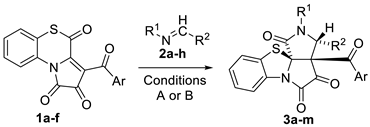
Abstract
1H-Pyrrole-2,3-diones, fused at [e]-side with a heterocycle, are suitable platforms for the synthesis of various angular polycyclic alkaloid-like spiroheterocycles. Recently discovered sulfur-containing [e]-fused 1H-pyrrole-2,3-diones (aroylpyrrolobenzothiazinetriones) tend to exhibit unusual reactivity. Based on these peculiar representatives of [e]-fused 1H-pyrrole-2,3-diones, we have developed an approach to an unprecedented 6/5/5/5-tetracyclic alkaloid-like spiroheterocyclic system of benzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole via their reaction with Schiff bases and carbodiimides. The experimental results have been supplemented with DFT computational studies. The synthesized alkaloid-like 6/5/5/5-tetracyclic compounds have been tested for their biotechnological potential as growth stimulants in the green algae Chlorella vulgaris.
1. Introduction
To improve the clinical success, reduce the undesirable side effects caused by the binding promiscuity of drug candidates, and speed up the lead optimization process, it is necessary to look for ways to expand the medicinal chemistry synthetic toolbox to be able to target more complex three-dimensional (3D) chemical space [1,2,3,4]. The 3D shape of a molecule is the most important factor determining its biological activity [5,6,7]. Due to these, angular polycyclic alkaloid-like spirocycles are attractive objects for drug discovery and related studies [8].
[e]-Fused 1H-pyrrole-2,3-diones (FPDs) (Figure 1) are versatile starting materials for the synthesis of various heterocyclic systems [9,10,11,12], including angular polycyclic alkaloid-like spiroheterocycles (for example, 6/6/5/5- [13], 6/6/5/6- [14], 6/6/5/6/6- [15], 6/6/5/6/5- [15], 6/5/7/5- [16], 6/5/7/6- [17], 6/6/5/7/6- [18], 6/7/5/6- [19], 5/6/5/6-systems [20] and some others (Figure 1)).

Figure 1.
Selected examples of angular polycyclic alkaloid-like spiroheterocyclic systems, synthesized on the basis of FPDs.
Exploring the scope of the recently discovered by us nucleophile-induced ring contraction reaction in FPDs [21], we unexpectedly found an approach to an unprecedented angular 6/5/5/5-tetracyclic alkaloid-like spiroheterocyclic system of benzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole (Figure 2). Such a 6/5/5/5-tetracyclic framework is a quite interesting one, since it is present in natural products (retigeranic acid, a sesterterpene from Himalayan lichens Lobaria retigera and Lobaria subretigeria [22]) and synthetic biologically active molecules [23,24] (Figure 2).
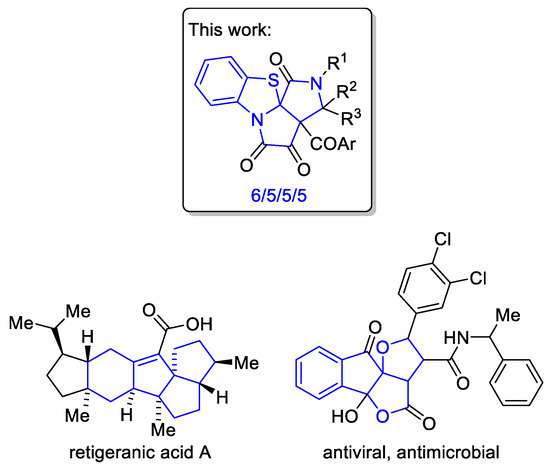
Figure 2.
6/5/5/5-Tetracyclic alkaloid-like spiroheterocyclic system reported in this study and related compounds.
Thus, herein, we report the first synthetic approach to an unprecedented 6/5/5/5-tetracyclic alkaloid-like spiroheterocyclic system of benzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole (Figure 2) via the reaction of pyrrolobenzothiazines (FPDs incorporating 1,4-benzothiazine moiety) with Schiff bases and carbodiimides. The experimental results were supplemented with DFT computational studies to elucidate the mechanism and stereoselectivity of the reaction. The biotechnological potential of the reported benzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazoles as growth stimulants and promoters of pigments accumulation in the green algae Chlorella vulgaris was demonstrated.
2. Results and Discussion
2.1. Chemistry
Recently, we reported [25] a new class of FPDs, aroylpyrrolobenzothiazinetriones (APBTTs) 1 (Scheme 1). APBTTs 1 were found to react as oxadienes in a hetero-Diels–Alder reaction with electron-rich dienophiles (alkoxyolefins, styrene) (Scheme 1) [25], which is a quite common reactivity for other known types of FPDs and monocyclic 1H-pyrrole-2,3-diones [9,10,14,15]. However, under the action of mononucleophiles (amines and alcohols), APBTTs 1 were found to undergo a ring-contraction reaction (Scheme 1) [21], which greatly distinguished their reactivity from the reactivity of their 5-oxa- and 5-aza-analogues [10].

Scheme 1.
Reactivity APBTTs 1 in the reactions with electron-rich dienophiles and mononucleophiles.
5-Oxa- and 5-aza-analogues of APBTTs 1 seem not to react with Schiff bases and carbodiimides (C=N reagents) without thermal decomposition (for such reactions under thermal decomposition conditions, see the work presented in [12]). At the same time, monocyclic 1H-pyrrole-2,3-diones are known to react with carbodiimides as oxadienes in formal hetero-Diels–Alder reactions to produce the corresponding cycloadducts (Scheme 2) [26].
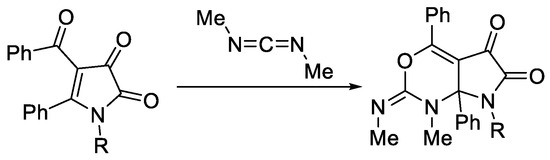
Scheme 2.
Reaction of monocyclic 1H-pyrrole-2,3-diones with carbodiimides.
Considering the tendency for the unusual reactivity of APBTTs 1, we studied their reaction with Schiff bases and carbodiimides under conditions without thermal decomposition of APBTTs 1 (for thermal decomposition of APBTTs 1, see the work presented in [27]).
To start with, we tested the reaction of APBTT 1a with N-benzylideneaniline 2a (Scheme 3). As a result, 6/5/5/5-tetracyclic product 3a was isolated by a simple crystallization from the reaction mixture in the yield of 52% (Scheme 3). Compound 3a was obtained as a single (3R*,3aS*,11aR*)-diastereomer, and its structure was unequivocally determined by a single crystal X-ray analysis (CCDC 2341688).
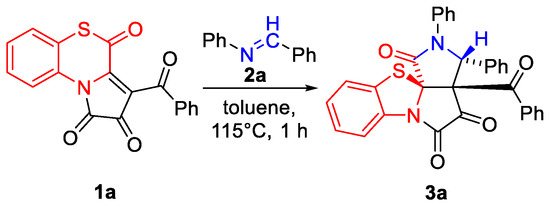
Scheme 3.
Reaction of APBTT 1a with N-benzylideneaniline 2a under test conditions.
Obviously, our hypothesis of the unusual reactivity of APBTTs 1 in reactions with C=N reagents was confirmed, which justified a more in-depth study of this transformation.
Next, we carried out a series of experiments to optimize the conditions of the reaction of APBTT 1a with Schiff base 2a (Table 1).

Table 1.
Optimization of the reaction of APBTT 1a with Schiff base 2a under various conditions 1.
According to Table 1, APBTT 1a and Schiff base 2a reacted most quickly in polar solvents (acetone, DMAA, DMSO, DMF, NMP (entries 1, 7–10, Table 1)), but the reaction proceeded unselectively (a difficult to identify mixture of products was observed), and only trace amounts of the target product 3a were formed. Interestingly, in acetonitrile (entry 2, Table 1), which is a polar solvent too, the reaction yield was much higher, which could indicate that the low yields of the product 3a in polar solvents were caused not only by the polarity of these solvents, but also by their specific solvation effects and their ability to react with the reaction intermediates. In nonpolar solvents (entries 3–6, 12, 13 Table 1), the reaction proceeded much slower and was more selective towards the product 3a. The best yield of the product 3a (HPLC yield of 90%) was observed in chloroform when heated for 5 h (entry 5, Table 1).
It should be mentioned that, during the optimization of the reaction of APBTT 1a with the Schiff base 2a at room temperature in anhydrous butyl acetate, acetonitrile, and acetone, we observed the formation of product 4a in significant amounts (Scheme 4). Under these conditions, the reaction proceeded very slowly (about a month), and obviously, side reactions took place. Since the reaction vials were not sealed, it could be assumed that atmospheric moisture affected the reaction (Scheme 4). Moreover, the reaction of APBTT 1a with the Schiff base 2a at room temperature in acetic acid, containing traces of water, produced compound 4a in 24 h in a very good isolated yield (86%) (Scheme 4).
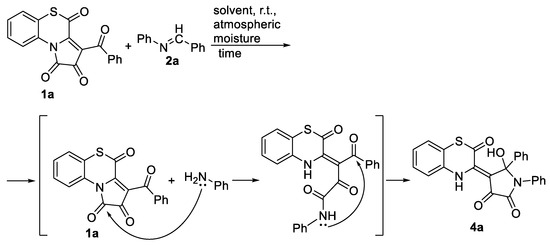
Scheme 4.
Reaction of APBTT 1a with N-benzylideneaniline 2a in the presence of atmospheric moisture at room temperature.
To prove our hypothesis of the formation of compound 4a, we carried out a reaction of APBTTs 1a,b with aniline 5a and benzylamine 5b (Scheme 5). As a result, we isolated target products 4a,b in yields of 95% and 46%, respectively. The structure of compound 4b was unequivocally confirmed by a single crystal X-ray analysis (CCDC 2341690).
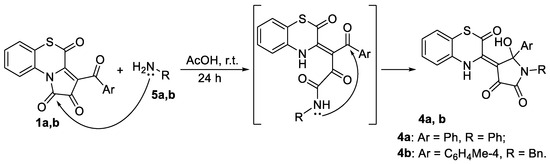
Scheme 5.
Reaction of APBTTs 1a,b with amines 5a,b.
Then, we performed reactions of APBTTs 1a–f with Schiff bases 2a–g and azine 2h to determine the reactant scope (Table 2).

Table 2.
The reaction of APBTTs 1a–f with Schiff bases 2a–g and azine 2h.
As a result, we found that the reaction of APBTTs 1 with Schiff bases 2 performed under optimized conditions (chloroform as the solvent) produced target products 3 in poor to very good HPLC yields (Conditions A, Table 2). However, our attempts to isolate products 3 from such reaction mixtures (in scale of 298 μmol) were unsuccessful. Moreover, we observed that compounds 3 underwent unfavorable transformations during our attempts to isolate and purify them by column chromatography. For these reasons, we replaced chloroform with benzene in these reactions. As a result, we noticed a decreasing tendency in the HPLC yields of products 3 (Conditions B, Table 2), which correlated with the optimization data for the test reaction of APBTT 1a with Schiff base 2a (Table 1). However, products 3 were easily isolated and purified by simple crystallization directly from the reaction mixtures (in scale of 298 μmol, benzene).
