DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandelli, D.; Kozlov, Y.N.; da Silva, C.A.R.; Carvalho, W.A.; Pescarmona, P.P.; Cella, D.d.A.; de Paiva, P.T.; Shul’pin, G.B. Oxidation of olefins with H2O2 catalyzed by gallium(III) nitrate and aluminum(III) nitrate in solution. J. Mol. Catal. A Chem. 2016, 422, 216–220. [Google Scholar] [CrossRef]
- Santos, I.C.M.S.; Gamelas, J.A.F.; Duarte, T.A.G.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Cavaleiro, A.M.V. Catalytic homogeneous oxidation of monoterpenes and cyclooctene with hydrogen peroxide in the presence of sandwich-type tungstophosphates [M4(H2O)2(PW9O34)2]n−, M = CoII, MnII and FeIII. J. Mol. Catal. A Chem. 2017, 426, 593–599. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Al-Jaloudi, R.; Abdullah, M.A.; Abughoush, M. Characterization of Olive Oil Volatile Compounds after Elution through Selected Bleaching Materials-Gas Chromatography-Mass Spectrometry Analysis. Molecules 2023, 28, 6444. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of Biogas Production from Orange Peel Waste by Leaching of Limonene. BioMed Res. Int. 2015, 2015, 494182. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska-Mizera, M.; Knitter, M.; Szymanowska, D.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Optical, mechanical, and antimicrobial properties of bio-based composites of poly(L-lactic acid) and D-limonene/β-cyclodextrin inclusion complex. J. App. Polym. Sci. 2022, 139, 52177. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Piss, M.; Szymanowska, D.; Mallardo, S.; Santagata, G.; Di Lorenzo, M.L. Linear low-density polyethylene modified with d-limonene/β-cyclodextrin inclusion complex: Antimicrobial composite for active food packaging. Polym. Eng. Sci. 2024, 64, 52–61. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Pais, R.; Simplício, A.L.; Mateus, M.; Duarte, C.M.M. Improvement of Aroma and Shelf-Life of Non-alcoholic Beverages Through Cyclodextrins-Limonene Inclusion Complexes. Food Bioprocess Technol. 2017, 10, 1297–1309. [Google Scholar] [CrossRef]
- Davaritouchaee, M.; Mosleh, I.; Dadmohammadi, Y.; Abbaspourrad, A. One-Step Oxidation of Orange Peel Waste to Carbon Feedstock for Bacterial Production of Polyhydroxybutyrate. Polymers 2023, 15, 697. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Stok, J.E.; Bell, S.G.; De Voss, J.J. Cymredoxin, a [2Fe-2S] ferredoxin, supports catalytic activity of the p-cymene oxidising P450 enzyme CYP108N12. Arch. Biochem. Biophys. 2023, 737, 109549. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Ji, G.; Li, A. Refinement of limonene epoxides from the light distillate of tire pyrolysis oil via catalytic epoxidation. Sep. Purif. Technol. 2023, 319, 124068. [Google Scholar] [CrossRef]
- Makrygenni, O.; Vanmairis, L.; Taourit, S.; Launay, F.; Shum Cheong Sing, A.; Proust, A.; Gérard, H.; Villanneau, R. Selective Formation of Epoxylimonene Catalyzed by Phosphonyl/Arsonyl Derivatives of Trivacant Polyoxotungstates at Low Temperature. Eur. J. Inorg. Chem. 2020, 2020, 605–612. [Google Scholar] [CrossRef]
