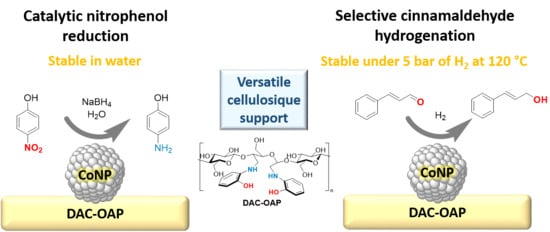
Schiff Base Functionalized Cellulose: Towards Strong Support-Cobalt Nanoparticles Interactions for High Catalytic Performances
Abstract
1. Introduction
2. Results and Discussion
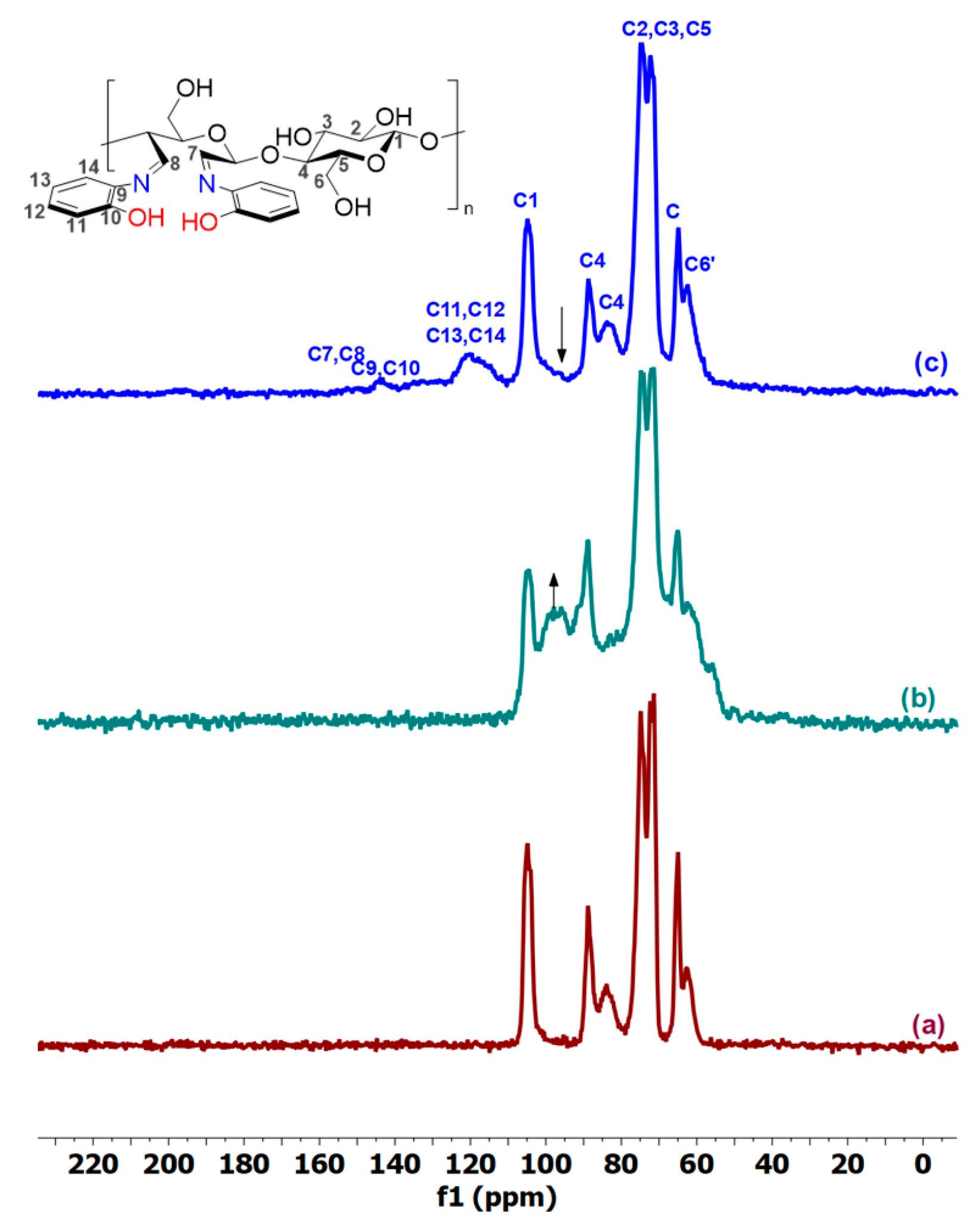
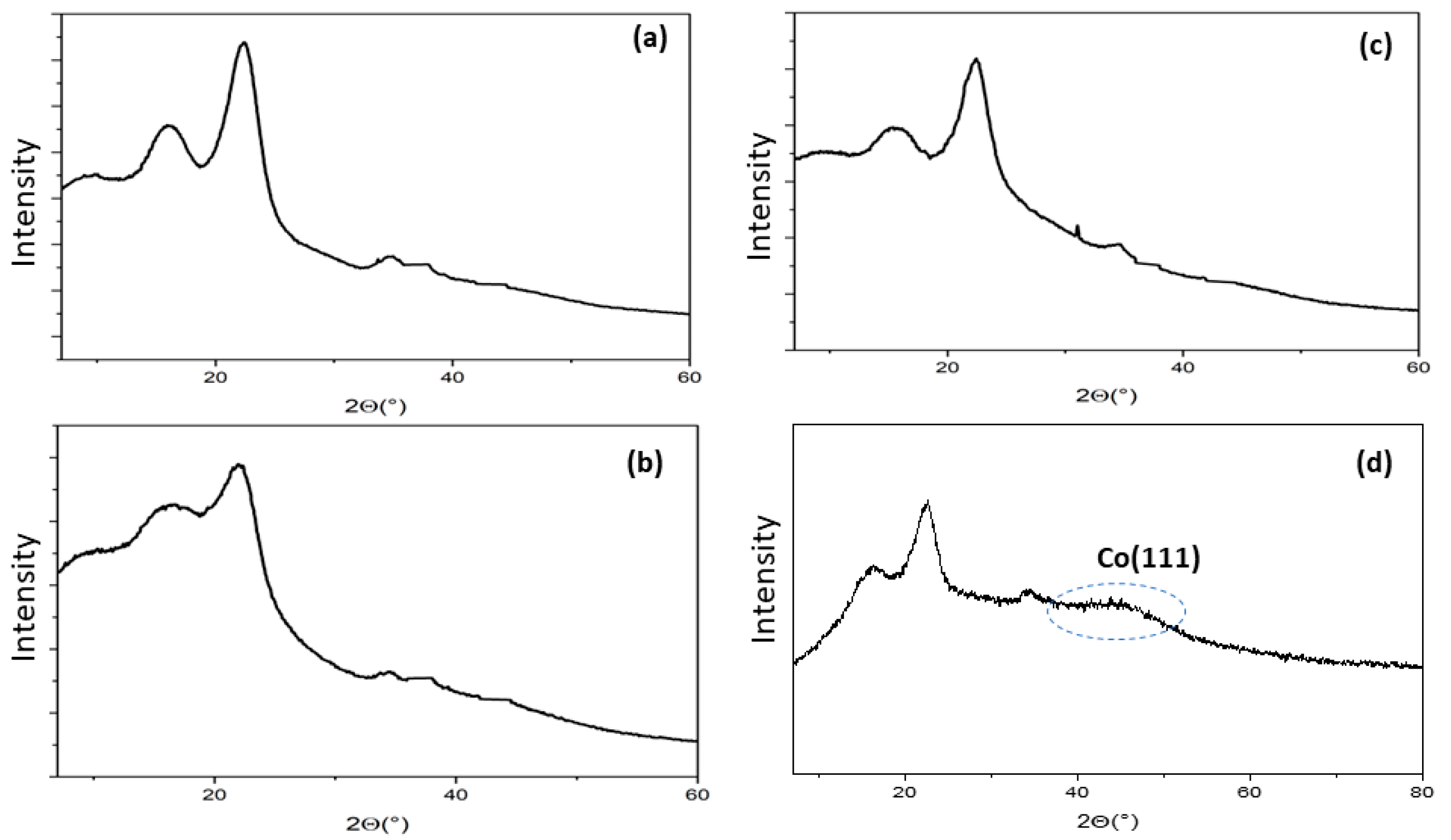
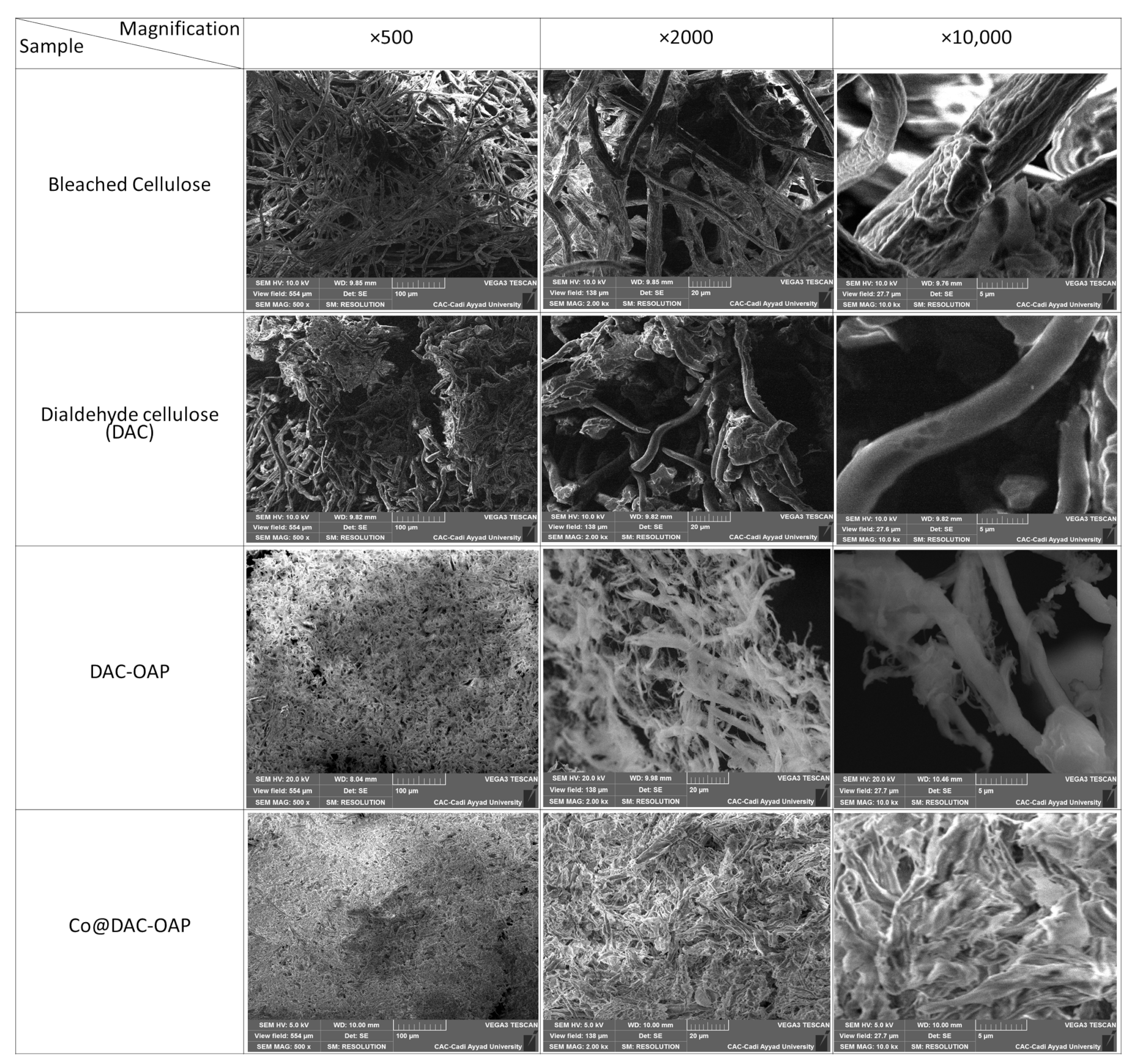
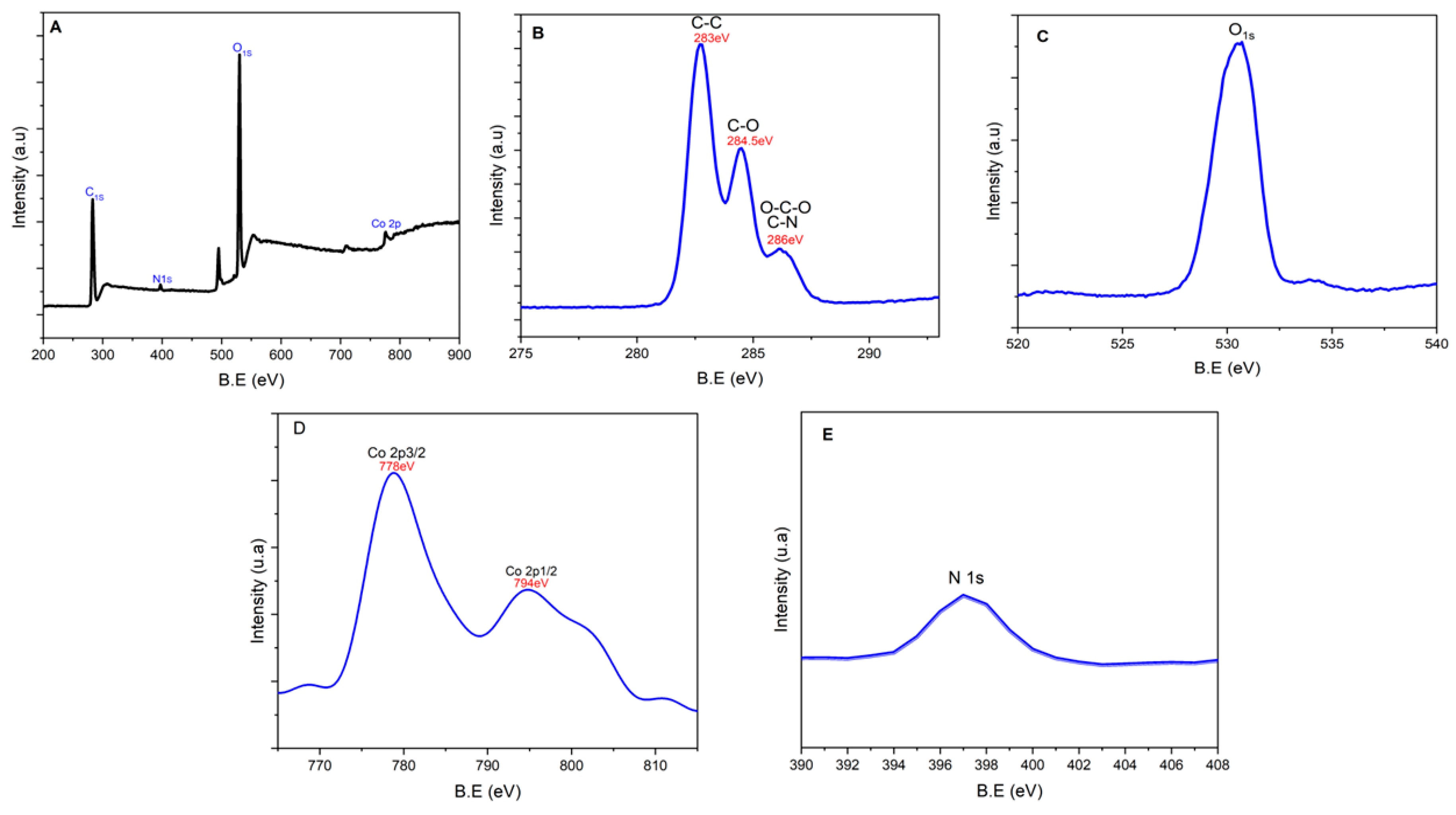
2.1. Structural and Morphological Characterization
2.2. Catalytic Activity of Co@DAC-OAP
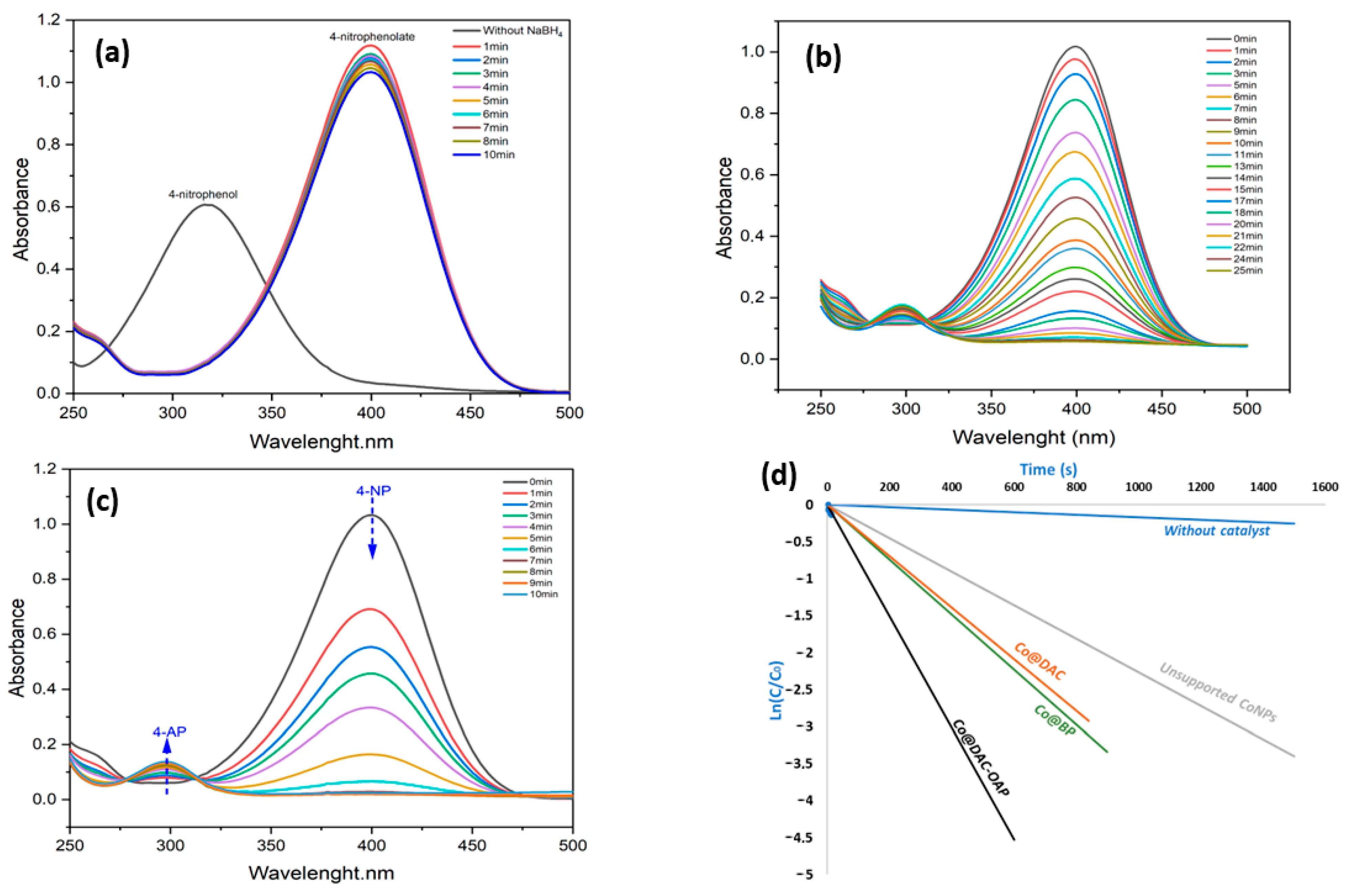
2.2.1. Reduction of 4-Nitrophenol (4-NP)
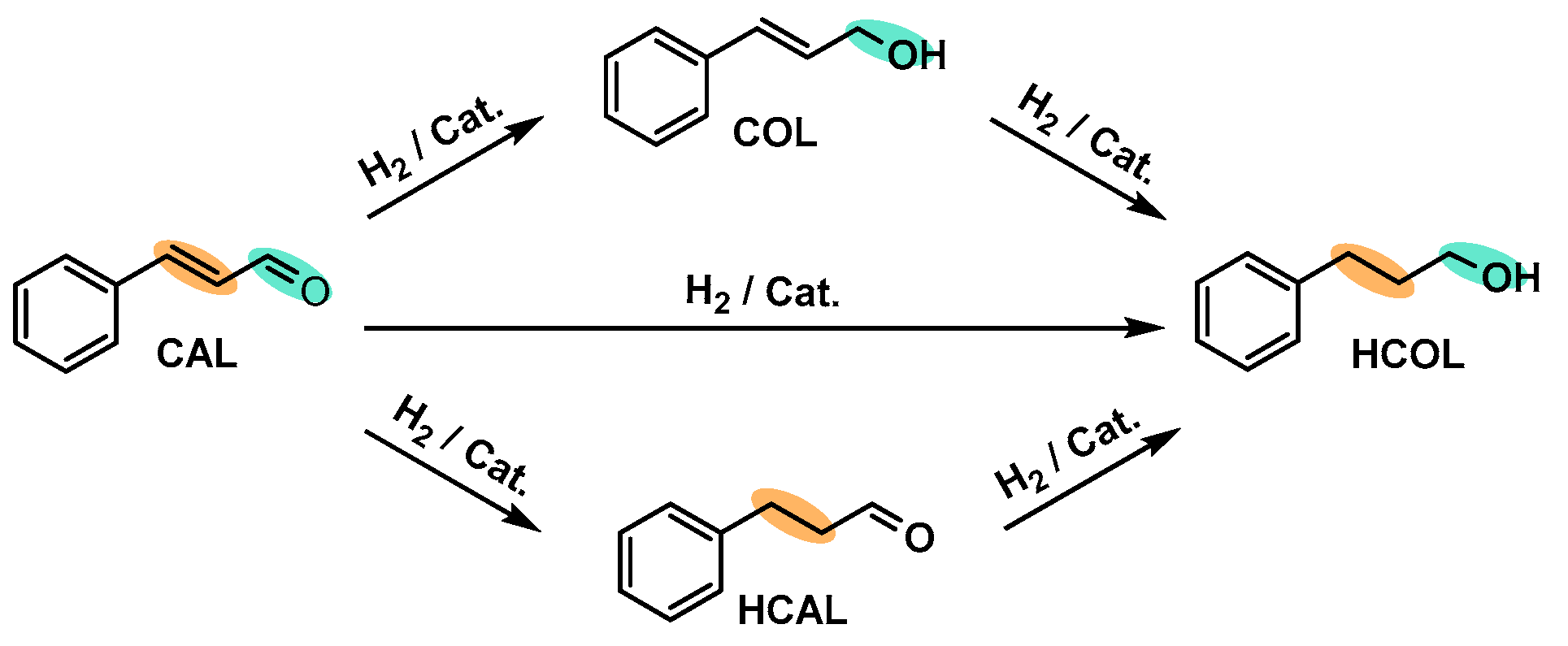
2.2.2. Selective Hydrogenation of Cinnamaldehyde
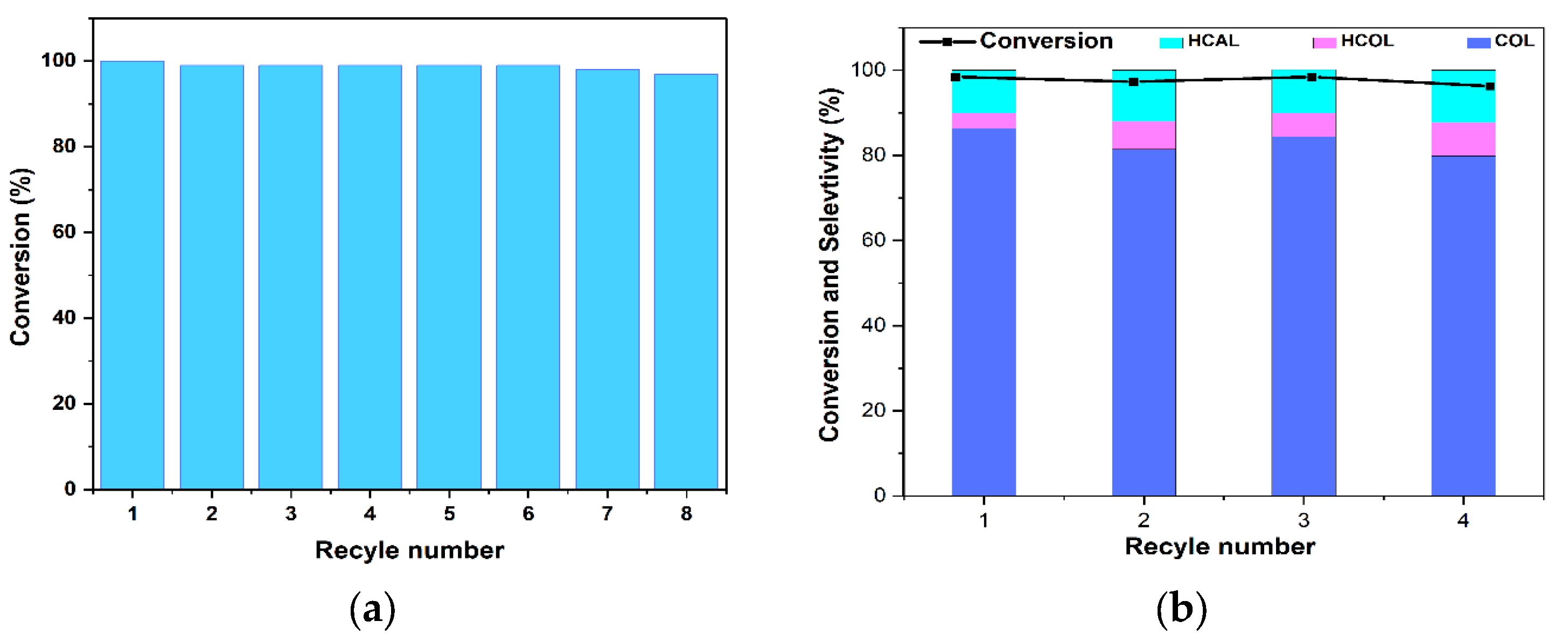
2.2.3. Study of Catalyst Recyclability
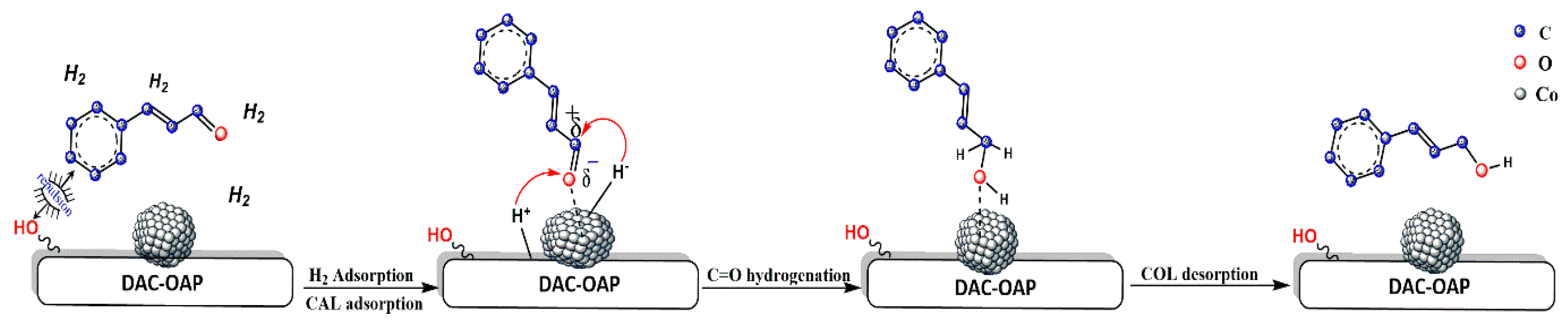
2.2.4. Origin of the Selectivity Observed during the Hydrogenation of Cinnamaldehyde Catalyzed by Co@DAC-OAP
3. Materials and Methods
3.1. Reagents
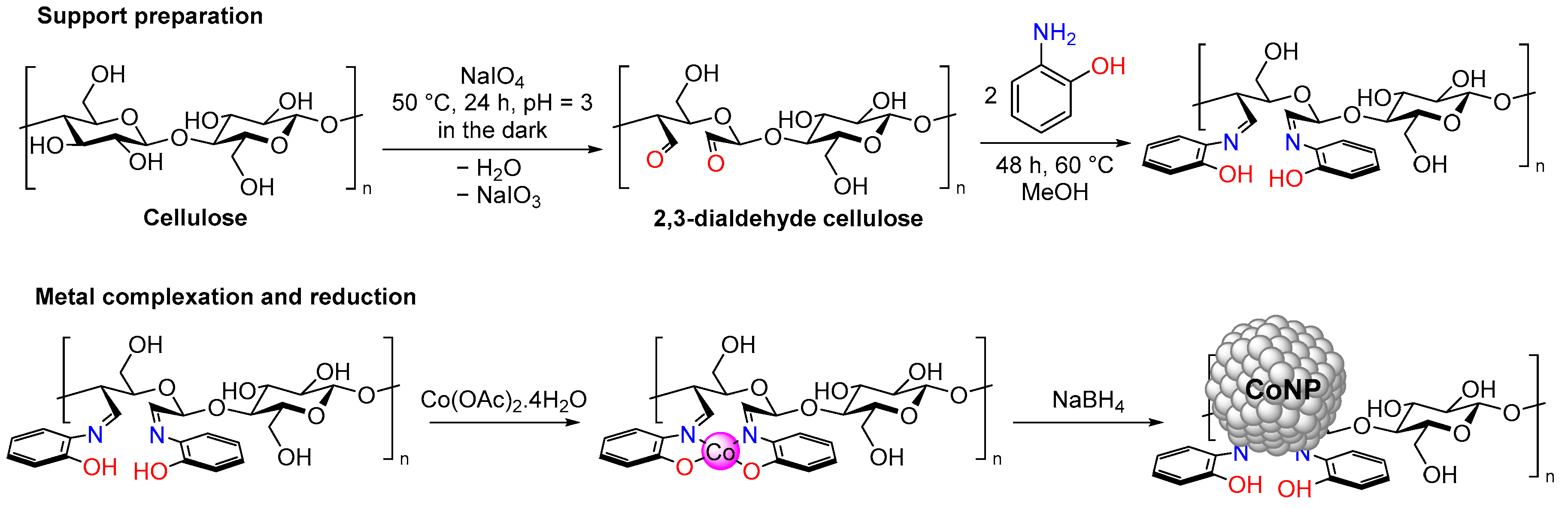
3.2. Catalyst Preparation
3.2.1. Preparation of Cellulose: Date Palm Pulp
3.2.2. Preparation of DAC (2,3-Dialdehyde Cellulose)
3.2.3. Preparation of DAC-OAP
3.2.4. Synthesis of Cellulosic Co(salen) Complex (Co@DAC-OAP)
3.2.5. Synthesis of Cellulosic Co@BP and Co@DAC
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, S.; Kwon, J.H.; Cho, K.Y.; Kim, D.H.; Eom, K.S. Enhanced activity and stability of Co-Ni-P-B catalyst for the hydrogen evolution reaction via predeposition of Co-Ni on a Cu substrate. Catal. Today 2021, 359, 35–42. [Google Scholar] [CrossRef]
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A Gen. 1997, 161, 59–78. [Google Scholar] [CrossRef]
- van Steen, E.; Claeys, M. Fischer-Tropsch catalysts for the biomass-to-liquid process. Chem. Eng. Technol. 2008, 31, 655–666. [Google Scholar] [CrossRef]
- Zhu, J.; Kailasam, K.; Fischer, A.; Thomas, A. Supported cobalt oxide nanoparticles as catalyst for aerobic oxidation of alcohols in liquid phase. ACS Catal. 2011, 1, 342–347. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Yang, X.; Hu, J.; Peng, L.; Bai, L.; Huo, Q.; Guan, J. Highly efficient single atom cobalt catalyst for selective oxidation of alcohols. Appl. Catal. A Gen. 2017, 543, 61–66. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. The catalytic activity of cobalt nanoparticles for low-temperature oxidation of carbon monoxide. Mater. Today Chem. 2019, 14, 100198. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Chen, R.; Ye, D.; Yang, Y.; Liao, Q. Kinetics of light assisted catalytic reduction of 4-NP over Ag/PDA. Chem. Eng. Sci. 2022, 259, 117778. [Google Scholar] [CrossRef]
- Gutiérrez, L.F.; Hamoudi, S.; Belkacemi, K. Synthesis of gold catalysts supported on mesoporous silica materials: Recent developments. Catalysts 2011, 1, 97–154. [Google Scholar] [CrossRef]
- Yu, X.; Williams, C.T. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 5765–5794. [Google Scholar] [CrossRef]
- Karimi, S.; Tavasoli, A.; Mortazavi, Y.; Karimi, A. Cobalt supported on Graphene—A promising novel Fischer-Tropsch synthesis catalyst. Appl. Catal. A Gen. 2015, 499, 188–196. [Google Scholar] [CrossRef]
- Yang, L.; Cao, N.; Du, C.; Dai, H.; Hu, K.; Luo, W.; Cheng, G. Graphene supported cobalt(0) nanoparticles for hydrolysis of ammonia borane. Mater. Lett. 2014, 115, 113–116. [Google Scholar] [CrossRef]