We also observed that the nature of the aryl substituents in the examined (Table 2) APBTTs 1 and Schiff bases 2 did not significantly affect the yields of the corresponding products 3. However, the reactions of APBTT 1a with 1-phenyl-N-(pyridin-2-yl)methanimine 2i, 1-((phenylimino)methyl)naphthalen-2-ol 2j, N-(4-nitrophenyl)-1-phenylmethanimine, N-mesityl-1-(4-nitrophenyl)methanimine 2k, N-(2-chlorophenyl)-1-phenylmethanimine 2l, and N,N-dimethyl-4-((phenylimino)methyl)aniline 2m did not produce the desired products 3, which was possibly caused by the presence of additional nucleophilic centers, o-substituents, or strong electron-withdrawing groups in the molecules of these Schiff bases. Moreover, the reaction with N-benzylmethanimine 2n did not give the corresponding product 3 too, possibly due to the absence of aromatic substituent at the CH part of this Schiff base, which could stabilize reaction intermediates.
Noteworthy, when synthesizing product 3d, we succeeded in isolating by-product 6d from the reaction mixture in the isolated yield of 2% (Scheme 6). The structure of compound 6d was unequivocally confirmed by a single crystal X-ray analysis (CCDC 2341691). Apparently, the formation of product 6d proceeded through an intermediate A (conditions for formation of compounds A were discussed in the work presented in [21]) and the hydrolysis of Schiff base 2a (Scheme 6).
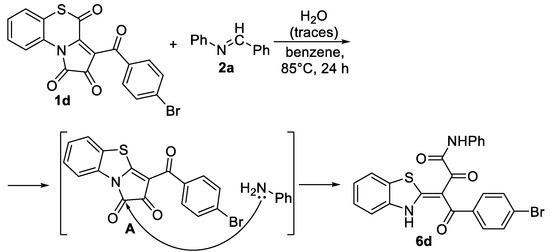
Scheme 6.
Formation of by-product 6e in the reaction of APBTT 1e with N-benzylideneaniline 2a.
Moreover, in cases of compounds 3j and 3k, we succeeded in isolating their stereoisomers, compounds 3′j and 3′k (isolated yields of 7 and 28%, respectively). Indeed, for structures of compounds 3, there are four possible diastereomers with (3R*,3aS*,11aR*), (3S*,3aS*,11aR*), (3S*,3aR*,11aR*), and (3R*,3aR*,11aR*) relative configurations (Figure 3). In our scope studies (Table 2), we isolated exclusively (3R*,3aS*,11aR*) diastereomers for compounds 3a–i,l,m. In our scope studies (Table 2), we were unable to detect and determine any other diastereomers by HPLC due to the absence of their reference samples; NMR spectra of crude reaction mixtures were also not suitable for these purposes. But, in cases of compounds 3j and 3k, we isolated both (3R*,3aS*,11aR*) diastereomers 3j,k and their stereoisomers 3′j,k with unknown relative configurations. Diastereomers 3′j,k were found to be unstable in HPLC studies, long NMR experiments in solutions and during our attempts to grow a crystal suitable for a single crystal X-ray analysis, which was possibly caused by their reaction with water.
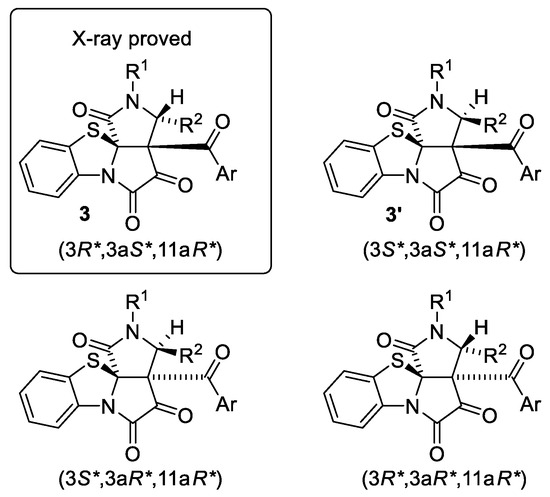
Figure 3.
Possible diastereomers of compounds 3.
The 1H NMR and IR spectra of compounds 3j,k and compounds 3′j,k are very similar and cannot be used for their identification relative to each other. But their 13C NMR spectra are quite different in the region of 62–82 ppm (where sp3 atoms—C3, C3a, C11a—appear), which is enough to distinguish them from each other. Fragments of their 13C NMR spectra in these regions look as follows:
13C NMR of 3j (100 MHz, DMSO-d6): δ = 79.3, 66.0, 65.2 ppm;
13C NMR of 3′j (100 MHz, DMSO-d6): δ = 79.2, 70.6, 64.2 ppm;
13C NMR of 3k (100 MHz, DMSO-d6): δ = 79.6, 65.7, 65.5 ppm;
13C NMR of 3′k (100 MHz, DMSO-d6): δ = 79.3, 70.8, 64.5 ppm.
However, this information is not enough to determine the relative configuration of compounds 3′.
Then, to elucidate the possible mechanism of the reaction and compare the thermodynamics and kinetics of possible stereoisomers formation, we performed computational DFT studies of a model reaction between the APBTT 1a and Schiff base 2a (Scheme 7). We proposed several reaction pathways for 1a → 3a transformation (Scheme 7), but the results of DFT calculations revealed only one, very energetically unprofitable, intermediate I2 on the potential energy surface and indicated that the formation of product 3a occurred directly from the orientation complex OC. The hypothetical transformation OC → 3a (via transition state TS (Figure 4)) is less thermodynamically profitable (by 4.7 kcal/mol in terms of Gibbs-free energies of reaction, Table 3, Figure 5) compared to the alternative hypothetical transformation OC → 3′a (via transition state TS’ (Figure 4)) but more kinetically favorable (by 1.6 kcal/mol in terms of Gibbs-free energies of activation, Table 3, Figure 5). Thus, diastereomer 3a is a kinetically controlled product, and diastereomer 3′a is a thermodynamically controlled one (Figure 5).
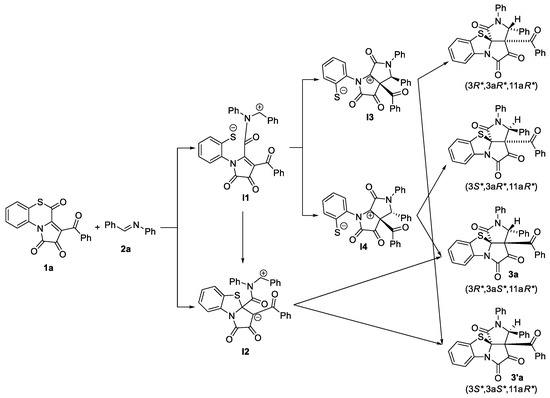
Scheme 7.
Proposed reaction pathways for 1a → 3a (or 3′a) transformation.

Figure 4.
Transition states structures for reactions leading from OC to adducts 3a and 3′a, according to the DFT calculations (M06-2X/6-31G* level of theory).

Table 3.
Calculated values of the total electronic energies, enthalpies, and Gibbs-free energies of reaction (ΔE, ΔH, and ΔG in kcal/mol) for elementary stages of 1a → 3a (or 3′a) transformation 1.
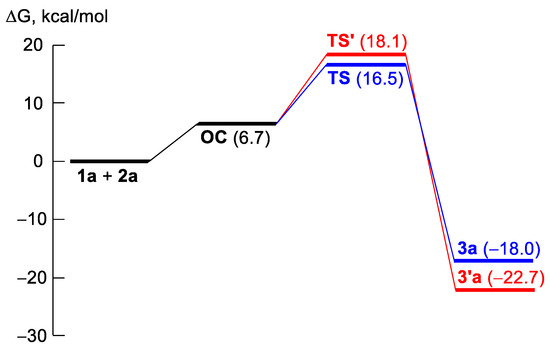
Figure 5.
Energy profile for the elementary stages of different pathways for 1a → 3a (or 3′a) transformation.
Since our computational studies revealed only intermediate-I2-like transition states TS and TS′ (their 3D structures are available in Supplementary Materials) in the reaction, and these transition states could afford only diastereomers (3R*,3aS*,11aR*) and (3S*,3aS*,11aR*) (Scheme 7), we suggest that compounds 3′j,k had (3S*,3aS*,11aR*) a relative configuration (Figure 3).
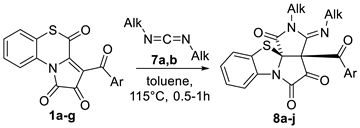
Then, in order to expand the reagent scope of the reaction, we studied the reactions of APBTTs 1 with carbodiimides 7.
First, we tested the reaction of APBTT 1a with N,N′-dicyclohexylcarbodiimide (DCC) 7a (Scheme 8). As we expected, 6/5/5/5-tetracyclic product 8a was isolated by a simple crystallization from the reaction mixture in the yield of 44% (Scheme 8). Compound 8a was obtained as a single diastereomer, and its structure was unequivocally determined by a single crystal X-ray analysis (CCDC 2341689).
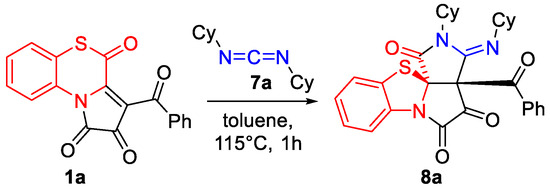
Scheme 8.
Reaction of APBTT 1a with DCC 7a under test conditions.
Next, we performed the optimization of the reaction conditions (Table 4). As a result, we did not observe any correlation between the polarity of the solvent and the yield of the product 8a. The best yield of the product 8a (HPLC yield of 96%) was observed in 1,4-dioxane when heated for 40 min (entry 6, Table 4). Our optimization studies at room temperature, as expected, revealed that reactions took place over 14 days, during which time both APBTT 1a and DCC 7a underwent side reactions with water.

Table 4.
Optimization of the reaction of APBTT 1a with DCC 7a under various conditions 1.
Then, we performed reactions of APBTTs 1a–g with carbodiimides 7a,b to determine the reactant scope (Table 5). Initially, we tried to perform the scope examination under optimal conditions in 1,4-dioxane (entry 6, Table 4). But under these conditions, our attempts to isolate products 8 from such reaction mixtures (in scale of 298 μmol) were unsuccessful, and we observed that compounds 8 underwent unfavorable transformations during our attempts to isolate and purify them by column chromatography. A similar situation was observed when we tried to apply acetonitrile as the reaction solvent (entry 2, Table 4). Thus, we had to carry out these reactions in toluene (entry 11, Table 4). In toluene, products 8 were easily isolated by simple crystallization from the reaction mixtures.

Table 5.
The reaction of APBTTs 1a–g with carbodiimides 7a,b.
As a result, we found that the reaction of APBTTs 1 with carbodiimides 7 performed in toluene produced target products 8 in fair to good HPLC yields (Table 5). We observed that the nature of the aryl substituents in examined (Table 5) APBTTs 1 did not significantly affect the yields of the corresponding products 8.
