Widely Targeted Metabolomics Analysis Reveals Metabolites Important for Antioxidant Properties and Quality Traits in Different Fruit Parts of Aurantii Fructus Immatures
Abstract
1. Introduction
2. Results
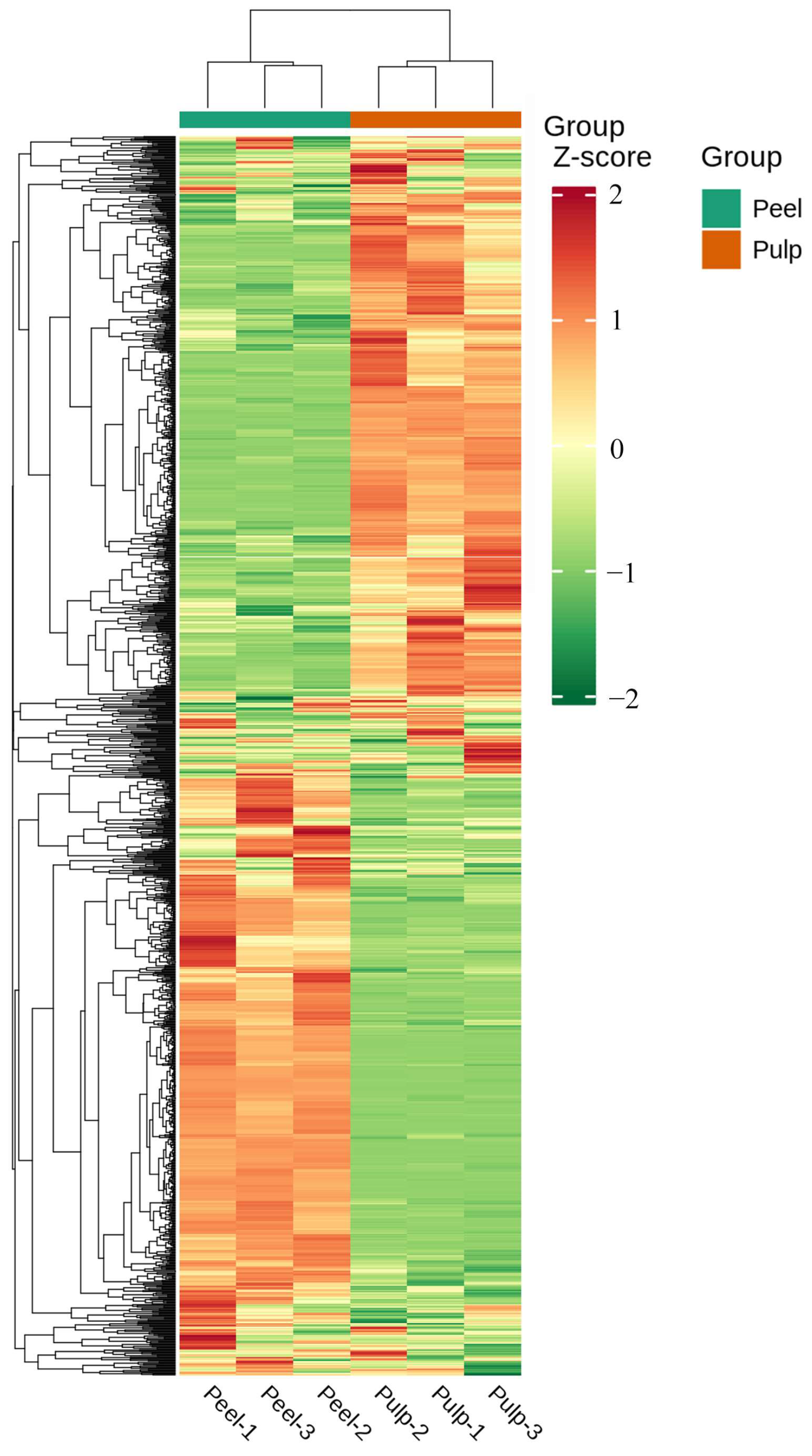
2.1. Metabolomic Profiling
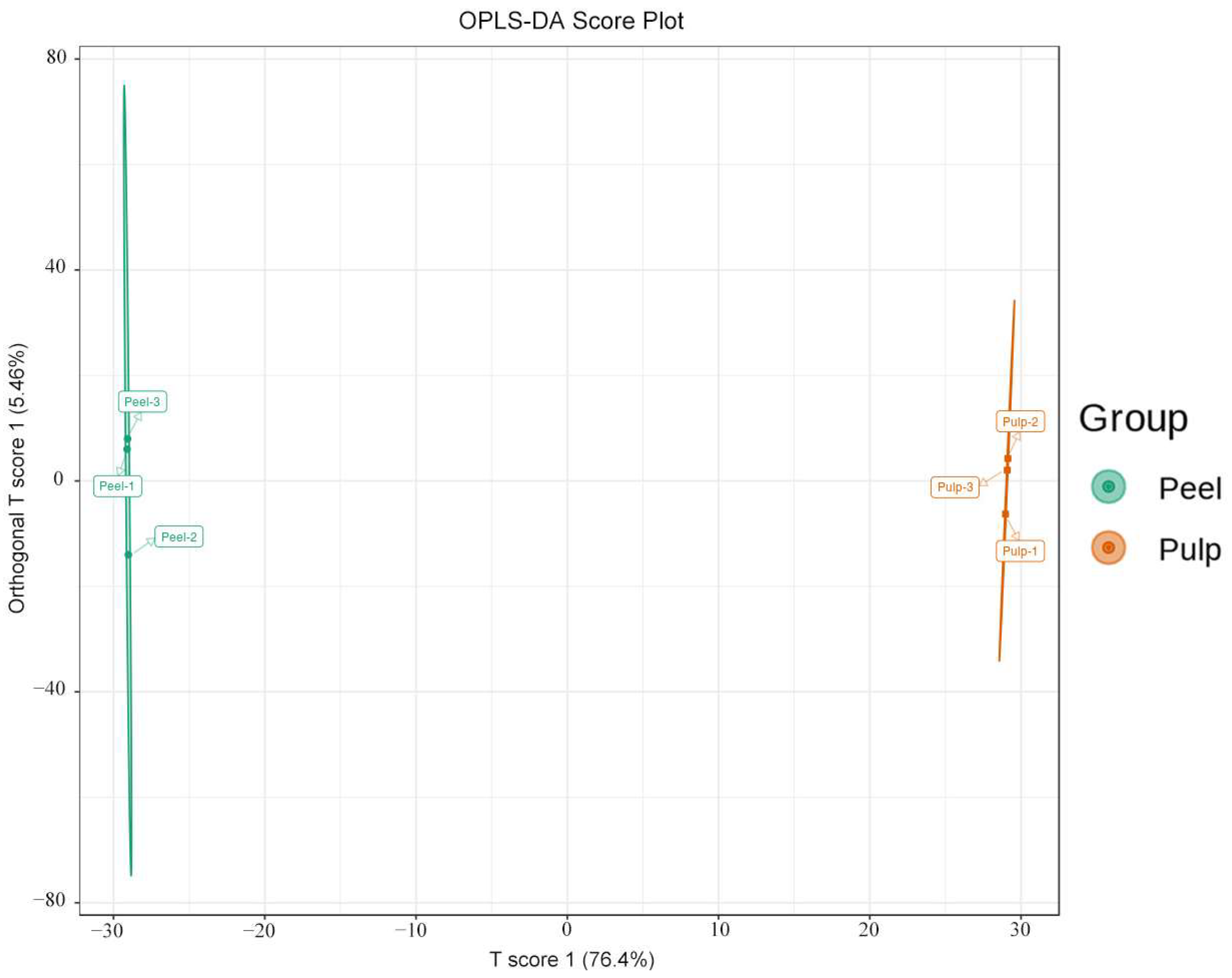
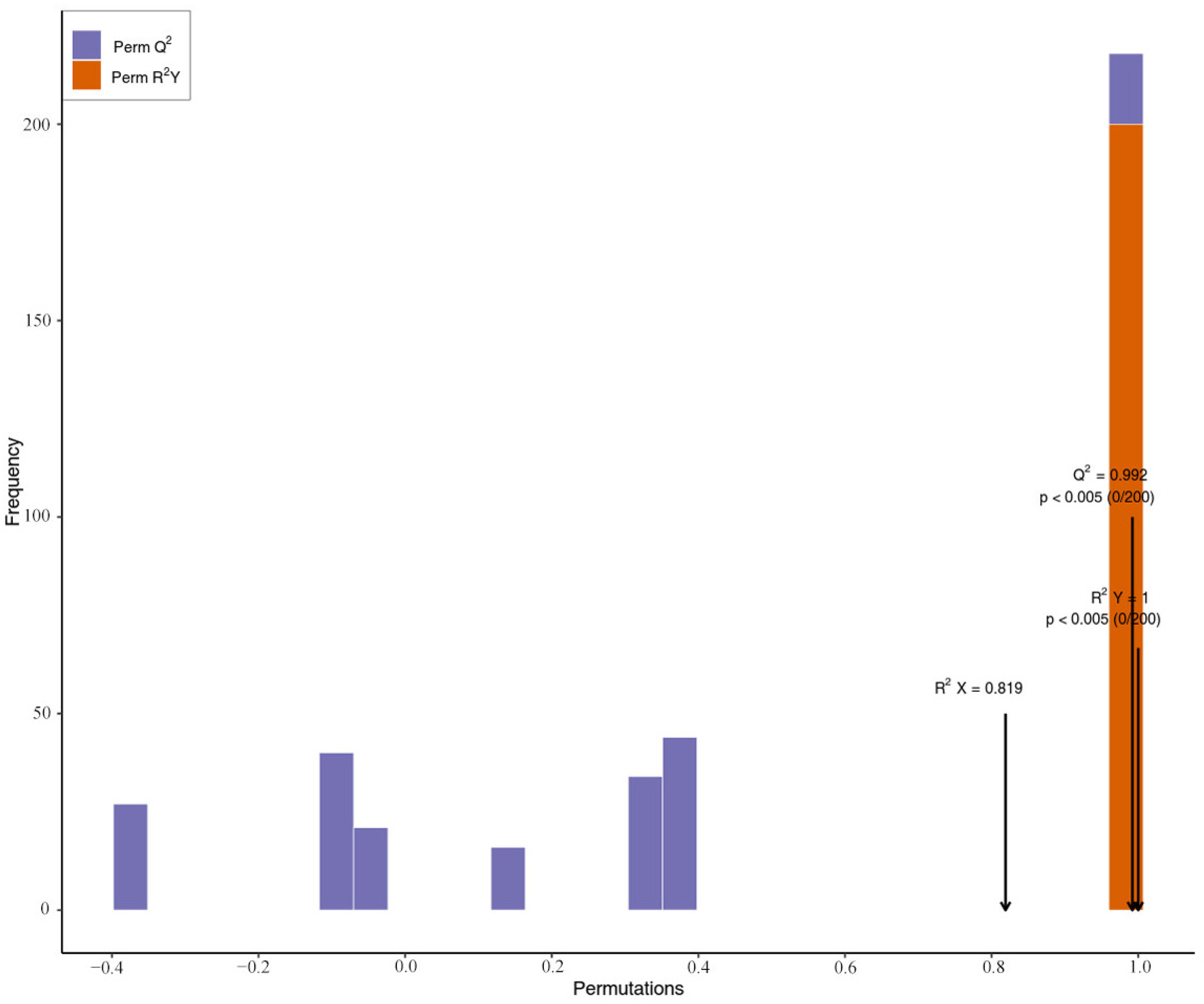
2.2. PCA and OPLS-DA for the Peel and Pulp of AFIs
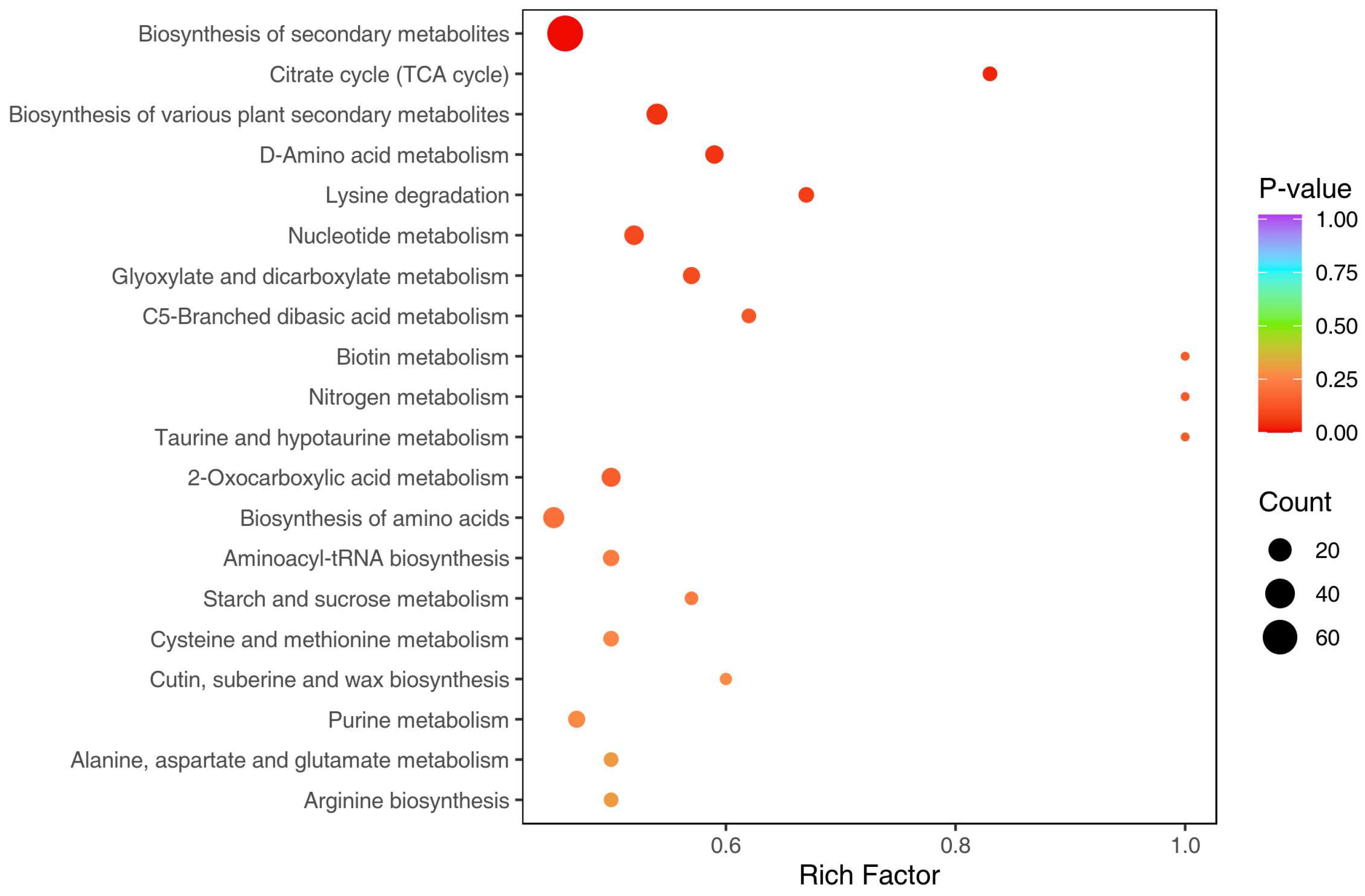
2.3. Differential Metabolite Screening, Functional Annotation, and Enrichment Analysis between Peel and Pulp
2.4. Antioxidant Activities of Different Fruit Parts in AFIs
3. Discussion
4. Materials and Methods
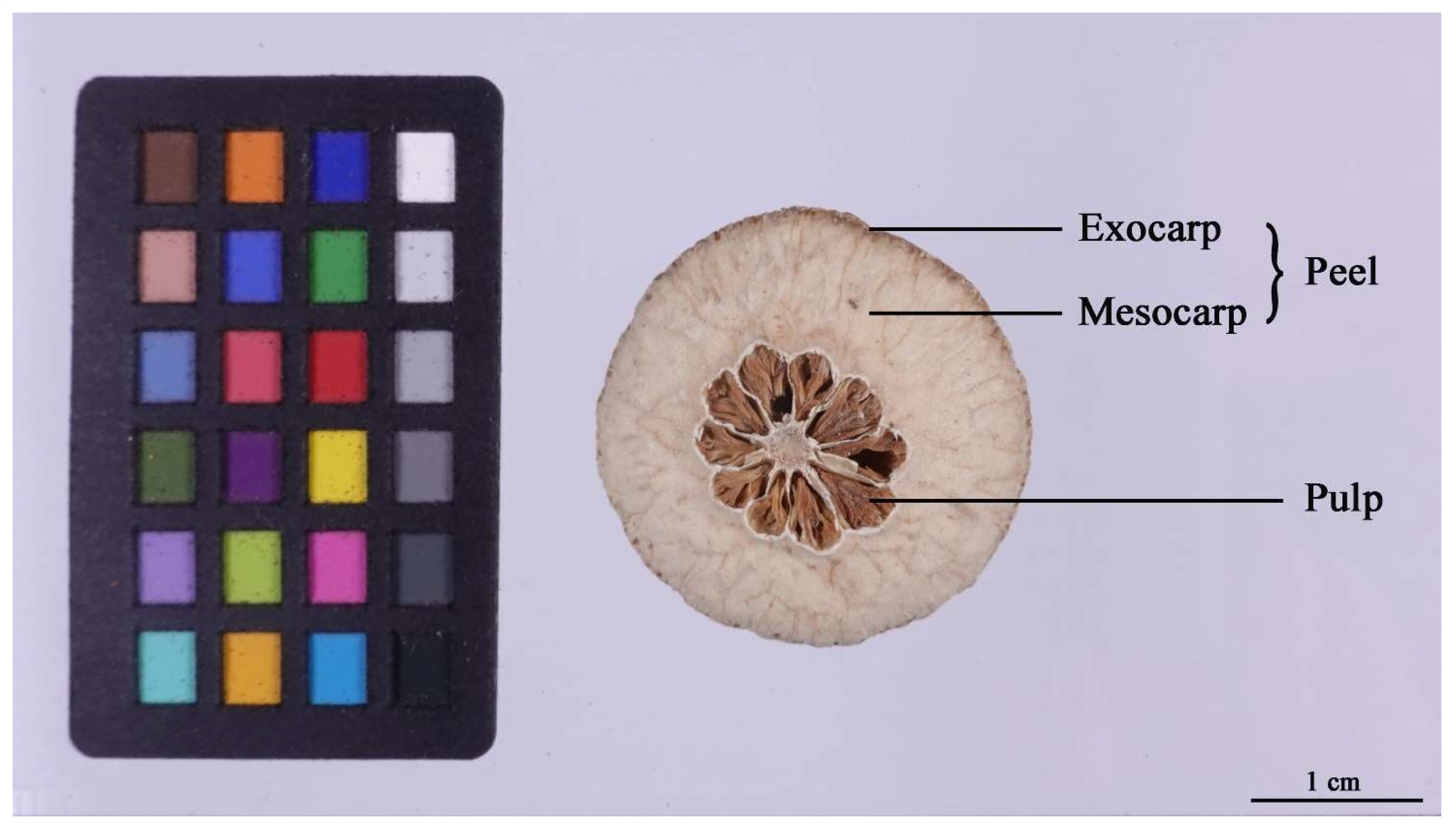
4.1. Plant Materials
4.2. Reagents
4.3. Widely Targeted Metabolomics Analysis
4.3.1. Sample Preparation and Extraction
4.3.2. UHPLC Conditions and ESI-Q TRAP-MS/MS
4.3.3. Analyzing Metabolites Qualitatively and Quantitatively
4.3.4. Multivariate Statistical Analysis
4.4. Determination of Antioxidant Capacities
4.4.1. Sample Preparation and Extraction
4.4.2. DPPH Assay
4.4.3. ABTS Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Jabri Karoui, I.; Marzouk, B. Characterization of Bioactive Compounds in Tunisian Bitter Orange (Citrus aurantium L.) Peel and Juice and Determination of Their Antioxidant Activities. Biomed Res. Int. 2013, 2013, 345415. [Google Scholar] [CrossRef]