- Zola, A.S.; Bidart, A.M.F.; Fraga, A.D.C.; Hori, C.E.; Sousa-Aguiar, E.F.; Arroyo, P.A. Cobalt supported on different zeolites for fischer-tropschsynthesis. Stud. Surf. Sci. Catal. 2007, 167, 129–134. [Google Scholar]
- Liu, J.; Wang, D.; Chen, J.F.; Zhang, Y. Cobalt nanoparticles imbedded into zeolite crystals: A tailor-made catalyst for one-step synthesis of gasoline from syngas. Int. J. Hydrogen Energy 2016, 41, 21965–21978. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Bidgoli, N.S.S.; Shafiei, N. Recent progresses in polymer supported cobalt complexes/nanoparticles for sustainable and selective oxidation reactions. Mol. Catal. 2020, 484, 110775. [Google Scholar] [CrossRef]
- de Cuadro, P.; Belt, T.; Kontturi, K.S.; Reza, M.; Kontturi, E.; Vuorinen, T.; Hughes, M. Cross-linking of cellulose and poly(ethylene glycol) with citric acid. React. Funct. Polym. 2015, 90, 21–24. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Lalanne-Tisné, M.; Eyley, S.; De Winter, J.; Favrelle-Huret, A.; Thielemans, W.; Zinck, P. Cellulose nanocrystals modification by grafting from ring opening polymerization of a cyclic carbonate. Carbohydr. Polym. 2022, 295, 119840. [Google Scholar] [CrossRef]
- Lalanne-Tisné, M.; Mees, M.A.; Eyley, S.; Zinck, P.; Thielemans, W. Organocatalyzed ring opening polymerization of lactide from the surface of cellulose nanofibrils. Carbohydr. Polym. 2020, 250, 116974. [Google Scholar] [CrossRef]
- Kalia, S.; Sabaa, M.W. Polysaccharide Based Graft Copolymers; Springer: Berlin/Heidelberg, Germany, 2013; p. 9783642365. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafted cellulose: A bio-based polymer for durable applications. Polym. Bull. 2018, 75, 2213–2242. [Google Scholar] [CrossRef]
- Martinelli, A.; Giannini, L.; Branduardi, P. Enzymatic Modification of Cellulose To Unlock Its Exploitation in Advanced Materials. ChemBioChem 2021, 22, 974–981. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging applications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- El Idrissi, N.; Belachemi, L.; Merle, N.; Zinck, P.; Kaddami, H. Comprehensive preparation and catalytic activities of Co/TEMPO-cellulose nanocomposites: A promising green catalyst. Carbohydr. Polym. 2022, 295, 119765. [Google Scholar] [CrossRef]
- Mou, K.; Li, J.; Wang, Y.; Cha, R.; Jiang, X. 2,3-Dialdehyde nanofibrillated cellulose as a potential material for the treatment of MRSA infection. J. Mater. Chem. B 2017, 5, 7876–7884. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.J.; Kulkarni, M.P. Oxidation of cellulose under controlled conditions. Polym. Degrad. Stab. 2002, 77, 25–27. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S.; El-Zahhar, A.A.; Haija, M.A.; Bondock, S. Synthesis of a Magnetic Nanoparticles/Dialdehyde Starch-Based Composite Film for Food Packaging. Starch-Staerke 2019, 71, 1800035. [Google Scholar] [CrossRef]
- Potthast, A.; Schiehser, S.; Rosenau, T.; Kostic, M. Oxidative modifications of cellulose in the periodate system—Reduction and beta-elimination reactions: 2nd ICC 2007, Tokyo, Japan. Holzforschung 2009, 63, 12–17. [Google Scholar] [CrossRef]