2.2. Biology
The green unicellular algae Chlorella vulgaris serves as a source of valuable metabolites for the food industry [28,29,30], agriculture [22,23,24,25,26,27,28,29,30,31,32,33,34], cosmetics [35,36,37], and biodiesel production [38,39,40,41,42]. There is a significant demand for chemical stimulants that promote the growth of this algae, as well as the accumulation of lipids [43,44,45], proteins [46,47], carbohydrates [48,49,50], and pigments [51] such as chlorophylls [52,53] and carotenoids [54,55,56,57,58]. We selected several synthesized compounds with favorable characteristics, such as high synthesis yield and improved solubility in polar solvents. These compounds were added in varying concentrations to C. vulgaris cultures, which were then cultivated, followed by the measurement of algae growth (cell concentration) and the accumulation of pigments.
The bioactivity study was conducted in two stages. Initially, a screening experiment in small volumes of algal cultures (96-well plates) was performed (Table 6). Two compounds (3a and 8j) that promoted the growth of C. vulgaris were further analyzed in a subsequent experiment. Algae were grown in 50-mL Erlenmeyer flasks in the presence of the tested compounds at concentrations ranging from 1 × 10−7 mol/L to 1 × 10−4 mol/L. Glucose served as a positive control due to its ability to enhance algae growth, while the negative control was culture fluid containing 1% DMSO, which was used for the dilution of the tested compounds.

Table 6.
The difference in algae cell concentration between cultures containing the compounds under study and the control cultures.
Both 3a and 8j increased the chlorophyll content in cells and/or algae growth at specific concentrations (Table 7 and Table 8). The most notable effect was the nearly 30% increase in chlorophyll content in cells in the presence of 1 × 10−4 mol/L 3a, although this was accompanied by a 17.8% decrease in cell growth. Chlorophylls are utilized as natural colorants in the food, cosmetics, and textile industries [59]. As C. vulgaris cells are enriched with chlorophyll (up to 4.5% of dry weight), it can be considered one of the most prominent natural sources of these pigments with substantial commercial potential [60]. Therefore, the synthesized compounds can be utilized to enhance the efficiency of algal chlorophyll production for industrial applications.

Table 7.
The impact of adding compound 3a on the concentration of Chlorella cells and the cellular content of pigments.

Table 8.
The impact of adding compound 8j on the concentration of Chlorella cells and the cellular content of pigments.
3. Materials and Methods
3.1. Synthetic Methods and Analytic Data of Compounds
3.1.1. General Information
1H and 13C NMR spectra (Supplementary Materials) were acquired on a Bruker Avance III 400 HD spectrometer (Bruker BioSpin AG, Faellanden, Switzerland) (at 400 and 100 MHz, respectively) in CDCl3 or DMSO-d6, using solvent residual signals (in 13C NMR, 77.00 for CDCl3, 39.52 for DMSO-d6; in 1H NMR, 7.26 for CDCl3, 2.50 for DMSO-d6) as internal standards. 19F NMR spectra (Supplementary Materials) were acquired on a Bruker Avance III 400 HD spectrometer (Bruker BioSpin AG, Faellanden, Switzerland) (at 376 MHz) in CDCl3 or DMSO-d6 using no internal standard. IR spectra were recorded on a Perkin–Elmer Spectrum Two spectrometer (PerkinElmer Inc., Waltham, MA, USA) from mulls in mineral oil. Melting points were measured on a Mettler Toledo MP70 apparatus (Mettler-Toledo (MTADA), Schwerzenbach, Switzerland). Elemental analyses were carried out on a Vario MICRO Cube analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). The reaction conditions were optimized using HPLC-UV on Hitachi Chromaster (Hitachi High-Tech, Tokyo, Japan) [NUCLEODUR C18 Gravity column (particle size 3 μm; eluent acetonitrile–water, flow rate 1.5 mL/min); Hitachi Chromaster 5430 diode array detector (λ 210–750 nm)]. The single crystal X-ray analyses of compounds 3a, 4b, 6d, 8a were performed on an Xcalibur Ruby diffractometer (Agilent Technologies, Wroclaw, Poland). The empirical absorption correction was introduced by a multi-scan method using the SCALE3 ABSPACK algorithm [61]. Using OLEX2 [62], the structures were solved with the SHELXT [63] or SUPERFLIP [64] program and refined by the full-matrix least-squares minimization in the anisotropic approximation for all non-hydrogen atoms with the SHELXL [65] program. Hydrogen atoms bound to carbon were positioned geometrically and refined using a riding model. The hydrogen atoms of NH and OH groups were refined independently with isotropic displacement parameters. The APBTTs 1a–g were obtained according to reported procedures [21,25]. The compounds 2a–n were obtained according to the reported procedures [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. Benzene, toluene, o-xylene, p-xylene, 1,4-dioxane, and THF for procedures with compounds 1 were distilled over Na before use. Acetone, butyl acetate, and chloroform for procedures with compounds 1 were distilled over P2O5 before the use. DMAA, DMF, DMSO, NMP, and acetonitrile for procedures with compounds 1 were dried over molecular sieves 4Å before the use. All procedures with APBTTs 1 were performed in an oven-dried glassware. All other solvents and reagents were purchased from commercial vendors and were used as received. Thin-layer chromatography (TLC) was performed on ALUGRAM Xtra SIL G/UV254 silica gel 60 plates (Macherey-Nagel, Düren, Germany) using EtOAC/toluene, 1:5 v/v, EtOAc, toluene as eluents; spots were visualized with iodine vapor and/or UV light (254, 365 nm) in the light of a TLC viewing cabinet Petrolaser TLC-254/365 Thin Layer Chromatography Dark Room (Petrolaser, St. Petersburg, Russia). In 13C NMR spectra of compounds 3a,c–g,i, 3′j, 8d–f,i,j, signals of some aromatic carbons could not be found.
3.1.2. Procedure to Compounds 3a–m
A mixture of APBTT 1 (0.298 mmol) and Schiff base 2 (0.298 mmol) in benzene (5 mL) was heated for 3–24 h at 85 °C (until the dark violet color characteristic of APBTT 1 disappeared and a transparent yellow solution formed). Then, the reaction mixture was cooled to room temperature.
- (3R*,3aS*,11aR*)-3a-Benzoyl-2,3-diphenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3a). The formed precipitate was filtered off. Then, the precipitate was stirred for 30 min at 40–45 °C in a mixture of toluene and ethanol (6:1 v/v, 3 mL). After that, the precipitate was filtered off and washed with a small amount of toluene (1 mL) and ethanol (1 mL) to produce compound 3a. Yield: 80.1 mg (52%); yellow solid; mp 133–135 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.84 (m, 1H), 7.62 (m, 1H), 7.54–7.47 (m, 7H), 7.37 (m, 1H), 7.34 (m, 2H), 7.29 (m, 2H), 7.23 (m, 4H), 7.13 (m, 1H), 6.65 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 192.8, 191.3, 165.1, 155.6, 136.2, 135.4, 134.9, 134.0, 133.7, 130.3, 128.9 (2C), 128.7 (2C), 128.6 (2C), 128.5 (2C), 128.3 (2C), 128.3, 126.5, 126.2, 124.4 (2C), 123.1, 117.4, 79.5, 66.0, 65.5 ppm. IR (mineral oil): 1763, 1716, 1678 cm−1. Anal. Calcd (%) for C31H20N2O4S: C 72.08; H 3.90; N 5.42. Found: C 72.23; H 3.98; N 5.43.
- (3R*,3aS*,11aR*)-3a-(4-Methylbenzoyl)-2,3-diphenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3b). The solvent was evaporated to 1 mL. The resulting precipitate was filtered off, washed with benzene (0.5 mL), and recrystallized from benzene (2 mL) to produce compound 3b. Yield: 28 mg (18%); yellow solid; mp 180–182 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.85 (m, 1 H), 7.52 (m, 3H), 7.43 (m, 2H), 7.34 (m, 2H), 7.30 (m, 3H), 7.27–7.17 (m, 6H), 7.13 (m, 1H), 6.67 (s, 1H), 2.33 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 191.7, 191.4, 170.2, 165.1, 155.6, 144.7, 136.2, 134.8, 134.0, 132.5, 130.3, 129.5 (2C), 128.9 (2C), 128.7, 128.6 (2C), 128.5 (2C), 128.3, 126.5, 126.3, 124.4 (2C), 123.2, 117.4, 114.5, 79.4, 65.9, 65.4, 21.0 ppm. IR (mineral oil): 1785, 1716, 1672 cm−1. Anal. Calcd (%) for C32H22N2O4S: C 72.44; H 4.18; N 5.28. Found: C 72.67; H 4.28; N 5.32.
- (3R*,3aS*,11aR*)-3a-(4-Fluorobenzoyl)-2,3-diphenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3c). The solvent was evaporated to 2.5 mL. The obtained mixture was frozen. The resulting precipitate was filtered off, washed with benzene (0.5 mL), and recrystallized from toluene (2 mL) to produce compound 3c. Yield: 40 mg (25%); yellow solid; mp 111–113 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.82 (m, 1H), 7.67 (m, 2H), 7.52 (m, 3H), 7.39 (m, 2H), 7.33 (m, 2H), 7.29 (m, 1H), 7.25 (m, 4H), 7.22 (m, 2H), 7.18–7.11 (m, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 191.6, 190.7, 165.2, 164.9 (d, J = 254.5 Hz), 155.8, 136.2, 134.8, 134.1, 132.0 (d, J = 10.1 Hz, 2C), 131.8 (d, J = 3.0 Hz), 130.7, 128.6 (2C), 128.6 (2C), 126.6, 126.1, 125.3, 124.4 (2C), 123.0, 117.6, 116.0 (d, J = 22.2 Hz, 2C), 79.9, 65.7, 65.5 ppm. 19F NMR (376 MHz, DMSO-d6): δ = −104.24 ppm. IR (mineral oil): 1735, 1697, 1658 cm−1. Anal. Calcd (%) for 2C31H19FN2O4S·C7H8: C 71.37; H 3.99; N 4.82. Found: C 71.51; H 4.08; N 4.99.
- (3R*,3aS*,11aR*)-3a-(4-Bromobenzoyl)-2,3-diphenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3d). The solvent was evaporated to 2.5 mL, the reaction mass was frozen. The resulting precipitate was filtered off, washed with benzene (0.5 mL), and recrystallized from toluene (2 mL) to produce compound 3d. Yield: 30 mg (17%); yellow solid; mp 207–209 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.81 (m, 1H), 7.75 (m, 2H), 7.53 (m, 5H), 7.33 (m, 1H), 7.29 (m, 2H), 7.26 (m, 4H), 7.22 (m, 2H), 7.13 (m, 1H), 6.57 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 192.2, 190.5, 165.08, 155.6, 136.1, 134.8, 134.2, 133.9, 131.8 (2C), 130.6 (2C), 128.8, 128.6 (2C), 128.5 (2C), 128.1, 127.7, 126.4, 126.0, 124.2 (2C), 122.8, 117.5, 79.8, 65.7, 65.4 ppm. IR (mineral oil): 1757, 1728, 1701 cm−1. Anal. Calcd (%) for C31H19BrN2O4S: C 62.53; H 3.22; N 4.70. Found: C 62.64; H 3.35; N 4.60.