- Kern, S.; Granier, T.; Dkhil, H.; Haupt, T.; Ellis, G.; Natsch, A. Stability of limonene and monitoring of a hydroperoxide in fragranced products. Flavour Fragr. J. 2014, 29, 277–286. [Google Scholar] [CrossRef]
- Christensson, J.B.; Johansson, S.; Hagvall, L.; Jonsson, C.; Börje, A.; Karlberg, A.T. Limonene hydroperoxide analogues differ in allergenic activity. Contact Dermat. 2008, 59, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kaade, W.; Méndez-Sánchez, C.; Güell, C.; De Lamo-Castellví, S.; Mestres, M.; Ferrando, M. Complexed Biopolymer of Whey Protein and Carboxymethyl Cellulose to Enhance the Chemical Stability of Lemon Oil-in-Water Emulsions. ACS Food Sci. Technol. 2022, 2, 41–48. [Google Scholar] [CrossRef]
- Francisco-Márquez, M.; Galano, A. Limonene: A scented and versatile tropospheric free radical deactivator. Int. J. Quantum Chem. 2023, 123, e27103. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Liang, X. Electrospun Polyvinyl Alcohol/d-Limonene Fibers Prepared by Ultrasonic Processing for Antibacterial Active Packaging Material. Molecules 2019, 24, 767. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Pant, A.; Pandey, R.K. Limonene Attenuates Oxidative Stress and Extends Longevity in Caenorhabditis elegans. Curr. Sci. 2019, 116, 959–965. [Google Scholar] [CrossRef]
- Sales, A.; Pastore, G.M.; Bicas, J.L. Optimization of limonene biotransformation to limonene-1,2-diol by Colletotrichum nymphaeae CBMAI 0864. Process Biochem. 2019, 86, 25–31. [Google Scholar] [CrossRef]
- De Oliveira, M.P.; Delolo, F.G.; Villarreal, J.A.A.; dos Santos, E.N.; Gusevskaya, E.V. Hydroformylation and one-pot hydroformylation/epoxy ring cleavage of limonene oxide: A sustainable access to biomass-based multi-functional fragrances. Appl. Catal. A Gen. 2021, 616, 118082. [Google Scholar] [CrossRef]
- Aissou, M.; Chemat-Djenni, Z.; Yara-Varón, E.; Fabiano-Tixier, A.-S.; Chemat, F. Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds. Comptes Rendus Chim. 2017, 20, 346–358. [Google Scholar] [CrossRef]
- Claudino, M.; Mathevet, J.-M.; Jonsson, M.; Johansson, M. Bringing d-limonene to the scene of bio-based thermoset coatings via free-radical thiol–ene chemistry: Macromonomer synthesis, UV-curing and thermo-mechanical characterization. Polym. Chem. 2014, 5, 3245–3260. [Google Scholar] [CrossRef]
- Hauenstein, O.; Reiter, M.; Agarwal, S.; Rieger, B.; Greiner, A. Bio-based polycarbonate from limonene oxide and CO2 with high molecular weight, excellent thermal resistance, hardness and transparency. Green Chem. 2016, 18, 760–770. [Google Scholar] [CrossRef]
- Li, C.; Sablong, R.J.; van Benthem, R.A.T.M.; Koning, C.E. Unique Base-Initiated Depolymerization of Limonene-Derived Polycarbonates. ACS Macro Lett. 2017, 6, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.C.; Silvino, C.A. A Brief Review of the Use of Limonene Derivative as a Promising Monomer in the Synthesis of Biodegradable Polymers. Rev. Virtual Química 2021, 13, 1017–1041. [Google Scholar] [CrossRef]
- Nunes, M.S.; Gomes, D.M.; Gomes, A.C.; Neves, P.; Mendes, R.F.; Paz, F.A.A.; Lopes, A.D.; Valente, A.A.; Gonçalves, I.S.; Pillinger, M. A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins. Catalysts 2021, 11, 1407. [Google Scholar] [CrossRef]