- Leguy, J.; Diallo, A.; Putaux, J.L.; Nishiyama, Y.; Heux, L.; Jean, B. Periodate Oxidation Followed by NaBH4 Reduction Converts Microfibrillated Cellulose into Sterically Stabilized Neutral Cellulose Nanocrystal Suspensions. Langmuir 2018, 34, 11066–11075. [Google Scholar] [CrossRef] [PubMed]
- Visanko, M.; Liimatainen, H.; Sirviö, J.A.; Haapala, A.; Sliz, R.; Niinimäki, J.; Hormi, O. Porous thin film barrier layers from 2,3-dicarboxylic acid cellulose nanofibrils for membrane structures. Carbohydr. Polym. 2014, 102, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Kuga, S. Ion-exchange chromatography by dicarboxyl cellulose gel. J. Chromatogr. A 2001, 919, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Dicarboxylic cellulose decorated with silver nanoparticles as sustainable antibacterial nanocomposite material. Environ. Nanotechnol. Monit. Manag. 2017, 8, 228–232. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, H.M. Cationization of periodate-oxidized cotton cellulose with choline chloride. Cellul. Chem. Technol. 2014, 48, 25–32. [Google Scholar]
- Tian, X.; Yan, D.; Lu, Q.; Jiang, X. Cationic surface modification of nanocrystalline cellulose as reinforcements for preparation of the chitosan-based nanocomposite films. Cellulose 2017, 24, 163–174. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Sirviö, J.A.; Liimatainen, H. Cationic nanocelluloses in dewatering of municipal activated sludge. Biochem. Pharmacol. 2016, 5, 86–92. [Google Scholar] [CrossRef]
- Huang, X.; Dognani, G.; Hadi, P.; Yang, M.; Job, A.E.; Hsiao, B.S. Cationic Dialdehyde Nanocellulose from Sugarcane Bagasse for Efficient Chromium(VI) Removal. ACS Sustain. Chem. Eng. 2020, 8, 4734–4744. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.; Zhang, H.; Zhu, Z.; Fu, S. Dialdehyde cellulose nanocrystals act as multi-role for the formation of ultra-fine gold nanoparticles with high efficiency. Int. J. Biol. Macromol. 2020, 163, 788–800. [Google Scholar] [CrossRef]
- Yu, C.; Wu, W.; Gao, M.; Liu, Y. Modified Cellulose with BINAP-Supported Rh as an Efficient Heterogeneous Catalyst for Asymmetric Hydrogenation. Catalysts 2022, 12, 83. [Google Scholar] [CrossRef]
- Guo, H.; Lei, B.; Yu, J.; Chen, Y.; Qian, J. Immobilization of lipase by dialdehyde cellulose crosslinked magnetic nanoparticles. Int. J. Biol. Macromol. 2021, 185, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, A.; Lin, L.; El-Sohaimy, S.A.; Li, Y.; Wu, L.; Zhang, H. Schiff base cross-linked dialdehyde cellulose/gelatin composite aerogels as porous structure templates for oleogels preparation. Int. J. Biol. Macromol. 2022, 224, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Strømme, M.; Lindh, J. A green and simple method for preparation of an efficient palladium adsorbent based on cysteine functionalized 2,3-dialdehyde cellulose. Cellulose 2016, 23, 2627–2638. [Google Scholar] [CrossRef]
- Ruan, C.Q.; Strømme, M.; Lindh, J. Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2017, 181, 200–207. [Google Scholar] [CrossRef]
- Kitkulnumchai, Y.; Ajavakom, A.; Sukwattanasinitt, M. Treatment of oxidized cellulose fabric with chitosan and its surface activity towards anionic reactive dyes. Cellulose 2008, 15, 599–608. [Google Scholar] [CrossRef]
