Film Coating of Small Molded Tablets for Pediatric Formulations with Rapid Disintegration and Bitterness-Masking Properties
Abstract
1. Introduction
2. Results and Discussion
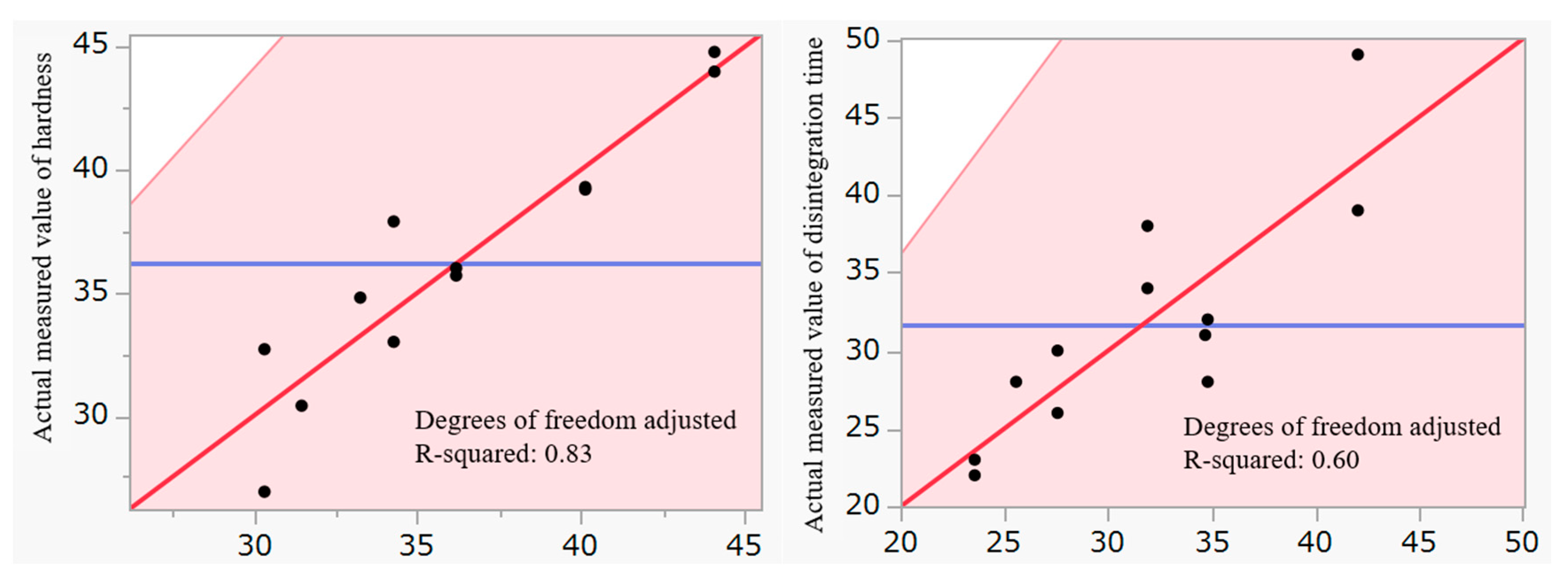
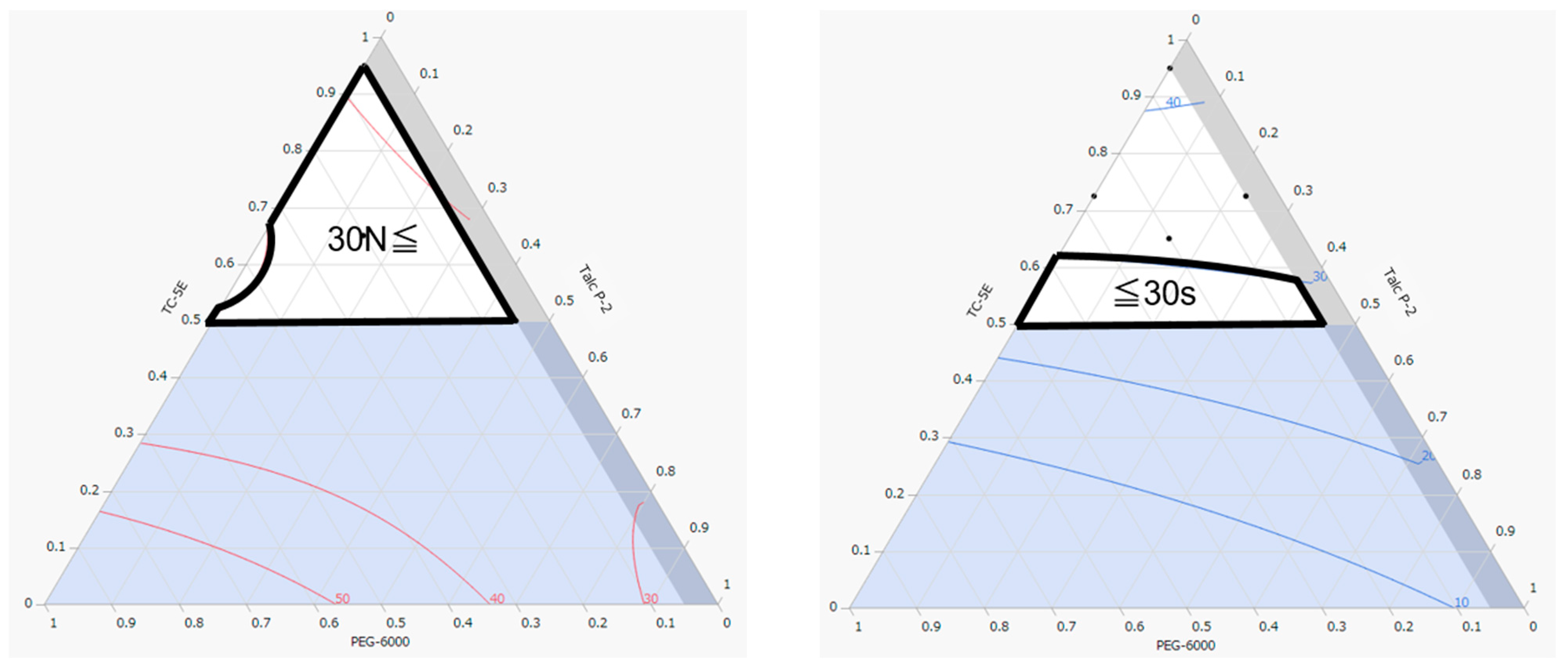
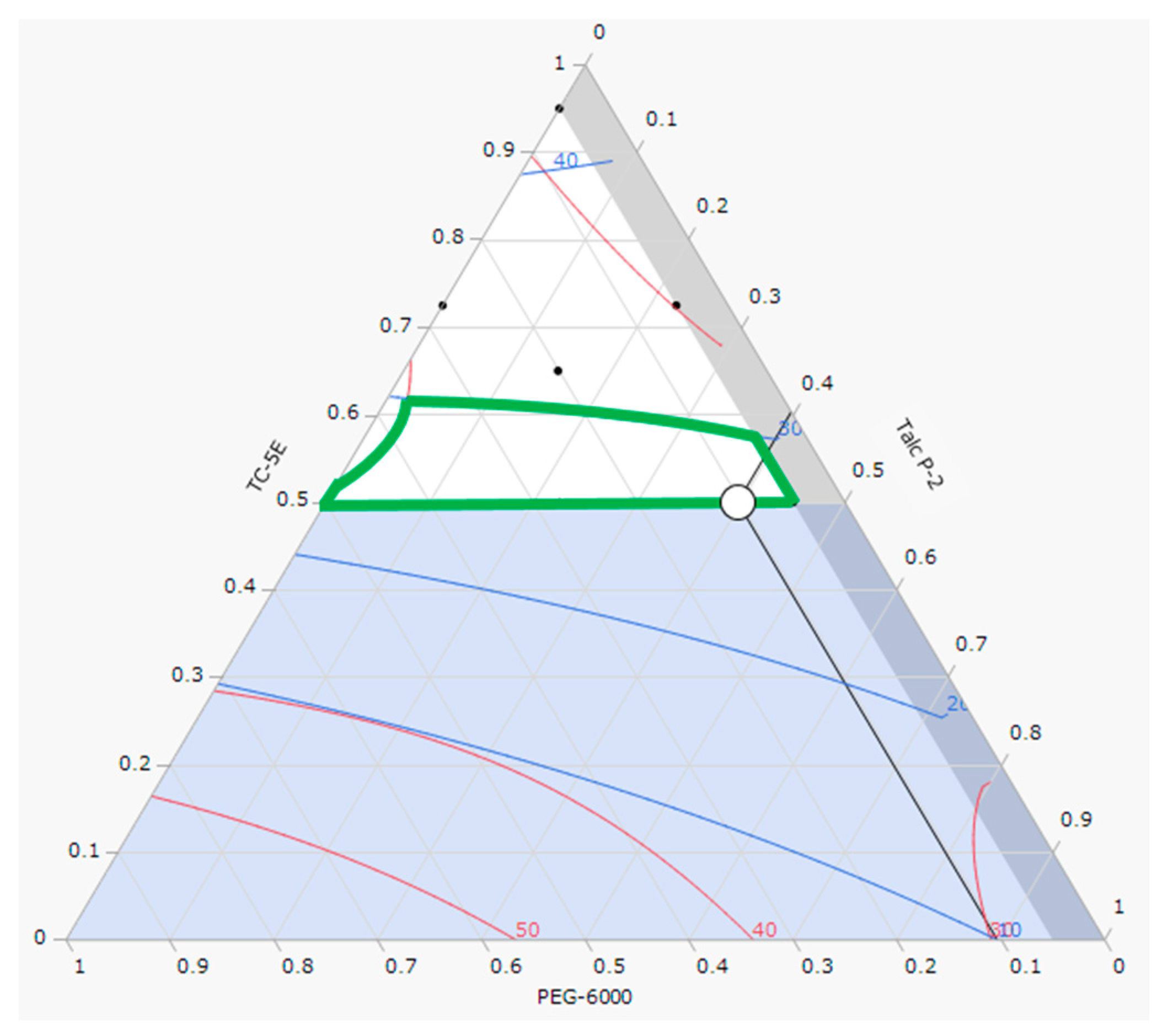
2.1. Optimization of Film Coating Formulation
2.2. Coating of 5 mg Dex Tablets
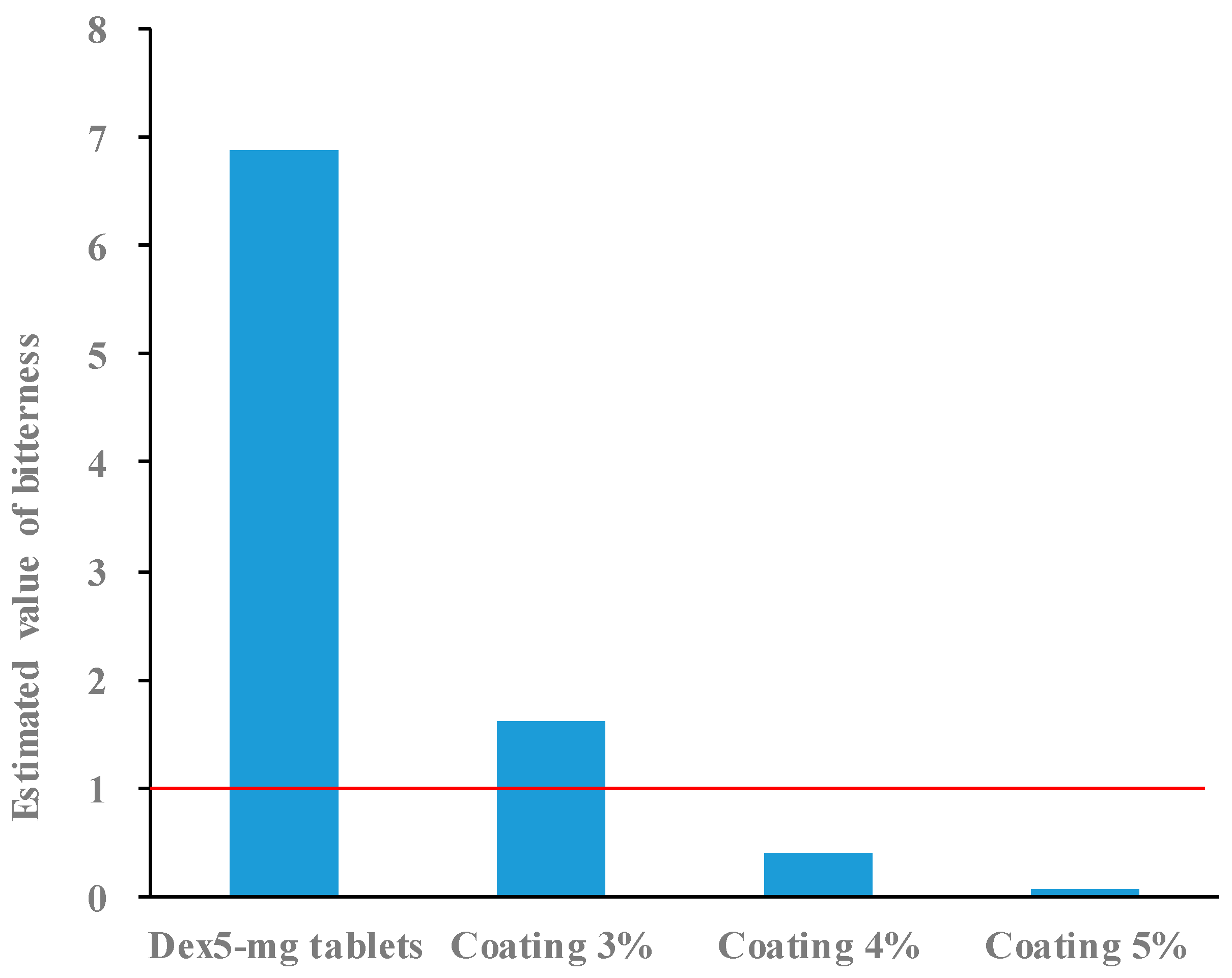
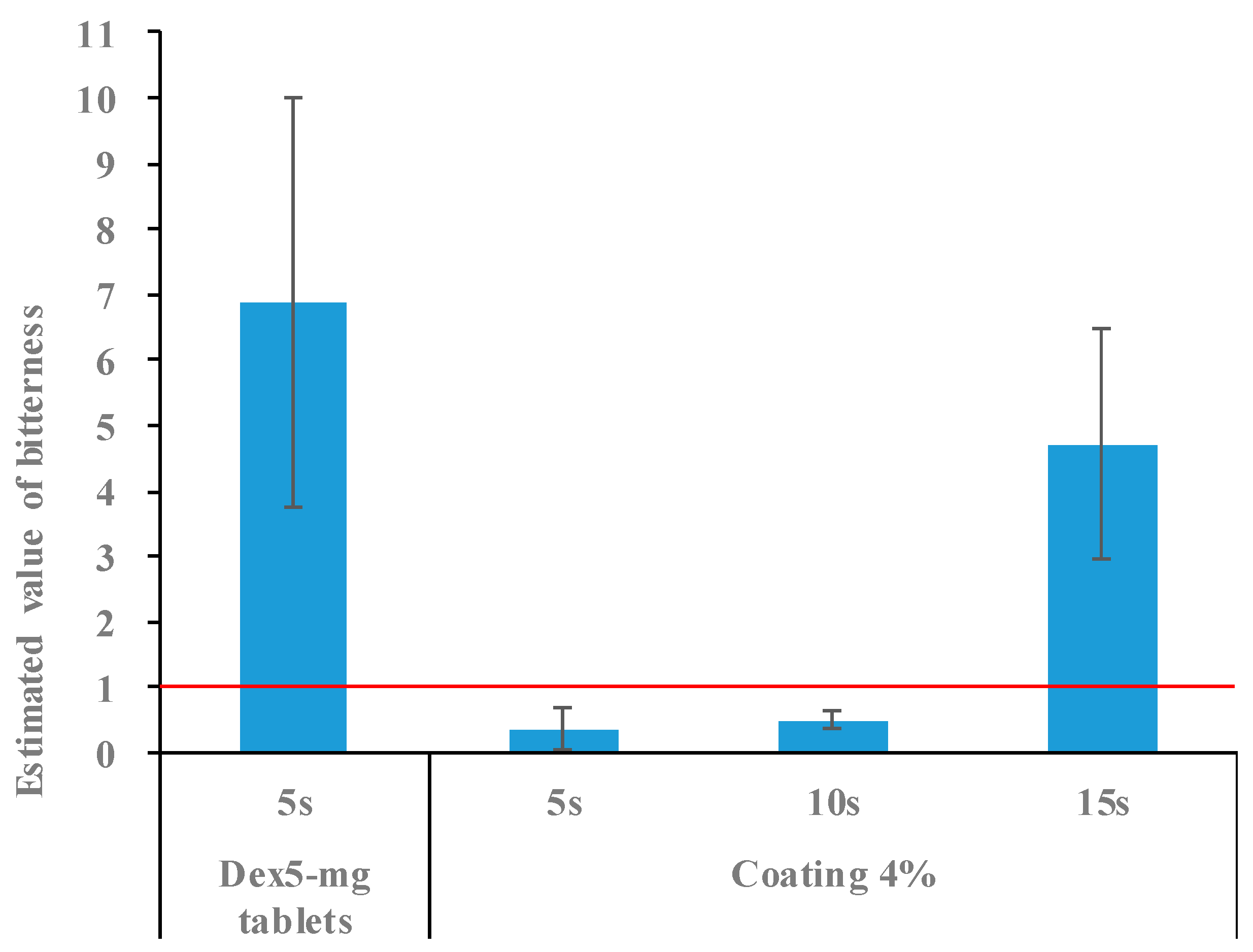
2.3. Bitterness of 5 mg Dex-Coated Tablets