- (3R*,3aS*,11aR*)-3a-(Furan-2-carbonyl)-2,3-diphenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3e). The resulting precipitate was filtered off, washed with benzene (1 mL) and recrystallized from toluene (2–3 mL). Then, the obtained crystals were stirred in a mixture of toluene and ethanol (5:1 v/v, 3 mL) at 50 °C for 10 min. Then, the precipitate was filtered off to produce compound 3e. Yield: 71 mg (47%); yellow solid; mp 147–149 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.88 (m, 1H), 7.64 (m, 1H), 7.49 (m, 2H), 7.43 (m, 1H), 7.32 (m, 3H), 7.26 (m, 4H), 7.18 (m, 4H), 7.11 (m, 1H), 6.80 (s, 1H), 6.71 (m, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 191.3, 176.5, 165.4, 156.0, 149.7, 149.4, 136.4, 135.1, 133.9, 129.9, 129.3 (2C), 128.4, 128.3, 128.1, 127.7, 126.3, 126.1, 125.3, 124.7 (2C), 122.8, 121.8, 116.6, 114.3, 78.7, 65.5, 62.8 ppm. IR (mineral oil): 1770, 1732, 1716, 1674 cm−1. Anal. Calcd (%) for 2C29H18N2O5S·C7H8: C 70.64; H 4.01; N 5.07. Found: C 70.82; H 4.11; N 5.00.
- (3R*,3aS*,11aR*)-2,3-Diphenyl-3a-(thiophene-2-carbonyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3f). The resulting precipitate was filtered off, washed with benzene (1 mL), and recrystallized from toluene (2–3 mL) to produce compound 3f. Yield: 70 mg (45%); yellow solid; mp 169–171 °C. 1H NMR (400 MHz, DMSO-d6): δ = 8.15 (m, 1H), 7.87 (m, 1H), 7.51 (m, 3H), 7.40 (m, 1H), 7.35 (m, 1H), 7.31 (m, 4H), 7.26 (m, 2H), 7.23 (m, 2H), 7.21 (m, 2H), 7.17 (m, 1H), 7.12 (m, 1H), 6.75 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 191.2, 182.1, 165.0, 155.2, 140.7, 137.9, 136.2, 134.7, 133.8, 130.3, 129.2, 128.9, 128.5, 128.4 (2C), 128.1 (2C), 126.3, 126.1, 125.2, 124.4 (2C), 123.0, 117.1, 79.1, 65.9, 64.6 ppm. IR (mineral oil): 1762, 1715, 1650 cm−1. Anal. Calcd (%) for 2C29H18N2O4S2·C7H8: C 68.64; H 3.90; N 4.93. Found: C 68.83; H 4.11; N 4.99.
- (3R*,3aS*,11aR*)-3a-Benzoyl-2-(3-nitrophenyl)-3-((E)-styryl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3g). The solvent was evaporated to 1 mL. The resulting precipitate was filtered off, washed with benzene (0.5 mL), and recrystallized from acetonitrile (1 mL) to produce compound 3g. Yield: 20 mg (12%); yellow solid; mp 212–214 °C. 1H NMR (400 MHz, DMSO-d6): δ = 8.51 (m, 1H), 8.07 (m, 1H), 7.96 (m, 1H), 7.87 (m, 1H), 7.69 (m, 1H), 7.62 (m, 1H), 7.50 (m, 5H), 7.36 (m, 2H), 7.29 (m, 2H), 7.24 (m, 3H), 6.73 (d, J 15.7 Hz, 1H), 6.18 (d, J 9.3 Hz, 1H), 6.02 (dd, J 15.7 Hz, J 9.8 Hz, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 193.3, 191.7, 165.4, 156.1, 147.9, 137.5, 137.4, 136.5, 136.0, 135.3, 134.1, 132.5, 130.4, 129.6 (2C), 129.2 (2C), 129.0, 128.6 (2C), 127.1 (2C), 126.9, 124.2, 123.6, 122.1, 121.6, 117.1, 78.9, 66.8, 64.2 ppm. IR (mineral oil): 1756, 1721, 1677 cm−1. Anal. Calcd (%) for C33H21N3O6S: C 67.45; H 3.60; N 7.15. Found: C 67.62; H 3.71; N 7.10.
- (3R*,3aS*,11aR*)-3a-Benzoyl-3-(4-iodophenyl)-2-phenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3h). The solvent was evaporated to 2.5 mL. The resulting precipitate was filtered off, washed with benzene (1 mL), and recrystallized from toluene (2–3 mL) to produce compound 3h. Yield: 54 mg (28%); yellow solid; mp 170–172 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.85 (m, 1H), 7.59 (m, 3H), 7.51 (m, 3H), 7.47 (m, 4H), 7.36 (m, 1H), 7.30 (m, 3H), 7.14 (m, 3H), 6.68 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 192.4, 191.5, 165.0, 155.3, 137.0 (2C), 136.0, 135.6, 134.7, 133.8, 133.5, 131.0, 130.0, 128.9 (2C), 128.5 (2C), 128.2 (2C), 128.2, 127.8, 126.5, 126.2, 124.4 (2C), 123.0, 117.1, 95.2, 79.1, 66.3, 64.7 ppm. IR (mineral oil): 1762, 1715, 1691 cm−1. Anal. Calcd (%) for C31H19IN2O4S: C 57.95; H 2.98; N 4.36. Found: C 58.16; H 3.10; N 4.28.
- (3R*,3aS*,11aR*)-3a-Benzoyl-3-(4-bromophenyl)-2-(4-methoxyphenyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3i). The reaction mixture was evaporated to dryness. Then, the residue was recrystallized from benzene (2–3 mL) to produce compound 3i. Yield: 52 mg (28%); yellow solid; mp 221–223 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.84 (m, 1H), 7.61 (m, 1H), 7.52 (m, 1H), 7.46 (m, 4H), 7.42 (m, 4H), 7.33 (m, 2H), 7.25 (m, 2H), 6.84 (m, 2H), 6.61 (s, 1H), 3.69 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 192.5, 191.7, 165.0, 157.5, 155.4, 135.7, 134.8, 133.7 (2C), 133.5, 131.3, 131.2 (2C), 130.1, 129.0 (2C), 128.8, 128.3 (2C), 126.3, 126.2 (2C), 123.1, 122.0, 117.1, 113.8 (2C), 79.1, 66.5, 64.9, 55.1 ppm. IR (mineral oil): 1761, 1729, 1710, 1689 cm−1. Anal. Calcd (%) for C32H21BrN2O5S: C 61.45; H 3.38; N 4.48. Found: C 61.63; H 3.50; N 4.40.
- (3R*,3aS*,11aR*)-3a-Benzoyl-2-(4-chlorophenyl)-3-(3,4-dimethoxyphenyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3j) and (3S*,3aS*,11aR*)-3a-benzoyl-2-(4-chlorophenyl)-3-(3,4-dimethoxyphenyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3′j). The reaction mixture was cooled to 10 °C. The resulting precipitate was filtered off (a mixture of products 3j and 3′j, 1:1). The mother liquor was evaporated to dryness. Then, the residue was recrystallized from ethanol (2–3 mL) at 60–65 °C to afform product 3j. Product 3j: Yield: 50 mg (28%); yellow solid; mp 238–240 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.84 (m, 1H), 7.62 (m, 1H), 7.58 (m, 2H), 7.50 (m, 5H), 7.34 (m, 4H), 6.80 (m, 3H), 6.56 (s, 1H), 3.69 (s, 3H), 3.60 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 193.0, 190.7, 165.0, 155.8, 148.9, 148.4, 135.6, 135.2, 134.8, 133.4, 130.6, 130.2, 128.7 (2C), 128.4 (2C), 128.4 (2C), 128.1, 126.2 (2C), 126.1, 125.5, 123.0, 121.4, 117.2, 112.5, 111.3, 79.4, 66.0, 65.2, 55.5, 55.2 ppm. IR (mineral oil): 1761, 1726, 1713, 1682 cm−1. Anal. Calcd (%) for C33H23ClN2O6S: C 64.86; H 3.79; N 4.58. Found: C 64.93; H 3.85; N 4.60. Product 3′j: Yield: 24 mg (13%, the mixture 3j:3′j, 1:1), orange solid; mp 224–226 °C (mixture 3j:3′j, 1:1). 1H NMR (400 MHz, DMSO-d6): δ = 7.80 (m, 1H), 7.62 (m, 2H), 7.56 (m, 2H), 7.36 (m, 5H), 7.30 (m, 2H), 6.77 (m, 1H), 6.71 (m, 3H), 6.47 (s, 1H), 3.59 (s, 3H), 3.54 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 191.0, 168.1, 165.7, 154.4, 148.7, 148.1, 135.7, 134.9, 134.4, 132.7, 130.7, 130.1, 129.0 (2C), 128.6 (2C), 127.7 (2C), 127.6, 125.9, 125.7 (2C), 122.43, 121.0, 115.8, 112.8, 111.4, 79.2, 70.6, 65.2, 55.3, 55.2 ppm. IR (mineral oil): 1761, 1713, 1680 cm−1. Anal. Calcd. (%) for C33H23ClN2O6S: C 64.86; H 3.79; N 4.58. Found: C 64.12; H 3.94; N 4.71.
- (3R*,3aS*,11aR*)-3a-Benzoyl-3-(3,4-dimethoxyphenyl)-2-phenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3k) and (3S*,3aS*,11aR*)-3a-benzoyl-3-(3,4-dimethoxyphenyl)-2-phenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3′k). The reaction mixture was cooled to 10 °C. The resulting precipitate was filtered off and recrystallized from benzene (2–3 mL) to give product 3’k. The mother liquor was evaporated to dryness, and the residue was recrystallized from toluene (2–3 mL) to produce product 3k. Product 3k: Yield: 40 mg (23%); yellow solid; mp 217–219 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.84 (m, 1H), 7.64 (m, 1H), 7.54 (m, 7H), 7.33 (m, 4H), 7.16 (m, 1H), 6.86 (s, 1H), 6.81 (m, 2H), 6.54 (s, 1H), 3.68 (s, 3H), 3.61 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 193.1, 190.6, 165.0, 155.9, 148.9, 148.5, 136.3, 135.4, 134.9, 133.5, 130.3, 128.7 (2C), 128.6 (2C), 128.5 (2C), 128.1, 126.4, 126.1, 125.8, 124.3 (2C), 123.0, 121.1, 117.3, 112.2, 111.3, 79.6, 65.7, 65.5, 55.5, 55.2 ppm. IR (mineral oil): 1759, 1728, 1715, 1689 cm−1. Anal. Calcd (%) for C33H24N2O6S: C 68.74; H 4.20; N 4.86. Found: C 68.90; H 4.27; N 4.79. Product 3′k: Yield: 48 mg (28%); orange solid; mp 247–249 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.82 (m, 3H), 7.62 (m, 1H), 7.52 (m, 3H), 7.40 (m, 2H), 7.32 (m, 4H), 7.16 (m, 1H), 6.73 (m, 2H), 6.67 (m, 1H), 6.46 (s, 1H), 3.59 (s, 3H), 3.52 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 191.4, 188.0, 165.8, 154.5, 148.7, 148.0, 136.0, 135.8, 134.5, 132.9, 130.3, 129.2 (2C), 128.7 (2C), 127.9 (2C), 126.6, 126.1, 125.8, 124.1 (2C), 123.0, 122.5, 121.0, 115.9, 112.7, 111.2, 79.3, 70.8, 64.5, 55.3, 55.2 ppm. IR (mineral oil): 1762, 1709, 1669 cm−1. Anal. Calcd (%) for C33H24N2O6S: C 68.74; H 4.20; N 4.86. Found: C 68.86; H 4.31; N 4.93.