- Lu, X.M.; Zhao, C.Y.; Shi, H.; Liao, Y.C.; Xu, F.; Du, H.J.; Xiao, H.; Zheng, J.K. Nutrients and Bioactives in Citrus Fruits: Different Citrus Varieties, Fruit Parts, and Growth Stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zeng, S.L.; Duan, L.; Ma, X.D.; Dou, L.L.; Wang, L.J.; Li, P.; Bi, Z.M.; Liu, E.H.P. Comparison of Aurantii Fructus Immaturus and Aurantii Fructus Based on Multiple Chromatographic Analysis and Chemometrics Methods. J. Chromatogr. A 2016, 1469, 96–107. [Google Scholar] [CrossRef]
- Li, J.M.; Luo, Y.; Zhan, L.L.; Gu, Y.Z.; Zhang, W.G.; Wen, Q.; Feng, Y.L.; Tan, J. Comprehensive Chemical Profiling of the Flowers of Citrus aurantium L. var. amara Engl. and Uncovering the Active Ingredients of Lipid Lowering. J. Pharm. Biomed. 2022, 211, 114621. [Google Scholar] [CrossRef]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef]
- Lee, S.H.; Yumnam, S.; Hong, G.E.; Raha, S.; Venkatarame Gowda Saralamma, V.; Lee, H.J.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H.; et al. Flavonoids of Korean Citrus aurantium L. Induce Apoptosis via Intrinsic Pathway in Human Hepatoblastoma HepG2 Cells: Citrus Flavonoids Induce Apoptosis in HepG2 Cells. Phytother. Res. 2015, 29, 1940–1949. [Google Scholar] [CrossRef]
- Wolffenbüttel, A.N.; Zamboni, A.; Becker, G.; Dos Santos, M.K.; Borille, B.T.; De Cássia Mariotti, K.; Fagundes, A.C.; De Oliveira Salomón, J.L.; Coelho, V.R.; Ruiz, L.V.; et al. Citrus Essential Oils Inhalation by Mice: Behavioral Testing, GCMS Plasma Analysis, Corticosterone, and Melatonin Levels Evaluation. Phytother. Res. 2018, 32, 160–169. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wan, L.; Wang, T.X.; Jiang, J.G. Citrus aurantium L. var. amara Engl. Inhibited Lipid Accumulation in 3T3-L1 Cells and Caenorhabditis Elegans and Prevented Obesity in High-Fat Diet-Fed Mice. Pharmacol. Res. 2019, 147, 104347. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Wang, T.X.; Jiang, J.G.; Huang, C.L.; Zhu, W. Bergaptol from Blossoms of Citrus aurantium L. var. amara Engl Inhibits LPS-Induced Inflammatory Responses and Ox-LDL-Induced Lipid Deposition. Food Funct. 2020, 11, 4915–4926. [Google Scholar] [CrossRef]
- Oliveira, S.A.C.; Zambrana, J.R.M.; Di Iorio, F.B.R.; Pereira, C.A.; Jorge, A.O.C. The Antimicrobial Effects of Citrus limonum and Citrus aurantium Essential Oils on Multi-Species Biofilms. Braz. Oral Res. 2013, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Wang, T.X.; Zhang, X.M.; Jiang, J.G. Various Antioxidant Effects Were Attributed to Different Components in the Dried Blossoms of Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 6087–6092. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Hu, Y.; Zhang, W.; Zhao, X.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Hypoglycemic and Hypolipidemic Effects of Neohesperidin Derived from Citrus aurantium L. in Diabetic KK-Ay Mice. Food Funct. 2015, 6, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.I.; Mahmoud, M.F.; Wink, M.; El-Shazly, A.M. Effect of Hesperidin and Neohesperidin from Bittersweet Orange (Citrus aurantium var. bigaradia) Peel on Indomethacin-Induced Peptic Ulcers in Rats. Environ. Toxicol. Pharmacol. 2014, 37, 907–915. [Google Scholar] [CrossRef]
- Liang, Z.; Sham, T.; Yang, G.; Yi, L.; Chen, H.; Zhao, Z. Profiling of Secondary Metabolites in Tissues from Rheum palmatum L. Using Laser Microdissection and Liquid Chromatography Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405, 4199–4212. [Google Scholar] [CrossRef]
