxyglucobrassicin 2 |
| Method | ABTS | DPPH | FRAP | TPC |
|---|---|---|---|---|
| B | 58.98 ± 2.28 f | 88.60 ± 3.13 e | 41.60 ± 2.22 e | 9.82 ± 0.58 d |
| B CD50 | 19.07 ± 0.24 b | 21.78 ± 0.72 b | 15.50 ± 1.08 b | 2.96 ± 0.38 a |
| B CD70 | 29.55 ± 0.74 d | 22.39 ± 0.38 b | 22.92 ± 1.73 d | 4.81 ± 0.46 c |
| BB | 44.79 ± 2.42 e | 66.03 ± 2.62 d | 47.79 ± 3.12 f | 13.06 ± 0.97 e |
| BB CD50 | 16.97 ± 0.76 a | 16.57 ± 0.56 a | 13.20 ± 0.62 a | 2.63 ± 0.14 a |
| BB CD70 | 24.07 ± 1.56 c | 24.34 ± 1.98 c | 19.72 ± 1.04 c | 3.50 ± 0.32 b |
| Material | WG (%) | °Bx | DM (%) |
|---|---|---|---|
| B | - | - | 12.87 ± 0.57 |
| BB | 14.88 ± 0.43 | 10.3 ± 0.2 | 11.95 ± 0.43 |
| Drying Method | Material | Model Parameters | Statistical Parameters | Drying Time [min] | |||||
|---|---|---|---|---|---|---|---|---|---|
| k | a | b | RMSE | Ve [%] | R2 | χ2 | |||
| Logistical Model | |||||||||
| CD50 | B | 0.0167 | 1962.7203 | 1870.3274 | 0.0165 | 5.1 | 0.9972 | 0.0003 | 290 |
| BB | 0.0110 | 1589.9455 | 1488.6300 | 0.0990 | 5.6 | 0.9959 | 0.0004 | 350 | |
| CD70 | B | 0.0388 | 2.4187 | 3.3763 | 0.0072 | 2.1 | 0.9995 | 0.0001 | 150 |
| BB | 0.0359 | 2.1818 | 3.1279 | 0.0093 | 2.9 | 0.9992 | 0.0001 | 170 | |
| Logarithmic Model | |||||||||
| CD50 | B | 0.0163 | 0.9270 | 0.0041 | 0.0113 | 3.8 | 0.9983 | 0.0001 | 290 |
| BB | 0.0110 | 0.9320 | 0.0000 | 0.0989 | 5.6 | 0.9959 | 0.0004 | 350 | |
| CD70 | B | 0.0330 | 1.0199 | 0.0000 | 0.0134 | 4.6 | 0.9982 | 0.0002 | 150 |
| BB | 0.0300 | 1.0149 | 0.0000 | 0.0153 | 5.5 | 0.9976 | 0.0003 | 170 | |
| Henderdon and Pabis Model | |||||||||
| CD50 | B | 0.0167 | 0.9525 | - | 0.01652 | 5.1 | 0.9972 | 0.0003 | 290 |
| BB | 0.0110 | 0.9322 | - | 0.0199 | 5.6 | 0.9959 | 0.0004 | 350 | |
| CD70 | B | 0.0326 | 1.0097 | - | 0.0135 | 4.0 | 0.9986 | 0.0002 | 150 |
| BB | 0.0297 | 1.0078 | - | 0.0150 | 4.8 | 0.9982 | 0.0003 | 170 | |
| Newton Model | |||||||||
| CD50 | B | 0.0179 | - | - | 0.0235 | 7.2 | 0.9972 | 0.0006 | 290 |
| BB | 0.0121 | - | - | 0.0331 | 9.3 | 0.9941 | 0.0012 | 350 | |
| CD70 | B | 0.0322 | - | - | 0.0139 | 4.2 | 0.9988 | 0.0002 | 150 |
| BB | 0.0294 | - | - | 0.0153 | 4.8 | 0.9984 | 0.0003 | 170 | |
| Page Model | |||||||||
| CD50 | B | 0.0297 | 0.8734 | - | 0.0075 | 2.3 | 0.9995 | 0.0001 | 290 |
| BB | 0.0224 | 0.8620 | - | 0.0203 | 5.7 | 0.9961 | 0.0004 | 350 | |
| CD70 | B | 0.0276 | 1.0338 | - | 0.0071 | 2.2 | 0.9995 | 0.0001 | 150 |
| BB | 0.0236 | 1.0624 | - | 0.0119 | 3.8 | 0.9987 | 0.0002 | 170 | |