- Kuznetsova, G.M.; Lobanova, T.V.; Rusina, I.F.; Kasaikina, O.T. Kinetic characteristics of initiated oxidation of limonene. Russ. Chem. Bull. 1996, 45, 1586–1591. [Google Scholar] [CrossRef]
- Wentzel, B.B.; Alsters, P.L.; Feiters, M.C.; Nolte, R.J.M. Mechanistic Studies on the Mukaiyama Epoxidation. J. Org. Chem. 2004, 69, 3453–3464. [Google Scholar] [CrossRef]
- Pena, A.; Veiga, S.; Sapelli, M.; Martínez, N.; Márquez, V.; Dellacassa, E.; Bussi, J. Limonene oxidation by molecular oxygen under solvent-free conditions: The influence of peroxides and catalysts on the reaction rate. React. Kinet. Mech. Catal. 2012, 107, 263–275. [Google Scholar] [CrossRef]
- Martinez, Q.H.; Amaya, Á.A.; Paez-Mozo, E.A.; Martinez, O.F.; Valange, S. Photo-assisted O-atom transfer to monoterpenes with molecular oxygen and a dioxoMo(VI) complex immobilized on TiO2 nanotubes. Catal. Today 2021, 375, 441–457. [Google Scholar] [CrossRef]
- Martínez, Q.H.; Paez-Mozo, E.A.; Martínez, O.F. Selective Photo-epoxidation of (R)-(+)- and (S)-(−)-Limonene by Chiral and Non-Chiral Dioxo-Mo(VI) Complexes Anchored on TiO2-Nanotubes. Top. Catal. 2021, 64, 36–50. [Google Scholar] [CrossRef]
- Martinez Quiñonez, H.; Amaya, Á.A.; Paez-Mozo, E.A.; Martinez Ortega, F. Aminothiazole Ligand-Type Dioxo-Mo(VI) Complex Anchored on TiO2 Nanotubes for Selective Oxidation of Monoterpenes with Light and O2. Top. Catal. 2022, 65, 1088–1101. [Google Scholar] [CrossRef]
- Naróg, D.; Szczepanik, A.; Sobkowiak, A. Iron(II, III)-Catalyzed Oxidation of Limonene by Dioxygen. Catal. Lett. 2008, 120, 320–325. [Google Scholar] [CrossRef]
- Szczepanik, A.; Sobkowiak, A. Manganese(II)-Induced Oxidation of Limonene by Dioxygen. Catal. Lett. 2008, 126, 261–267. [Google Scholar] [CrossRef]
- Liu, J.; Ji, X.; Wang, C.; Wang, L.; Jian, P. Beneficial Synergistic Intermetallic Effect in ZnCo2O4 for Enhancing the Limonene Oxidation Catalysis. Inorg. Chem. 2023, 62, 18750–18757. [Google Scholar] [CrossRef]
- Ciriminna, R.; Parrino, F.; De Pasquale, C.; Palmisano, L.; Pagliaro, M. Photocatalytic partial oxidation of limonene to 1,2 limonene oxide. Chem. Commun. 2018, 54, 1008–1011. [Google Scholar] [CrossRef]
- Wang, W.; Agustin, D.; Poli, R. Influence of ligand substitution on molybdenum catalysts with tridentate Schiff base ligands for the organic solvent-free oxidation of limonene using aqueous TBHP as oxidant. Mol. Catal. 2017, 443, 52–59. [Google Scholar] [CrossRef]
- Yuan, L.S.; Chandren, S.; Efendi, J.O.N.; Ho, C.S.; Nur, H. Hydrophobic effect of silica functionalized with silylated Ti-salicylaldimine complex on limonene oxidation by aqueous hydrogen peroxide. J. Chem. Sci. 2015, 127, 1905–1917. [Google Scholar] [CrossRef]
- Kala Raj, N.K.; Puranik, V.G.; Gopinathan, C.; Ramaswamy, A.V. Selective oxidation of limonene over sodium salt of cobalt containing sandwich-type polyoxotungstate [WCo3(H2O)2{W9CoO34}2]10−. Appl. Catal. A Gen. 2003, 256, 265–273. [Google Scholar] [CrossRef]
- Godhani, D.R.; Nakum, H.D.; Parmar, D.K.; Mehta, J.P.; Desai, N.C. Zeolite-Y immobilized Metallo-ligand complexes: A novel heterogenous catalysts for selective oxidation. Inorg. Chem. Commun. 2016, 72, 105–116. [Google Scholar] [CrossRef]
