Abstract
Layered double hydroxides (LDHs) are fascinating clay-like materials that display versatile properties, making them an extremely fertile playground for diverse applications, ranging from bio-compatible materials to the pharmaceutical industry to catalysis and photocatalysis. When intercalating organic and bio-organic species between the inorganic layers, such materials are named hybrid LDHs. The structure–property relation in these systems is particularly relevant, since most of the properties of the materials may be fine-tuned if a comprehensive understanding of the microscopic structure in the interlamellar space is achieved, especially with respect to the reorganization under water uptake (swelling). In this work, we combined experiments and simulations to rationalize the behavior of LDHs intercalating three carboxylates, the general structure of which can be given as ·O (with = succinate, aspartate, or glutamate and X representing increasing water content). Following this strategy, we were able to provide an interpretation of the different shapes observed for the experimental water adsorption isotherms and for the evolution of the infrared carboxylate band of the anions. Apart from small differences, due to the different reorganization of the conformational space under confinement, the behavior of the two amino acids is very similar. However, such behavior is quite different in the case of succinate. We were able to describe the different response of the anions, which has a significant impact on the isotherm and on the size of the interlamellar region, in terms of a different interaction mechanism with the inorganic layer.
1. Introduction
Minerals, in particular natural clays, have been used as anti-inflammatories, antiseptics, cicatrizers, and cosmetics since prehistory [1]. Their use as active principles (for instance, as gastrointestinal protectors) and in therapeutic activity and aesthetic medicine is widely known. The scientific research in this field has continuously evolved, and the most recent advances have led to the development of new bio-compatible materials inspired by nature and based on synthetic approaches. The knowledge of the molecular structure and of the mechanisms related to the action of clays has pushed even further their potential use in the pharmaceutical industry, in particular for the delivery of drugs and bio-active molecules [2]. Among these materials, layered double hydroxides (LDHs) possess low or null toxicity, good bio-compatibility, and the possibility of leading to controlled release. LDHs comprise inorganic layers having a positively charged surface and packing together, including water and negative ions in the interlamellar region to allow for a neutral environment. Anions of very different nature can be intercalated to obtain hybrid systems, i.e., inorganic, organic, and biological molecules and macromolecules (proteins and DNA) [3,4,5], leading to bio-inorganic hybrid materials [6,7,8,9,10,11,12]. LDHs intercalating molecules with a negative charge born by carboxylate groups, such as deprotonated carboxylic acids, have been systematically synthesized since the 1970s [13], and it has been suggested that they can provide stabilization, transport, and release of drugs, such as ibuprofen, containing such groups [14].
The link between the adsorption of an intercalating anion and the expansion of the interlayer distance depending on the hydration conditions has been extensively documented in the literature, based on X-ray diffraction measurements, for small inorganic anions [15], depollutants [16], and organic dyes [17]. In such systems, the adsorption process and its kinetics are key to optimizing the use of LDHs for water remediation.
Among the possible intercalates containing carboxylate groups, amino acids have attracted increasing attention for the production of hybrid materials [18,19,20]. Natural anionic clays might have played a role in concentrating amino acid units, providing a propitious environment for the polymerization leading to polypeptides, the first step towards the origin of life under abiotic conditions [21]. Aspartate, the deprotonated form of aspartic acid, has a deprotonated side chain at neutral and basic pH, and it was present in the first naturally synthesized proteins [22]. It has been suggested that in the mechanism of the anion–surface interaction, mediated by intercalated water molecules, the amino group in amino acids may play a role in anchoring the surface [23]. If we compare the structure of succinate and aspartate, the only structural difference is the absence/presence of an amino group (see Figure 1). Studying the differences in the binding modes of these two molecules containing carboxylate units would, therefore, provide some information for understanding such a mechanism.
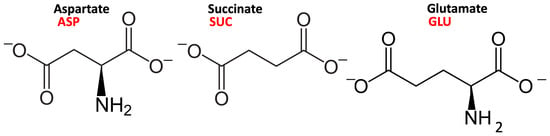
Figure 1.
The structure and protonation state of the L--aspartate, succinate, and L--glutamate anions, with the definition of the abbreviations used in the text.
It has been shown that the conformations adopted by intercalated biomolecules in solution can either be retained or modified, inducing the unfolding of the secondary, tertiary, or quaternary structure [4], and that intercalated DNA is stabilized compared with bulk water [24]. Given the importance of the structure of biomolecules to deliver their biological function, understanding the way in which nanoconfinement within LDHs affects the configuration, the dynamics, and the reactivity of intercalated species is key to the rational design of hybrid materials.
Experimental work based on X-ray diffraction techniques has provided information on the structural parameters of the inorganic host (distance between the cations within two layers, or interlayer spacing), and vibrational spectroscopy has provided information about the evolution of the vibrational spectrum of the intercalated guests upon hydration [25,26]. However, it is rather complex to achieve a molecular description of the system, especially with respect to the disordered interlamellar space (water structure, anion hydration, etc.). The structure of LDHs intercalating some carboxylic acids [27], amino acids [28], and DNA [24] has been studied by means of molecular dynamics (MD) simulations. Indeed, significant differences have been observed in the structure of the interlamellar region based on the species considered, which motivates us to push further the analysis to rationalize the effect of the anion charge and structure on the local organization. For instance, different amino acids can lead to different orientations with respect to the inorganic layer, and the water structure is strongly dependent on the balance between the interactions with the hydrophilic and hydrophobic groups.
Thanks to the availability of reliable and transferable force fields, such as the widely used Clay force field [29], Monte Carlo and molecular dynamics methods have been successfully applied to analyze the interlayer structure and dynamics [30]. Although remarkable insights were provided by applying these methods to LDHs intercalating carboxylates and amino acids [23,26,27,31,32], we are not aware of previous theoretical work explicitly examining how their different molecular structures can induce differences in the adsorption mechanism inside the material and for different hydration states. As it was already mentioned, from the experimental point of view, the focus so far has been on the synthesis of the material, on proving that the anions are actually intercalated, and on the adsorption kinetics.
The results in the literature strongly hint at the complexity of the local environment in LDHs. In this work, we propose to tackle this challenging problem by means of the interplay between theory and experiments. We propose an approach based on molecular dynamics simulations and on the synthesis and characterization of LDHs intercalating three different anions, choosing different hydration states along the experimental adsorption isotherms and providing an atomistic interpretation of the evolution of the structure–property relationship. In addition to the aforementioned succinate and aspartate, we included a third intercalate, namely, glutamate, which is in all similar to aspartate but displays a longer side chain (Figure 1).