- Yi, L.; Liang, Z.T.; Peng, Y.; Yao, X.; Chen, H.B.; Zhao, Z.Z. Tissue-Specific Metabolite Profiling of Alkaloids in Sinomenii Caulis Using Laser Microdissection and Liquid Chromatography–Quadrupole/Time of Flight-Mass Spectrometry. J. Chromatogr. A 2012, 1248, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Li, J.; Ju, M.; Yu, H.; Zha, L.; Peng, H.; Wang, J.; Peng, D.; Gui, S. Untargeted Metabolomics Approach Reveals the Tissue-Specific Markers of Balloon Flower Root (Platycodi Radix) Using UPLC-Q-TOF/MS. Microchem. J. 2021, 168, 106447. [Google Scholar] [CrossRef]
- Witzell, J.; Gref, R.; Näsholm, T. Plant-Part Specific and Temporal Variation in Phenolic Compounds of Boreal Bilberry (Vaccinium myrtillus) Plants. Biochem. Syst. Ecol. 2003, 31, 115–127. [Google Scholar] [CrossRef]
- Castro-Alves, V.; Kalbina, I.; Nilsen, A.; Aronsson, M.; Rosenqvist, E.; Jansen, M.A.K.; Qian, M.; Öström, Å.; Hyötyläinen, T.; Strid, Å. Integration of Non-Target Metabolomics and Sensory Analysis Unravels Vegetable Plant Metabolite Signatures Associated with Sensory Quality: A Case Study Using Dill (Anethum graveolens). Food Chem. 2021, 344, 128714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chu, S.; Gui, S.; Qin, Y.; Xu, R.; Shan, T.; Peng, H. Tissue-Specific Metabolite Profiling of Fallopia multiflora (Heshouwu) and Fallopia multiflora var. angulata by Mass Spectrometry Imaging and Laser Microdissection Combined with UPLC-Q/TOF-MS. J. Pharm. Biomed. 2021, 200, 114070. [Google Scholar] [CrossRef]