- Oliveira, P.; Machado, A.; Ramos, A.M.; Fonseca, I.M.; Braz Fernandes, F.M.; Botelho do Rego, A.M.; Vital, J. Anchoring manganese acetylacetonate complex on MCM-41: Catalytic testing on limonene oxidation. Catal. Commun. 2007, 8, 1366–1372. [Google Scholar] [CrossRef]
- Bonon, A.J.; Kozlov, Y.N.; Bahú, J.O.; Filho, R.M.; Mandelli, D.; Shul’pin, G.B. Limonene epoxidation with H2O2 promoted by Al2O3: Kinetic study, experimental design. J. Catal. 2014, 319, 71–86. [Google Scholar] [CrossRef]
- Michel, T.; Cokoja, M.; Sieber, V.; Kühn, F.E. Selective epoxidation of (+)-limonene employing methyltrioxorhenium as catalyst. J. Mol. Catal. A Chem. 2012, 358, 159–165. [Google Scholar] [CrossRef]
- Młodzik, J.; Wróblewska, A.; Makuch, E.; Wróbel, R.J.; Michalkiewicz, B. Fe/EuroPh catalysts for limonene oxidation to 1,2-epoxylimonene, its diol, carveol, carvone and perillyl alcohol. Catal. Today 2016, 268, 111–120. [Google Scholar] [CrossRef]
- Wróblewska, A.; Makuch, E.; Młodzik, J.; Koren, Z.C.; Michalkiewicz, B. Oxidation of limonene over molybdenum dioxide-containing nanoporous carbon catalysts as a simple effective method for the utilization of waste orange peels. React. Kinet. Mech. Catal. 2018, 125, 843–858. [Google Scholar] [CrossRef]
- Modi, C.K.; Gade, B.G.; Chudasama, J.A.; Parmar, D.K.; Nakum, H.D.; Patel, A.L. Synthesis, spectral investigation and catalytic aspects of entrapped VO(IV) and Cu(II) complexes into the supercages of zeolite-Y. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.K.; Butani, P.M.; Thumar, N.J.; Jasani, P.M.; Padaliya, R.V.; Sandhiya, P.R.; Nakum, H.D.; Khan, M.N.; Makwana, D. Oxy-functionalization of olefins with neat and heterogenized binuclear V(IV)O and Fe(II) complexes: Effect of steric hindrance on product selectivity and output in homogeneous and heterogeneous phase. Mol. Catal. 2019, 474, 110424. [Google Scholar] [CrossRef]
- Santos, J.S.D.; Faria, A.L.; Amorin, P.M.; Luna, F.M.L.; Caiado, K.L.; Silva, D.O.; Sartoratto, P.P.; Assis, M.D. Iron(III) Porphyrin Covalently Supported onto Magnetic Amino-Functionalized Nanospheres as Catalyst for Hydrocarbon and Herbicide Oxidations. J. Braz. Chem. Soc. 2012, 23, 1411–1420. [Google Scholar] [CrossRef]
- Madadi, M.; Rahimi, R. Zeolite-immobilized Mn(III), Fe(III) and Co(III) complexes with 5,10,15,20-tetra(4-methoxyphenyl)porphyrin as heterogeneous catalysts for the epoxidation of (R)-(+)-limonene: Synthesis, characterization and catalytic activity. React. Kinet. Mech. Catal. 2012, 107, 215–229. [Google Scholar] [CrossRef]
- Modi, C.K.; Chudasama, J.A.; Nakum, H.D.; Parmar, D.K.; Patel, A.L. Catalytic oxidation of limonene over zeolite-Y entrapped oxovanadium (IV) complexes as heterogeneous catalysts. J. Mol. Catal. A Chem. 2014, 395, 151–161. [Google Scholar] [CrossRef]
- Godhani, D.R.; Nakum, H.D.; Parmar, D.K.; Mehta, J.P.; Desai, N.C. Zeolite Y encaged Ru(III) and Fe(III) complexes for oxidation of styrene, cyclohexene, limonene, and α-pinene: An eye-catching impact of H2SO4 on product selectivity. J. Mol. Catal. A Chem. 2017, 426, 223–237. [Google Scholar] [CrossRef]
