Mechanistic Insights into NDMA Adsorption onto Selected Pollutants and Their Removal via Direct Rapid Sand Filtration and After Enhanced Coagulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. NDMA and Water Pollution Parameter Removal Regulations in Various Rapid Sand Filtration Water Systems
2.1.1. NDMA Removal Regulations in Terms of Rapid Sand Filtration of Simulated Supernatants for Blank Water System
2.1.2. NDMA and Water Pollutant Parameter Removal Regulations in Terms of Rapid Sand Filtration of Simulated Supernatants for Various Single-Component Systems
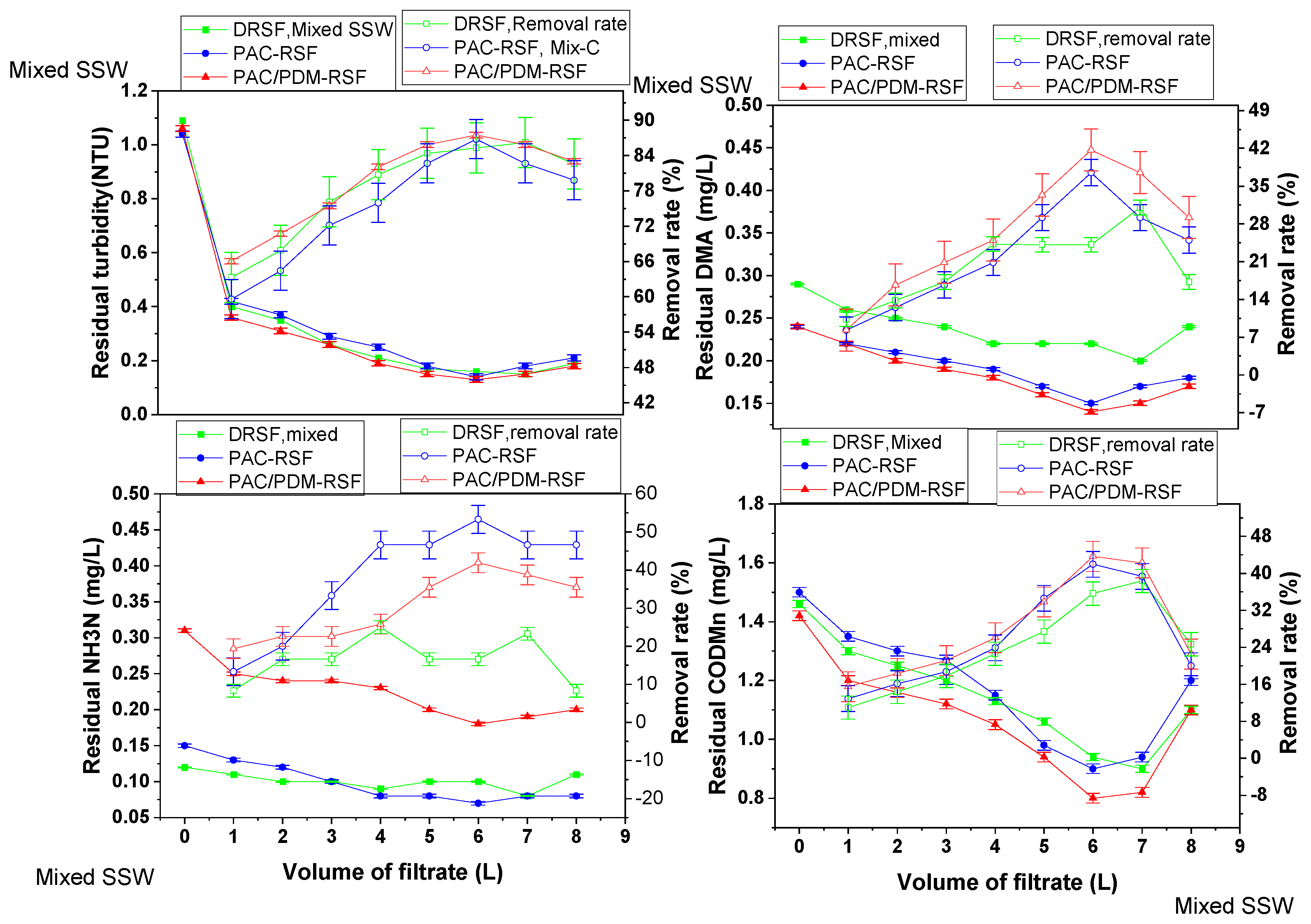
2.1.3. NDMA and Water Pollution Parameter Removal Regulations in Rapid Sand Filtration of Simulated Supernatants for Mixed Multi-Component Systems
2.2. NDMA and Pollutant Parameter Removal Rate Change Tendency and Their Correlation in Various Rapid Sand Filtration Water Systems
2.2.1. Correlation in Rapid Sand Filtration of Simulated Supernatant Directly for Single-Component and Mixed Multi-Component Systems
2.2.2. Correlation in Rapid Sand Filtration of Simulated Supernatant After Coagulation for Single-Component and Mixed Multi-Component Simulation Systems
2.2.3. Coordination Effects of NDMA Removal in Mixed Simulation Water Systems