- Li, W.; Wen, L.C.; Chen, Z.C.; Zhang, Z.L.; Pang, X.L.; Deng, Z.C.; Liu, T.; Guo, Y.F. Study on Metabolic Variation in Whole Grains of Four Proso Millet Varieties Reveals Metabolites Important for Antioxidant Properties and Quality Traits. Food Chem. 2021, 357, 129791. [Google Scholar] [CrossRef] [PubMed]
- Gui, A.H.; Gao, S.W.; Zheng, P.C.; Feng, Z.H.; Liu, P.P.; Ye, F.; Wang, S.P.; Xue, J.J.; Xiang, J.; Ni, D.J.; et al. Dynamic Changes in Non-Volatile Components during Steamed Green Tea Manufacturing Based on Widely Targeted Metabolomic Analysis. Foods 2023, 12, 1551. [Google Scholar] [CrossRef]
- Wang, Z.H.; Gan, S.; Sun, W.J.; Chen, Z.D. Widely Targeted Metabolomics Analysis Reveals the Differences of Nonvolatile Compounds in Oolong Tea in Different Production Areas. Foods 2022, 11, 1057. [Google Scholar] [CrossRef]
- Ma, Y.B.; Li, J.H.; Li, J.L.; Yang, L.; Wu, G.L.; Liu, S.Y. Comparative Metabolomics Study of Chaenomeles speciosa (Sweet) Nakai from Different Geographical Regions. Foods 2022, 11, 1019. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, X.; Zheng, J.; Zhao, C.; Wang, D.; Xu, Y.; Sun, C. A Multifunctional True Caffeoyl Coenzyme A O-MethyltransFerase Enzyme Participates in the Biosynthesis of Polymethoxylated Flavones in Citrus. Plant Physiol. 2023, 192, 2049–2066. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, X.; Long, C.; Du, Y.; Li, J.; Yue, J.; Pan, S. Variations of Flavonoid Composition and Antioxidant Properties among Different Cultivars, Fruit Tissues and Developmental Stages of Citrus Fruits. Chem. Biodivers. 2020, 17, e1900690. [Google Scholar] [CrossRef]
