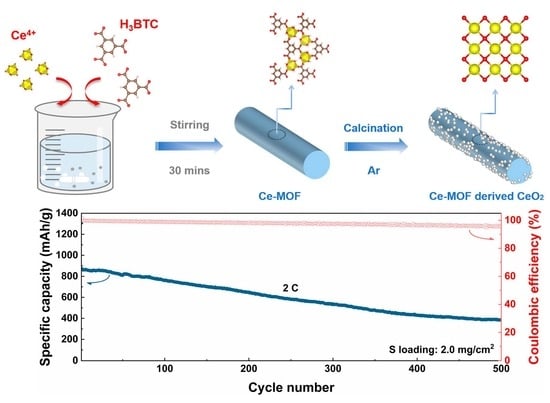
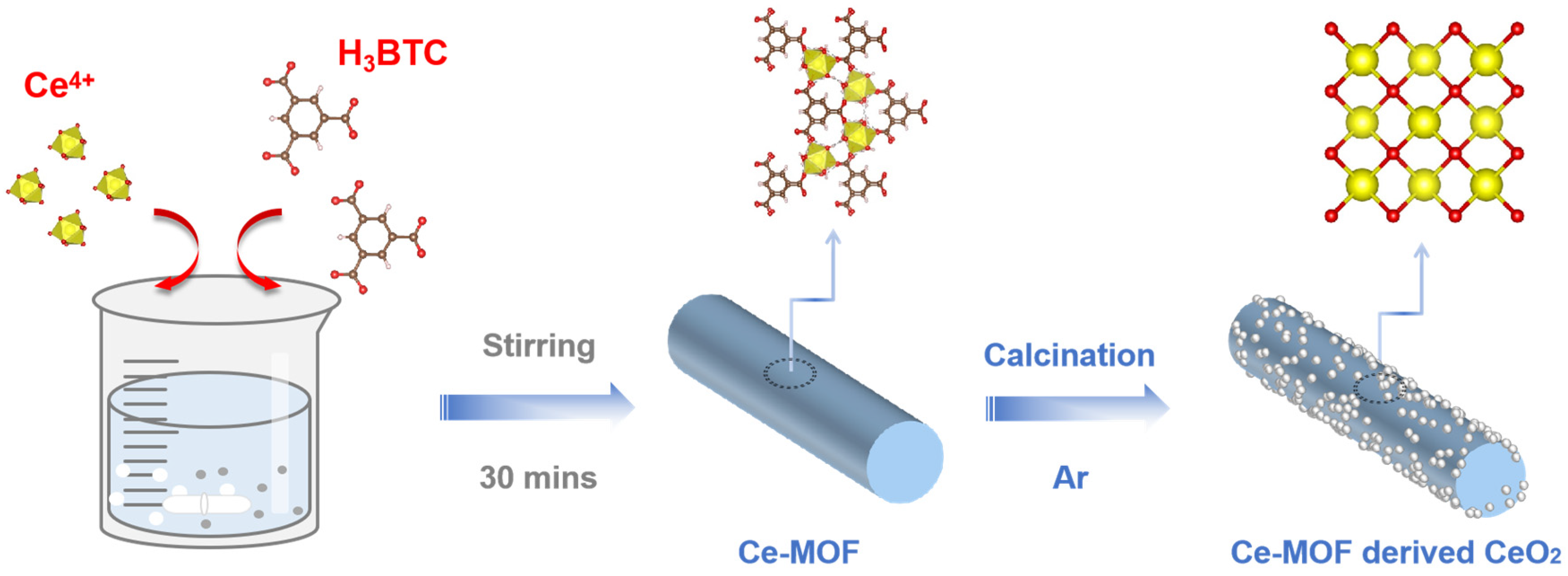
MOF-Derived CeO2 Nanorod as a Separator Coating Enabling Enhanced Performance for Lithium–Sulfur Batteries
Abstract
1. Introduction
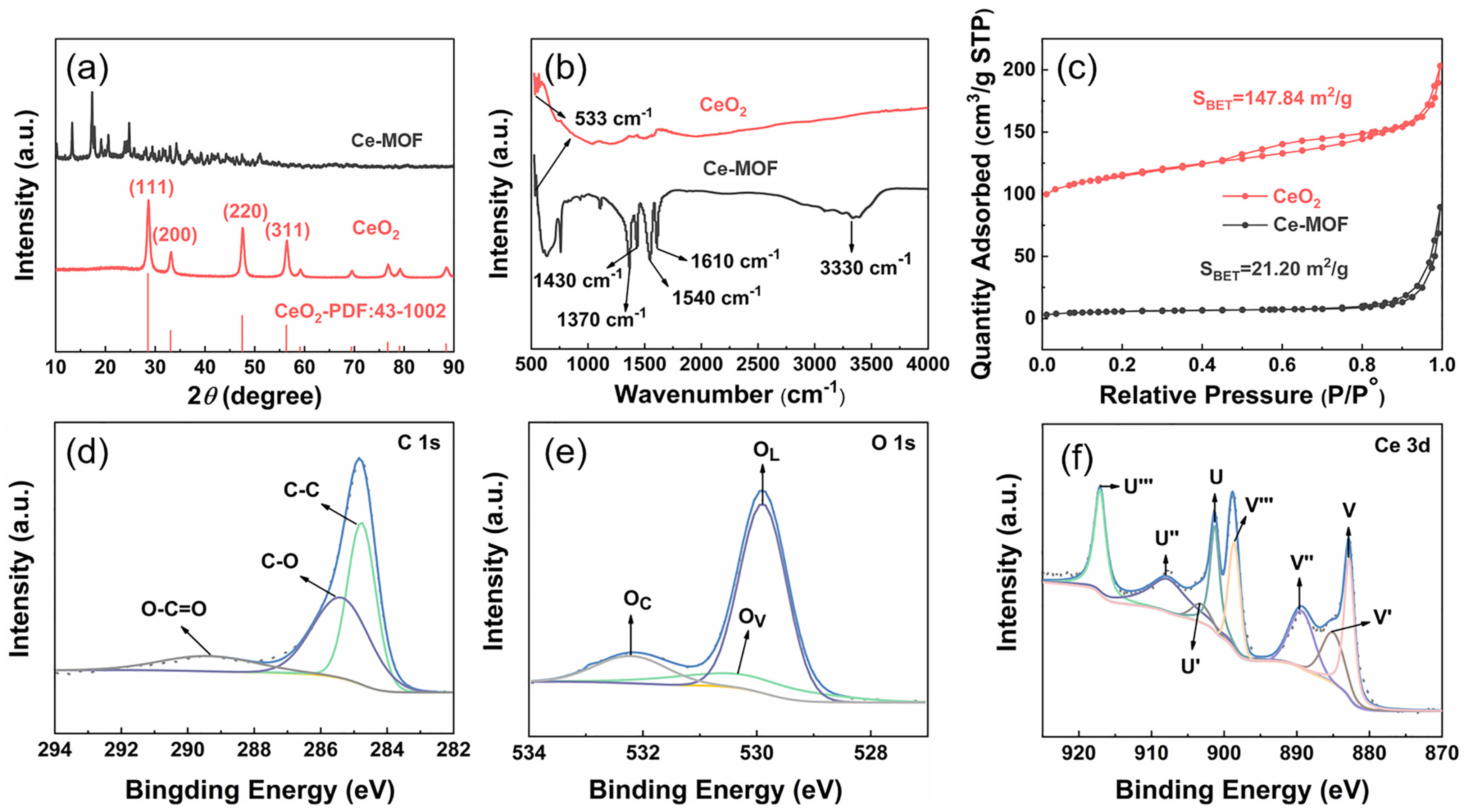
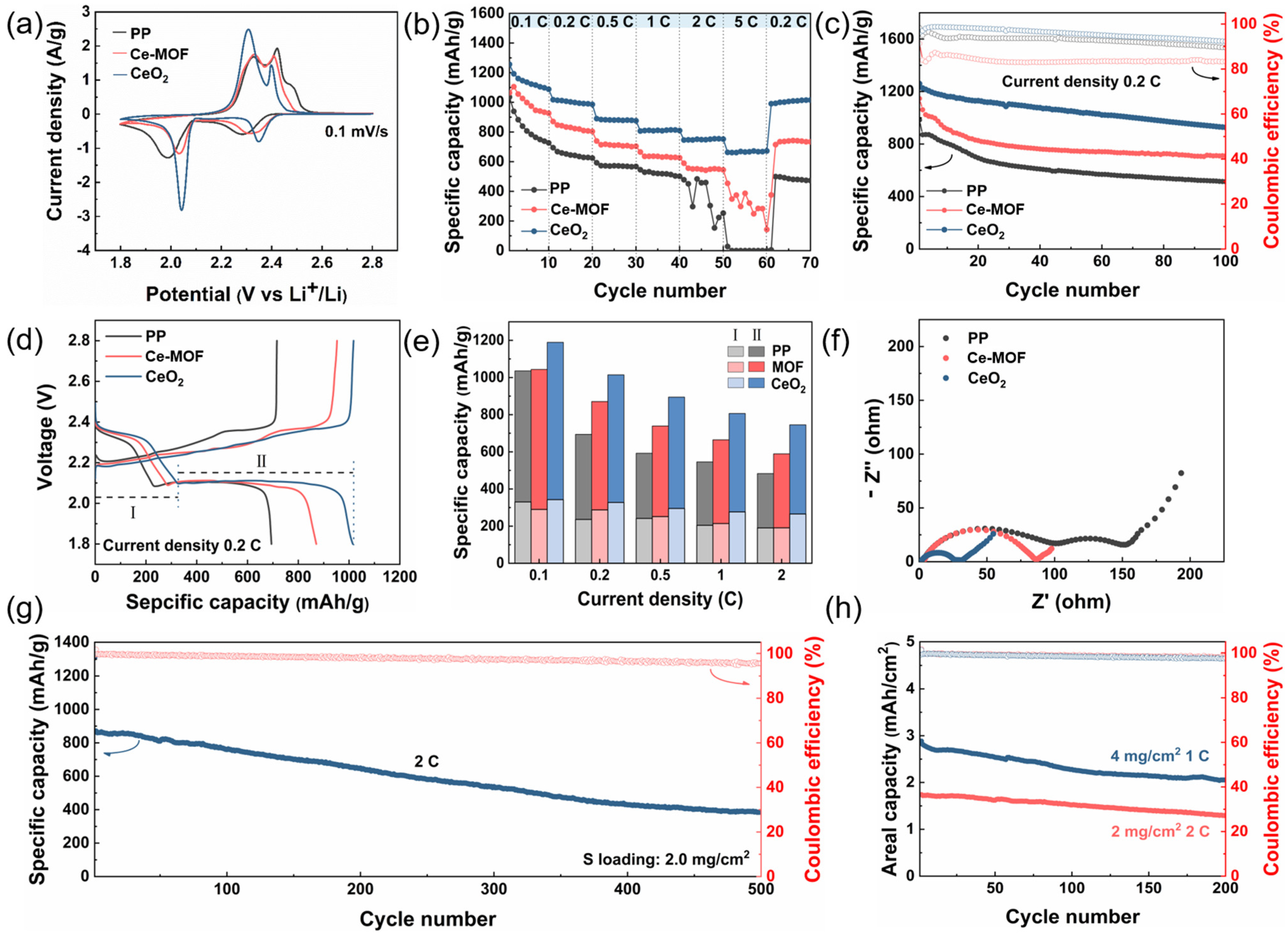
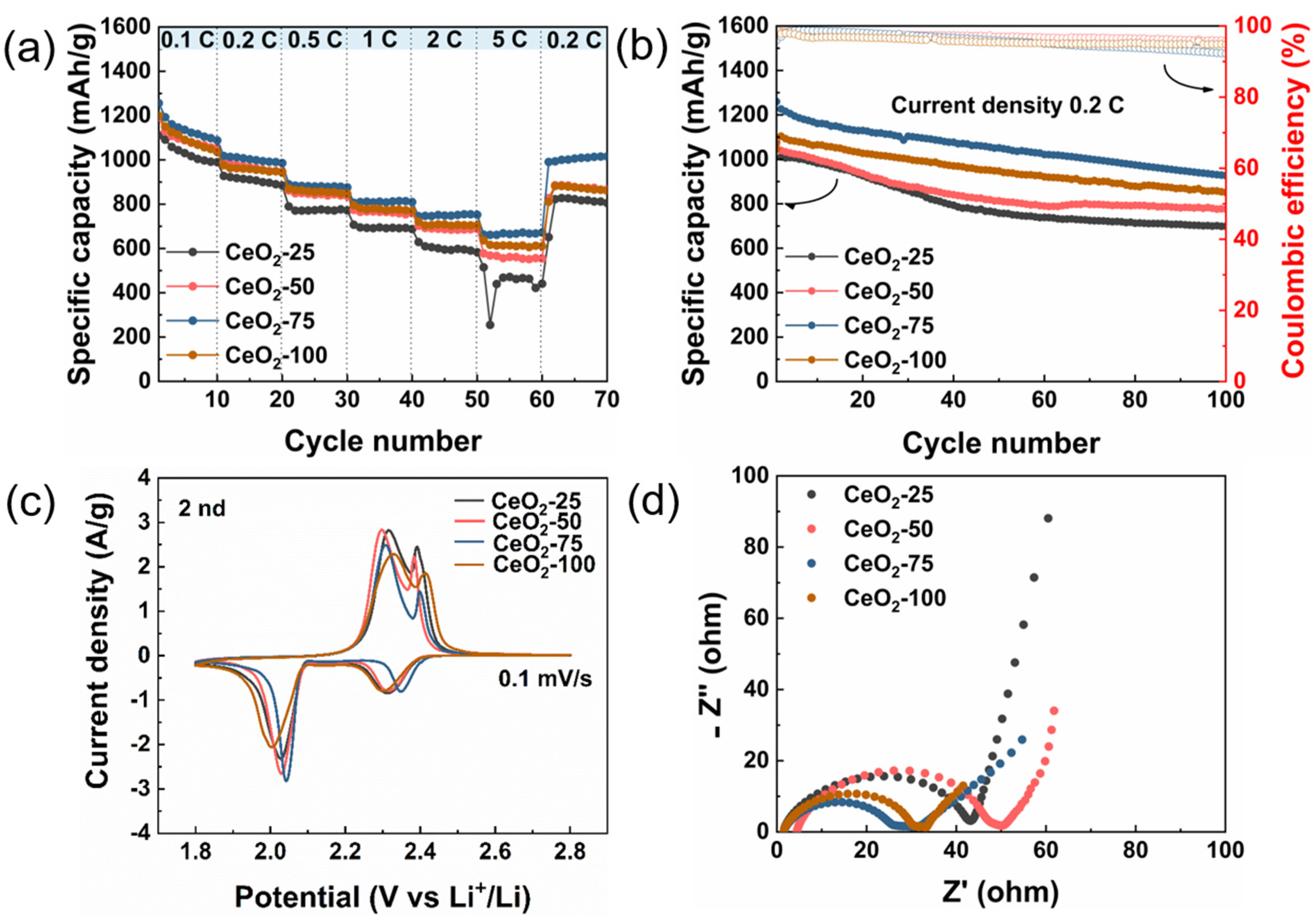
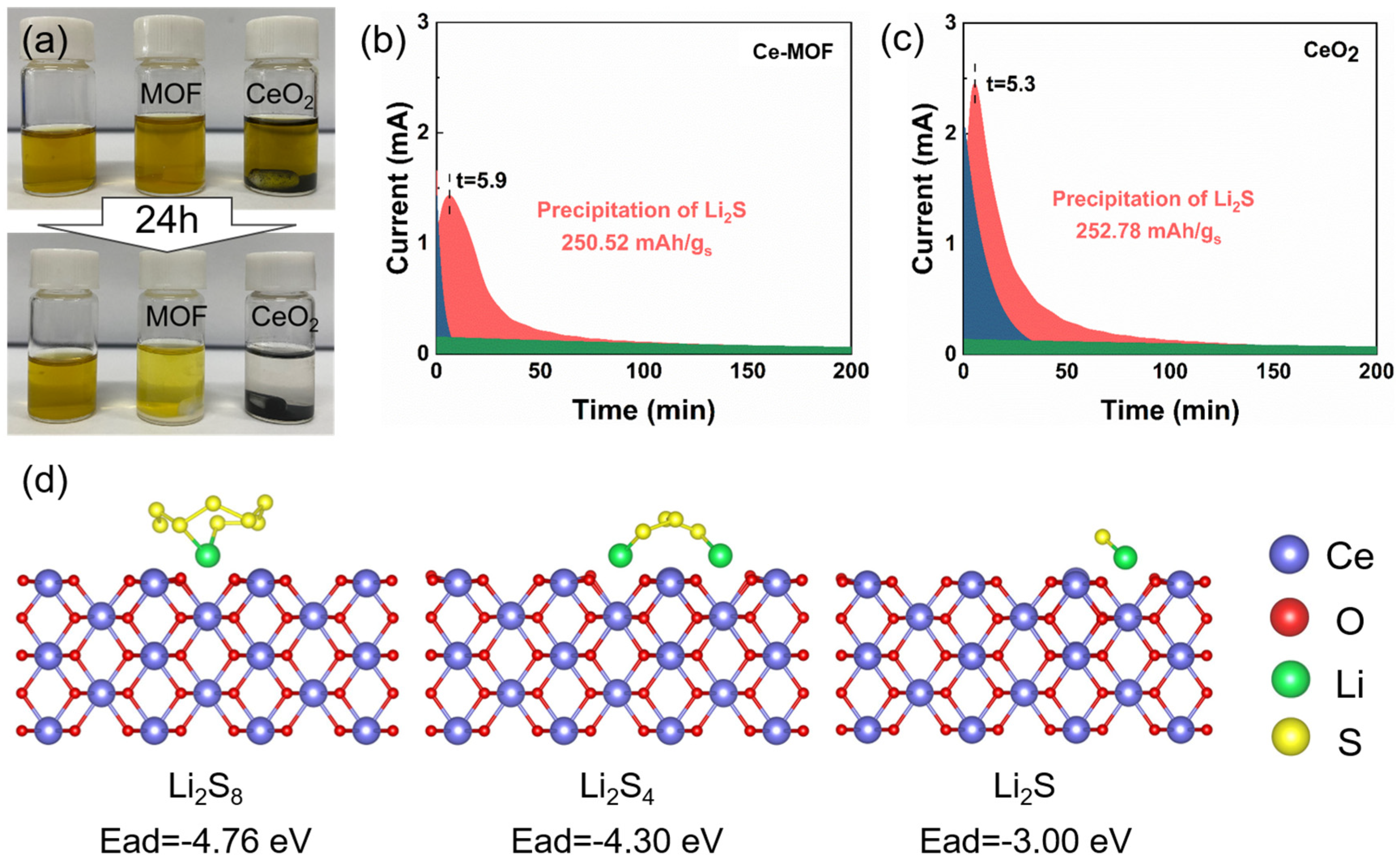
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of the MOF
3.2. Synthesis of the CeO2
3.3. Synthesis of CeO2 Coating Separator
3.4. Sulfur Cathode Preparation
3.5. Electrochemical Measurements
3.6. Materials Characterization
3.7. Polysulfides Adsorption Experiment
3.8. Measurement of the Li2S Nucleation
3.9. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, H.; Yan, K.; Li, W.; Zheng, G.; Kong, D.; Seh, Z.W.; Narasimhan, V.K.; Liang, Z.; Cui, Y. Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 2014, 7, 3381–3390. [Google Scholar] [CrossRef]
- Ansari, Y.; Zhang, S.; Wen, B.; Fan, F.; Chiang, Y.M. Stabilizing Li–S battery through multilayer encapsulation of sulfur. Adv. Energy Mater. 2019, 9, 1802213. [Google Scholar] [CrossRef]
- Pei, F.; Lin, L.; Fu, A.; Mo, S.; Ou, D.; Fang, X.; Zheng, N. A two-dimensional porous carbon-modified separator for high-energy-density Li-S batteries. Joule 2018, 2, 323–336. [Google Scholar] [CrossRef]