2.3. Surface Charge Changes in Single-Component and Mixed Multi-Component Systems: Insights from Zeta Potentials
2.4. NDMA Removal Mechanism Analysis via Adsorption in Filtration Water Systems
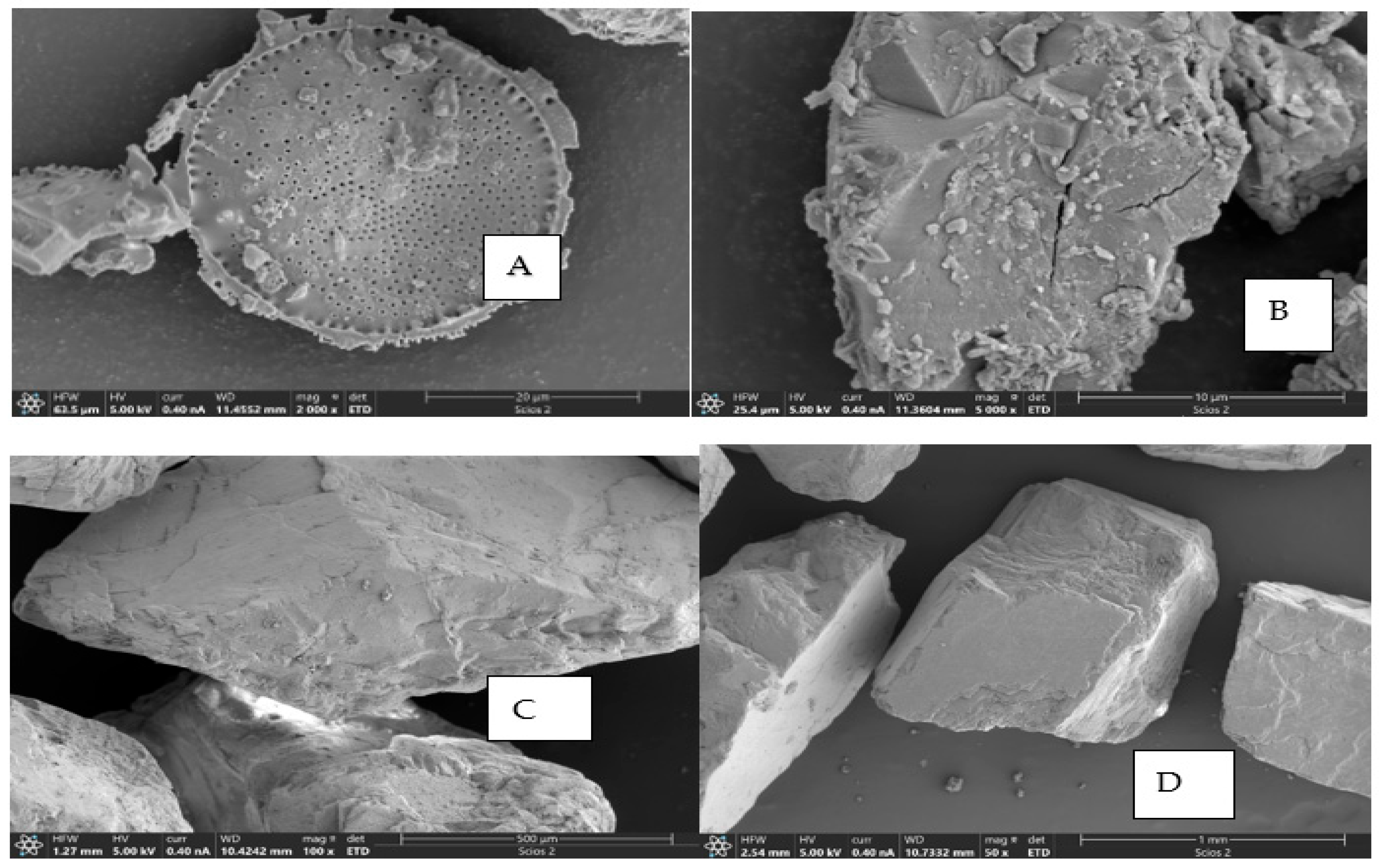
2.4.1. Adsorption Mechanism of NDMA on Water Pollutants Based on Surface Microstructure and Functional Group Analysis
2.4.2. Adsorption of NDMA Directly on Sand with Both NDMA and Water Pollution Parameters Based on Microstructure, Functional Group, and Surface Charge Interactions
3. Materials and Experimental Methods
3.1. Materials
3.1.1. Selection of Materials
3.1.2. Preparation of Materials
Preparation of Coagulants
Determination of Initial Conditions for Supernatants in Rapid Sand Filtration
3.1.3. Formulation of Simulated Supernatants Directly and After Enhanced Coagulation in Simulated Raw Water Systems
- (1)
- Blank water systems: A total of 80 µL of NDMA working standard from 0.1 g/L NDMA standard solution with a concentration of 80 µg/L was used in 100 mL of a volumetric flask. Then, 10 mL of the NDMA working standard was withdrawn with a micropipette, diluted by 1000-fold, in 10 L of deionized water to obtain the simulated supernatant water sample directly with an 80 ng/L final concentration for blank systems, while the simulated Yangtze River raw water with a 100 ng/L final concentration was also prepared following the above procedure for the supernatant after coagulation.
- (2)
- Single-component systems: Based on the summary in Table 1, the appropriate amount of each water pollution parameter material was weighed and dissolved in 13 L of deionized water spiked with either 80 ng/L or 100 ng/L of NDMA, respectively, for simulated supernatants directly and after coagulation. The various single-component water systems were homogenized and then allowed to stand for 20 min. DTA-NDMA, HAs-NDMA, DMA-NDMA, and NH4NO3-NDMA were used for rapid sand filtration directly and rapid sand filtration after enhanced coagulation operations.
- (3)
- Mixed multi-component systems: Based on the summary in Table 1, an appropriate amount of all water pollution parameters, namely diatomite (DTA), humic acid salt (HAs), dimethyl amine (DMA), and ammonium nitrate (NH4NO3), was weighed and dissolved in 13 L of deionized water spiked with either 80 ng/L or 100 ng/L of NDMA, respectively, for the supernatants simulated directly and after coagulation. The mixed multi-component water systems were homogenized and then allowed to stand for 20 min, which were used for rapid sand filtration directly and rapid sand filtration after enhanced coagulation.