The LDH inorganic precursor derives from the hydrotalcite mineral [33,34,35,36], and its chemical formula can be given as [Mg4Al2(OH)12]CO3·4H2O. In this compound, the cations are bridged by units coordinated at the octahedral positions, forming sheets packing into a layered structure similar to that of the mineral brucite [37]. The sheets carry net positive charges that must be neutralized by intercalated anions (the carbonate anions in hydrotalcite), and such anions coexist with structural water in the interlayer region. While the hydrotalcite inorganic precursor can be synthesized by using a coprecipitation method, the hybrid LDHs are obtained by anionic exchange from this material [38,39]. According to this procedure, the hydrotalcite precursor undergoes a first anionic exchange with perchlorate anions in ethanaloic medium in order to increase the interlayer spacing [15], as well as to replace the carbonate anion with an easily exchangeable anion. A second anionic exchange is then carried out in water, under nitrogen flow to prevent contamination, where the perchlorate is finally replaced by the organic anion of interest. The advantages of this approach are that (i) the first step does not require any precaution regarding carbonate pollution coming from atmospheric CO2 and (ii) it provides the same inorganic host (same layer composition and same sheet dimensions) for all hybrid LDHs. Following this synthetic step, in this work, experiments under controlled humidity provided information on the adsorption isotherm and the evolution of the interlamellar space upon hydration, as well as some specific changes in the infrared spectra of the three intercalates. The analysis of our MD simulations delivered a molecular picture unravelling the observed experimental trends.
2. Materials and Methods
2.1. Experimental Procedure
2.1.1. Chemicals
MilliQ water purged with nitrogen to remove carbon dioxide and L--amino acids were used throughout this work. Di-sodium succinate, sodium aspartate monohydrate, sodium glutamate monohydrate, aluminum chloride hexahydrate (AlCl3 · 6H2O; ≥99.0%), magnesium chloride (MgCl2 · 6 H2O; ≥99.0%), and sodium carbonate (Na2CO3; ≥99.5%) were purchased from Sigma-Aldrich. Perchloric acid (HClO4; 60%) was purchased from Riedel-de haën. Sodium hydroxide (NaOH; 1M) was purchased from Corto Erba reagents groups. All products were used as received without any further treatment.
2.1.2. Synthesis of LDHs
Hybrid LDHs were obtained via a double anionic exchange procedure. The starting material was a carbonated Mg2Al LDH, synthesized according to the “coprecipitation at constant pH” method previously described [38,39]. In a first exchange, the carbonate anion was replaced with perchlorate (which has a low affinity to the interlayer space) in an ethanol–acid mixture [15]; in brief, 6 mmol of LDH was dispersed in 25 mL of ethanol at 50 °C under nitrogen flow, and a mixture of perchlorate acid (986 L; 60%) and ethanol (25 mL; 99%) was slowly added to the initial mixture ensuring an excess of perchlorate (the ratio was equal to 1.5). Finally, the mixture was stirred for 1 h under nitrogen flow; then, the slurry was centrifuged, washed with ethanol three times, and dried in air for one night. The anion of interest (succinate, aspartate, or glutamate) was intercalated by a second ion exchange, realized this time under nitrogen flow, in aqueous solution at pH = 10 (to ensure amino acids were present in anionic form). Then, 1 mmol of perchlorated LDH was added to 100 mL of an aqueous solution containing a small excess of the desired anion. The mixture was stirred at 60 °C for 30 min. Afterwards, the mixture was centrifuged, and the solid was dried in nitrogen atmosphere for one night.
2.1.3. Characterization of LDHs
The synthesis of the carbonated LDHs and the successive anion exchanges were monitored by powder X-ray diffraction (PXRD) to follow the evolution of the interlayer spacing and by infrared spectroscopy to ensure the total replacement of the spectral signature of the exchanged anions. Diffraction patterns were recorded with a Panalytical X’Pert Pro MPD diffractometer in reflection geometry by using a tube with Cu radiation (K = 1.5406 Å), a Ge(111) incident-beam monochromator, 0.02 rad Soller slits, programmable divergence and antiscatter slits (the irradiated area was fixed to 10 × 10 mm), and an X’Celerator detector. Data were collected from finely ground samples with a sample holder spinner and continuous rotation of sample to improve statistical representation of the sample. Infrared spectra were recorded in the 400–4000 spectral region with a Nicolet 8700 spectrometer equipped with an ATR accessory (GladiATR with diamond crystal; Pike Technologies, Fitchburg, WI, USA) and a DTGS detector. The spectral resolution was set to 4 , and 100 scans were averaged to increase the signal-to-noise ratio. Diffraction patterns for the different LDHs are provided as Supplementary Materials (Figure S1).
2.1.4. Hydration of LDHs
The LDHs’ hydration profiles were studied thanks to water vapor adsorption–desorption isotherms. The experiments were conducted at 298 K by using a MicrotracBEL Belsorp-Max volumetric adsorption analyzer equipped with three pressure sensors (1.33 bar, 13.3 mbar, and 0.133 mbar). Long acquisition times (3 days per isotherm) were required because of the slow equilibrium kinetics. The investigated sample was outgassed under vacuum (residual pressure of Pa). Volumetric measurements were normalized by considering the molar mass of water-free LDHs (M = 277.1, 235.6, 243.2, and 250.2 g· for , succinate, aspartate, and glutamate, respectively) in order to derive the number of adsorbed water compound per chloride ion for increasing relative humidity (RH = P/ (298 K), with P the water pressure and = 31 mbar the saturation water pressure at 298 K). Mid-infrared diffuse reflectance spectra under controlled water pressure were recorded with a Nicolet 8700 spectrometer, equipped with an MCT detector. The spectra in diffuse reflectance mode were collected by using an environmental cell with a Harrick Praying Mantis. All powder samples were finely ground and dispersed in KBr (2.5 wt%). The relative humidity was controlled by using an in-house dynamic system mixing a dry and a wet flow controlled by using a mass flow meter. With such a setup, an RH between 0 and 100% can be generated with an uncertainty of 0.3–0.5%, but the RH range is limited to 0–80 % due to the hygroscopic nature of the KBr matrix.
2.2. Simulations
Molecular dynamics simulations were run for LDH materials having three different intercalated anions (L--aspartate (ASP), succinate (SUC), and L--glutamate (GLU)), under different humidity conditions. The experiments carried out under controlled humidity gave access to the number of water molecules per anion, as well as to the interlamellar space to be used to build the starting configurations corresponding to the experimental conditions. In the case of aspartate and glutamate, experiments, and in particular nuclear magnetic resonance and elemental analysis [40], allowed us to define the protonation state of the amino acid molecules, in agreement with other work carried out under similar pH conditions [26,41,42,43]. The LDH material was described by using the Clay force field (ClayFF) [29], which is based on an ionic (non-bonded) description of the metal–hydroxyl interactions and can be combined with other model potentials. The SPC/E force field was used to simulate water [44], and the Amber force field [45,46] was employed to simulate the intercalated organic anions.
All MD simulations were conducted by using Amber16 [47] and visualized with VMD [48]. The initial cell was chosen to have three layers, to provide three layers and three interlamellar regions in the simulation box, thus improving statistics. After adjusting the interlamellar space to the experimental value for each set of conditions (anion + increasing amounts of water), we replicated the initial cell in the x- and y-directions (with z being the axis orthogonal to the layers). We collect in Table 1 the abbreviations used for the studied systems, the number of molecules per anion (corresponding to the hydration content), and the interlamellar spacing.

Table 1.
Definition of the simulated systems.
The minimization and equilibration protocol and the details about the simulation boxes are fully described in the Supplementary Materials section. In the production run, for each system, MD simulations in parallelepipedal boxes with periodic boundary conditions were run in the NPT ensemble at a temperature of 300 K and a pressure of 1 bar, using Berendsen’s barostat [49], for 10 ns. All quantities measured along the production runs were averaged over the three interlamellar regions of the simulation box.