- Chung, S.-H.; Chang, C.-H.; Manthiram, A. A core–shell electrode for dynamically and statically stable Li–S battery chemistry. Energy Environ. Sci. 2016, 9, 3188–3200. [Google Scholar] [CrossRef]
- Cheng, P.; Guo, P.; Sun, K.; Zhao, Y.; Liu, D.; He, D. CeO2 decorated graphene as separator modification material for capture and boost conversion of polysulfide in lithium-sulfur batteries. J. Membr. Sci. 2021, 619, 118780. [Google Scholar] [CrossRef]
- Xiao, D.; Lu, C.; Chen, C.; Yuan, S. CeO2-webbed carbon nanotubes as a highly efficient sulfur host for lithium-sulfur batteries. Energy Storage Mater. 2018, 10, 216–222. [Google Scholar] [CrossRef]
- Hong, X.-J.; Song, C.-L.; Yang, Y.; Tan, H.-C.; Li, G.-H.; Cai, Y.-P.; Wang, H. Cerium based metal–organic frameworks as an efficient separator coating catalyzing the conversion of polysulfides for high performance lithium–sulfur batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef]
- Gueon, D.; Yoon, J.; Hwang, J.T.; Moon, J.H. Microdomain sulfur-impregnated CeO2-coated CNT particles for high-performance Li-S batteries. Chem. Eng. J. 2020, 390, 124548. [Google Scholar] [CrossRef]
- Ma, L.; Chen, R.; Zhu, G.; Hu, Y.; Wang, Y.; Chen, T.; Liu, J.; Jin, Z. Cerium oxide nanocrystal embedded bimodal micromesoporous nitrogen-rich carbon nanospheres as effective sulfur host for lithium–sulfur batteries. ACS Nano 2017, 11, 7274–7283. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Z.; Wang, D.; Zhang, F.; Jin, J. Covalent bond glued sulfur nanosheet-based cathode integration for long-cycle-life Li–S batteries. Nano Lett. 2013, 13, 6244–6250. [Google Scholar] [CrossRef]
- Hwa, Y.; Seo, H.K.; Yuk, J.-m.; Cairns, E.J. Freeze-dried sulfur–graphene oxide–carbon nanotube nanocomposite for high sulfur-loading lithium/sulfur cells. Nano Lett. 2017, 17, 7086–7094. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.H.; Rao, H.; Jokhakar, D.; Parikh, V.P.; Palanisamy, M.; Pol, V.G. Polysulfide shuttle mitigation through a tailored separator for critical temperature energy-dense lithium–sulfur batteries. Sustain. Energy Fuels 2022, 6, 5591–5599. [Google Scholar] [CrossRef]
- Xue, W.; Yan, Q.-B.; Xu, G.; Suo, L.; Chen, Y.; Wang, C.; Wang, C.-A.; Li, J. Double-oxide sulfur host for advanced lithium-sulfur batteries. Nano Energy 2017, 38, 12–18. [Google Scholar] [CrossRef]
- Li, N.; Xie, Y.; Peng, S.; Xiong, X.; Han, K. Ultra-lightweight Ti3C2Tx MXene modified separator for Li–S batteries: Thickness regulation enabled polysulfide inhibition and lithium ion transportation. J. Energy Chem. 2020, 42, 116–125. [Google Scholar] [CrossRef]