- Shi, J.Y.; Cai, W.J.; Lin, W.D.; Zhang, S.; Luo, R. Comparison between Peel and Pulp of Aurantii Fructus Immaturus by UPLC Fingerprint and Multicomponent Quantitative Analysis. China J. Chin. Mater. Med. 2021, 46, 4446–4455. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and Ripening Impact on Phenolic Composition and Antioxidant Activity of Mandarin (Citrus reticulate Blanco) and Bitter Orange (Citrus aurantium L.) Seeds Extracts. Ind. Crop. Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Chen, H.F.; Zhang, W.G.; Yuan, J.B.; Li, Y.G.; Yang, S.L.; Yang, W.L. Simultaneous Quantification of Polymethoxylated Flavones and Coumarins in Fructus aurantii and Fructus aurantii Immaturus using HPLC–ESI-MS/MS. J. Pharm. Biomed. 2012, 59, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism Against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic Acid: Pharmacological and Toxicological Aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, X.; Zhu, Z.; Gong, Z.; Tang, W.; Hu, Y.; Cheng, C.; Wang, H.; Sarwar, A.; Chen, Y.; et al. STING Activation in Macrophages by Vanillic Acid Exhibits Antineoplastic Potential. Biochem. Pharmacol. 2023, 213, 115618. [Google Scholar] [CrossRef]
- Ooghe, W.C.; Ooghe, S.J.; Detavernier, C.M.; Huyghebaert, A. Characterization of Orange Juice (Citrus sinensis) by Polymethoxylated Flavones. J. Agric. Food Chem. 1994, 42, 2191–2195. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical Structures, Bioactivities and Molecular Mechanisms of Citrus Polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- De Moraes Barros, H.R.; De Castro Ferreira, T.A.P.; Inés Genovese, M. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus Polymethoxyflavones Attenuate Metabolic Syndrome by Regulating Gut Microbiome and Amino Acid Metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, Y.; Jin, F.; Zhang, J.; Ji, F.; Bai, Y.; Pei, D. Metabolome and Transcriptome Profiling Unveil the Mechanisms of Polyphenol Synthesis in the Developing Endopleura of Walnut (Juglans regia L.). Int. J. Mol. Sci. 2022, 23, 6623. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.J.; Hu, S.Q.; Li, T.; Zhang, J.Q.; Wang, B.S.; Liu, C.L. Metabolomics Analysis Reveals the Differences between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Front. Plant Sci. 2022, 13, 933849. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, M.Z.; Chu, S.S.; Zhao, Y.J.; Fang, Q.Y.; Cheng, M.E.; Peng, H.S.; Huang, L.Q. Colour, Chemical Compounds, and Antioxidant Capacity of Astragali Radix based on Untargeted Metabolomics and Targeted Quantification. Phytochem. Anal. 2022, 33, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Litewski, S.; Koss-Mikołajczyk, I.; Kusznierewicz, B. Comparative Analysis of Phytochemical Profiles and Selected Biological Activities of Various Morphological Parts of Ligustrum vulgare. Molecules 2024, 29, 399. [Google Scholar] [CrossRef] [PubMed]
- Sarega, N.; Imam, M.U.; Ooi, D.J.; Chan, K.W.; Md Esa, N.; Zawawi, N.; Ismail, M. Phenolic Rich Extract from Clinacanthus nutans Attenuates Hyperlipidemia-Associated Oxidative Stress in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 4137908. [Google Scholar] [CrossRef]




| Type | Number | Percentage |
|---|---|---|
| Flavonoids | 241 | 34.7 |
| Lignans and coumarins | 114 | 16.4 |
| Phenolic acids | 77 | 11.1 |