Infrared spectra were computed by averaging the spectra obtained for each anion by using a 1 ns trajectory. The absorption spectrum of a molecule can be evaluated from MD trajectories by using a formulation stemming from Kubo’s relationship [50]. According to this development, the infrared intensity can be expressed in terms of the Fourier transform of the molecular dipole time correlation function as
where ) is a pre-factor taking into account the quantum nature of the time correlation function [51,52]. An explicit treatment of this factor was not included in our calculation, since this goes beyond the purposes of our analysis. The maximum entropy method was used to compute the Fourier transform [53].
3. Results and Discussion
The experiments conducted under controlled humidity provided the adsorption isotherms of the three LDHs intercalating aspartate, glutamate, and succinate, which are displayed in Figure 2. Interestingly, whereas the two curves measured when ASP and GLU are intercalated present an extremely similar shape, when SUC is intercalated, the curve presents one plateau at low humidity and then a very abrupt increase (roughly between two and six water molecules per anion) followed by a smoother increase. The results for ASP are qualitatively consistent with those in Ref. [26].
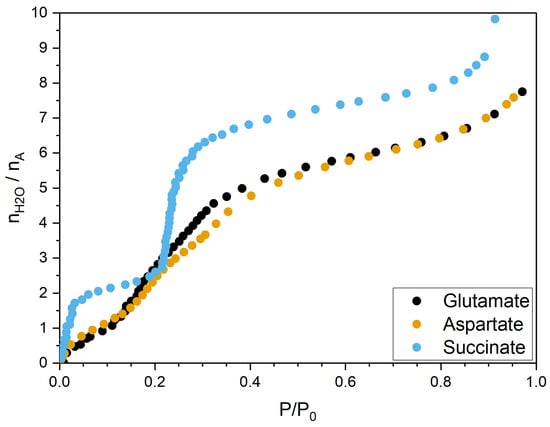
Figure 2.
Water adsorption isotherms for LDHs intercalating aspartate, glutamate, and succinate. Relative humidity is given as , with corresponding to RH = 100%. Water adsorbed is given as number of water molecules per intercalated anion.
Following the behavior of the adsorption isotherm for the material intercalating succinate, the interlamellar space changes dramatically between the anhydrous system and the first hydration state considered (see the last column in Table 1), while the LDHs intercalating ASP and GLU undergo a slower transition towards a wider spacing.
To unveil the effect of increasing hydration on the molecular properties of the intercalated anions, we monitored the evolution of the carboxylate stretch band in the infrared spectrum. This band, comprising two sub-bands (symmetric (S) and antisymmetric (AS) stretches, at about 1400 and 1600 , respectively) is sensitive to the environment (e.g., hydrogen bonds with water, with the hydroxyl groups on the surface, and among anions) and to coupling with other modes, as the N-H bend for the amino acid systems. It also has the advantage of not overlapping with IR bands of other portions of the system (water and OH groups). Our experimental and computational results are reported in Figure 3.
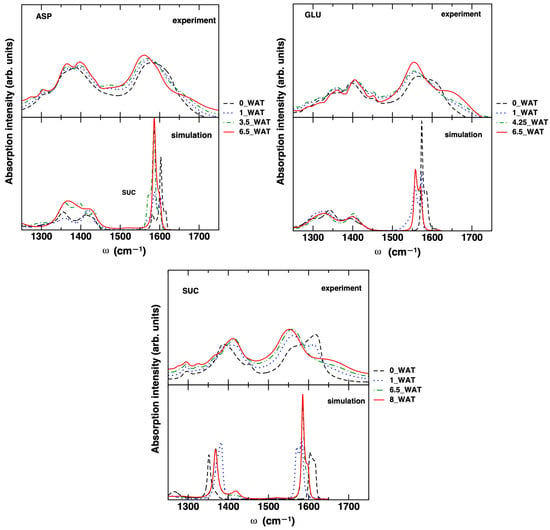
Figure 3.
Experimental and simulated carboxylate stretch band of the three intercalated anions for the different hydration states considered for LDHs. The computed bands were shifted (see text).
For the computed spectra, the assignment of the bands was confirmed by analyzing the Fourier transform of the atomic velocities of the C and O atoms of the carboxyl groups and of the N atoms (not reported for the sake of simplicity; see Ref. [54] for further details). The computed bands were shifted by −200 in all systems to match the experimental result. Such a shift is not surprising, since we neglected the pre-factor in Equation (1) and we used a standard force field without specific reparametrization of the intramolecular potential. The value of the shift is consistent with what some of us found for the C=O stretch of similar molecules in water solution [54,55] by using different simulation methods (molecular mechanics-based MD, Born–Oppenheimer MD, and hybrid quantum mechanics–molecular mechanics dynamics) and Equation (1). The poor description of the band shape for the AS stretch band is also related to some limitations of the computational approach. This particular aspect would require significant efforts [56] that would be beyond the scope of the present work. On the other hand, the value of the splitting between the S and AS (sub-)bands is in fairly good agreement with the experiments, as it can be observed in Table 2, especially for ASP and GLU. In addition, the trend with increasing hydration qualitatively matches the experimental trend, as does the shape of the S stretch band, which is quite wide and most likely results from a superposition of multiple bands for ASP and GLU, whereas it is narrower for SUC. Such band may be affected by coupling with the vibrations of the − groups, as some of us showed in Ref. [55] for the aspartate anion in water solution, a coupling which does not occur in the case of succinate.

Table 2.
Shifts between the asymmetric and the symmetric stretch carboxylate bands: comparison between experiments and simulations.
In the systems intercalating ASP and GLU, we see the AS band progressively red-shifting, while the S band is much less affected. In the case of ASP, the measured bands are in good agreement with the published results in Ref. [26]. It is worth noting that for these two systems, a shoulder appears in the highest hydration state in the higher-frequency region of the experimental data. In past work on the carboxylate bands of ASP in water [55], we found that this shoulder is present when explicit water molecules are included in the solvation shell of the anion, whereas it is not present when a continuum model is considered. We were able to show that the shoulder is due to the coupling of the AS stretch of the carboxylate unit with the bend vibration of water. In the present work, the molecular mechanics force field used is most likely not able to catch this fine feature. We can safely assume that the presence of the shoulder acts as a probe of a more hydrated environment of the C=O oscillator. The band gap for the system intercalating SUC is overestimated compared with experiments but the trend is qualitatively the same. In moving from the anhydrous system to the hydrated systems, the S stretch band is blue-shifted and the AS band red-shifted. From this analysis, we can conclude that our simulations are able to catch the most important features in the spectral evolution of the carboxylate band as the water content increases in the LDH material and that the bands of the system intercalating SUC are more significantly affected than in the cases of ASP- and GLU- intercalated LDHs.
To analyze this behavior, we performed a study of the evolution of the local structure in the galleries upon hydration. For the system intercalating ASP, it has already been discussed in the literature that the anions reorient themselves when the water content increases, starting from a situation in which they lie parallel with respect to the layer in the anhydrous system [26,31]. In studies considering comparable values of the interlamellar spacing in LDHs, a monolayer arrangement of the anions in the galleries has been hypothesized, in agreement with what we observe [43,57,58,59].