3.2. Water Pollution Parameters and NDMA in Simulated Supernatants in Rapid Sand Filtration Directly and After Enhanced Coagulation Operations
3.2.1. Selection and Set-Up of Rapid Sand Filters
3.2.2. Water Pollutants and NDMA Removal by Coagulation of Simulated Raw Water Operations
3.2.3. Water Pollution and NDMA Removal by Rapid Sand Filtration Directly and After Enhanced Coagulation Operations
3.3. Basic Water Pollution Parameters and NDMA Instruments and Measurements
3.3.1. Basic Water Pollution Parameter Analysis
3.3.2. NDMA Measurements
3.3.3. Scan Electron Microscope Analysis
4. Conclusions
- There was a positive correlation for NDMA-HAs, NDMA-DMA, NDMA-NH4NO3, and NDMA-DTA in both the direct and after enhanced coagulation filtration systems. The correlation order was discovered to vary to different degrees depending on the microstructure, polar functional function, and surface charges of pollutants, as well as coagulation functions on sand pores and surfaces.
- The highest NDMA removal rates in the blank system obtained using RSF directly and after the enhanced coagulation of simulated raw water were 10.29% and 12.84%, indicating that the direct adsorption of NDMA onto sand and the effects of coagulant functions increased the NDMA removal rate. In single-component systems, NDMA removal varied between 13.66 and 14.90% (direct filtration) and 15.12–21.03% (after enhanced coagulation), influenced by pollutant properties and enhanced by coagulation.
- The highest NDMA removal rates in mixed multi-component systems were 42.50% (direct filtration) and 53.30% (after coagulation), surpassing those in single-component systems. This increase highlights the synergistic adsorption effects among pollutants, though competitive interactions on sand surfaces were limited.
- The adsorption analysis conducted via the surface morphology, functional groups, and polarity of water pollutant, alongside coagulation-induced surface charge modifications, revealed their collective role in enhancing NDMA adsorption.
- The NDMA removal mechanism assumption via adsorption was verified. Initially, NDMA adsorption onto major water pollutants depends on their microstructure, polar functional groups, and surface charge interactions with sand surfaces with limited competitive removal effects. However, coagulation—particularly enhanced coagulation—further strengthens the adsorption of both NDMA only and NDMA with pollutants on sand surfaces, improving overall removal efficiency. This study provides new insights into the NDMA removal mechanisms enabled by rapid sand filtration and encourages the wider application of enhanced coagulation coupled with filtration as a viable and sustainable technology for NDMA removal, benefiting researchers and water professionals.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Li, D.; Chen, C.; Liu, X.; Xiong, L. Health Risk Assessment of N-Nitrosodimethylamine in Drinking Water in Nanjing, Jiangsu Province. J. Environ. Occup. Med. 2022, 39, 890–894. [Google Scholar] [CrossRef]