| Lipids | 75 | 10.8 |
| Alkaloids | 63 | 9.1 |
| Others | 32 | 4.6 |
| Amino acids and derivatives | 30 | 4.3 |
| Organic acids | 23 | 3.3 |
| Terpenoids | 22 | 3.2 |
| Nucleotides and derivatives | 18 | 2.6 |
| Peel | Pulp | |
|---|---|---|
| DPPH (IC50) | 0.124 ± 0.009 | 0.237 ± 0.017 *** |
| ABTS (IC50) | 0.130 ± 0.008 | 0.211 ± 0.010 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, Z.; Xu, X.; Zhao, R.; Zhang, S.; Luo, R. Widely Targeted Metabolomics Analysis Reveals Metabolites Important for Antioxidant Properties and Quality Traits in Different Fruit Parts of Aurantii Fructus Immatures. Molecules 2024, 29, 1733. https://doi.org/10.3390/molecules29081733
Zhang S, Liu Z, Xu X, Zhao R, Zhang S, Luo R. Widely Targeted Metabolomics Analysis Reveals Metabolites Important for Antioxidant Properties and Quality Traits in Different Fruit Parts of Aurantii Fructus Immatures. Molecules. 2024; 29(8):1733. https://doi.org/10.3390/molecules29081733
Chicago/Turabian StyleZhang, Shuo, Ze Liu, Xinyu Xu, Ruihua Zhao, Shujiang Zhang, and Rong Luo. 2024. "Widely Targeted Metabolomics Analysis Reveals Metabolites Important for Antioxidant Properties and Quality Traits in Different Fruit Parts of Aurantii Fructus Immatures" Molecules 29, no. 8: 1733. https://doi.org/10.3390/molecules29081733
APA StyleZhang, S., Liu, Z., Xu, X., Zhao, R., Zhang, S., & Luo, R. (2024). Widely Targeted Metabolomics Analysis Reveals Metabolites Important for Antioxidant Properties and Quality Traits in Different Fruit Parts of Aurantii Fructus Immatures. Molecules, 29(8), 1733. https://doi.org/10.3390/molecules29081733