In order to follow anion reorientation, similarly to Ref. [26], we monitored the angle formed by a vector joining the two carbon atoms of the carboxylate groups with respect to the normal to the surface. To rationalize our results, we present them in terms of populations of orientational states, computed as percentages. We count anions in a parallel orientation when is larger than and in an intermediate/perpendicular orientation otherwise. The results are reported in Table 3.

Table 3.
Orientational states for the anions with increasing hydration.
All systems undergo a reorientation with the increase in the amount of water in the interlamellar space, and for each system, we observe an intermediate state for which the number of anions lying horizontally decreases drastically, followed by hydration states for which a slight increase in this population is seen. Therefore, this descriptor alone cannot account for the differences observed in the three systems. As a matter of fact, the underlying interpretation is still blurred by the fact that the choice of a global descriptor of reorientation, such as the vector joining the C atoms of the carboxylate groups, may hide some relevant degrees of freedom, particularly those related to the conformational space of the anions. To complete this analysis, we monitored the dihedral distributions (reported as Supplementary Materials, Figures S3–S5) of the C-C-C-C dihedral in SUC and the C--- dihedral in the amino acids, defined in Figure 4, Figure 5 and Figure 6, panel (a), allowing us to recognize the more stable conformers, anti and gauche [55,60,61].
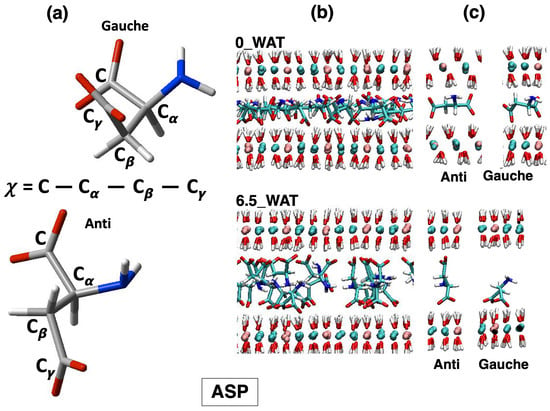
Figure 4.
LDH intercalating aspartate anions. (a) Structures of the anions in the relevant conformations observed on average along the MD simulations of LDHs and definition of the dihedral angles computed to describe such conformations. (b) Snapshots illustrating the organization of the anions in the anhydrous system and in the most hydrated state. (c) Most relevant configurations observed for the anions in the interlamellar space and in the corresponding hydration state.
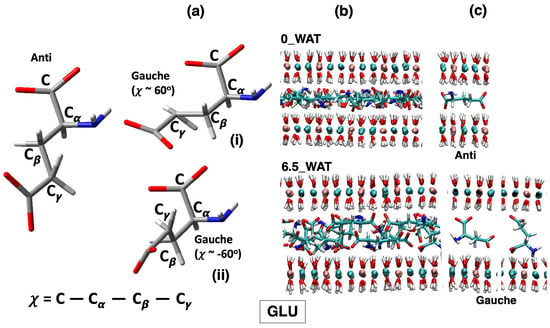
Figure 5.
LDH intercalating glutamate anions. (a) Structures of the anions in the relevant conformations observed on average along the MD simulations of LDHs and definition of the dihedral angles computed to describe such conformations. (b) Snapshots illustrating the organization of the anions in the anhydrous system and in the most hydrated state. (c) Most relevant configurations observed for the anions in the interlamellar space and in the corresponding hydration state.
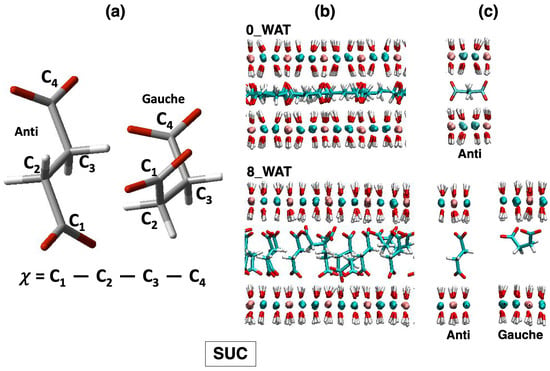
Figure 6.
LDH intercalating succinate anions. (a) Structures of the anions in the relevant conformations observed on average along the MD simulations of LDHs and definition of the dihedral angles computed to describe such conformations. (b) Snapshots illustrating the organization of the anions in the anhydrous system and in the most hydrated state. (c) Most relevant configurations observed for the anions in the interlamellar space and in the corresponding hydration state.
In the case of aspartate, both the anti and gauche conformations are present in all hydration states. An illustration of the change in the global organization of the interlamellar space is depicted in panel (b) of Figure 4, while some structures extracted from those snapshots show ASP in anti and gauche conformations interacting with the surface. These illustrations also show that such interaction is driven both by the carboxylate oxygens and by the N atom, which is an important confirmation of the findings reported in Ref. [23]. To shed more light on this observation, we computed the number density of N atoms along the z-axis, orthogonal to the surface, as shown in Figure 7. In order to facilitate the description, we chose to report in Figure 7 our results for the anhydrous systems as well as for the most hydrated states, and the collection of all our results is provided as Supplementary Materials, Figure S2.
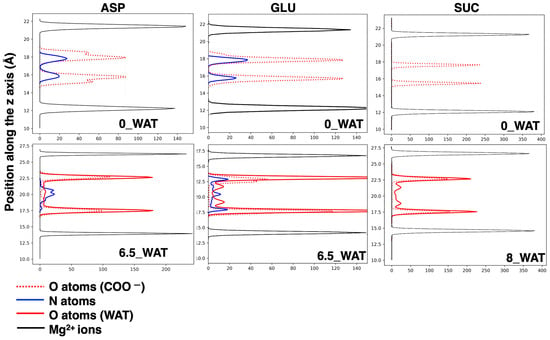
Figure 7.
Number densities along the axis perpendicular to the surfaces of relevant atoms in the interlamellar space, shown for the anhydrous systems (top plots) and for the higher hydration states (bottom plots) for the materials intercalating ASP, GLU, and SUC (from left to right). The distributions of the z positions of the Mg2+ cations are displayed as a reference.
The carboxylate oxygen atoms and the nitrogen atoms from the amino groups sit next to the surface in the anhydrous system and in the lower hydration state. However, our analysis reveals that most of the N atoms are found in the middle of the interlamellar region in the highest hydration state (6.5_WAT), as the snapshot in Figure 4b displays. When ASP is in anti, the two carboxylate groups interact with two different surfaces. Conversely, when it is in gauche (a more compact configuration) they both lie next to the same surface, whereas the − group can reorient itself toward the middle of the interface.