- (3R*,3aS*,11aR*)-3a-Benzoyl-2-benzyl-3-(4-bromophenyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3l). The solvent was evaporated. The resulting mass was stirred in acetonitrile (2 mL) at 80 °C for 5 min. Then, the obtained mixture was cooled to room temperature, and the formed precipitate was filtered off to produce compound 3l. Yield: 60 mg (33%); yellow solid; mp 204–206 °C. 1H NMR (400 MHz, DMSO-d6): δ = 7.79 (m, 1H), 7.59 (m, 3H), 7.54 (m, 1H), 7.37 (m, 9H), 7.16 (m, 2H), 7.09 (m, 2H), 5.58 (s, 1H), 4.95 (d, J 15.2 Hz, 1H), 3.71 (d, J 14.7 Hz, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 192.7, 191.2, 166.1, 155.1, 134.7, 134.6, 134.0, 133.8, 132.6, 131.9 (2C), 130.7, 129.8, 128.8 (2C), 128.7 (2C), 128.3 (2C), 128.3, 128.1 (2C), 128.0, 127.6, 126.3, 123.2, 122.6, 117.3, 78.9, 65.6, 63.9, 45.4 ppm. IR (mineral oil): 1760, 1719, 1672 cm−1. Anal. Calcd (%) for C32H21BrN2O4S: C 63.06; H 3.47; N 4.60. Found: C 63.14; H 3.58; N 4.63.
- (3R*,3aS*,11aR*)-3a-Benzoyl-2-(benzylideneamino)-3-phenyl-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (3m). The solvent was evaporated to 1 mL. The resulting precipitate was filtered off, stirred in benzene (2–3 mL) at 85 °C for 10 min, and then filtered off to produce compound 3m. Yield: 42 mg (26%); yellow solid; mp 224–226 °C. 1H NMR (400 MHz, DMSO-d6): δ = 8.63 (s, 1H), 7.81 (m, 1H), 7.65 (m, 1H), 7.55 (m, 7H), 7.43 (m, 3H), 7.35 (m, 5H), 7.29 (2H), 6.54 (s, 1H) ppm. 13C NMR (100 MHz, DMSO-d6): δ = 192.4, 190.4, 162.3, 156.6, 155.5, 134.9, 134.6, 133.7, 133.5, 132.9, 131.3, 130.4, 129.0, 128.9 (2C), 128.8 (2C), 128.8 (2C), 128.7 (2C), 128.3, 128.0 (2C), 127.5 (2C), 126.1, 123.0, 117.5, 78.3, 65.3, 64.7 ppm. IR (mineral oil): 1757, 1725, 1686 cm−1. Anal. Calcd (%) for C32H21N3O4S: C 70.71; H 3.89; N 7.73. Found: C 70.93; H 3.97; N 7.81.
3.1.3. Procedure to Compounds 8a–j
A mixture of APBTT 1 (0.298 mmol) and carbodiimide 7 (0.313 mmol) in toluene (5 mL) was heated for 1 h at 115 °C (until the dark violet color characteristic of APBTT 1 disappeared and a transparent yellow solution formed). Then, the reaction mixture was cooled to room temperature. The resulting precipitate was filtered off and stirred in ethanol (2 mL) at 45–50 °C for 30 min. Then, the precipitate was filtered off and recrystallized from toluene (5 mL) to produce the corresponding compound 8.
- (3aS*,11aR*)-3a-Benzoyl-2-cyclohexyl-3-(cyclohexylimino)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8a). Yield: 69 mg (44%); yellow solid; mp 264–266 °C. 1H NMR (400 MHz, CDCl3): δ = 7.87 (m, 1H), 7.68 (m, 3H), 7.49 (m, 2H), 7.25 (m, 3H), 4.31 (m, 1H), 3.41 (m, 1H), 2.32 (m, 2H), 1.86 (s, 3H), 1.73 (m, 4H), 1.51 (m, 1H), 1.42 (m, 3H), 1.36 (m, 2H), 1.21 (m, 2H), 0.91 (m, 2H), 0.38 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.7, 187.7, 168.0, 154.1, 141.6, 135.1, 134.8, 133.8, 129.3, 129.1 (2C), 128.7 (2C), 128.5, 126.9, 122.6, 118.0, 77.6, 65.4, 60.6, 54.0, 34.1, 32.1, 28.2, 27.8, 25.9, 25.8, 25.7, 25.2, 23.9, 23.8 ppm. IR (mineral oil): 1779, 1749, 1722, 1677 cm−1. Anal. Calcd (%) for C31H31N3O4S: C 68.74; H 5.77; N 7.76. Found: C 68.85; H 5.83; N 7.71.
- (3aS*,11aR*)-2-Cyclohexyl-3-(cyclohexylimino)-3a-(4-methylbenzoyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8b). Yield: 25 mg (15%); yellow solid; mp 230–232 °C. 1H NMR (400 MHz, CDCl3): δ = 7.87 (m, 1H), 7.59 (m, 2H), 7.29 (m, 2H), 7.24 (m, 3H), 4.33 (m, 1H), 3.45 (m, 1H), 2.46 (s, 3H), 2.31 (m, 2H), 1.86 (m, 3H), 1.75 (m, 1H), 1.69 (m, 4H), 1.53 (m, 1H), 1.44 (m, 3H), 1.39 (m, 1H), 1.35 (m, 1H), 1.29 (m, 1H), 1.20 (m, 2H), 0.93 (m, 2H), 0.47 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.1, 187.8, 168.1, 154.1, 146.6, 141.8, 134.8, 131.2, 129.8, 129.4, 129.0, 128.9, 128.5, 128.2, 126.8, 125.3, 122.5, 118.0, 77.7, 60.5, 54.0, 34.1, 32.2, 28.2, 27.8, 25.9, 25.8, 25.7, 25.2, 24.0, 23.7, 21.8 ppm. IR (mineral oil): 1788, 1744, 1729, 1674 cm−1. Anal. Calcd (%) for C32H33N3O4S: C 69.17; H 5.99; N 7.56. Found: C 69.55; H 6.12; N 7.66.
- (3aS*,11aR*)-2-Cyclohexyl-3-(cyclohexylimino)-3a-(4-fluorobenzoyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8c). Yield: 18 mg (11%); yellow solid; mp 228–230 °C. 1H NMR (400 MHz, CDCl3): δ = 7.92 (m, 1H), 7.79 (m, 2H), 7.33 (m, 2H), 7.23 (m, 3H), 4.36 (m, 1H), 3.47 (m, 1H), 2.36 (m, 2H), 1.92 (m, 3H), 1.80 (m, 1H), 1.74 (m, 3H), 1.60–1.47 (m, 4H), 1.45–1.37 (m, 2H), 1.29–1.19 (m, 2H), 1.00 (m, 2H), 0.52 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 189.1, 187.4, 168.0, 166.7 (d, J = 261.6 Hz), 154.0, 141.5, 134.7, 131.5 (d, J = 10.1 Hz, 2C), 130.2 (d, J = 3.0 Hz), 129.1, 128.6, 127.0, 122.6, 118.1, 116.5 (d, J = 24.2 Hz, 2C), 77.5, 65.3, 60.6, 54.1, 34.1, 32.3, 28.3, 27.8, 25.9, 25.8, 25.6, 25.1, 23.9, 23.8 ppm. 19F NMR (376 MHz, DMSO-d6): δ = −100.03 ppm. IR (mineral oil): 1789, 1749, 1726, 1673 cm−1. Anal. Calcd (%) for C31H30FN3O4S: C 66.53; H 5.40; N 7.51. Found: C 66.68; H 5.51; N 7.60.
- (3aS*,11aR*)-3a-(4-Bromobenzoyl)-2-cyclohexyl-3-(cyclohexylimino)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8d). Yield: 57 mg (31%); yellow solid; mp 180–182 °C. 1H NMR (400 MHz, CDCl3): δ = 7.92 (m, 1H), 7.71 (m, 2H), 7.61 (m, 2H), 7.32 (m, 1H), 7.26 (m, 2H), 4.35 (m, 1H), 3.46 (m, 1H), 2.36 (m, 2H), 1.91 (m, 3H), 1.80 (m, 1H), 1.74 (m, 2H), 1.60 (m, 3H), 1.48 (m, 1H), 1.46–1.31 (m, 3H), 1.25 (m, 2H), 1.02 (m, 2H), 0.55 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 189.6, 187.3, 167.9, 153.9, 141.3, 134.7, 132.5 (2C), 130.7, 130.0 (2C), 129.0, 128.6, 127.0, 122.6, 118.1, 77.4, 65.2, 60.5, 54.1, 34.1, 32.3, 28.2, 27.8, 25.8, 25.8, 25.6, 25.1, 23.9, 23.8 ppm. IR (mineral oil): 1791, 1789, 1752, 1726, 1672 cm−1. Anal. Calcd (%) for C31H30BrN3O4S: C 60.00; H 4.87; N 6.77. Found: C 60.14; H 4.97; N 6.65.
- (3aS*,11aR*)-2-Cyclohexyl-3-(cyclohexylimino)-3a-(furan-2-carbonyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8e). Yield: 71 mg (45%); yellow solid; mp 251–253 °C. 1H NMR (400 MHz, CDCl3): δ = 7.91 (m, 1H), 7.60 (s, 1H), 7.51 (m, 1H), 7.28 (m, 3H), 6.72 (s, 1H), 4.34 (m, 1H), 3.60 (br.s, 1H), 2.34 (m, 2H), 1.85 (m, 4H), 1.73 (d, J = 12, 3H), 1.63 (m, 2H), 1.52 (m, 2H), 1.38 (m, 3H), 1.22 (m, 3H), 0.95–0.62 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 186.6, 168.2, 149.4 (2C), 140.5, 134.9, 129.3, 128.4, 126.7, 122.6, 120.9, 118.0, 114.2, 77.7, 77.2, 54.1, 34.0, 28.3, 27.8, 25.9, 25.9, 25.7, 25.2, 24.0, 23.8 ppm. IR (mineral oil): 1775, 1745, 1721, 1667 cm−1. Anal. Calcd (%) for C29H29N3O5S: C 65.52; H 5.50; N 7.90. Found: C 65.71; H 5.58; N 8.03.
- (3aS*,11aR*)-2-Cyclohexyl-3-(cyclohexylimino)-3a-(thiophene-2-carbonyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8f). Yield: 96 mg (59%); yellow solid; mp 274–276 °C. 1H NMR (400 MHz, CDCl3): δ = 7.91 (m, 2H), 7.55 (m, 1H), 7.32 (m, 2H), 7.23 (m, 2H), 4.39 (m, 1H), 3.70 (m, 1H), 2.38 (m, 2H), 1.94 (m, 3H), 1.83 (m, 1H), 1.76 (m, 3H), 1.60 (m, 2H), 1.54 (m, 2H), 1.42 (m, 2H), 1.29 (m, 2H), 1.13 (m, 2H), 0.75 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 170.7, 168.0, 154.0, 141.3, 137.4 (2C), 134.8, 133.1, 129.2, 128.6, 128.5, 126.9, 122.6, 118.0, 77.8, 77.2, 60.4, 54.1, 34.2, 32.3, 28.3, 27.8, 25.9, 25.8, 25.7, 25.2, 24.0, 23.8 ppm. IR (mineral oil): 1779, 1747, 1724, 1682, 1657 cm−1. Anal. Calcd (%) for C29H29N3O4S2: C 63.60; H 5.34; N 7.67. Found: C 63.83; H 5.41; N 7.54.