- Shah, A.D.; Mitch, W.A. Halonitroalkanes, Halonitriles, Haloamides, and N-Nitrosamines: A Critical Review of Nitrogenous Disinfection Byproduct Formation Pathways. Environ. Sci. Technol. 2012, 46, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Carra, I.; Fernandez Lozano, J.; Autin, O.; Bolton, J.R.; Jarvis, P. Disinfection By-Product Formation during UV/Chlorine Treatment of Pesticides in a Novel UV-LED Reactor at 285 Nm and the Mitigation Impact of GAC Treatment. Sci. Total Environ. 2020, 712, 136413. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. N-Nitrosodimethylamine (NDMA) and Its Precursors in Water and Wastewater: A Review on Formation and Removal. Chemosphere 2018, 191, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Al-Hussaini, A.J.M. Solvation Descriptors for N-Nitrosodialkylamines; Calculation of Some of Their Properties of Environmental Significance. J. Environ. Monit. 2002, 4, 743–746. [Google Scholar] [CrossRef]
- USEPA. Six-Year Review 3 Technical Support Document for Disinfectants/Disinfection Byproducts Rules; EPA-810-R-16-013; U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- Edzwald, J.K.; Becker, W.C.; Tambini, S.J. Organics, Polymers, and Performance in Direct Filtration. J. Environ. Eng. 1987, 113, 167–185. [Google Scholar] [CrossRef]
- Edzwald, J.K. Water Quality & Treatment, A Handbook on Drinking Water, 6th ed.; Edzwald, J.K., Ed.; AWWA: Denver, CO, USA, 2011; ISBN 9780071630108. [Google Scholar]
- Lee, W.; Westerhoff, P. Dissolved Organic Nitrogen Removal during Water Treatment by Aluminum Sulfate and Cationic Polymer Coagulation. Water Res. 2006, 40, 3767–3774. [Google Scholar] [CrossRef]
- Alzahrani, F.; Collins, A.R.; Erfanian, E. Drinking Water Quality Impacts on Health Care Expenditures in the United States. Water Resour. Econ. 2020, 32, 100162. [Google Scholar] [CrossRef]
- Adam Branson, F.C. Staff Report Name: National Standard for Drinking Water Quality Released. CH2023-009, 1–13. 2023. Available online: https://fas.usda.gov/data/china-national-standard-drinking-water-quality-released (accessed on 30 April 2025).
- Fujioka, T.; Kodamatani, H.; Minh Tran, H.D.; Fujioka, A.; Hino, K.; Yoshikawa, T.; Inoue, D.; Ikehata, K. Degradation of N-Nitrosamines and 1,4-Dioxane Using Vacuum Ultraviolet Irradiation (UV254+185 Nm or UV172 Nm). Chemosphere 2021, 278, 130326. [Google Scholar] [CrossRef]
- Glover, C.M.; Verdugo, E.M.; Trenholm, R.A.; Dickenson, E.R.V. N-Nitrosomorpholine in Potable Reuse. Water Res. 2019, 148, 306–313. [Google Scholar] [CrossRef]
- Golea, D.M.; Jarvis, P.; Jefferson, B.; Moore, G.; Sutherland, S.; Parsons, S.A.; Judd, S.J. Influence of Granular Activated Carbon Media Properties on Natural Organic Matter and Disinfection By-Product Precursor Removal from Drinking Water. Water Res. 2020, 174, 115613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Olukowi, O.M.; Xie, Y.; Adebayo, I.O.; Zhang, Y. Coagulation Efficiency and Removal Mechanism for Composite Coagulant Polyaluminium Chloride/Polydimethyldiallylammonium Chloride in Treating Lightly Micro-Polluted Raw Water of Yangtze River in Autumn. Water SA 2024, 50, 121–130. [Google Scholar] [CrossRef]
- Adebayo, I.O.; Olukowi, O.O.; Zhiyuan, Z.; Zhang, Y. Comparisons of Coagulation Efficiency of Conventional Aluminium Sulfate and Enhanced Composite Aluminium Sulfate/Polydimethyldiallylammonium Chloride Coagulants Coupled with Rapid Sand Filtration. J. Water Process Eng. 2021, 44, 102322. [Google Scholar] [CrossRef]