As in the case of ASP, for GLU, both the anti and gauche conformations are present in all hydrated systems, but in the anhydrous system, only the anti conformer is present. In addition, in the case of aspartate, the proximity of the amino group to the carboxylate group at the carbon and the resulting steric hindrance only allow for one gauche orientation (−). However, the presence of a longer side chain in glutamate allows for two possibilities ( = − and = ), presented as structures (i) and (ii) in Figure 5a and as extractions from MD simulations, interacting with the surface, in Figure 5c. In all cases, the N atoms interact strongly with the surfaces, as can be retrieved by the analysis of the number density along z, in the middle panel of Figure 7. In the highest hydration state, part of the N atoms are also present in the middle of the interlamellar space, but the population interacting with the surface is still present, unlike the LDH intercalating ASP. On the one hand, the anions lying perpendicularly to the surface display N in the center of the interlayer; on the other hand, those in gauche conformations may have it either interacting with the surface or in the middle of the interlayer (see illustration in panel (c) of Figure 5). We can conclude that notwithstanding the similar structure of the two amino acids, the different length of the side chain triggers a different exploration of the conformational space under confinement, as it was observed by using MD to study these anions intercalated in the same material but in a different protonation state [32]. To complete the analysis of the number density (ASP and GLU), we note that the O atoms of the water molecules are found, in all cases, in close proximity to the carboxylate O atoms, a finding that is fully consistent with the reported affinity of water for the carboxylate groups, in LDHs as in water solutions [27,28,55,60]. In the higher hydration state, some water molecules are also present in the middle of the interlayer.
Finally, in the system intercalating SUC, the gauche configuration is not observed in the anhydrous system and in the lowest hydration state, but it gets slightly more populated in the highest hydration states (corresponding to a higher population of anions with parallel orientation; see an illustration in Figure 6c). However, the majority of the anions switch from an anti configuration, with the molecule horizontal to the surface (low hydration and narrower interlayer), to an anti configuration with perpendicular orientation as soon as the uptake of water has allowed for a wider interlamellar spacing. This is the key difference in the response of succinate to hydration, compared with the amino acids, since the latter can adapt to the changes in the confined space with additional degrees of freedom, enhanced by the interaction of the amino group with the surface and with water. As for the amino acids, water molecules sit next to the carboxylates of SUC (right panel of Figure 7), but some of them appear inside the gallery at higher hydration. The general picture that we draw is in agreement with the behavior of SUC in Zn/Al- and Al/Cr-based LDHs, in which a contraction of the interlayer is observed (which is interpreted as a reorientation of the anion) under thermal treatment [57].
The collection of the results that we presented, as well as some observations based on visual inspection of the trajectories, allow us to introduce some comments on the binding modes of the carboxylate moieties, by analogy with their binding modes in the presence of a metal [62,63,64,65]. Surely, such units are key to driving the interaction between molecules containing carboxylate units and the layer, as it has been clearly shown by extracting a one-dimensional electron density map from X-ray diffraction experiments [66]. In our case, the dramatic change in the orientational state of SUC corresponds to a change from bidentate bridging binding (each O of one carboxylate interacts with a different surface) to bidentate chelating (both O interacting with the same surface). When ASP or GLU are intercalated, both types of binding are present under all hydration conditions, and an abrupt change of interaction modes does not take place.
In the 1980s, Deacon and Phillips provided an extensive investigation of the relative position of the anti-symmetric and symmetric stretch bands for complexes based on acetate and trifluoroacetate complexes [62]. The AS-S band gap was correlated with the type of binding; compared with the free anion, a larger band gap band would be observed for monodentate binding, a smaller band gap for bidentate chelating, and a similar band gap for bidentate bridging modes. Those criteria have been extensively used in the literature to assign the type of binding of a wide variety of compounds. However, the authors themselves warned against applying this classification without caution, especially in the case of complex systems and for different experimental conditions. Among more recent studies, we chose to report two that represent the kind of controversy that is still related to the correlation between the band gap and the binding mode. In 2015, Sutton et al. [65] performed a computational study of complexes of acetate and formate with different cations in an aqueous environment. For these carboxylates, the predictions by Deacon and Phillips were fulfilled, but the main correlation was between the band gap and the geometrical parameters of the carboxylate groups. As a matter of fact, the distortion of the C-O bond and of the O-C-O angle upon complexation would explain the evolution of the band gap with the different modes of binding. On the other hand, in 2010, Martinez et al. [64] performed an experimental investigation of the AS-S band gap for manganese complexes of carboxylates having a more complicated structure, and their conclusions do not support the prediction of the evolution of the band gap with binding modes, but they do prove that the experimental conditions are key to correctly assigning the C-O stretch band, since a change in the coordination sphere of the metal can be observed.
Considering that in our case, the interaction takes place with a charged surface (and not with a metal center), that coupling with the motions involving the amino/methyl groups occurs in ASP and GLU, and that a final answer on the correlation between binding modes and the S vs. AS band gap does not clearly emerge from the literature, the results that we obtained hint at a stronger effect on the band gap and shape in the case of SUC. The band gap decreasing with the increase in hydration, for all systems, is both due to the conformational reorganization of the anions and their interactions with water.
To shed more light on the interactions of the anions with the local environment, including water and the surface, we conclude our discussion by presenting the results of the computed radial distribution functions (RDFs) for some relevant atom–atom interactions. For the LDHs intercalating ASP and GLU, we observed interesting changes with the increase in the water content, as displayed in Figure 8.
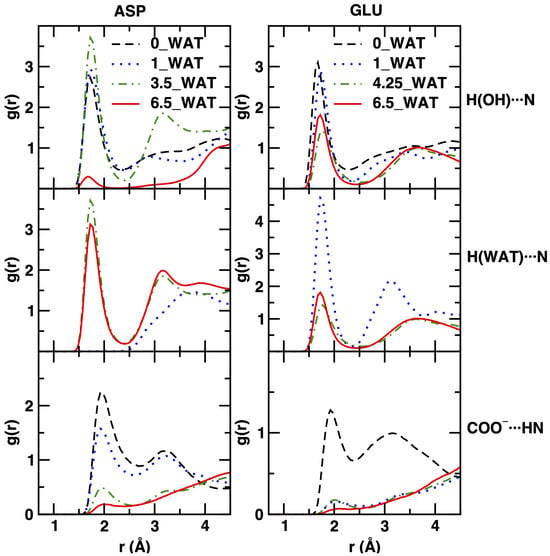
Figure 8.
Radial distribution functions for atom–atom interactions, displayed for distances consistent with the size of the interlamellar space. On the left column, we show results for the system intercalating ASP and on the right column for that intercalating GLU. From top to bottom: interactions between the H atom of the hydroxyl groups on the surface with the N atom of the intercalates; interactions of the H atom of water with N; interactions between the oxygen atoms of the carboxylate unit of one intercalate with the H atom at the N site of another one.
The interactions between the amino acids and the layers, monitored by using the RDFs of the H atom of the hydroxyl groups of the surfaces and the N atom of the anions, generally decrease when the water content (and thus the interlamellar space size) increases; in agreement with the density increase of N atoms in the center of the gallery, this effect is stronger in the case of the aspartate intercalate. In addition, for this system, the 3.5_WAT state seems to suggest slightly stronger interactions. We recall that in this hydration state, we observed an intermediate behavior with respect to the orientation of the anions. Regarding the interactions with water, we note that they stay more or less the same, with the exception of the 1_WAT system intercalating GLU (as above, this is the case for which an intermediate reorientational behavior was observed).