- Cui, Z.; Zu, C.; Zhou, W.; Manthiram, A.; Goodenough, J.B. Mesoporous titanium nitride-enabled highly stable lithium-sulfur batteries. Adv. Mater. 2016, 28, 6926–6931. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Manthiram, A. Hollow cobalt sulfide polyhedra-enabled long-life, high areal-capacity lithium-sulfur batteries. Nano Energy 2017, 33, 124–129. [Google Scholar] [CrossRef]
- Ye, B.; Feng, C.; Zhu, G.; Wang, S.; Fakhri, A. Feather duster liked CeO2 as efficient adsorber host material for advanced lithium–sulfur batteries. J. Alloys Compd. 2020, 823, 153743. [Google Scholar] [CrossRef]
- Wang, S.; Gao, F.; Zhao, Y.; Liu, N.; Tan, T.; Wang, X. Two-dimensional CeO2/RGO composite-modified separator for lithium/sulfur batteries. Nanoscale Res. Lett. 2018, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.; Jeon, Y.; Piao, Y. Phosphorus-doped graphene nanosheets anchored with cerium oxide nanocrystals as effective sulfur hosts for high performance lithium–sulfur batteries. Nanoscale 2019, 11, 13758–13766. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wang, S.; Liu, Y.; Ding, Y.; He, G.; Jiang, X.; Xiao, W.; Yu, G. Scalable high-areal-capacity Li–S batteries enabled by sandwich-structured hierarchically porous membranes with intrinsic polysulfide adsorption. Nano Lett. 2020, 20, 6922–6929. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, W.; Zou, M.; Wang, Y.; Chen, Y.; Xu, L.; Wu, H.; Cao, A. 3D, mutually embedded MOF@ carbon nanotube hybrid networks for high-performance lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1800013. [Google Scholar] [CrossRef]