- Mehta, J.P.; Parmar, D.K.; Nakum, H.D.; Godhani, D.R.; Desai, N.C. Enhanced catalytic oxidation of monoterpenes by zeolite-Y entrapped iron complex: Spectral studies and mechanistic vision. J. Porous Mater. 2018, 25, 1649–1658. [Google Scholar] [CrossRef]
- Nunes, M.S.; Gomes, D.M.; Gomes, A.C.; Neves, P.; Mendes, R.F.; Paz, F.A.A.; Lopes, A.D.; Pillinger, M.; Valente, A.A.; Gonçalves, I.S. A Molybdenum(VI) Complex of 5-(2-pyridyl-1-oxide)tetrazole: Synthesis, Structure, and Transformation into a MoO3-Based Hybrid Catalyst for the Epoxidation of Bio-Olefins. Catalysts 2023, 13, 565. [Google Scholar]
- Gawarecka, A.; Wróblewska, A. Limonene oxidation over Ti-MCM-41 and Ti-MWW catalysts with t-butyl hydroperoxide as the oxidant. React. Kinet. Mech. Catal. 2018, 124, 523–543. [Google Scholar] [CrossRef]
- Becerra, J.-A.; González, L.-M.; Villa, A.-L. A bio-inspired heterogeneous catalyst for the transformation of limonene from orange peel waste biomass into value-added products. Catal.Today 2018, 302, 250–260. [Google Scholar] [CrossRef]
- Abrantes, M.; Bruno, S.M.; Tomé, C.; Pillinger, M.; Gonçalves, I.S.; Valente, A.A. Epoxidation of DL-limonene using an indenyl molybdenum(II) tricarbonyl complex as catalyst precursor. Catal. Commun. 2011, 15, 64–67. [Google Scholar] [CrossRef]
- Oliveira, P.; Machado, A.; Ramos, A.M.; Fonseca, I.; Fernandes, F.M.B.; Rego, A.M.B.d.; Vital, J. MCM-41 anchored manganese salen complexes as catalysts for limonene oxidation. Microporous Mesoporous Mater. 2009, 120, 432–440. [Google Scholar] [CrossRef]
- Oliveira, P.; Ramos, A.M.; Fonseca, I.; Botelho do Rego, A.; Vital, J. Oxidation of limonene over carbon anchored transition metal Schiff base complexes: Effect of the linking agent. Catal. Today 2005, 102–103, 67–77. [Google Scholar]
- De Fátima Teixeira Gomes, M.; Antunes, O.A.C. Oxidation of limonene catalyzed by MnIII(Salen)Cl-H2O. Catal. Lett. 1996, 38, 133–134. [Google Scholar] [CrossRef]
- Cubillos, J.; Vásquez, S.; Montes de Correa, C. Salen manganese (III) complexes as catalysts for R-(+)-limonene oxidation. Appl. Catal. A Gen. 2010, 373, 57–65. [Google Scholar] [CrossRef]
- Lima, L.F.; Corraza, M.L.; Cardozo-Filho, L.; Márquez-Alvarez, H.; Antunes, O.A.C. Oxidation of limonene catalyzed by Metal(Salen) complexes. Braz. J. Chem. Eng. 2006, 23, 83–92. [Google Scholar] [CrossRef][Green Version]
- Joseph, T.; Halligudi, S.B. Oxyfunctionalization of limonene using vanadium complex anchored on functionalized SBA-15. J. Mol. Catal. A Chem. 2005, 229, 241–247. [Google Scholar] [CrossRef]
- Gonçalves, J.A.; Bueno, A.C.; Gusevskaya, E.V. Palladium-catalyzed oxidation of monoterpenes: Highly selective syntheses of allylic ethers from limonene. J. Mol. Catal. A Chem. 2006, 252, 5–11. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LDJayaweera, S.; ADias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M.R. Synergistic Activity of Thymol with Commercial Antibiotics against Critical and High WHO Priority Pathogenic Bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.L.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, N.; Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Carvalho, P.J.; Russina, O.; Triolo, A.; Paccou, L.; Guinet, Y.; Hedoux, A.; et al. Non-Ideality in Thymol + Menthol Type V Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 2203–2211. [Google Scholar] [CrossRef]