- Huang, X.; Hadi, P.; Joshi, R.; Alhamzani, A.G.; Hsiao, B.S. A Comparative Study of Mechanism and Performance of Anionic and Cationic Dialdehyde Nanocelluloses for Dye Adsorption and Separation. ACS Omega 2023, 8, 8634–8649. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Fischer, N.; Claeys, M. Capturing the interconnectivity of water-induced oxidation and sintering of cobalt nanoparticles during the Fischer-Tropsch synthesis in situ. J. Catal. 2019, 374, 199–207. [Google Scholar] [CrossRef]
- Claeys, M.; Dry, M.E.; van Steen, E.; van Berge, P.J.; Booyens, S.; Crous, R.; van Helden, P.; Labuschagne, J.; Moodley, D.J.; Saib, A.M. Impact of process conditions on the sintering behavior of an alumina-supported cobalt Fischer-Tropsch catalyst studied with an in situ magnetometer. ACS Catal. 2015, 5, 841–852. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Remans, T.J.; Van Bavel, A.P.; Dugulan, A.I. Direct evidence of water-assisted sintering of cobalt on carbon nanofiber catalysts during simulated fischer-tropsch conditions revealed with in situ mössbauer spectroscopy. J. Am. Chem. Soc. 2010, 132, 8540–8541. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.P.; Remediakis, I.N.; Bengaard, H.; Nørskov, J.K. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar] [CrossRef]
- Butova, V.V.; Polyakov, V.A.; Erofeeva, E.A.; Rusalev, Y.V.; Gritsai, M.A.; Ozhogin, I.V.; Borodkin, G.S.; Kirsanova, D.Y.; Gadzhimagomedova, Z.M.; Guda, A.A.; et al. Cobalt nanoparticles embedded in porous N-doped carbon support as a superior catalyst for the p-nitrophenol reduction. Appl. Surf. Sci. 2022, 592, 153292. [Google Scholar] [CrossRef]
- Hasan, Z.; Cho, D.W.; Chon, C.M.; Yoon, K.; Song, H. Reduction of p-nitrophenol by magnetic Co-carbon composites derived from metal organic frameworks. Chem. Eng. J. 2016, 298, 183–190. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Priya, V.S.; Balakumar, V.; Prabavathi, S.L.; Muthuraj, V. Noble metal nanoparticles (Mx = Ag, Au, Pd) decorated graphitic carbon nitride nanosheets for ultrafast catalytic reduction of anthropogenic pollutant, 4-nitrophenol. Environ. Res. 2022, 212, 113185. [Google Scholar] [CrossRef] [PubMed]
- Mejía, Y.R.; Bogireddy, N.K.R. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef] [PubMed]
- Aislabie, J.; Lloyd-Jones, G. A review of bacterial degradation of pesticides. Aust. J. Soil Res. 1995, 33, 925–942. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Areo, F.I. Removal of phenol and 4-nitrophenol from wastewater using a composite prepared from clay and Cocos nucifera shell: Kinetic, equilibrium and thermodynamic studies. Resour. Environ. Sustain. 2021, 3, 100020. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, X.; Sheng, Y.; Chen, X.; Wang, X.; Liang, C. Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol. Chin. J. Chem. Eng. 2023, 62, 46–56. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Geng, P.; Li, Q. Recent Advances in Selective Hydrogenation of Cinnamaldehyde over Supported Metal-Based Catalysts. ACS Catal. 2020, 10, 2395–2412. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Prakash, M.G.; Elangovan, T.; Viswanathan, B. Selective hydrogenation of cinnamaldehyde over cobalt supported on alumina, silica and titania. Catal. Lett. 2012, 142, 87–94. [Google Scholar]