- Geng, P.; Wang, L.; Du, M.; Bai, Y.; Li, W.; Liu, Y.; Chen, S.; Braunstein, P.; Xu, Q.; Pang, H. MIL-96-Al for Li–S Batteries: Shape or Size? Adv. Mater. 2022, 34, 2107836. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.A.; Yuan, X.; Chen, Y.; Hu, J.; Mu, Q.; Ma, Y.; Zhao, X.; Miao, L.; Ahn, J.-H.; Peng, Y. Anchoring MOF-derived CoS2 on sulfurized polyacrylonitrile nanofibers for high areal capacity lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 1298–1306. [Google Scholar] [CrossRef]
- Deng, N.; Wang, L.; Feng, Y.; Liu, M.; Li, Q.; Wang, G.; Zhang, L.; Kang, W.; Cheng, B.; Liu, Y. Co-based and Cu-based MOFs modified separators to strengthen the kinetics of redox reaction and inhibit lithium-dendrite for long-life lithium-sulfur batteries. Chem. Eng. J. 2020, 388, 124241. [Google Scholar] [CrossRef]
- Jiang, G.; Zheng, N.; Chen, X.; Ding, G.; Li, Y.; Sun, F.; Li, Y. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem. Eng. J. 2019, 373, 1309–1318. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Fang, T.; Zhao, Y.; Wang, Q.; Zhang, J.; Zhou, Z. MOF-derived Co3O4 polyhedrons as efficient polysulfides barrier on polyimide separators for high temperature lithium–sulfur batteries. Nanomaterials 