- Bergua, F.; Castro, M.; Lafuente, C.; Artal, M. Thymol+l-menthol eutectic mixtures: Thermophysical properties and possible applications as decontaminants. J. Mol. Liq. 2022, 368, 120789. [Google Scholar] [CrossRef]
- Benito, C.; Alcalde, R.; Atilhan, M.; Aparicio, S. High-pressure properties of type V Natural Deep Eutectic Solvents: The case of menthol: Thymol. J. Mol. Liq. 2023, 376, 121398. [Google Scholar] [CrossRef]
- Kamatou GP, P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Di Cesare Mannelli, L.; Mazzanti, G.; Bartolini, A.; Ghelardini, C. Menthol: A natural analgesic compound. Neurosci. Lett. 2002, 322, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Suzuki, N.; Tsugami, Y.; Nii, T.; Kobayashi, K.; Isobe, N. Menthol application on healthy and inflamed goat udders changes antimicrobial components in milk. Anim. Sci. J. 2023, 94, e13832. [Google Scholar] [CrossRef] [PubMed]
- Peel, J.; John, K.; Page, J.; Jeffries, O.; Heffernan, S.M.; Tallent, J.; Waldron, M. Topical application of isolated menthol and combined menthol-capsaicin creams: Exercise tolerance, thermal perception, pain, attentional focus and thermoregulation in the heat. Eur. J. Sport Sci. 2023, 23, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M.; Ringer, K.L.; McConkey, M.E.; Croteau, R. Monoterpene metabolism. Cloning, expression, and characterization of menthone reductases from peppermint. Plant Physiol. 2005, 137, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Rydel-Ciszek, K.; Pacześniak, T.; Chmielarz, P.; Sobkowiak, A. Bio-Inspired Iron Pentadentate Complexes as Dioxygen Activators in the Oxidation of Cyclohexene and Limonene. Molecules 2023, 28, 2240. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Molecular Orbital Theory. In Molecular Orbitals and Organic Chemical Reactions; Wiley, John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–57. [Google Scholar]
- Ahmed, L.; Omer, R.; Qader, I.; Koparir, P. Theoretical Analysis of the Reactivity of Carmustine and Lomustine Drugs. J. Phys. Chem. Funct. Mater. 2022, 5, 84–96. [Google Scholar]
- Eryilmaz, S.; Gul, M.; Inkaya, E. Investigation of global reactivity descriptors of some perillaldehyde derivatives in different solvents by DFT method. Indian J. Chem. Technol. 2019, 26, 235–238. [Google Scholar]
- Mali, S.N.; Anand, A.; Zaki, M.E.A.; Al-Hussain, S.A.; Jawarkar, R.D.; Pandey, A.; Kuznetsov, A. Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives. Molecules 2023, 28, 2801. [Google Scholar] [CrossRef]
- Boukabcha, N.; Benmohammed, A.; Belhachemi, M.H.M.; Goudjil, M.; Yahiaoui, S.; Megrouss, Y.; Djafri, A.; Khelloul, N.; Benyehlou, Z.D.; Djafri, A.; et al. Spectral investigation, TD-DFT study, Hirshfeld surface analysis, NCI-RDG, HOMO-LUMO, chemical reactivity and NLO properties of 1-(4-fluorobenzyl)-5-bromolindolin-2,3-dione. J. Mol. Struct. 2023, 1285, 135492. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 1986, 83, 8440–8441. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Absolute electronegativity and hardness: Applications to organic chemistry. J. Org. Chem. 1989, 54, 1423–1430. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Roy, D.R.; Chattaraj, P.K. Electrophilicity index as a possible descriptor of biological activity. Bioorganic Med. Chem. 2004, 12, 5533–5543. [Google Scholar] [CrossRef] [PubMed]