- Wang, J.; de Ridder, D.; van der Wal, A.; Sutton, N.B. Harnessing Biodegradation Potential of Rapid Sand Filtration for Organic Micropollutant Removal from Drinking Water: A Review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2086–2118. [Google Scholar] [CrossRef]
- Okaikue-Woodi, F.E.K.; Cherukumilli, K.; Ray, J.R. A Critical Review of Contaminant Removal by Conventional and Emerging Media for Urban Stormwater Treatment in the United States. Water Res. 2020, 187, 116434. [Google Scholar] [CrossRef] [PubMed]
- Atabaki, M.; Idris, J. Performance of Activated Carbon in Water Filters. Water Resour. 2014, 52, 1–19. [Google Scholar]
- Zhang, W.; Gago-Ferrero, P.; Gao, Q.; Ahrens, L.; Blum, K.; Rostvall, A.; Björlenius, B.; Andersson, P.L.; Wiberg, K.; Haglund, P.; et al. Evaluation of Five Filter Media in Column Experiment on the Removal of Selected Organic Micropollutants and Phosphorus from Household Wastewater. J. Environ. Manag. 2019, 246, 920–928. [Google Scholar] [CrossRef]
- Ripperger, S.; Gosele, W.; Alt, C.; Loewe, T. Filtration, 1. Fundamentals. In Ullman’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2012; pp. 678–708. [Google Scholar]
- Fleming, E.C.; Pennington, J.C.; Wachob, B.G.; Howe, R.A.; Hill, D.O. Removal of N-Nitrosodimethylamine from Waters Using Physical-Chemical Techniques. J. Hazard. Mater. 1996, 51, 151–164. [Google Scholar] [CrossRef]
- Zhu, J.H.; Yan, D.; Rong Xai, J.; Ma, L.L.; Shen, B. Attempt to Adsorb N-Nitrosamines in Solution by Use of Zeolites. Chemosphere 2001, 44, 949–956. [Google Scholar] [CrossRef]
- Dai, X.; Zou, L.; Yan, Z.; Millikan, M. Adsorption Characteristics of N-Nitrosodimethylamine from Aqueous Solution on Surface-Modified Activated Carbons. J. Hazard. Mater. 2009, 168, 51–56. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, W.; Lin, D. Sorption Characteristics of N-Nitrosodimethylamine onto Biochar from Aqueous Solution. Bioresour. Technol. 2015, 179, 359–366. [Google Scholar] [CrossRef]
- Yilun, X. Study on Enhanced Coagulation Coupling Filtration to Remove NDMA. Master’s Thesis, Nanjiang University of Science and Technology, Nanjing, China, 2019; pp. 1–120. [Google Scholar]
- Zhou, Z. Enhanced Coagulation coupled with Filtration using Composite PAC/PDM in Treatment of Yangtze River in Autumn. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2022; pp. 1–124. [Google Scholar]
- Olukowi, O.M.; Xie, Y.; Zhou, Z.; Adebayo, I.O.; Zhang, Y. Performance Improvement and Mechanism of Composite PAC/PDMDAAC Coagulant via Enhanced Coagulation Coupled with Rapid Sand Filtration in the Treatment of Micro-Polluted Surface Water. J. Environ. Chem. Eng. 2022, 10. [Google Scholar] [CrossRef]
- Davies, M. Some Electrical and Optical Aspectts of Molecular Behaviour, 1st ed.; Pergamon Press Ltd.: London, UK, 1965. [Google Scholar]
- Nyoman Rupiasih, N.; Vidyasagar, P.B. A Review: Compositions, Structures, Properties and Applications of Humic Substances. J. Adv. Sci. Technol. 2005, 8, 16–25. [Google Scholar]
- Kamaloo, E.; Aaron Deskins, N.; Kazantzis, N.; Thompson, R.W. Molecular Modeling of Adsorbed NDMA and Water in MFI Zeolites. Microporous Mesoporous Mater. 2013, 182, 198–206. [Google Scholar] [CrossRef]