- (3aS*,11aR*)-3a-(4-Chlorobenzoyl)-2-cyclohexyl-3-(cyclohexylimino)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8g). Yield: 43 mg (25%); yellow solid; mp 248–250 °C. 1H NMR (400 MHz, CDCl3): δ = 7.92 (m, 1H), 7.69 (m, 2H), 7.53 (m, 2H), 7.30 (m, 3H), 4.35 (m, 1H), 3.46 (m, 1H), 2.36 (m, 2H), 1.92 (m, 3H), 1.80 (m, 1H), 1.74 (m, 3H), 1.58 (m, 2H), 1.48 (m, 2H), 1.39 (m, 2H), 1.26 (m, 2H), 1.01 (m, 2H), 0.53 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ = 189.4, 187.4, 167.9, 153.9, 142.0, 141.3, 134.7, 132.1, 130.0 (2C), 129.5 (2C), 129.1, 128.6, 127.0, 122.6, 118.1, 77.5, 65.3, 60.5, 54.1, 34.1, 32.3, 28.3, 27.8, 25.9, 25.8, 25.6, 25.1, 23.9, 23.8 ppm. IR (mineral oil): 1791, 1789, 1751, 1726, 1674 cm−1. Anal. Calcd (%) for C31H30ClN3O4S: C 64.63; H 5.25; N 7.29. Found: C 64.69; H 5.19; N 7.37.
- (3aS*,11aR*)-3a-Benzoyl-2-isopropyl-3-(isopropylimino)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8h). Yield: 89 mg (65%); yellow solid; mp 207–207 °C. 1H NMR (400 MHz, CDCl3): δ = 7.94 (m, 1H), 7.74 (m, 3H), 7.56 (m, 2H), 7.35–7.25 (m, 3H), 4.78 (m, 1H), 3.79 (m, 1H), 1.54 (m, 6H), 1.30 (m, 3H), 0.50 (d, J = 8 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.3, 187.8, 167.9, 154.1, 141.4, 135.2, 134.8, 133.8, 129.2, 129.2 (2C), 128.8 (2C), 128.5, 126.9, 122.6, 118.1, 77.6, 66.7, 52.8, 46.1, 24.4, 22.0, 18.8, 18.4 ppm. IR (mineral oil): 1786, 1749, 1724, 1669 cm−1. Anal. Calcd (%) for C25H23N3O4S: C 65.06; H 5.02; N 9.10. Found: C 65.17; H 5.09; N 9.18.
- (3aS*,11aR*)-2-Isopropyl-3-(isopropylimino)-3a-(4-methylbenzoyl)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8i). Yield: 106 mg (75%); yellow solid; mp 216–218 °C. 1H NMR (400 MHz, CDCl3): δ = 7.94 (m, 1H), 7.67 (m, 2H), 7.37 (m, 2H), 7.35–7.25 (m, 3H), 4.79 (m, 1H), 3.84 (m, 1H), 2.52 (s, 3H), 1.55 (m, 6H), 1.31 (m, 3H), 0.53 (m, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 189.8, 187.8, 167.9, 154.1, 146.8, 141.6, 134.8, 131.2, 129.9, 129.3, 129.0, 128.5, 126.9 (2C), 122.6, 118.0 (2C), 115.0, 52.7, 46.1, 24.4, 22.1, 21.8, 18.8, 18.4 ppm. IR (mineral oil): 1786, 1751, 1727 cm−1. Anal. Calcd (%) for C26H25N3O4S: C 65.67; H 5.30; N 8.84. Found: C 65.81; H 5.26; N 8.80.
- (3aS*,11aR*)-3a-(4-Chlorobenzoyl)-2-isopropyl-3-(isopropylimino)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-trione (8j). Yield: 106 mg (72%); yellow solid; mp 228–230 °C. 1H NMR (400 MHz, CDCl3): δ = 7.64 (m, 1H), 7.41 (m, 2H), 7.30 (m, 1H), 7.06–6.96 (m, 4H), 4.48 (m, 1H), 3.49 (m, 1H), 1.25 (d, J = 4 Hz, 6H), 1.02 (d, J = 8 Hz, 3H), 0.29 (d, J = 8 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 189.1, 187.4, 167.7, 153.9, 142.1, 141.2, 134.7, 132.0, 131.3, 130.1 (2C), 129.6, 129.0, 128.6 (2C), 127.0, 122.6, 118.1, 52.8, 46.2, 24.4, 22.2, 18.8, 18.4 ppm. IR (mineral oil): 1786, 1750, 1726, 1664 cm−1. Anal. Calcd (%) for C25H22ClN3O4S: C 60.54; H 4.47; N 8.47. Found: C 60.76; H 4.58; N 8.55.
3.1.4. Procedures to Compounds 4a,b,6d
- 5-Hydroxy-4-(2-oxo-2H-benzo[b][1,4]thiazin-3(4H)-ylidene)-1,5-diphenylpyrrolidine-2,3-dione (4a). Method 1. Acetic acid (3 mL) was added to the mixture of APBTT 1a (100 mg, 0.298 mmol) and N-benzylideneaniline 2a (54 mg, 0.298 mmol). The mixture was stirred for 24 h at room temperature. The resulting precipitate was filtered off and washed with acetone (2 mL) to produce compound 4a. Yield: 110 mg (86%); red solid; mp 179–181 °C. Method 2. Aniline 5a (27.2 μL, 0.298 mmol) was added to the mixture of acetic acid (3 mL) and APBTT 1a (100 mg, 0.298 mmol). The mixture was stirred for 24 h at room temperature. The resulting precipitate was filtered off and washed with acetone (2 mL) to produce compound 4a. Yield: 121 mg (95%); red solid; mp 179–181 °C. 1H NMR (400 MHz, DMSO-d6): δ = 14.05 (s, 1H), 7.60 (m, 1H), 7.52 (m, 2H), 7.38 (m, 1H), 7.34 (m, 2H), 7.22 (m, 3H), 7.18 (m, 1H), 7.16 (s, 1H), 7.13 (m, 1H), 7.11 (s, 1H), 7.04 (m, 2H) ppm. IR (mineral oil): 3467, 3169, 3083, 1723, 1684, 1632 cm−1. Anal. Calcd (%) for C24H16N2O4S: C 67.28; H 3.76; N 6.54. Found: C 67.39; H 3.81; N 6.47.
- 1-Benzyl-5-hydroxy-4-(2-oxo-2H-benzo[b][1,4]thiazin-3(4H)-ylidene)-5-(4-methylphenyl)pyrrolidine-2,3-dione (4b). Benzylamine 5b (15.6 μL, 0.143 mmol) was added to the mixture of acetic acid (3 mL) and APBTT 1a (50 mg, 0.143 mmol). The mixture was stirred for 24 h at room temperature. The resulting precipitate was filtered off and washed with acetone (1 mL) to produce compound 4b. Yield: 63 mg (46%); orange solid; mp 162–164 °C. 1H NMR (400 MHz, DMSO-d6): δ = 14.24 (s, 1H), 7.55 (m, 1H), 7.46 (m, 2H), 7.31 (m, 3H), 7.12 (m, 3H), 7.03 (m, 4H), 6.67 (s, 1H), 4.20 (dd, J 64.1 Hz, J 15.2 Hz, 2H), 2.24 (s, 3H) ppm. 13C NMR (400 MHz, DMSO-d6): δ = 183.1, 177.4, 160.4, 138.8, 137.8, 137.0, 136.3, 129.5, 128.6, 128.1 (2C), 127.8 (2C), 127.5 (2C), 126.3, 126.1, 125.9 (2C), 125.5, 120.5, 119.4, 109.9, 88.6, 42.5, 20.5 ppm. IR (mineral oil): 3474, 3192, 3058, 1720, 1640 cm−1. Anal. Calcd (%) for C26H20N2O4S: C 68.41; H 4.42; N 6.14. Found: C 68.56; H 4.47; N 6.20.
- (Z)-3-(Benzo[d]thiazol-2(3H)-ylidene)-4-(4-bromophenyl)-2,4-dioxo-N-phenylbutanamide (6d). This product was a by-product in the synthesis of compound 3d. Product 6d was precipitated in the first fraction upon recrystallization of the main product from toluene. Yield: 3 mg (2%); colorless crystals; mp 179–181 °C. 1H NMR (400 MHz, CDCl3): δ = 13.76 (br.s, 1H), 10.39 (s, 1H), 8.07 (m, 1H), 7.53 (m, 3H), 7.43 (m, 3H), 7.29 (m, 2H), 7.21 (m, 3H), 7.02 (m, 1H) ppm. IR (mineral oil): 3278, 3136, 1718, 1672, 1635 cm−1. Anal. Calcd (%) for C23H15BrN2O3S: C 57.63; H 3.15; N 5.84. Found: C 57.86; H 3.24; N 5.91.
3.2. Computational Details
The DFT calculations for all model structures were carried out at the M06-2X/6-31G* level of theory with the help of the Gaussian-09 program package [81]. No symmetry restrictions have been applied during the geometry optimization procedure. The Hessian matrices were calculated analytically for all optimized model structures to prove the location of correct minima or saddle points (transition states) on the potential energy surface. The Cartesian atomic coordinates for all model structures are presented in attached xyz-files (Supplementary Materials).
3.3. Biology
3.3.1. Screening of Substances in 96-Well Plates
Chlorella vulgaris (strain IMBR-19, obtained from the A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS, Sevastopol, Russia) was cultivated in BG-11 medium. BG-11 medium was prepared using following solutions: Solution #1: Na2-EDTA—20 mg in 20 mL; Solution #2: citric acid—120 mg and iron(III) citrate—120 mg in 20 mL; Solution #3: K2HPO4∙3H2O—800 mg in 20 mL; Solution #4. MgSO4∙7H2O—1.5 g in 20 mL; Solution #5: CaCl2∙2H2O—720 mg in 20 mL; Solution #6: Na2CO3—400 mg in 20 mL; Solution #7: NaNO3—15 g in 100mL; Solution #8: H3BO3—57.2 mg, MnCl2∙4H2O—36.2 mg, ZnSO4∙7H2O—4.4 mg, CuSO4∙5H2O—1.58 mg, Na2MoO4∙2H2O—7.8 mg, and 1 mL of 0.988 g/L Co(NO3)2∙6H2O in 20 mL. All of the solutions were prepared using Milli-Q water. To obtain 500 mL of BG-11 medium, 500 µL of solutions #1–6 and #8 and 5 mL of solution #7 were added to 400 mL of water. Then, the volume was adjusted to 500 mL. A solution of D(+)-glucose in BG-11 (6 g/L) was used for the preparation of positive controls.
Cultures of C. vulgaris were maintained and cultivated in aseptic conditions, with the only exception being DMSO solutions of tested substances that were not sterilized upon dilution.