- Zaklika, J.; Hładyszowski, J.; Ordon, P. From the Electron Density Gradient to the Quantitative Reactivity Indicators: Local Softness and the Fukui Function. ACS Omega 2022, 7, 7745–7758. [Google Scholar] [CrossRef]
- Omer, R.; Koparir, P.; Qader, I.N.; Ahmed, L. Structure reactivity analysis for Phenylalanine and Tyrosine. Cumhur. Sci. J. 2021, 42, 576–585. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Buczynski, J.B.; Bell, S.G.; Stok, J.E.; De Voss, J.J. CYP108N14: A Monoterpene Monooxygenase from Rhodococcus globerulus. Arch. Biochem. Biophys. 2024, 752, 109852. [Google Scholar] [CrossRef] [PubMed]
- Neaţu, F.; Culică, G.; Florea, M.; Parvulescu, V.I.; Cavani, F. Synthesis of Terephthalic Acid by p-Cymene Oxidation using Oxygen: Toward a More Sustainable Production of Bio-Polyethylene Terephthalate. ChemSusChem 2016, 9, 3102–3112. [Google Scholar] [CrossRef]
- Jakaria, M.; Cho, D.Y.; Ezazul Haque, M.; Karthivashan, G. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxid. Med. Cell Longev. 2018, 2018, 1209801. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Q.; Shang, Z.; Liu, J.; Zhou, S.; Dang, J.; Liu, L.; Lange, I.; Srividya, N.; Lange, B.M.; et al. Functional Characterization and Structural Insights Into Stereoselectivity of Pulegone Reductase in Menthol Biosynthesis. Front. Plant Sci. 2021, 12, 780970. [Google Scholar] [CrossRef] [PubMed]
- Kani, İ.; Taşkınlar, İ.; Uzel, Z.; Avan, İ. Catalytic oxidation of thymol and carvacrol with Mn(II)-benzoylbenzoate-bipyridine complex. Polyhedron 2024, 249, 116772. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, version 16, revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Scuseria, G.E. Predicting Band Gaps with Hybrid Density Functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Moto Ongagna, J.; Tamafo Fouegue, A.D.; Ateba Amana, B.; Mouzong D’ambassa, G.; Zobo Mfomo, J.; Mbaze Meva’A, L.; Bikele Mama, D. B3LYP, M06 and B3PW91 DFT assignment of nd8 metal-bis-(N-heterocyclic carbene) complexes. J. Mol. Model. 2020, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Trung, N.Q.; Mechler, A.; Hoa, N.T.; Vo, Q.V. Calculating bond dissociation energies of X−H (X = C, N, O, S) bonds of aromatic systems via density functional theory: A detailed comparison of methods. R. Soc. Open Sci. 2022, 9, 220177. [Google Scholar] [CrossRef]
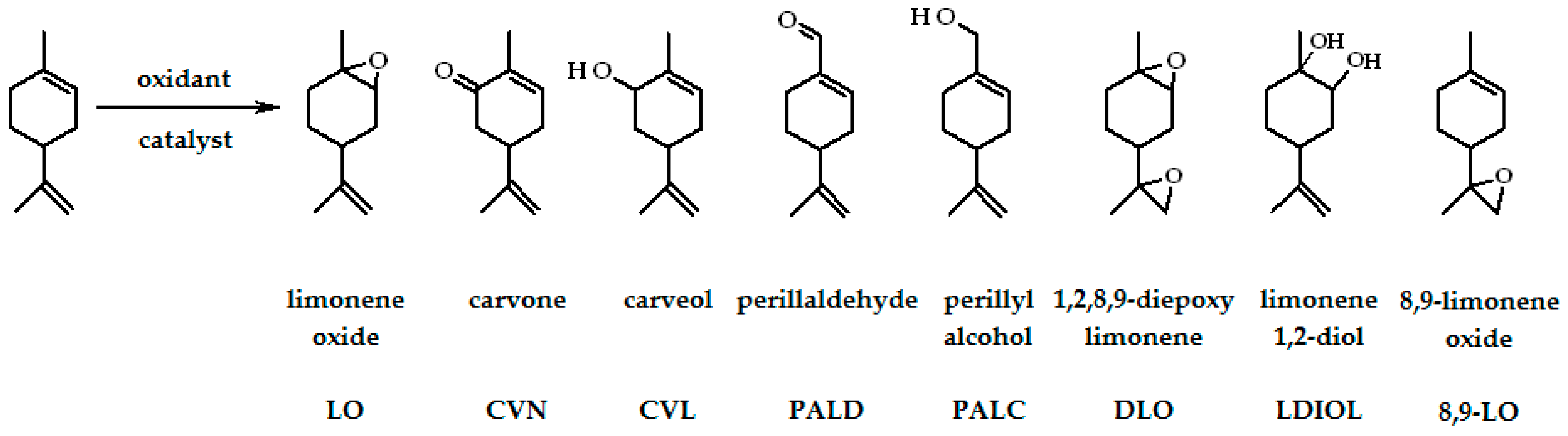

| Entry | Catalysts | Oxidants | Solvents | Oxidations Products | Ref. |
|---|---|---|---|---|---|
| 1 | MoCl2O2(Bipy)/TiO2-NT | O2/(λ = 360 nm) | MeCN | LO, DLO, CVN | [30] |
| 2 | MoO2(L1–L4)/TiO2-NT | O2/(λ = 360 nm) | MeCN | LO, DLO, CVN | [31,32] |
| 3 | CYP108N12 | O2 | Tris-HCl | PALC, PALD | [9] |
| 4 | [Fe(bpy)2]2+, [Fe(bpy)2]3+ | air, O2 | MeCN | LO, CVN, CVL, PALD | [33] |
| 5 | [Mn(bpy)2]2+ | air, O2 | MeCN | LO, CVN, CVL, PALD, PALC | [34] |