| Method | Water Activity [-] | Density [kg/m3] |
|---|---|---|
| B | 0.987 ± 0.007 e | 240.94 ± 5.05 d |
| B CD50 | 0.469 ± 0.008 c | 111.34 ± 4.00 b |
| B CD70 | 0.436± 0.001 a | 84.16 ± 1.73 a |
| BB | 0.382 ± 0.008 e | 288.90 ± 5.98 d |
| BB CD50 | 0.354 ± 0.010 d | 116.16 ± 2.95 b |
| BB CD70 | 0.459 ± 0.005 b | 93.75 ± 2.87 c |
| Color Parameters | B | B CD50 | B CD70 | BB | BB CD50 | BB CD70 |
|---|---|---|---|---|---|---|
| L | 32.54 ± 1.09 e | 18.35 ± 0.59 c | 15.75 ± 0.91 b | 23.86 ± 0.56 d | 16.74 ± 0.40 b | 14.62 ± 1.02 a |
| A | −5.12 ± 0.61 a | −1.90 ± 0.16 b | −0.66 ± 1.12 b | 1.35 ± 0.04 c | 2.85 ± 0.53 d | 3.40 ± 0.26 e |
| B | 6.04 ± 0.76 c | 10.24 ± 0.38 e | 7.95 ± 0.33 d | 1.36 ± 0.11 a | 7.45 ± 0.55 d | 5.56 ± 0.71 b |
| C* | 5.60 ± 0.78 b | 10.15 ± 0.38 e | 7.91 ± 0.34 d | 1.79 ± 0.08 a | 7.64 ± 0.54 d | 5.86 ± 0.68 b |
| BI | 1.99 ± 2.09 a | 62.31 ± 3.2 c | 61.85 ± 10.59 c | 12.03 ± 0.69 b | 80.45 ± 6.17 e | 77.62 ± 9.87 d |
| ∆E | - | 15.15 b | 17.48 c | 11.80 a | 17.75 c | 19.85 d |
| Code | Material | Type of Drying |
|---|---|---|
| B | Broccoli | - |
| B CD50 | Broccoli | Convective drying at 50 °C |
| B CD70 | Broccoli | Convective drying at 70 °C |
| BB | Broccoli after impregnation with beetroot juice | - |
| BB CD50 | Broccoli after impregnation with beetroot juice | Convective drying at 50 °C |
| BB CD70 | Broccoli after impregnation with beetroot juice | Convective drying at 70 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kręcisz, M.; Kolniak-Ostek, J.; Stępień, B.; Combrzyński, M. Bio-Compounds, Antioxidant Activity, and Phenolic Content of Broccoli After Impregnation with Beetroot Juice. Molecules 2025, 30, 2143. https://doi.org/10.3390/molecules30102143
Kręcisz M, Kolniak-Ostek J, Stępień B, Combrzyński M. Bio-Compounds, Antioxidant Activity, and Phenolic Content of Broccoli After Impregnation with Beetroot Juice. Molecules. 2025; 30(10):2143. https://doi.org/10.3390/molecules30102143
Chicago/Turabian StyleKręcisz, Magdalena, Joanna Kolniak-Ostek, Bogdan Stępień, and Maciej Combrzyński. 2025. "Bio-Compounds, Antioxidant Activity, and Phenolic Content of Broccoli After Impregnation with Beetroot Juice" Molecules 30, no. 10: 2143. https://doi.org/10.3390/molecules30102143
APA StyleKręcisz, M., Kolniak-Ostek, J., Stępień, B., & Combrzyński, M. (2025). Bio-Compounds, Antioxidant Activity, and Phenolic Content of Broccoli After Impregnation with Beetroot Juice. Molecules, 30(10), 2143. https://doi.org/10.3390/molecules30102143