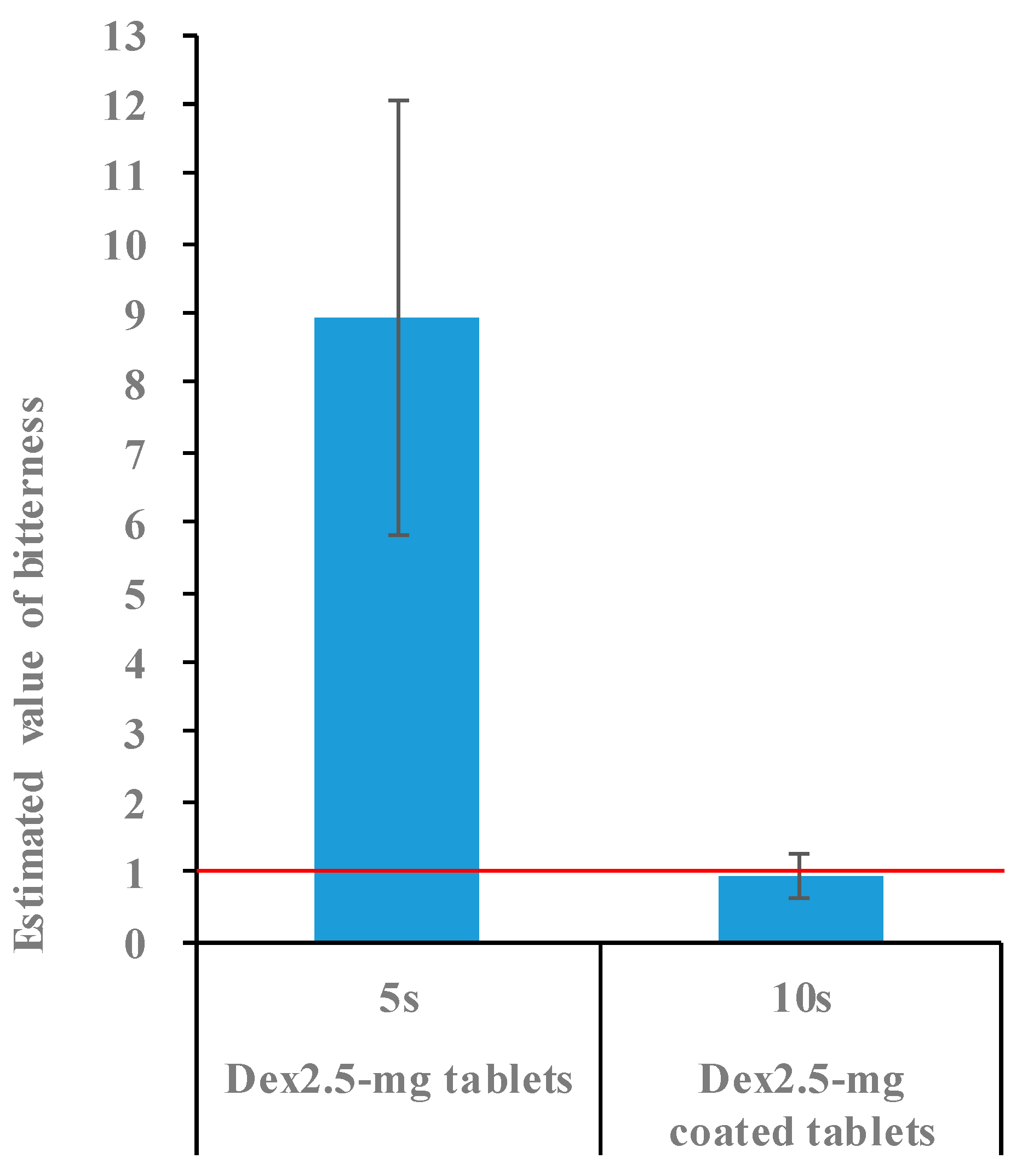
2.4. Bitterness of 2.5 mg Dex Coated Tablets
3. Materials and Methods
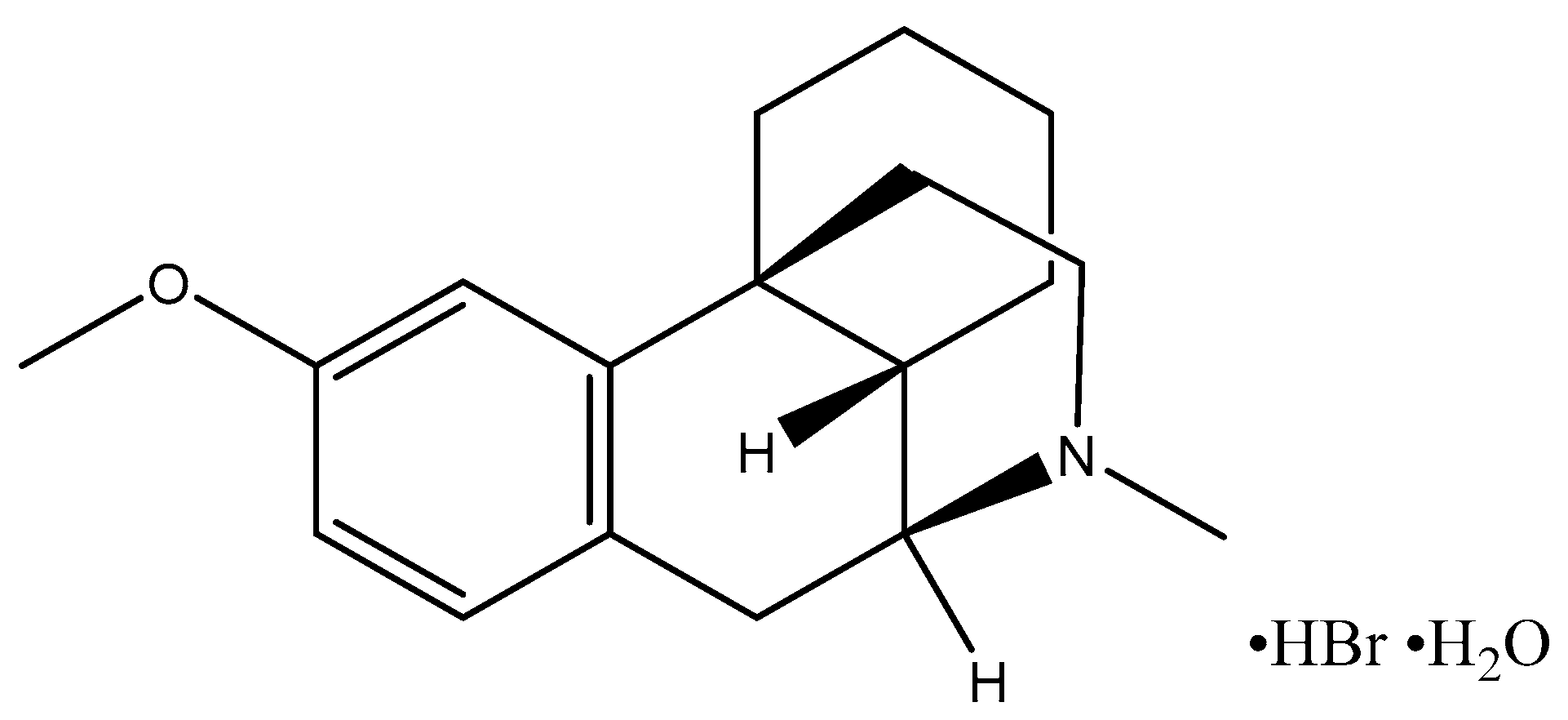
3.1. Materials
3.2. Mixture Design for Film-Coating Formulation
3.3. Statistical Analysis of Responses
3.4. Optimization of the Film-Coating Formulation
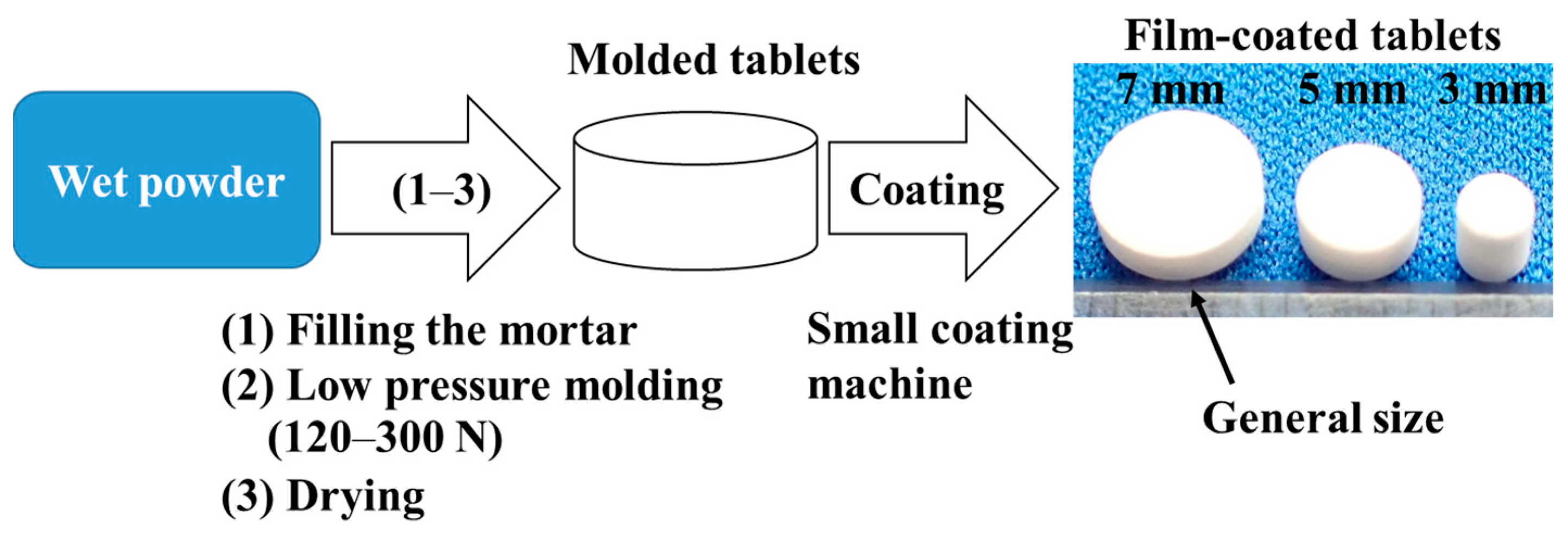
3.5. Preparation of Molded Tablets
3.6. Film Coating of Molded Tablets
3.7. Tablet Hardness
3.8. Disintegration Time