The most intriguing result is related to the evolution of the anion–anion interactions, through the (weak) hydrogen bond interactions between the charged O atoms of the carboxylate groups and the acidic H atom attached to the N atom of the amino acids. Two very structured peaks are observed here within the distances corresponding to one interlamellar space (up to 4–5 Å). This interaction, well characterized in the anhydrous system and in the 1_WAT systems, gradually fades away with the increase in hydration, as the first peak loses intensity and structuring. Our results are thus consistent with the hypothesis that lower hydration leads to much stronger COO−⋯HN interactions, bringing the amino acid units to the proximity that might finally lead to chemical reactivity (i.e., polypeptides formation) in natural systems [23,26,67]. Differences between the behavior of the aspartate and glutamate anions upon the increase in hydration are mostly observed in the shape and in the evolution of the second, much broader peak. This peak disappears at higher hydration conditions in the case of the LDH intercalating aspartate, while it not only stays but becomes more structured in the case of glutamate. However, in both cases, the evolution of the RDF shapes can be interpreted as a sign of weaker proximity along the COO−⋯HN direction, which is more sudden in the case of aspartate and more regular in the case of glutamate (especially for the first peak). We recall here that this interpretation needs to be kept at a qualitative level, since we are applying definitions that are based on spherical symmetry (i.e., the RDF) to a system with a different topology.
Our inspection of the interactions of the carboxylates, both with the surface and with the water molecules, and of water-water interactions are reported in Figure 9 for the three anions considered.
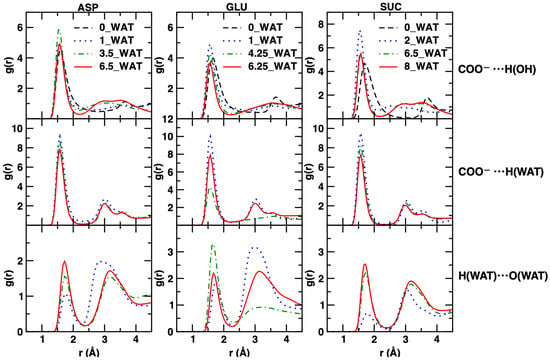
Figure 9.
Radial distribution functions for atom–atom interactions, displayed for distances consistent with the size of the interlamellar space. In the left column, we show results for the system intercalating ASP; in the middle column, those for the system intercalating GLU, and in the right column, those for the system intercalating SUC. From top to bottom: interactions between the H atom of the hydroxyl groups on the surface with the O atoms of the carboxyl groups of the intercalates; interactions of the H atom of water with the O atoms of the carboxyl groups of the intercalates; interactions between the H and the O atoms of water molecules.
Our results converge in pointing to a strong interaction established between the carboxylate groups of all systems and with both the water molecules and the surface. The structure of water around those groups is generally very similar in all cases, even under lower hydration. This can be safely interpreted as a fingerprint of a local hydrogen bond network that is well established in all situations. However, it is interesting to note that in the case of SUC, a drastic change is observed in the first peak of the RDF characterizing the intermolecular H⋯O interactions in water. In the 2_WAT system, such peak is smaller than in the case of the most hydrated states. With the help of our results for the number density along z, we can estimate that the local network of water hydrogen bonds is only recovered when water molecules start populating the middle region of the interlayer (4.25_WAT and 6.5_WAT), while it is weakened if water is only present next to the surface (2_WAT).
4. Conclusions
In this work, we presented a joint experimental and molecular dynamics study of the properties of LDH hybrid materials intercalating aspartate, succinate, and glutamate anions. In particular, we modeled the anhydrous systems and three different hydration states corresponding to the experimental conditions under which the measurements were carried out.
We compared the experimental and simulated carboxyl stretch bands, finding that the S and AS band gap and its evolution with increasing hydration are nicely accounted for by our model. A stronger effect is observed in the case of succinate, whereas a decrease in the band gap (but smaller effects on the band shape) are seen for aspartate and glutamate. We were able to relate the strong effect measured for succinate with a different reaction to increasing the water content in the material. SUC reorients itself completely, from lying horizontally with respect to the surfaces to lying perpendicularly, mostly keeping an anti conformation and moving from a bridging bidentate mode to bridging chelating with respect to the surface. The presence of the − group, an additional source of interaction with the surface and with water, and the more complex conformational space explored by ASP and GLU, lead to partial reorganization in the interlayer and to the presence of both anti and gauche conformers.
To summarize, we were able to show that relying on a single descriptor (the angle formed by the vector joining the C atoms of the groups and the z-axis) does not provide conclusive answers on the reorganization of the intercalated species, since this is not the only degree of freedom in non-rigid structures such as those of the three anions considered. The picture arising from our investigation is in agreement with the observed shape of the hydration isotherms, which are similar and featureless for ASP and GLU, whereas for SUC, the curve presents a plateau after the first steps of the water uptake and then a very steep rise, followed by a much slower increase. Once the switch in the two populations of succinate is made, the gallery can more easily pack water molecules, since the perpendicular orientation of the anions and their simple molecular structure allow for more room. On the other hand, the presence of an additional source of intermolecular interactions (with the surface, with water, and with the other anions) and the richer configurational space in ASP and GLU lead to a smoother, more regular change when increasing the water content.
The analysis of radial distribution functions, restricted to distances corresponding to the size of the interlamellar space, as well as the number distribution of significant atoms along the z-axis (perpendicular to the surface) provided information on the most important intermolecular interactions that are active in the confined systems, on the position of the polar groups, and on how they evolve with increasing water content. The amino group of the amino acids can act as a hydrogen bond acceptor, from the surface and from water, but under anhydrous conditions, it acts as a hydrogen bond donor to the carboxylate group of a nearby anion. The latter situation becomes increasingly less favorable with increasing hydration, a phenomenon that was already mentioned in the literature and claimed to be a possible path to the synthesis of peptide bonds in abiotic conditions and low water content [23,26].
Our findings show that combining computational and experimental work can significantly increase our knowledge of the microscopic structure of hybrid LDHs, making further steps toward a rational design of their properties. Increasing attention is being paid in the literature to hybrids containing carboxylate anions for applications in the electro- and photo-catalysis domain [68,69,70,71]. The role of these groups may impact the reaction kinetics by acting as a Lewis base [69]. Preliminary, non published results by some of us confirm the role played by the carboxylate groups in modulating the interaction of intercalates with the surface, which can be strongly affected by the electronic state of the molecules, as it was suggested by Xiao et al. [72]. This synergistic approach can lead to a constructive interplay between simulation and experiment, trigger the improvement of the models through fine tuning based on measured properties, and promote novel analytical and synthetic discoveries in the field of hybrid materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29081853/s1. References [29,44,45,46,47,48,49,73,74,75] are cited in Supplementary Materials.
Author Contributions
Conceptualization, E.A., A.C., A.B.P., C.C. and F.I.; Methodology, E.A. and F.I.; Investigation, V.K.P., E.A. and F.I.; Data curation, V.K.P., E.A. and F.I.; Writing – original draft, F.I.; Writing – review & editing, E.A., A.C., A.B.P. and C.C.; Project administration, F.I.; Funding acquisition, F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Chemistry Institute of the CNRS (IEA project NBioS) and by the FEDER program (project COMETE@CNRS).