2019, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Elhussein, E.A.A.; Şahin, S.; Bayazit, Ş.S. Preparation of CeO2 nanofibers derived from Ce-BTC metal-organic frameworks and its application on pesticide adsorption. J. Mol. Liq. 2018, 255, 10–17. [Google Scholar] [CrossRef]
- Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Q.; Fang, H.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Ce–UiO-66 Derived CeO2 Octahedron Catalysts for Efficient Ketonization of Propionic Acid. Ind. Eng. Chem. Res. 2020, 59, 17269–17278. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N.; Chen, D.; Yang, Y. A facile synthesis for cauliflower like CeO2 catalysts from Ce-BTC precursor and their catalytic performance for CO oxidation. Appl. Surf. Sci. 2017, 423, 771–779. [Google Scholar] [CrossRef]
- Wang, R.; Luo, C.; Wang, T.; Zhou, G.; Deng, Y.; He, Y.; Zhang, Q.; Kang, F.; Lv, W.; Yang, Q.H. Bidirectional catalysts for liquid–solid redox conversion in lithium–sulfur batteries. Adv. Mater. 2020, 32, 2000315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, Y.; Feng, X.; Yu, S.-H.; Seok, J.; Muller, D.A.; Abruña, H.D. Sulfur encapsulation by MOF-derived CoS2 embedded in carbon hosts for high-performance Li–S batteries. J. Mater. Chem. A 2019, 7, 21128–21139. [Google Scholar] [CrossRef]