3.9. Bitterness Evaluation of Coated Tablets
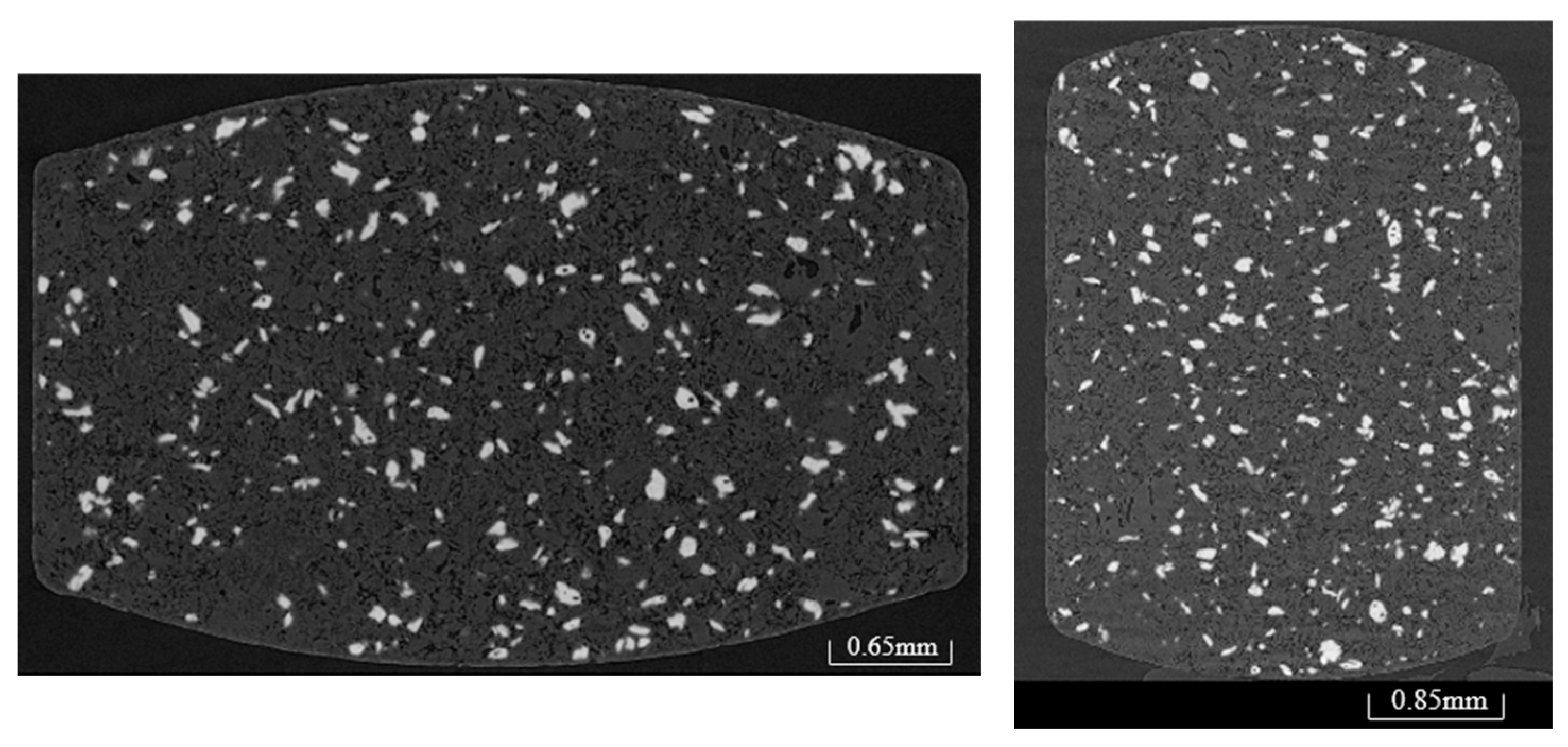
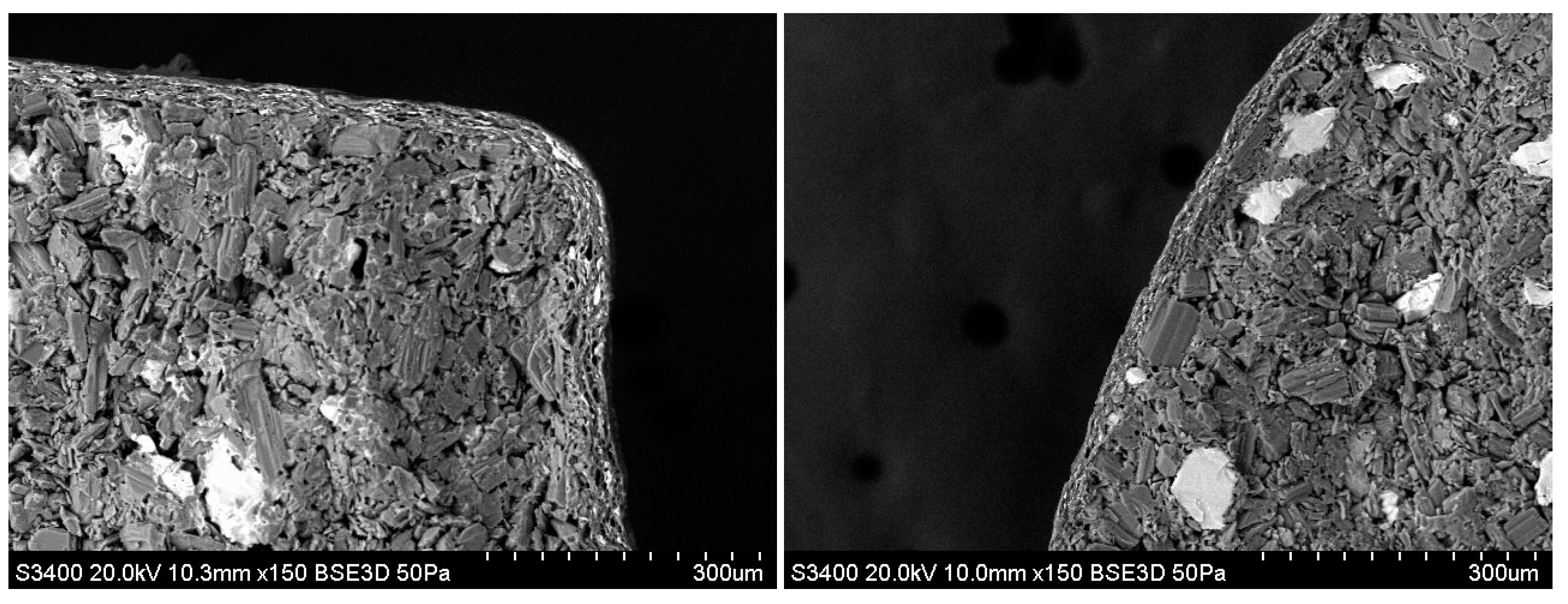
3.10. X-Ray CT
3.11. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| Dex | Dextromethorphan hydrobromide hydrate |
| HPMC | Hydroxypropyl methylcellulose |
References
- PUBLIC LAW 108–155—DEC. 3, 2003. PEDIATRIC RESEARCH EQUITY ACT OF 2003. Available online: https://www.congress.gov/108/plaws/publ155/PLAW-108publ155.pdf (accessed on 6 May 2025).