- Dos Santos, A.P.; Levin, Y. Like-Charge Attraction between Metal Nanoparticles in a 11 Electrolyte Solution. Phys. Rev. Lett. 2019, 122, 248005. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Qiang, X.; Dong, H.L.; Huo, J.; Tan, Z.J. Multivalent Ion-Mediated Attraction between Like-Charged Colloidal Particles: Nonmonotonic Dependence on the Particle Charge. ACS Omega 2021, 6, 9876–9886. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, T.; Guo, B.; Jiang, S.; Cao, M.; Zheng, X. Adsorption Characteristics of Dissolved Organic Nitrogen on Aquifer Porous Media: The Role of Media Particle Size. ACS EST Water 2024, 4, 2170–2180. [Google Scholar] [CrossRef]
- Barry, E.; Burns, R.; Chen, W.; De Hoe, G.X.; De Oca, J.M.M.; De Pablo, J.J.; Dombrowski, J.; Elam, J.W.; Felts, A.M.; Galli, G.; et al. Advanced Materials for Energy-Water Systems: The Central Role of Water/Solid Interfaces in Adsorption, Reactivity, and Transport. Chem. Rev. 2021, 121, 9450–9501. [Google Scholar] [CrossRef]
- Bradford, S.A.; Bettahar, M.; Simunek, J.; van Genuchten, M.T. Straining and Attachment of Colloids in Physically Heterogeneous Porous Media. Vadose Zone J. 2004, 3, 384–394. [Google Scholar] [CrossRef]
- Weber-Shirk, M.L.; Dick, R.I. Physical-Chemical Mechanisms in Slow Sand Filters. J. Am. Water Work. Assoc. 1997, 89, 87–100. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of Humic and Fulvic Acids onto a Range of Adsorbents in Aqueous Systems, and Their Effect on the Adsorption of Other Species: A Review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Goulet, P.J.G.; Garrido, J.J. Characterization of the Porous Structure of Different Humic Fractions. Coll. Surf. A Physicochem. Eng. Asp. 2005, 256, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Sheng, X.; Li, N.; Ping, Q. Adsorption and Release Kinetics, Equilibrium, and Thermodynamic Studies of Hymexazol onto Diatomite. ACS Omega 2020, 5, 29504–29512. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of Aqueous Silica Nanoparticle Surfaces and the Mechanism of Selective Peptide Adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef]
- Ho, L.; Grasset, C.; Hoefel, D.; Dixon, M.B.; Leusch, F.D.L.; Newcombe, G.; Saint, C.P.; Brookes, J.D.; Supe, E. Assessing Granular Media Filtration for the Removal of Chemical Contaminants from Wastewater. Water Res. 2011, 45, 3461–3472. [Google Scholar] [CrossRef]
- Chung, J.; Yoon, Y.; Kim, M.; Lee, S.B.; Kim, H.J.; Choi, C.K. Removal of Radio N-Nitrosodimethylamine (NDMA) from Drinking Water by Coagulation and Powdered Activated Carbon (PAC) Adsorption. Drink. Water Eng. Sci. 2009, 2, 49–55. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Shadman, H.R.; Habibi, M.; Didandeh, M.; Nikzad, A.; Golmohammadi, M.; Maleki, R.; Suwaileh, W.A.; Khataee, A.; Zargar, M.; et al. Elucidating the Sorption Mechanisms of Environmental Pollutants Using Molecular Simulation. Ind. Eng. Chem. Res. 2023, 62, 3373–3393. [Google Scholar] [CrossRef]
- Gans, W.; Boeyens, S. (Eds.) Intermolecular Interactions, 1st ed.; Springer: New York, NY, USA, 1998; ISBN 9781003803287. [Google Scholar]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Burlington, UK, 2011; Volume 7, ISBN 9780123751829. [Google Scholar]