Substances to be tested were diluted to 1 × 10−3, 1 × 10−4, and 1 × 10−5 mol/L in 99% DMSO before the experiment. Poorly soluble substances were either kept for 16 h on a rotator or treated by ultrasound using an ultrasound homogenizer equipped with a 3 mm probe (VCX-130, Sonics and Materials, Newtown, CT, USA).
Starter cultures of C. vulgaris were prepared as follows: stock culture cells (2 mL, in exponential growth phase) were washed two times with BG-11 medium by centrifugation (20 min, 350 g). The cells were then diluted in 10 mL of BG-11, and the cell concentration was measured using a hemocytometer.
In the wells of 96-well culture plates, BG-11 medium, starter culture of C. vulgaris, and tested substances diluted in DMSO were combined, resulting in a total volume of 300 µL. The resulting number of cells was 5 × 104 cells/well, with a resulting volume fraction of DMSO at 1%. The resulting concentrations of tested substances were 1 × 10−5, 1 × 10−6, and 1 × 10−7 mol/L. In negative control and positive control wells, pure DMSO was added. BG-11 with glucose was added to the positive control wells, resulting in a concentration of glucose of 2 g/L. All substances were tested in duplicate (2 wells for each concentration). In the edge and corner wells of the plates, sterile distilled water was added. The plates were sealed with a gas-permeable film.
All cultures were maintained for 5 days in a humid chamber at +28 °C at 150 rpm under cyclic illumination consisting of 12 h on: 12 h off. The light intensity was 100 μmol·m−2·s−1. The lighting unit was an array of evenly distributed white LEDs with a cooling device preventing well heating, positioned below the culture plates (bottom illumination).
After the end of cultivation, the contents of the wells were mixed using a multichannel pipette, and the cell concentration was assessed by measuring the absorbance at 750 nm.
3.3.2. Evaluation of Lead Substances in 50-mL Flasks
Substances to be tested were diluted to 1 × 10−2, 1 × 10−3, 1 × 10−4, and 1 × 10−5 mol/L in 99% DMSO before the experiment. Starter cultures of C. vulgaris were prepared as follows: stock culture cells (12 mL, in exponential growth phase) were washed twice with BG-11 medium by centrifugation (15 min, 450 g). The cells were then diluted in 10 mL of BG-11, and the cell concentration was measured using a hemocytometer.
In the 50-mL Erlenmeyer flasks, BG-11 medium, the starter culture of C. vulgaris, and tested substances diluted in DMSO were combined, resulting in a total volume of 30 mL. The resulting number of cells was 1 × 107 cells/flask, with a resulting volume fraction of DMSO at 1%. The resulting concentrations of tested substances were 1 × 10−4, 1 × 10−5, 1 × 10−6, and 1 × 10−7 mol/L. In the negative control and positive control flasks, pure DMSO was added. BG-11 with glucose was added to the positive control flasks, resulting in a concentration of glucose of 2 g/L. All substances were tested in triplicate (three flasks for each concentration). The flasks were sealed with gas-permeable cellulose caps. All cultures were maintained for 5 days in a humid chamber at +28 °C at 150 rpm under cyclic illumination consisting of 12 h on: 12 h off. The light intensity was 100 μmol·m−2·s−1. The lighting unit was an array of evenly distributed white LEDs with a cooling device preventing overheating, positioned below the culture plates (bottom illumination).
3.3.3. Cell Count and Pigments Analysis
The flask contents were carefully mixed, and then 10 mL of cell culture was transferred into 15-mL centrifuge tubes. The cells were washed twice with 10 mL of water by centrifugation (15 min, 450 g). The washed cells were diluted in 10 mL of water, and the cell concentration was measured using a hemocytometer.
Pigment extraction was carried out as follows: two milliliters of cell culture were transferred to centrifuge tubes. The cells were washed by centrifugation two times at 7000× g for 10 min and concentrated two-fold. After the second wash, the sediment was vortexed for 1 min, and then 90% methanol was added. The tubes were heated at +60 °C for 30 min in a solid-state thermostat. Then, the samples were cooled to room temperature and centrifuged at 10,000× g for 10 min. The absorbance of the supernatant containing extracted pigments was measured at 665, 652, and 470 nm. The concentrations of chlorophylls and carotenoids were calculated as described in the papers [82,83]. After that, the concentration of pigments in micrograms per 1 × 107 cells was calculated.
4. Conclusions
An approach to a new 6/5/5/5-tetracyclic alkaloid-like spiroheterocyclic system of benzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole 3, 8 was developed on the basis of a reaction of 3-aroylpyrrolo[2,1-c][1,4]benzothiazine-1,2,4-triones 1 with Schiff bases 2 and carbodiimides 7. This reaction proceeded as a nucleophile-induced ring contraction—intramolecular cyclization cascade. The formation of the benzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazoles 3, 8 was found to be diastereoselective, with the exception of compounds 3k,j, 3′k,j. Compounds 3a, 8j were found to promote the growth of Chlorella vulgaris and to increase the chlorophyll content in its cells.
5. Patents
The method for preparing products 8 has been patented [84].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29092089/s1, The following are available online, copies of NMR spectra for new compounds 3a–m, 3′j,k, 4a,b, 6d, 8a–j, details of DFT calculations, Cartesian atomic coordinates for all model structures, ORTEP images of X-ray crystal structures.
Author Contributions
Conceptualization, E.E.K.; methodology, E.E.K., E.A.L. (synthetic chemistry) and A.D.N. (biology); investigation, E.E.K., E.A.L. (synthetic chemistry), M.V.D. (X-ray analysis), A.S.N. (DFT calculations), A.D.N. and P.V.K. (biology); writing—original draft preparation, E.E.K., E.A.L., A.S.N., A.D.N. and P.V.K.; writing—review and editing, E.E.K., E.A.L., A.S.N., M.V.D. and P.V.K.; supervision, E.E.K., P.V.K. and A.N.M.; project administration, E.E.K.; funding acquisition E.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (RSF-22-73-00088).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The presented data are available in this article.
Acknowledgments
A.S.N. is grateful to the RUDN University Scientific Projects Grant System (project № 021342-2-000) for the support of the DFT calculations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. Med. Chem. Commun. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef]
- Cotman, A.E. Escaping from Flatland: Stereoconvergent Synthesis of Three-Dimensional Scaffolds via Ruthenium(II)-Catalyzed Noyori–Ikariya Transfer Hydrogenation. Chem. Eur. J. 2021, 27, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Galloway, W.; Isidro-Llobet, A.; Spring, D. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhang, K.Y.J. Advances in the Development of Shape Similarity Methods and Their Application in Drug Discovery. Front. Chem. 2018, 6, 315. [Google Scholar] [CrossRef]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Gerry, C.J.; Schreiber, S.L. Recent achievements and current trajectories of diversity-oriented synthesis. Curr. Opin. Chem. Biol. 2020, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sano, T. Synthesis of Heterocyclic Compounds Containing Nitrogen Utilizing Dioxopyrrolines. J. Synth. Org. Chem. Jpn. 1984, 42, 340–354. [Google Scholar] [CrossRef]
- Konovalova, V.V.; Shklyaev, Y.V.; Maslivets, A.N. Reactions of fused pyrrole-2,3-diones with dinucleophiles. ARKIVOC 2015, i, 48–69. [Google Scholar] [CrossRef]
- Konovalova, V.V.; Maslivets, A.N. Synthesis of Spiro Compounds Based on 1H-Pyrrole-2,3-Diones. Mini-Rev. Org. Chem. 2019, 16, 173–192. [Google Scholar] [CrossRef]
- Lystsova, E.A.; Khramtsova, E.E.; Maslivets, A.N. Acyl(imidoyl)ketenes: Reactive Bidentate Oxa/Aza-Dienes for Organic Synthesis. Symmetry 2021, 13, 1509. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Facile approach to alkaloid-like 6/6/5/5-tetracyclic spiroheterocycles via 1,3-dipolar cycloaddition reaction of fused 1H-pyrrole-2,3-diones with nitrones. Tetrahedron Lett. 2020, 61, 151595. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Hetero-Diels-Alder Reaction of 3-Aroylpyrrolo[2,1-c][1,4]benzoxazines with Styrene. Synthesis of Pyrano[4′,3′:2,3]pyrrolo[2,1-c][1,4]benzoxazines. Russ. J. Org. Chem. 2017, 53, 1851–1856. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Dmitriev, M.V.; Slepukhin, P.A.; Maslivets, A.N. Two Concurrent Pathways of the Reaction Pyrrolobenzoxazinetriones with Cyclic Alkoxyolefins. Synthesis of Alkaloid-Like Pentacyclic 6/6/5/6/5- and 6/6/5/6/6-Angularly Fused Heterocycles. Russ. J. Org. Chem. 2017, 53, 74–81. [Google Scholar] [CrossRef]
- Chervyakov, A.V.; Maslivets, A.N. Synthesis of Angular [1,2,5]Oxadiazolo[3,4-b]pyrazino[1′,2′:1,2]pyrrolo[2,3-e][1,4]diazepine by Stepwise Reaction of Pyrrolo[1,2-a]pyrazinetrione with 3,4-Diaminofurazan. Russ. J. Org. Chem. 2018, 54, 512–513. [Google Scholar] [CrossRef]
- Chervyakov, A.V.; Maslivets, A.N. Reaction of pyrrolo[1,2-a]pyrazinetriones with o-phenylenediamine. Synthesis of angular benzo[b]pyrazino[1′,2′:1,2]pyrrolo[2,3-e][1,4]diazepines. Russ. J. Org. Chem. 2016, 52, 610–611. [Google Scholar] [CrossRef]
- Mashevskaya, I.V.; Duvalov, A.V.; Kol’tsova, S.V.; Maslivets, A.N. Unusual reaction of condensed 2,3-dihydro-2,3-pyrrolediones with o-phenylenediamine. Chem. Heterocycl. Compd. 2000, 36, 617–618. [Google Scholar] [CrossRef]
- Tsuda, Y.; Ohshima, T.; Hosoi, S.; Kaneuchi, S.; Kiuchi, F.; Toda, J.; Sano, T. Synthesis of Erythrina and related alkaloids. XLIV. Total synthesis of homoerythrinan alkaloids, schelhammericine and 3-epischelhammericine. Chem. Pharm. Bull. 1996, 44, 500–508. [Google Scholar] [CrossRef]
- Sano, T.; Toda, J.; Shoda, M.; Yamamoto, R.; Ando, H.; Isobe, K.; Hosoi, S.; Tsuda, Y. Synthesis of erythrina and related alkaloids. XXXVI. Studies toward total synthesis of non-aromatic Erythrina alkaloids. 6. Synthesis of 8-oxo-γ-erythroidine and 8-oxocycloerythroidine, isomers of the natural alkaloids. Chem. Pharm. Bull. 1992, 40, 3145–3156. [Google Scholar] [CrossRef][Green Version]