- Paediatric Regulation. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/paediatric-medicines-overview/paediatric-regulation (accessed on 6 May 2025).
- 10-Year Report to the European Commission. Available online: https://health.ec.europa.eu/system/files/2020-06/paediatrics_10_years_ema_technical_report_0.pdf (accessed on 6 May 2025).
- Partial Amendment of the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (Pharmaceutical and Medical Device Act) of 2022. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000179749_00006.html (accessed on 6 May 2025).
- Ranmal, S.R.; Nhouchi, Z.; Keeley, A.; Adler, L.; Lavarde, M.; Pensé-Lhéritier, A.-M.; Tuleu, C. Taste assessment for paediatric drug Development: A comparison of bitterness taste aversion in children versus Naïve and expert young adult assessors. Int. J. Pharm. 2023, 647, 123494. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Meissner, T.; Máyatepek, E.; Wargenau, M.; Breitkreutz, J.; Bosse, H.M.; Klingmann, V. Acceptability of small-sized oblong tablets in comparison to syrup and mini-tablets in infants and toddlers: A randomized controlled trial. Eur. J. Pharm. Biopharm. 2021, 166, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Development of Paediatric Medicines: Points to Consider in Formulation. Available online: https://cdn.who.int/media/docs/default-source/medicines/norms-and-standards/guidelines/trs970/annex5trs-970.pdf?sfvrsn=699cdb68_8&download (accessed on 6 May 2025).