- Liu, B.; Lu, L.; Cai, T.; Iwatani, K. Selective hydrogenation of cinnamaldehyde over Raney cobalt catalysts modified with salts of heteropolyacids. Appl. Catal. A Gen. 1999, 180, 105–111. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Yang, X.; Tao, Z.; Ren, X.; Lv, B. The role of surface NH groups on the selective hydrogenation of cinnamaldehyde over Co/BN catalysts. Appl. Surf. Sci. 2019, 492, 736–745. [Google Scholar] [CrossRef]
- Cheng, S.; Lu, S.; Liu, X.; Li, G.; Al, S. Enhanced Activity of Alkali-Treated ZSM-5 Zeolite-Supported. Molecules 2023, 28, 1730. [Google Scholar] [CrossRef]
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Zhang, L.; Du, R.; Dai, Y.; Tan, Z. Preparation of 2,3-dialdehyde microcrystalline cellulose particles crosslinked with ε-poly-L-lysine and their antibacterial activity. Cellulose 2021, 28, 2833–2847. [Google Scholar] [CrossRef]
- Lindh, J.; Carlsson, D.O.; Strømme, M.; Mihranyan, A. Convenient one-pot formation of 2,3-dialdehyde cellulose beads via periodate oxidation of cellulose in water. Biomacromolecules 2014, 15, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Azzam, F.; Galliot, M.; Putaux, J.L.; Heux, L.; Jean, B. Surface peeling of cellulose nanocrystals resulting from periodate oxidation and reductive amination with water-soluble polymers. Cellulose 2015, 22, 3701–3714. [Google Scholar] [CrossRef]
- El Nokab, M.E.H.; Habib, M.H.; Alassmy, Y.A.; Abduljawad, M.M.; Alshamrani, K.M.; Sebakhy, K.O. Solid State NMR a Powerful Technique for Investigating Sustainable/Renewable Cellulose-Based Materials. Polymers 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, S.; Hietala, S.; Hummel, M.; Maunu, S.L.; Sixta, H. Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydr. Polym. 2019, 207, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Leguy, J.; Nishiyama, Y.; Jean, B.; Heux, L. Ultrastructural Characterization of the Core-Shell Structure of a Wide Range of Periodate-Oxidized Cellulose from Different Native Sources by Solid-State 13 C CP-MAS NMR. ACS Sustain. Chem. Eng. 2019, 7, 412–420. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; Heux, L.; Sbirrazzuoli, N. Partial periodate oxidation and thermal cross-linking for the processing ofthermosetall-cellulose composites. Compos. Sci. Technol. 2015, 117, 54–61. [Google Scholar] [CrossRef]
- Guigo, N.; Mazeau, K.; Putaux, J.L.; Heux, L. Surface modification of cellulose microfibrils by periodate oxidation and subsequent reductive amination with benzylamine: A topochemical study. Cellulose 2014, 21, 4119–4133. [Google Scholar] [CrossRef]
- Arthur, J.L.; Min, K.S.; Miller, J.S. Molecular tristability of CoII(salen)-based (salen = N,N’-ethylene-bis(salicylideniminato) compounds. J. Magn. Magn. Mater. 2019, 489, 165375. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.; Saha, B.; Qadir, M.; Gafur, M.; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Q.; Wu, C.H.; Zhu, H.; Mendoza-Garcia, A.; Shen, B.; Guo, J.; Sun, S. Stable Cobalt Nanoparticles and Their Monolayer Array as an Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 7071–7074. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Zheng, R.K.; Zhang, X.X.; Xiao, J.Q. A Simple Method to Prepare Uniform Co Nanoparticles. IEEE Trans. Magn. 2003, 39, 2764–2766. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2019, 135, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, C.; Su, P.; Shen, Z.; Liu, H.; Wang, G.; Liu, S.; Liu, J. Metal-organic-framework-derived formation of Co–N-doped carbon materials for efficient oxygen reduction reaction. J. Energy Chem. 2020, 40, 137–143. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Wang, X.W.; Wu, K.L.; Yue, Y.X.; Zhao, M.L.; Cheng, J.; Ming, J.; Yu, C.J.; Wei, X.W. Co0.85Se bundle-like nanostructure catalysts for hydrogenation of 4-nitrophenol to 4-aminophenol. New J. Chem. 2014, 38, 6147–6151. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P.; Bergeret, G.; Gallezot, P.; Size, P.; Measurements, D. Particle Size and Dispersion Measurements. Handb. Heretogeneous Catal. 2 Wiley-VCH 2008, 38, 738–765. [Google Scholar]
- Wu, G.; Lu, C.; Wu, X.; Zhang, S.; He, F.; Ling, L. X-ray photoelectron spectroscopy investigation into thermal degradation and stabilization of polyacrylonitrile fibers. J. Appl. Polym. Sci. 2004, 94, 1705–1709. [Google Scholar] [CrossRef]
- Davydov, V.; Rakhmanina, A.; Kireev, I.; Alieva, I.; Zhironkina, O.; Strelkova, O.; Dianova, V.; Samani, T.D.; Mireles, K.; Yahia, L.H.; et al. Solid state synthesis of carbon-encapsulated iron carbide nanoparticles and their interaction with living cells. J. Mater. Chem. B 2014, 2, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zeng, Y.; Ma, X.; Wang, H.; Gao, L.; Zhong, H.; Meng, Q. Cobalt nanoparticles embedded into N-doped carbon from metal organic frameworks as highly active electrocatalyst for oxygen evolution reaction. Polymers 2019, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.C.; Venditti, R.; Pawlak, J.; Gilbert, R.; Zauscher, S.; Kadla, J.F. Chemical force microscopy of cellulosic fibers. Carbohydr. Polym. 2005, 62, 369–378. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Hu, Y.; Guo, R. Mesoporous C, N-codoped TiO2 hybrid shells with enhanced visible light photocatalytic performance. RSC Adv. 2017, 7, 15513–15520. [Google Scholar] [CrossRef]
- He, H.; Hu, Y.; Chen, S.; Zhuang, L.; Ma, B.; Wu, Q. Preparation and Properties of A Hyperbranch-Structured Polyamine adsorbent for Carbon Dioxide Capture. Sci. Rep. 2017, 7, 3913. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Zhao, P.; Zheng, J.; Yang, M.; Huo, D.; Hou, C. ZIF-67 MOF-derived Co nanoparticles supported on N-doped carbon skeletons for the amperometric determination of hydrogen peroxide. Microchim. Acta 2021, 188, 1–10. [Google Scholar] [CrossRef]
- Mondal, A.; Mondal, A.; Adhikary, B.; Mukherjee, D.K. Cobalt nanoparticles as reusable catalysts for reduction of 4-nitrophenol under mild conditions. Bull. Mater. Sci. 2017, 40, 321–328. [Google Scholar] [CrossRef]
- Ma, H.; Wang, H.; Wu, T.; Na, C. Highly active layered double hydroxide-derived cobalt nano-catalysts for p-nitrophenol reduction. Appl. Catal. B Environ. 2016, 180, 471–479. [Google Scholar] [CrossRef]