- Lystsova, E.A.; Dmitriev, M.V.; Maslivets, A.N.; Khramtsova, E.E. Nucleophile-induced ring contraction in pyrrolo[2,1-c][1,4]benzothiazines: Access to pyrrolo[2,1-b][1,3]benzothiazoles. Beilstein J. Org. Chem. 2023, 19, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.R.; Hudlický, T. Retigeranic acid. In Total Synthesis of Natural Products. At the Frontiers of Organic Chemistry, 1st ed.; Li, J.J., Corey, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–258. [Google Scholar] [CrossRef]
- Yu, X.Y.; Finn, J.; Hill, J.M.; Wang, Z.G.; Keith, D.; Silverman, J.; Oliver, N. A series of heterocyclic inhibitors of phenylalanyl-tRNA synthetases with antibacterial activity. Bioorg. Med. Chem. Lett. 2004, 14, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Goudreau, N.; Cameron, D.R.; Déziel, R.; Haché, B.; Jakalian, A.; Malenfant, E.; Naud, J.; Ogilvie, W.W.; O’Meara, J.; White, P.W.; et al. Optimization and determination of the absolute configuration of a series of potent inhibitors of human papillomavirus type-11 E1–E2 protein–protein interaction: A combined medicinal chemistry, NMR and computational chemistry approach. Bioorg. Med. Chem. 2007, 15, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- Khramtsova, E.E.; Lystsova, E.A.; Dmitriev, M.V.; Maslivets, A.N.; Jasiński, R. Reaction of Aroylpyrrolobenzothiazinetriones with Electron-Rich Dienophiles. ChemistrySelect 2021, 6, 6295–6301. [Google Scholar] [CrossRef]
- Kollenz, G.; Penn, G.; Ott, W.; Peters, K.; Peters, E.-M.; von Schnering, H.G. Reaktionen mit cyclischen Oxalylverbindungen, XXIII. Zur Reaktion heterocyclischer Fünfring-2,3-dione mit Carbodiimiden—Eine Synthesemöglichkeit für hetero-analoge 7-Desazapurin-Systeme. Chem. Ber. 1984, 117, 1310–1329. [Google Scholar] [CrossRef]
- Lystsova, E.A.; Novikov, A.S.; Dmitriev, M.V.; Maslivets, A.N.; Khramtsova, E.E. Approach to Pyrido[2,1-b][1,3]benzothiazol-1-ones via In Situ Generation of Acyl(1,3-benzothiazol-2-yl)ketenes by Thermolysis of Pyrrolo[2,1-c][1,4]benzothiazine-1,2,4-triones. Molecules 2023, 28, 5495. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of Microalgal Biomass for Innovative Food Production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef] [PubMed]
- Al-Hammadi, M.; Güngörmüşler, M. New insights into Chlorella vulgaris applications. Biotechnol. Bioeng. 2024, 121, 1486–1502. [Google Scholar] [CrossRef]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energ. Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Moreira, J.B.; Vaz, B.d.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.d.; Costa, J.A.V.; Morais, M.G.d. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Thaher, M.I.; AbdulQuadir, M.; Mahata, C.; Al Jabri, H. Utilization of nitrogen-rich agricultural waste streams by microalgae for the production of protein and value-added compounds. Curr. Opin. Green Sustain. Chem. 2023, 41, 100797. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef] [PubMed]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Chlorella vulgaris as a green biofuel factory: Comparison between biodiesel, biogas and combustible biomass production. Bioresour. Technol. 2019, 273, 237–243. [Google Scholar] [CrossRef]
- Mallick, N.; Mandal, S.; Singh, A.K.; Bishai, M.; Dash, A. Green microalga Chlorella vulgaris as a potential feedstock for biodiesel. J. Chem. Technol. Biotechnol. 2021, 87, 137–145. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Thirugnanasambantham, R.; Elango, T.; Elangovan, K. Chlorella vulgaris sp. microalgae as a feedstock for biofuel. Mater. Today Proc. 2020, 33, 3182–3185. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 2010, 1, 47–58. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Farooq, W.; Naqvi, S.R.; Sajid, M.; Shrivastav, A.; Kumar, K. Monitoring lipids profile, CO2 fixation, and water recyclability for the economic viability of microalgae Chlorella vulgaris cultivation at different initial nitrogen. J. Biotechnol. 2020, 345, 30–39. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, T.Y.; Chang, Y.B.; Kuo, C.M.; Lin, C.S. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A multifunctional dietary supplement with diverse medicinal properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Kashirskikh, E.; Prosekov, A.; Michaud, P.; Babich, O. Food Proteins: Potential Resources. Sustainability 2023, 15, 5863. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef] [PubMed]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Enhancing Digestibility of Chlorella vulgaris Biomass in Monogastric Diets: Strategies and Insights. Animals 2023, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.; Tadda, M.A.; Zhao, Y.; Farmanullah, F.; Chu, B.; Li, X.; He, Y. Microalgae bioactive carbohydrates as a novel sustainable and eco-friendly source of prebiotics: Emerging health functionality and recent technologies for extraction and detection. Front. Nutr. 2022, 9, 806692. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energ. Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Li, X.; Ge, B.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Structure, Functions and Potential Medicinal Effects of Chlorophylls Derived from Microalgae. Mar. Drugs 2024, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosyn. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Wichuk, K.; Brynjólfsson, S.; Fu, W. Biotechnological production of value-added carotenoids from microalgae: Emerging technology and prospects. Bioengineered 2014, 5, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Wase, N.; Tu, B.; Rasineni, G.K.; Cerny, R.; Grove, R.; Adamec, J.; Black, P.; DiRusso, C.C. Remodeling of Chlamydomonas metabolism using synthetic inducers results in lipid storage during growth. Plant Physiol. 2019, 181, 1029–1049. [Google Scholar] [CrossRef] [PubMed]
- Mutaf-Kılıç, T.; Demir, A.; Elibol, M.; Oncel, S.Ş. Microalgae pigments as a sustainable approach to textile dyeing: A critical review. Algal Res. 2023, 76, 103291. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.42.74a; Rigaku Oxford Diffraction: Wroclaw, Poland, 2022.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Chapuis, G. SUPERFLIP–a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- da Silva-Filho, L.C.; Lacerda Júnior, V.; Constantino, M.G.; da Silva, G.V.J. Fast and Efficient Synthesis of Pyrano[3,2-c]quinolines Catalyzed by Niobium(V) Chloride. Synthesis 2008, 2008, 2527–2536. [Google Scholar] [CrossRef]
- Singal, K.K.; Singh, B.; Rehalia, K.S. Studies in 1-aza-1,3-butadienes: A novel synthesis of substituted thiadiazine 1-oxides through Diels-Alder cycloaddition of N-sulfinylanilines and open-chain conjugated azomethines. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1988, 27B, 843–845. [Google Scholar] [CrossRef]
- Ye, J.-H.; Bellotti, P.; Paulisch, T.O.; Daniliuc, C.G.; Glorius, F. Visible-Light-Induced Cycloaddition of α-Ketoacylsilanes with Imines: Facile Access to β-Lactams. Angew. Chem. Int. Ed. 2021, 60, 13671. [Google Scholar] [CrossRef]
- Subashini, A.; Veeramani, V.; Thamaraiselvi, K.; Crochet, A.; Rose, P.; Philip, R.; Babu, R.R.; Ramamurthi, K. Synthesis, growth and characterization of benzylideneaniline compounds: N-(4-bromobenzylidene)-4-fluoroaniline and N-(4-bromobenzylidene)-4-methoxyaniline. Opt. Mater. 2021, 117, 111081. [Google Scholar] [CrossRef]
- Dave, M.A.; Mahabal, N.B. Synthesis of 3-amino-1,4-diarylazetidin-2-ones. Part I J. Indian. Chem. Soc. 1993, 70, 172–173. [Google Scholar]
- Giraud, L.; Grelier, S.; Grau, E.; Garel, L.; Hadziioannou, G.; Kauffmann, B.; Cloutet, É.; Cramail, H.; Brochon, C. Synthesis and Characterization of Vanillin-Based π-Conjugated Polyazomethines and Their Oligomer Model Compounds. Molecules 2022, 27, 4138. [Google Scholar] [CrossRef]
- Karami, K.; Alinaghi, M.; Amirghofran, Z.; Lipkowski, J.; Momtazi-borojeni, A.A. A saccharinate-bridged palladacyclic dimer with a Pd–Pd bond: Experimental and molecular docking studies of the interaction with DNA and BSA and in vitro cytotoxicity against human cancer cell lines. New J. Chem. 2018, 42, 574–586. [Google Scholar] [CrossRef]
- Pradhan, S.; Thiyagarajan, S.; Gunanathan, C. Ruthenium(ii)-catalysed 1,2-selective hydroboration of aldazines. Org. Biomol. Chem. 2021, 19, 7147–7151. [Google Scholar] [CrossRef] [PubMed]
- Confair, D.N.; Greenwood, N.S.; Mercado, B.Q.; Ellman, J.A. Rh(III)-Catalyzed Imidoyl C–H Carbamylation and Cyclization to Bicyclic [1,3,5]Triazinones. Org. Lett. 2020, 22, 8993–8997. [Google Scholar] [CrossRef] [PubMed]
- Hisaindee, S.; Al-Kaabi, L.; Ajeb, S.; Torky, Y.; Iratni, R.; Saleh, N.; AbuQamar, S.F. Antipathogenic effects of structurally-related Schiff base derivatives: Structure–activity relationship. Arab. J. Chem. 2015, 8, 828–836. [Google Scholar] [CrossRef]
- Porai-Koshits, B.A.; Remizov, A.L. Synthesis and properties of azomethines from weakly basic aromatic amines. Zhurn. Obshch. Khim. 1954, 24, 372–375. (In Russian) [Google Scholar]
- Zhizhko, P.A.; Zhizhin, A.A.; Zarubin, D.N.; Ustynyuk, N.A.; Lemenovskii, D.A.; Shelimov, B.N.; Kustov, L.M.; Tkachenko, O.P.; Kirakosyan, G.A. Oxo/imido heterometathesis of N-sulfinylamines and carbonyl compounds catalyzed by silica-supported vanadium oxochloride. J. Catal. 2011, 283, 108–118. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, J. Synthesis of New C2-Symmetric Chiral Bisamides from (1S,2S)-Cyclohexane-1,2-dicarboxylic Acid. HCA 2014, 97, 1396–1405. [Google Scholar] [CrossRef]
- Benbouguerra, K.; Chafaa, S.; Chafai, N.; Mehri, M.; Moumeni, O.; Hellal, A. Synthesis, spectroscopic characterization and a comparative study of the corrosion inhibitive efficiency of an α-aminophosphonate and Schiff base derivatives: Experimental and theoretical investigations. J. Mol. Struct. 2018, 1157, 165–176. [Google Scholar] [CrossRef]
- Miiller, E.; Kettler, R.; Wiessler, M. Synthesen von α-C-funktionellen N-Nitrosodialkylaminen: Ester und Ether von 1-[(Alkyl)(nitroso)amino]alkoholen. Liebigs Ann. Chem. 1984, 1984, 1468–1493. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Xiong, J.-Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.-K.; Choi, J.; Kim, J.O.; Jeon, B.-H. Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Maslivets, A.N.; Khramtsova, E.E.; Lystsova, E.A. Method of Preparing 3a-aroyl-2-isopropyl-3-(isopropylimino)-3,3a-dihydrobenzo[d]pyrrolo[3′,4′:2,3]pyrrolo[2,1-b]thiazole-1,4,5(2H)-triones Exhibiting Antioxidant Activity. Russia Patent RU2810075C1, 21 December 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).