- Saxena, K.; Brighu, U. Comparison of Floc Properties of Coagulation Systems: Effect of Particle Concentration, Scale and Mode of Flocculation. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Mota, M.H.; Patil, P.S. The Effect of Alum as Filter Conditioner on the Performance of Conventional Rapid Sand Filter. Int. J. Eng. Adv. Technol. 2020, 9, 1916–1920. [Google Scholar] [CrossRef]
- Gans, W.; Boeyens, J.C.A. Standard Methods for the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 2012; Volume 51, ISBN 0875532357. [Google Scholar]
- Karweik, D.H.; Meyers, H. Spectrophotometric Determination of Secondary Amines. Anal. Chem. 1979, 51, 319–320. [Google Scholar] [CrossRef]
- Munch, J.; Bassett, M. EPA-521: Solid Phase Extraction and Capillary Column Gas Chromatography with Large Volume Injection and Chemical Ionization Tandem Mass Spectrometry (MS/MS). Natl. Expo. Res. 2004, 1–47. [Google Scholar]





| Rapid Sand Filtration Directly and After Enhanced Coagulation for Single-Component System | ||||
|---|---|---|---|---|
| N = 9 | NDMA-DTA | NDMA-HAs | NDMA-DMA | NDMA-NH4NO3 |
| DTA, turbidity– RSF-SSD | ŧ_b = 0.889, p = 0.001 | |||
| RSF-SSEC | ŧ_b = 0.833, p = 0.002 | |||
| HAs, CODMn– RSF-SSD | ŧ_b = 0.9845, p = 0.000 | |||
| RSF-SSEC | ŧ_b = 0.889, p = 0.001 | |||
| DMA– RSF-SSD | ŧ_b = 0.915, p = 0.000 | |||
| RSF-SSEC | ŧ_b = 0.889, p = 0.001 | |||
| NH4NO3– RSF-SSD | ŧ_b = 0.944, p = 0.001 | |||
| RSF-SSEC | ŧ_b = 0.915, p = 0.001 | |||
| Rapid Sand Filtration Directly and After Enhanced Coagulation for Mixed Multi-Component System | ||||
| DTA, turbidity– RSF-SSD | ŧ_b = 0.786, p = 0.000 | |||
| RSF-SSEC | ŧ_b = 1.000 p = 0.000 | |||
| HAs, CODMn RSF-SSD | ŧ_b = 0.994, p = 0.000 | |||
| RSF-SSEC | ŧ_b = 1.000, p = 0.000 | |||
| DMA RSF-SSD | ŧ_b = 0.800, p = 0.003 | |||
| RSF-SSEC | ŧ_b = 1.000, p = 0.000 | |||
| NH4NO3 RSF-SSD | ŧ_b = 0.868, p = 0.001 | |||
| RSF-SSEC | ŧ_b = 0.915, p = 0.036 | |||
| DTA (g/L) Turbidity | HAs (g/L) CODMn | NH4NO3/(g/L) | DMA (g/L) | NDMA/(ng/L) | |
|---|---|---|---|---|---|
| Direct simulated supernatant | 0.01 | 0.0022 | 0.10 | 0.001 | 80.00 |
| Simulated supernatant after enhanced coagulation | 0.06 | 0.0024 | 0.16 | 0.001 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olukowi, O.M.; Tian, T.; Yan, X.; Zhang, Y. Mechanistic Insights into NDMA Adsorption onto Selected Pollutants and Their Removal via Direct Rapid Sand Filtration and After Enhanced Coagulation. Molecules 2025, 30, 2094. https://doi.org/10.3390/molecules30102094
Olukowi OM, Tian T, Yan X, Zhang Y. Mechanistic Insights into NDMA Adsorption onto Selected Pollutants and Their Removal via Direct Rapid Sand Filtration and After Enhanced Coagulation. Molecules. 2025; 30(10):2094. https://doi.org/10.3390/molecules30102094
Chicago/Turabian StyleOlukowi, Olubunmi M., Tian Tian, Xie Yan, and Yuejun Zhang. 2025. "Mechanistic Insights into NDMA Adsorption onto Selected Pollutants and Their Removal via Direct Rapid Sand Filtration and After Enhanced Coagulation" Molecules 30, no. 10: 2094. https://doi.org/10.3390/molecules30102094
APA StyleOlukowi, O. M., Tian, T., Yan, X., & Zhang, Y. (2025). Mechanistic Insights into NDMA Adsorption onto Selected Pollutants and Their Removal via Direct Rapid Sand Filtration and After Enhanced Coagulation. Molecules, 30(10), 2094. https://doi.org/10.3390/molecules30102094