Data Availability Statement
The diffraction spectra of the materials, further details about the computational procedure, the distribution of the dihedral angles describing the conformations of the anions, and the plots of the number densities computed for all hydration states are provided as Supplementary Materials.
Acknowledgments
The authors thank Fabien Pascale for his skillful assistance and the Explor HPC center, projects 2019CPMXX1332 and 2018CPMXX0851, for computing time.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Desigaux, L.; Belkacem, M.B.; Richard, P.; Cellier, J.; Léone, P.; Cario, L.; Leroux, F.; Taviot-Guého, C.; Pitard, B. Self-assembly and characterization of layered double hydroxide/DNA hybrids. Nano Lett. 2006, 6, 199–204. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Lu, S.; He, J.; Wang, Y. Colloidal assembly of proteins with delaminated lamellas of layered metal hydroxide. Langmuir 2009, 25, 10704–10710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Chapter 14.1—Layered Double Hydroxides (LDH). In Handbook of Clay Science; Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Del Hoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Carlino, S. The intercalation of carboxylic acids into layered double hydroxides: A critical evaluation and review of the different methods. Solid State Ion 1997, 98, 73–84. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Ren, L.; Evans, D.G.; Duan, X. Synthesis of layered double hydroxide anionic clays intercalated by carboxylate anions. Mater. Chem. Phys. 2004, 85, 207–214. [Google Scholar] [CrossRef]
- Iyi, N.; Yamada, H.; Sasaki, T. Deintercalation of carbonate ions from carbonate-type layered double hydroxides (LDHs) using acid–alcohol mixed solutions. Applied Clay Science 2011, 54, 132–137. [Google Scholar] [CrossRef]
- Lee, S.M.; Tiwari, D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: An overview. Appl. Clay Sci. 2012, 59-60, 84–102. [Google Scholar] [CrossRef]
- Darmograi, G.; Prelot, B.; Layrac, G.; Tichit, D.; Martin-Gassin, G.; Salles, F.; Zajac, J. Study of Adsorption and Intercalation of Orange-Type Dyes into Mg–Al Layered Double Hydroxide. J. Phys. Chem. C 2015, 119, 23388–23397. [Google Scholar] [CrossRef]
- Whilton, N.T.; Vickers, P.J.; Mann, S. Bioinorganic clays: Synthesis and characterization of amino-andpolyamino acid intercalated layered double hydroxides. J. Mater. Chemistry 1997, 7, 1623–1629. [Google Scholar] [CrossRef]
- Nakayama, H.; Wada, N.; Tsuhako, M. Intercalation of amino acids and peptides into Mg–Al layered double hydroxide by reconstruction method. Int. J. Pharm. 2004, 269, 469–478. [Google Scholar] [CrossRef]
- Aisawa, S.; Takahashi, S.; Ogasawara, W.; Umetsu, Y.; Narita, E. Direct intercalation of amino acids into layered double hydroxides by coprecipitation. J. Solid State Chem. 2001, 162, 52–62. [Google Scholar] [CrossRef]
- Bernal, J.D. The physical basis of life. Proc. Phys. Soc. B 1949, 62, 597. [Google Scholar] [CrossRef]
- Meister, A. Biochemistry of the Amino Acids, Volume II; Academic Press: London, UK, 1965. [Google Scholar]
- Erastova, V.; Degiacomi, M.T.; G Fraser, D.; Greenwell, H.C. Mineral surface chemistry control for origin of prebiotic peptides. Nat. Commun. 2017, 8, 2033. [Google Scholar] [CrossRef]
- Thyveetil, M.A.; Coveney, P.V.; Greenwell, H.C.; Suter, J.L. Computer simulation study of the structural stability and materials properties of DNA-intercalated layered double hydroxides. J. Am. Chem. Soc. 2008, 130, 4742–4756. [Google Scholar] [CrossRef] [PubMed]
- Rives, V. Characterisation of Layered Double Hydroxides and Their Decomposition Products. Mater. Chem. Phys. 2002, 75, 19–25. [Google Scholar] [CrossRef]
- Grégoire, B.; Erastova, V.; Geatches, D.L.; Clark, S.J.; Greenwell, H.C.; Fraser, D.G. Insights into the behaviour of biomolecules on the early Earth: The concentration of aspartate by layered double hydroxide minerals. Geochim. Cosmochim. Acta 2016, 176, 239–258. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kalinichev, A.G.; Kirkpatrick, R.J. Molecular dynamics simulation of the energetics and structure of layered double hydroxides intercalated with carboxylic acids. J. Phys. Chem. C 2007, 111, 13517–13523. [Google Scholar] [CrossRef]
- Newman, S.P.; Di Cristina, T.; Coveney, P.V.; Jones, W. Molecular dynamics simulation of cationic and anionic clays containing amino acids. Langmuir 2002, 18, 2933–2939. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Liu, X.; Cygan, R.T. Introduction to a special issue on molecular computer simulations of clays and clay–water interfaces: Recent progress, challenges, and opportunities. Clays Clay Miner 2016, 64, 335–336. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Padma Kumar, P.; James Kirkpatrick, R. Molecular dynamics computer simulations of the effects of hydrogen bonding on the properties of layered double hydroxides intercalated with organic acids. Philos. Mag. 2010, 90, 2475–2488. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S. Energy and structure of bonds in the interaction of organic anions with layered double hydroxide nanosheets: A molecular dynamics study. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Manasse, E. Idrotalcite e piroaurite. Atti Soc. Toscana Sci. Nat 1915, 24, 92. [Google Scholar]
- Allmann, R. The Crystal Structure of Pyroaurite. Acta Crystallogr. B Struct. Sci. 1968, 24, 972–977. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Segregation and Cation-Ordering in Sjögrenite and Pyroaurite. Mineral. Mag. 1969, 37, 338–342. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal Structures of Some Double Hydroxide Minerals. Mineral. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. Layered Double Hydroxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 119. [Google Scholar]
- Di Bitetto, A.; André, E.; Carteret, C.; Durand, P.; Kervern, G. Probing the Dynamics of Layered Double Hydroxides by Solid-State 27Al NMR Spectroscopy. J. Phys. Chem. C 2017, 121, 7276–7281. [Google Scholar] [CrossRef]
- Grégoire, B.; Ruby, C.; Carteret, C. Structural Cohesion of MII-MIII Layered Double Hydroxides Crystals: Electrostatic Forces and Cationic Polarizing Power. Cryst. Growth Des. 2012, 12, 4324–4333. [Google Scholar] [CrossRef]
- Fahel, J. Intercalation de Dicarboxylates et D’Acides Aminés dans des Hydroxydes Doubles Lamellaires: Relation Composition-Structure. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2016. [Google Scholar]