- Chen, F.; Xi, P.; Ma, C.; Shao, C.; Wang, J.; Wang, S.; Liu, G.; Zeng, Z. In situ preparation, characterization, magnetic and catalytic studies of surfactant free RGO/FexCo100-x nanocomposites. Dalt. Trans. 2013, 42, 7936–7942. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Ozay, H.; Ozay, O.; Aktas, N. A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols. Appl. Catal. B Environ. 2010, 101, 137–143. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, Z.; Li, Y.; Wang, F.; Zhong, H.; Chen, Q. Synthesis of novel two-phase Co@SiO2 nanorattles with high catalytic activity. Inorg. Chem. 2014, 53, 9073–9079. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Srivastava, S.K.; Singh, S.B. Synthesis, magnetic properties and catalytic activity of hierarchical cobalt microflowers. J. Nanosci. Nanotechnol. 2012, 12, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Long, J.; Zhang, W.; Liu, X.; Wei, D. MOFs-Derived Co@CN bi-functional catalysts for selective transfer hydrogenation of α,β-unsaturated aldehydes without use of base additives. Mater. Chem. Front. 2017, 1, 2005–2012. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.B.; Xu, L.Y.; Zhang, Y.J.; Qin, Y.H.; Liang, C.F. Selective hydrogenation of cinnamaldehyde over ZSM-5 supported Co catalysts. React. Kinet. Mech. Catal. 2013, 110, 207–214. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Jiang, L.; Cheng, Y.; Liu, Y.; Wei, Z. Highly Efficient Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over CoRe/TiO2 Catalyst. Molecules 2023, 28, 3336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Li, C.; Armbrüster, M.; Peng, Z.; Liang, C. Cobalt Silicides Nanoparticles Embedded in N-Doped Carbon as Highly Efficient Catalyst in Selective Hydrogenation of Cinnamaldehyde. ChemistrySelect 2018, 3, 1658–1666. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Gogoi, P.; Chilukuri, S.V. Active and Recyclable Gold Metal Nanoparticles Catalyst Supported on Nitrogen-Doped Mesoporous Carbon for Chemoselective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol. Chem. Asian J. 2021, 16, 2702–2722. [Google Scholar] [CrossRef]
- Liu, H.; Mei, Q.; Li, S.; Yang, Y.; Wang, Y.; Liu, H.; Zheng, L.; An, P.; Zhang, J.; Han, B. Selective hydrogenation of unsaturated aldehydes over Pt nanoparticles promoted by the cooperation of steric and electronic effects. Chem. Commun. 2018, 54, 908–911. [Google Scholar] [CrossRef]
- Liu, C.; Abroshan, H.; Yan, C.; Li, G.; Haruta, M. One-Pot Synthesis of Au11(PPh2Py)7Br3 for the Highly Chemoselective Hydrogenation of Nitrobenzaldehyde. ACS Catal. 2016, 6, 92–99. [Google Scholar] [CrossRef]
- Castelbou, J.L.; Szeto, K.C.; Barakat, W.; Merle, N.; Godard, C.; Taoufik, M.; Claver, C. A new approach for the preparation of well-defined Rh and Pt nanoparticles stabilized by phosphine-functionalized silica for selective hydrogenation reactions. Chem. Commun. 2017, 53, 3261–3264. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, A.; Habibi, Y.; Kaddami, H.; Dufresne, A. Physico-chemical characterization of palm from Phoenix Dactylifera-L, preparation of cellulose whiskers and natural rubber-based nanocomposites. J. Biobased Mater. Bioenergy 2009, 3, 81–90. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Achterweust, M.; Janssen, H.G.; Westphal, Y.; Schols, H.A. Periodate oxidation of plant polysaccharides provides polysaccharide-specific oligosaccharides. Carbohydr. Polym. 2022, 291, 119540. [Google Scholar] [CrossRef] [PubMed]