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. J. Pediatr. 2013, 163, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Kreeftmeijer-Vegter, A.R.; de Meijer, M.; Wegman, K.A.M.; van Veldhuizen, C.K.M. Development and evaluation of age-appropriate film-coated tablets of levamisole for paediatric use (2–18 years). Expert Opin. Drug Deliv. 2013, 10, 293–300. [Google Scholar] [CrossRef] [PubMed]
- van Riet-Nales, D.A.; de Neef, B.J.; Schobben, A.F.A.M.; Ferreira, J.A.; Egberts, T.C.G.; Rademaker, C.M.A. Acceptability of different oral formulations in infants and preschool children. Arch. Dis. Child. 2013, 98, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Swedrowska, M.; Ingham, S.; Tomlin, S.; Forbes, B. Recommendations for crushing Circadin® (melatonin) tablets for safe and reliable delivery via pediatric nasogastric tubes. Int. J. Pharm. 2021, 594, 120151. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Furuishi, T.; Yonemochi, E. Optimization and advantages of molded tablets using trehalose as a binder. Chem. Pharm. Bull. 2023, 71, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Publication of Patent Applications (JP 2018-193360). Available online: https://www.j-platpat.inpit.go.jp/c1801/PU/JP-2018-071450/10/ja (accessed on 6 May 2025).
- Owusu-Ware, S.K.; Boateng, J.S.; Chowdhry, B.Z.; Antonijevic, M.D. Glassy state molecular mobility and its relationship to the physico-mechanical properties of plasticized hydroxypropyl methylcellulose (HPMC) films. Int. J. Pharm. 2019, 10, 100033. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, Center for Drug Evaluation and Research (CDER). “Guidance for Industry: Orally Disintegrating Tablets”, U.S. Department of Health and Human Services, USA. 2008. Available online: https://cir.nii.ac.jp/crid/1372539179752994305 (accessed on 6 May 2025).
- Derringer, G.; Suich, R.J. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic tongues—A review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef] [PubMed]
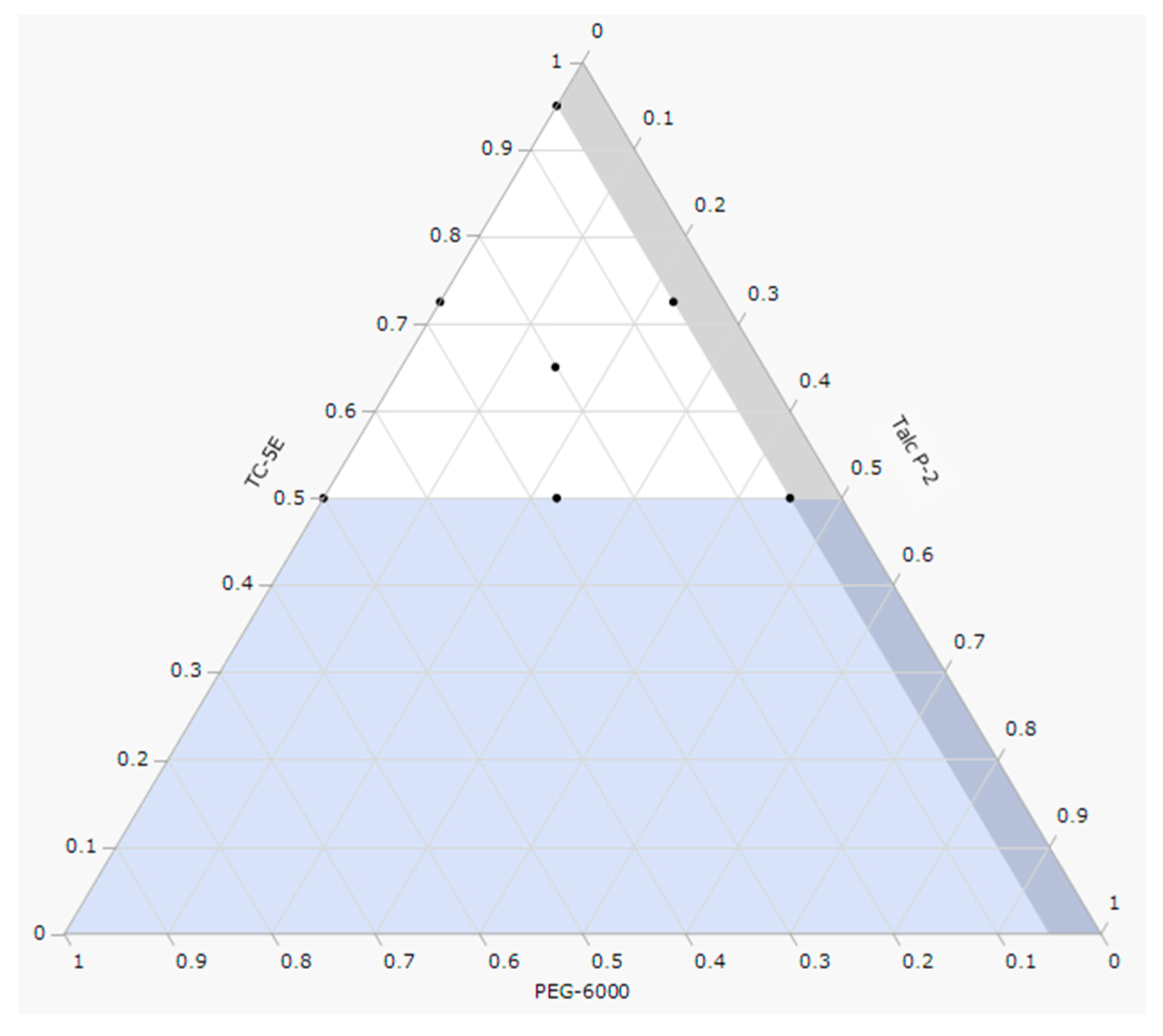
| No. | Factor | |||
|---|---|---|---|---|
| TC-5E (%) (X1) | PEG-6000 (%) (X2) | Talc P-2 (%) (X3) | Repeat | |
| 1 | 50.0 | 5.0 | 45.0 | — |
| 2 | 72.5 | 5.0 | 22.5 | — |
| 3 | 95.0 | 5.0 | 0.0 | — |
| 4 | 72.5 | 27.5 | 0.0 | — |
| 5 | 95.0 | 5.0 | 0.0 | No. 3 |
| 6 | 50.0 | 27.5 | 22.5 | — |
| 7 | 50.0 | 50.0 | 0.0 | — |
| 8 | 65.0 | 20.1 | 14.9 | — |
| 9 | 50.0 | 5.0 | 45.0 | No. 1 |
| 10 | 50.0 | 50.0 | 0.0 | No. 7 |
| 11 | 65.0 | 20.1 | 14.9 | No. 8 |
| 12 | 72.5 | 5.0 | 22.5 | No. 2 |
| No. | Response | |
|---|---|---|
| Hardness, N (Y1) | Disintegration Time, s (Y2) | |
| 1 | 36.0 | 26 |
| 2 | 39.2 | 28 |
| 3 | 44.8 | 39 |
| 4 | 30.4 | 31 |
| 5 | 44.0 | 49 |
| 6 | 34.8 | 28 |
| 7 | 26.9 | 23 |
| 8 | 37.9 | 34 |
| 9 | 35.7 | 30 |
| 10 | 32.7 | 22 |
| 11 | 33.0 | 28 |
| 12 | 39.3 | 32 |
| Item | Estimated Value (N) | Standard Error (N) | t-Value | p-Value |
|---|---|---|---|---|
| TC-5E | 44.086588 | 1.40353 | 31.41 | <0.0001 * |
| PEG-6000 | 68.931864 | 21.16757 | 3.26 | 0.0116 * |
| Talc P-2 | 27.402006 | 3.451996 | 7.94 | <0.0001 * |
| TC-5E × PEG-6000 | −102.4309 | 38.54277 | −2.66 | 0.0289 * |
| Item | Estimated Value (s) | Standard Error (s) | t-Value | p-Value |
|---|---|---|---|---|
| TC-5E | 42.047464 | 3.088938 | 13.61 | <0.0001 * |
| PEG-6000 | −14.56181 | 46.58634 | −0.31 | 0.762 |
| Talc P-2 | 11.464492 | 7.597276 | 1.51 | 0.1697 |