- Reinholdt, M.X.; Kirkpatrick, R.J. Experimental Investigations of Amino Acid Layered Double Hydroxide Complexes: Glutamate-Hydrotalcite. Chem. Mater. 2006, 18, 2567–2576. [Google Scholar] [CrossRef]
- Choi, G.; Yang, J.H.; Park, G.Y.; Vinu, A.; Elzatahry, A.; Yo, C.H.; Choy, J.H. Intercalative Ion-Exchange Route to Amino Acid Layered Double Hydroxide Nanohybrids and Their Sorption Properties. Eur. J. Inorg. Chem. 2015, 2015, 925–930. [Google Scholar] [CrossRef]
- Wu, W.; Song, L.; Li, Y.C.; Zhang, F.; Zeng, R.C.; Li, S.Q.; Zou, Y.H. Synthesis of glutamate intercalated Mg-Al layered double hydroxides: Influence of stirring and aging time. J. Dispers. Sci. 2021, 42, 2154–2162. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces; Pullman, B., Ed.; Springer: Dordrecht, The Netherlands, 1981; Volume 14, pp. 331–342. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T.; et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- McQuarrie, D.A. Statistical Mechanics; University Science Books: Sausalito, CA, USA, 2000. [Google Scholar]
- Egorov, S.; Skinner, J. Semiclassical approximations to quantum time correlation functions. Chem. Phys. Lett. 1998, 293, 469–476. [Google Scholar] [CrossRef]
- Ramirez, R.; López-Ciudad, T.; Kumar P, P.; Marx, D. Quantum corrections to classical time-correlation functions: Hydrogen bonding and anharmonic floppy modes. J. Chem. Phys. 2004, 121, 3973–3983. [Google Scholar] [CrossRef] [PubMed]
- Press, W.H. (Ed.) Numerical Recipes in C++: The Art of Scientific Computing, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2002. [Google Scholar]
- Ingrosso, F.; Monard, G.; Hamdi Farag, M.; Bastida, A.; Ruiz-López, M.F. Importance of polarization and charge transfer effects to model the infrared spectra of peptides in solution. J. Chem. Theory Comput. 2011, 7, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Porwal, V.K.; Carof, A.; Ingrosso, F. Hydration effects on the vibrational properties of carboxylates: From continuum models to QM/MM simulations. J. Comp. Chem. 2023, 44, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.M.V.; Tasinato, N.; Barone, V.; Amadei, A.; Zanetti-Polzi, L.; Daidone, I. Modeling amino-acid side chain infrared spectra: The case of carboxylic residues. Phys. Chem. Chem. Phys. 2020, 22, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- Prevot, V.; Forano, C.; Besse, J.P.; Abraham, F. Syntheses and Thermal and Chemical Behaviors of Tartrate and Succinate Intercalated Zn3Al and Zn2Cr Layered Double Hydroxides. Inorg. Chem. 1998, 37, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wei, M.; Evans, D.G.; Duan, X. Preparation and Investigation of Thermolysis of L-Aspartic Acid-Intercalated Layered Double Hydroxide. J. Phys. Chem. B 2004, 108, 12381–12387. [Google Scholar] [CrossRef]
- Feng, Y.J.; Williams, G.R.; Leroux, F.; Taviot-Gueho, C.; O’Hare, D. Selective Anion-Exchange Properties of Second-Stage Layered Double Hydroxide Heterostructures. Chem. Mater. 2006, 18, 4312–4318. [Google Scholar] [CrossRef]
- Sutton, C.C.; Franks, G.V.; da Silva, G. Modeling the antisymmetric and symmetric stretching vibrational modes of aqueous carboxylate anions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.; Pflüger, F.; Ghomi, M. Aspartate: An interesting model for analyzing dipole-ion and ion pair interactions through its oppositely charged amine and acid groups. J. Comp. Chem. 2020, 41, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.; Phillips, R. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Monodentate versus bidentate carboxylate binding in magnesium and calcium proteins: What are the basic principles? J. Phys. Chem. B 2004, 108, 4546–4557. [Google Scholar] [CrossRef]
- Martínez, D.; Motevalli, M.; Watkinson, M. Is there really a diagnostically useful relationship between the carbon–oxygen stretching frequencies in metal carboxylate complexes and their coordination mode? Dalton Trans. 2010, 39, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.C.; da Silva, G.; Franks, G.V. Modeling the IR spectra of aqueous metal carboxylate complexes: Correlation between bonding geometry and stretching mode wavenumber shifts. Chem. Eur. J. 2015, 21, 6801–6805. [Google Scholar] [CrossRef] [PubMed]
- Cherif, N.F.; Constantino, V.R.L.; Hamdaoui, O.; Leroux, F.; Taviot-Guého, C. New insights into two ciprofloxacin-intercalated arrangements for layered double hydroxide carrier materials. New J. Chem. 2020, 44, 10076–10086. [Google Scholar] [CrossRef]
- Grégoire, B.; Greenwell, H.C.; Fraser, D.G. Peptide Formation on Layered Mineral Surfaces: The Key Role of Brucite-like Minerals on the Enhanced Formation of Alanine Dipeptides. ACS Earth Space Chem. 2018, 2, 852–862. [Google Scholar] [CrossRef]
- Dong, Y.; Komarneni, S.; Zhang, F.; Wang, N.; Terrones, M.; Hu, W.; Huang, W. “Structural instability” induced high-performance NiFe layered double hydroxides as oxygen evolution reaction catalysts for pH-near-neutral borate electrolyte: The role of intercalates. Appl. Catal. B. 2020, 263, 118343. [Google Scholar] [CrossRef]
- Li, C.F.; Zhao, J.W.; Xie, L.J.; Wu, J.Q.; Ren, Q.; Wang, Y.; Li, G.R. Surface-Adsorbed Carboxylate Ligands on Layered Double Hydroxides/Metal–Organic Frameworks Promote the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 18129–18137. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Li, L.; Yuan, C.; Weng, T.C. Boosting the Electrocatalytic Activity of Nickel-Iron Layered Double Hydroxide for the Oxygen Evolution Reaction byTerephthalic Acid. Catalysts 2022, 12, 258. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Q.N.; Yang, Y.B.; Guo, P.F.; Feng, W.X.; Jia, Y.; Wang, K.; Wang, W.T.; He, Z.H.; Liu, Z.T. Enhancing Water Oxidation of Ru Single Atoms via Oxygen-Coordination Bonding with NiFe Layered Double Hydroxide. ACS Catal. 2023, 13, 2771–2779. [Google Scholar] [CrossRef]
- Xiao, F.N.; Wang, K.; Wang, F.B.; Xia, X.H. Highly stable and luminescent layered hybrid materials for sensitive detection of TNT explosives. Anal. Chem. 2015, 87, 4530–4537. [Google Scholar] [CrossRef] [PubMed]
- Leach, A.R. Molecular Modelling: Principles and Applications, 2nd ed.; Prentice Hall: Harlow, UK; New York, NY, USA, 2001. [Google Scholar]
- Kräutler, V.; van Gunsteren, W.F.; Hünenberger, P.H. A Fast SHAKE Algorithm to Solve Distance Constraint Equations for Small Molecules in Molecular Dynamics Simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Andrea, T.A.; Swope, W.C.; Andersen, H.C. The Role of Long Ranged Forces in Determining the Structure and Properties of Liquid Water. J. Chem. Phys. 1983, 79, 4576–4584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).