- Pandeirada, C.O.; Hageman, J.A.; Janssen, H.G.Y.; Westphal, Y.; Schols, H.A. Identification of plant polysaccharides by MALDI-TOF MS fingerprinting after periodate oxidation and thermal hydrolysis. Carbohydr. Polym. 2022, 292, 119685. [Google Scholar] [CrossRef] [PubMed]
- She, Q.; Li, J.; Lu, Y.; Lin, S.; You, R. In situ synthesis of silver nanoparticles on dialdehyde cellulose as reliable SERS substrate. Cellulose 2021, 28, 10827–10840. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, Q.; Huo, D.; Hou, C.; Zhou, J.; Luo, H.; Yang, M. New Application of Old Methods: Development of Colorimetric Sensor Array Based on Tollen’s Reagent for the Discrimination of Aldehydes Based on Tollen’s Reagent. Analytica Chim. Acta 2020, 1096, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
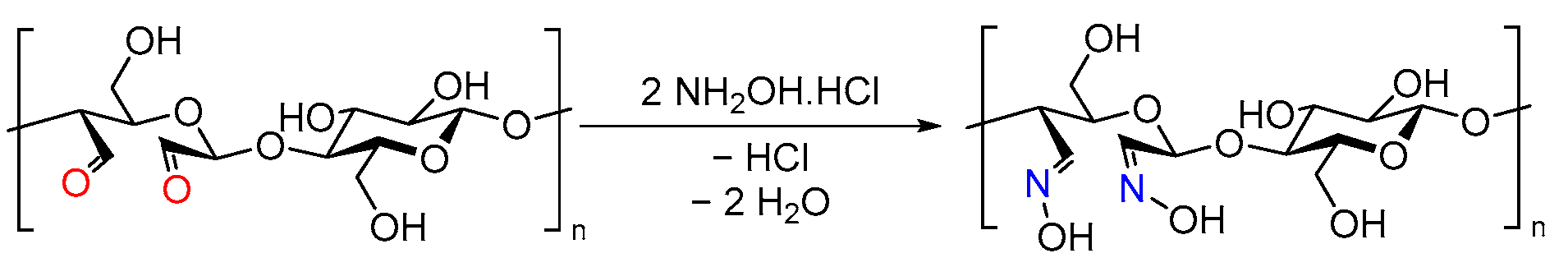


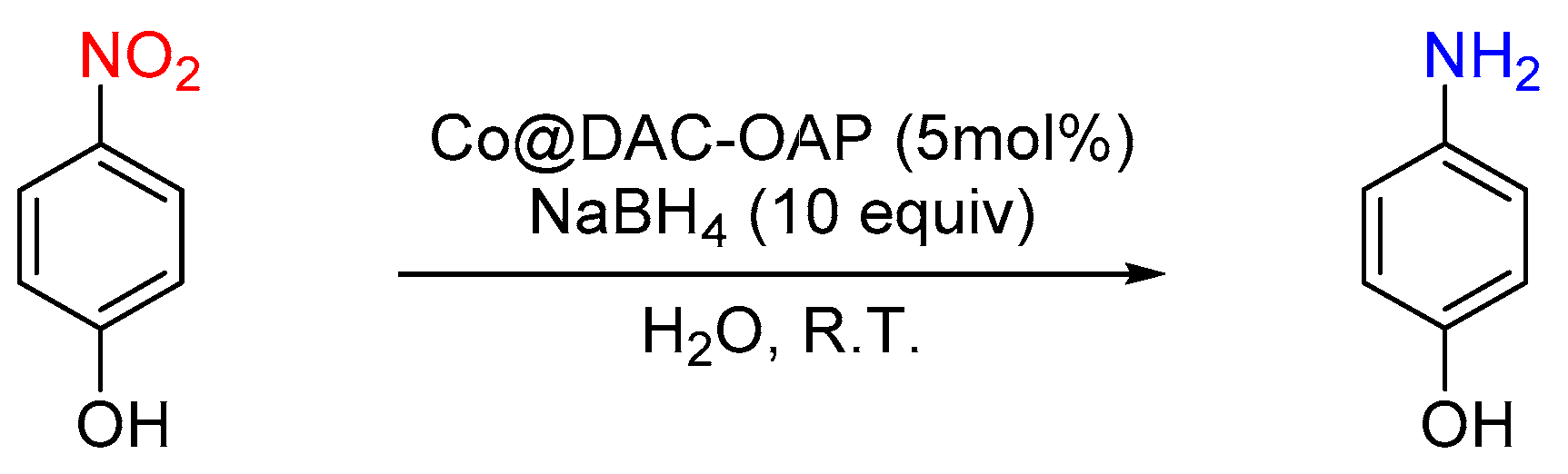
| Entry | Co:CAL | Substrate | Tem. (°C) | P(H2) (bar) | Time (h) | Conv. (%) | Sel. (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| COL | HCAL | HCOL | |||||||
| 1 | 0:100 | CAL | 120 | 5 | 7 h | 4.5 | 48.9 | 51.1 | 0 |
| 2 | 1:100 | CAL | 120 | 3 | 7 h | 50.3 | 74 | 9.16 | 16.5 |
| 2 * | 1:100 | CAL | 120 | 3 | 7 h | 49 | 90.6 | Traces | 9.3 |
| 3 | 1:100 | CAL | 120 | 5 | 7 h | 96 | 81.5 | 6.5 | 12 |
| 4 | 1:100 | CAL | 120 | 5 | 5 h | 70 | 71 | 14 | 15 |
| 5 | 1:100 | CAL | 120 | 5 | 3 h | 36 | 58 | 30 | 12 |
| 6 | 1:100 | CAL | 120 | 10 | 5 h | 80.8 | 65.6 | 24.8 | 9.6 |
| 7 | 1:100 | CAL | 120 | 20 | 5 h | 99 | 44.3 | 12.4 | 43.3 |
| 8 | 1:100 | CAL | 140 | 10 | 3 h | 48.8 | 27.6 | 54 | 18.4 |
| 9 | 1:100 | CAL | 140 | 10 | 5 h | 94.9 | 53.6 | 10.4 | 36 |
| 10 | 1:100 | CAL | 140 | 10 | 7 h | 100 | 24 | 0 | 76 |
| 11 | 1:100 | CAL | 60 | 5 | 5 h | <5 | 0 | 100 | 0 |
| 12 | 1:100 | CAL | 80 | 5 | 5 h | 17% | 12 | 83 | 5 |
| 13 | 1:100 | CAL | 100 | 5 | 5 h | 48 | 44 | 16 | 40 |
| 14 | 1:100 | CAL | 140 | 5 | 5 h | 89 | 78.6 | 11 | 10.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitbella, H.; Belachemi, L.; Merle, N.; Zinck, P.; Kaddami, H. Schiff Base Functionalized Cellulose: Towards Strong Support-Cobalt Nanoparticles Interactions for High Catalytic Performances. Molecules 2024, 29, 1734. https://doi.org/10.3390/molecules29081734
Aitbella H, Belachemi L, Merle N, Zinck P, Kaddami H. Schiff Base Functionalized Cellulose: Towards Strong Support-Cobalt Nanoparticles Interactions for High Catalytic Performances. Molecules. 2024; 29(8):1734. https://doi.org/10.3390/molecules29081734
Chicago/Turabian StyleAitbella, Hicham, Larbi Belachemi, Nicolas Merle, Philippe Zinck, and Hamid Kaddami. 2024. "Schiff Base Functionalized Cellulose: Towards Strong Support-Cobalt Nanoparticles Interactions for High Catalytic Performances" Molecules 29, no. 8: 1734. https://doi.org/10.3390/molecules29081734
APA StyleAitbella, H., Belachemi, L., Merle, N., Zinck, P., & Kaddami, H. (2024). Schiff Base Functionalized Cellulose: Towards Strong Support-Cobalt Nanoparticles Interactions for High Catalytic Performances. Molecules, 29(8), 1734. https://doi.org/10.3390/molecules29081734