| TC-5E × PEG-6000 | 33.429604 | 84.82631 | 0.39 | 0.7038 |
| Mixing Purpose | Raw Material | Ratio (wt%) |
|---|---|---|
| Coating Base | TC-5E | 50.0 |
| Plasticizer | PEG-6000 | 10.3 |
| Lubricant | Talc P-2 | 39.7 |
| Solvent | Purified water | (a) |
| Total (excluding solvent) | 100 | |
| Response | ||
|---|---|---|
| Hardness, N | Disintegration Time, s | |
| Predicted values for film-coated tablets (①) | 35 (33–38) | 27 (21–33) |
| Actual results for uncoated tablets (②) | 29 | 11 |
| Expected increase after coating (①–②) | 6 (4–8) | 16 (10–22) |
| Response | ||
|---|---|---|
| Hardness, N | Disintegration Time, s | |
| Uncoated tablets (①) | 10.9 | 12 |
| Coated tablets (②) | 15.7 | 23 |
| Difference (②–①) | 4.8 | 11 |
| Response | ||
|---|---|---|
| Hardness, N | Disintegration Time, s | |
| Uncoated tablets (①) | 16.0 | 13 |
| Coated tablets (②) | 22.9 | 29 |
| Difference (②–①) | 6.9 | 16 |
| Factor | Range | |
|---|---|---|
| Lower Limit (%) | Upper Limit (%) | |
| TC-5E (X1) | 50 | 95 |
| PEG-6000 (X2) | 5 | 50 |
| Talc P-2 (X3) | 0 | 45 |
| Mixing Purpose | Raw Material | Ratio (wt%) | |
|---|---|---|---|
| Placebo Tablets | 5 or 2.5 mg Dex Tablets | ||
| Diluent | Pearlitol 25C | 89.6 | 81.3 |
| Binder | Trehalose P | 10.4 | 10.4 |
| Active component | Dex | — | 8.3 |
| Solvent | 99% ethanol | 5.0 (a) | 5.0 (a) |
| Purified water | 7.1 (a) | 7.1 (a) | |
| Total (excluding solvent) | 100 | 100 | |
| Task | Setting Item | Setting Value |
|---|---|---|
| Preheating | Supply air temperature (°C) | 65 |
| Supply air volume (m3/min) | 0.4 | |
| Static pressure inside pan | −10 | |
| Pan rotation speed (rpm) | 5 | |
| Exhaust temperature (°C) | 45 | |
| Spraying | Supply air temperature (°C) | 65 |
| Supply air volume (m3/min) | 0.4 | |
| Static pressure inside pan | −10 | |
| Pan rotation speed (rpm) | 25 | |
| Liquid velocity (g/min) | 1.2 | |
| Spray flow meter (NL/min) | 40 | |
| Spray pressure (mPa s) | 0.12 | |
| Exhaust temperature (°C, approximate) | ≥43 °C | |
| Drying | Supply air temperature (°C) | 65 |
| Supply air volume (m3/min) | 0.4 | |
| Static pressure inside pan | −10 | |
| Pan rotation speed (rpm) | 5 | |
| Exhaust temperature (°C) | 47 | |
| Cooling | Supply air temperature (°C) | OFF |
| Supply air volume (m3/min) | 0.4 | |
| Static pressure inside pan | −10 | |
| Pan rotation speed (rpm) | 5 | |
| Exhaust temperature (°C) | ≤40 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, Y.; Furuishi, T.; Yonemochi, E. Film Coating of Small Molded Tablets for Pediatric Formulations with Rapid Disintegration and Bitterness-Masking Properties. Molecules 2025, 30, 2142. https://doi.org/10.3390/molecules30102142
Takahashi Y, Furuishi T, Yonemochi E. Film Coating of Small Molded Tablets for Pediatric Formulations with Rapid Disintegration and Bitterness-Masking Properties. Molecules. 2025; 30(10):2142. https://doi.org/10.3390/molecules30102142
Chicago/Turabian StyleTakahashi, Yuki, Takayuki Furuishi, and Etsuo Yonemochi. 2025. "Film Coating of Small Molded Tablets for Pediatric Formulations with Rapid Disintegration and Bitterness-Masking Properties" Molecules 30, no. 10: 2142. https://doi.org/10.3390/molecules30102142
APA StyleTakahashi, Y., Furuishi, T., & Yonemochi, E. (2025). Film Coating of Small Molded Tablets for Pediatric Formulations with Rapid Disintegration and Bitterness-Masking Properties. Molecules, 30(10), 2142. https://doi.org/10.3390/molecules30102142