- Abdelkader, A.A.; Rodene, D.D.; Norouzi, N.; Alzharani, A.; Weeraratne, K.S.; Gupta, R.B.; El-Kaderi, H.M. Multifunctional Electrocatalytic Cathodes Derived from Metal–Organic Frameworks for Advanced Lithium-Sulfur Batteries. Chem.–A Eur. J. 2020, 26, 13896–13903. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, C.; Yan, Y.; Yan, T.; Cheng, C.; Sun, D.; Zhang, L. Utilizing the Built-in Electric Field of p–n Junctions to Spatially Propel the Stepwise Polysulfide Conversion in Lithium–Sulfur Batteries. Adv. Mater. 2021, 33, 2105067. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, W.; Chen, Y.; Fan, H.; Su, D.; Wang, G. MOF-derived porous N–Co3O4@N–C nanododecahedra wrapped with reduced graphene oxide as a high capacity cathode for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 2797–2807. [Google Scholar] [CrossRef]
- Li, W.; Qian, J.; Zhao, T.; Ye, Y.; Xing, Y.; Huang, Y.; Wei, L.; Zhang, N.; Chen, N.; Li, L. Boosting High-Rate Li–S Batteries by an MOF-Derived Catalytic Electrode with a Layer-by-Layer Structure. Adv. Sci. 2019, 6, 1802362. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, X.; Li, B.; Shu, H.; Luo, L.; Xia, W.; Chen, M.; Zeng, P.; Yang, X.; Gao, P. NiMoO4 Nanosheets Anchored on N–S Doped Carbon Clothes with Hierarchical Structure as a Bidirectional Catalyst toward Accelerating Polysulfides Conversion for Li–S Battery. Adv. Funct. Mater. 2021, 31, 2101285. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and Catalytic Conversion of Polysulfides by In Situ Built TiO2-MXene Heterostructures for Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1900219. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J. Catalytic oxidation of Li2S on the surface of metal sulfides for Li–S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Qin, J.; Wang, H.; Lai, X.; Shi, P.; Chen, C.; Sun, D. MOF-Derived CeO2 Nanorod as a Separator Coating Enabling Enhanced Performance for Lithium–Sulfur Batteries. Molecules 2024, 29, 1852. https://doi.org/10.3390/molecules29081852
Xiao H, Qin J, Wang H, Lai X, Shi P, Chen C, Sun D. MOF-Derived CeO2 Nanorod as a Separator Coating Enabling Enhanced Performance for Lithium–Sulfur Batteries. Molecules. 2024; 29(8):1852. https://doi.org/10.3390/molecules29081852
Chicago/Turabian StyleXiao, Hao, Jian Qin, Haodong Wang, Xiaoxu Lai, Pei Shi, Chi Chen, and Dan Sun. 2024. "MOF-Derived CeO2 Nanorod as a Separator Coating Enabling Enhanced Performance for Lithium–Sulfur Batteries" Molecules 29, no. 8: 1852. https://doi.org/10.3390/molecules29081852
APA StyleXiao, H., Qin, J., Wang, H., Lai, X., Shi, P., Chen, C., & Sun, D. (2024). MOF-Derived CeO2 Nanorod as a Separator Coating Enabling Enhanced Performance for Lithium–Sulfur Batteries. Molecules, 29(8), 1852. https://doi.org/10.3390/molecules29081852