| 6 | TiO2-P25 | O2 | MeCN | LO | [36] |
| 7 | ZnCo2O4, isobutyraldehyde | O2 | MeCN | LO | [35] |
| 8 | Pd(OAc)2/PTSA/BQ, Na2PdCl4/PTSA/BQ | O2 | MeOH, ethanol, 2-Propanol | allylic ethers | [63] |
| 9 | NiAl-HT | O2 | Limonene | LO, CVN, CVL | [29] |
| 10 | [MoO2(SAP)]2, [MoO2(SATP)]2 | t-Bu-OOH | Limonene | LO, LDIOL | [37] |
| 11 | Ti:OTMS | H2O2 | Limonene | CVN, CVL, LO | [38] |
| 12 | Na10[Co5W19O70H4]·44H2O | air, H2O2 | MeCN, MeOH, acetone | LO, CVN, CVL | [39] |
| 13 | [M4(H2O)2(PW9O34)2]n−, M–CoII, MnII, FeIII | H2O2 | MeCN | LDIOL, CVN, CVL | [2] |
| 14 | [(C18H37)2N(CH3)2]3PW4O20, | H2O2 | Tire pyrolysis oil | LO, DLO, LDIOL | [10] |
| 15 | (nBu4N)3[NaHAsW9O33[P(O)R]2] (R = t-Bu or CH2CH2COOH) (n-Bu4N)3-[NaHPW9O34[As(O)p-C6H4NH2]2] | H2O2 | MeCN | LO, DLO, LDIOL | [11] |
| 16 | Co(II)-Y, Cu(II)-Y with Schiff base ligands | H2O2 | MeCN | CVN, CVL, LO, LDIOL | [40] |
| 17 | [Mn(acac)2APTS]@MCM-41 | H2O2 | Acetone–t-butanol | LO, CVL, CVN, polymer | [41] |
| 18 | Al2O3 | H2O2 | Ethyl acetate | LO, DLO, 8,9-LO | [42] |
| 19 | Ga(NO3)3, Al(NO3)3 | H2O2 | Ethyl acetate, THF | LO, DLO, LDIOL, 8,9-LO | [1] |
| 20 | MTO:L5-7 | H2O2 | CH2Cl2 | LO, 8,9-LO, DLO, CVL, CVN | [43] |
| 21 | carbon EuroPh with Fe | H2O2 | MeOH | PALC, CVL, CVN, LO, LDIOL | [44] |
| 22 | [VO(L8)H2O]-Y, [Cu(L8)H2O]-Y | H2O2 | MeCN | LDIOL, CVL, CVN, LO | [46] |
| 23 | [VO(sal2bz)]2, [VO(sal2bz)]2-Y [Fe(sal2bz)(H2O)2]2⋅2H2O, [Fe(sal2bz)(H2O)2]2-Y | H2O2 | MeCN | LDIOL, PALC, CVN, CVL | [47] |
| 24 | Mn(III)/Fe(III)/Co(III)/L9/Y/ammonium acetate | H2O2 | MeCN | LO, 8,9-LO | [49] |
| 25 | [FeII(L10)2(H2O)2]-Y | H2O2 | MeCN | CVN, CVL | [52] |
| 26 | RuY, FeY, 3Y–6Y | H2O2 | MeCN | CVN, CVL, LO, LDIOL | [51] |
| 27 | γ-Fe2O3/SiO2-NHFeP | m-CPBA, H2O2 | MeCN | LO, CVN, CVL | [48] |
| 28 | MoO2-EuroPh | H2O2, t-Bu-OOH | MeOH | CVN, CVL, LO, PALC | [45] |
| 29 | [VO(VFCH)2]-Y, [VO(VTCH)2]-Y, [VO(SFCH)·H2O]-Y, [VO(STCH)·H2O]-Y | H2O2, t-Bu-OOH | MeCN | LO, LDIOL, CVN, CVL | [50] |
| 30 | [MoO3(Hpto)]∙H2O | t-Bu-OOH | α,α,α- trifluorotoluene | LO, LDIOL, DLO | [53] |
| 31 | [MoO3(Hpytz)] | t-Bu-OOH | LO, LDIOL, DLO | [26] | |
| 32 | Ti-MCM-41, Ti-MWW | t-Bu-OOH | MeOH | LO, CVN, CVL, PALC | [54] |
| 33 | FePcCl16-NH2-SiO2 | t-Bu-OOH | Acetone | CVN, LO, CVL | [55] |
| 34 | cobalt(II)-(acac)-carbon-based catalysts | t-Bu-OOH | Acetone–t-butanol | LO, CVN, CVL, polymer | [58] |
| 35 | (η5-C9H7)Mo(CO)3Me | t-Bu-OOH | Decane, t-butanol | LO, DLO, LDIOL | [56] |
| 36 | MCM-41-Mn(4-OHsalen), MCM-41Mn(4-OHsalhd), MCM-41 Mn(4-OHsalophen) | t-Bu-OOH | Acetone–t-butanol | LO, CVN, CVL, polymer | [57] |
| 37 | Mn(III)-Jacobsen-type catalysts | KHSO5 | Acetone–H2O | DLO | [60] |
| 38 | Mn(Salen)Cl∙H2O | PhIO | CH2Cl2 | LO, CVN, PALD | [59] |
| 39 | M(Salen)Cl∙H2O M = MnII, NiII, CoII | PhIO, NaOCl | Acetone, MeCN, CH2Cl2, ethyl acetate | LO, CVN, CVL | [61] |
| 40 | VO(Salten)-SBA-15 | UHP | MeCN | LO, CVN, CVL, carvacrol | [62] |
| Gas | H2O | MeCN | MeOH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | EHOMO, [eV] | ELUMO, [eV] | Egap, [eV] | |
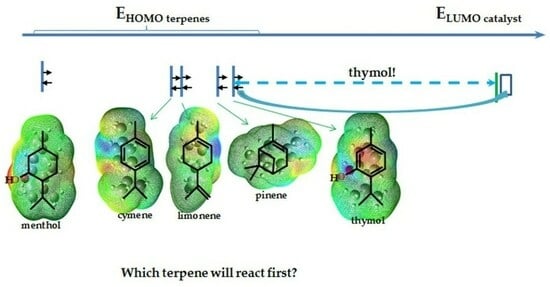
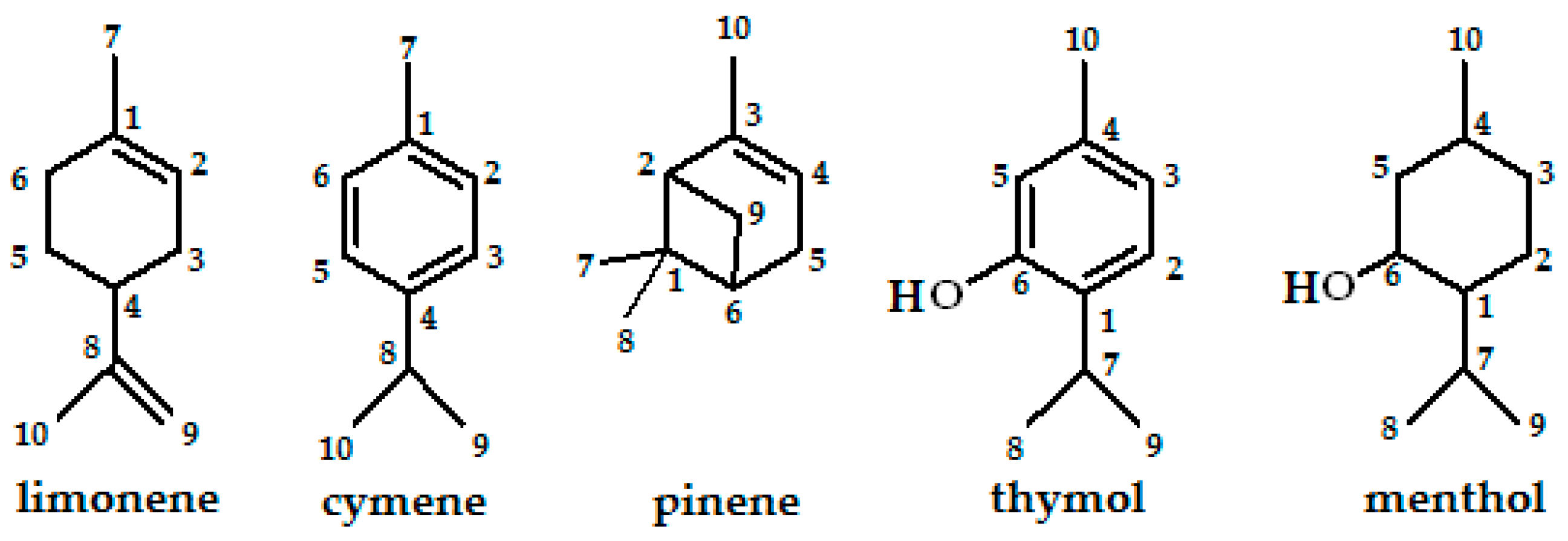
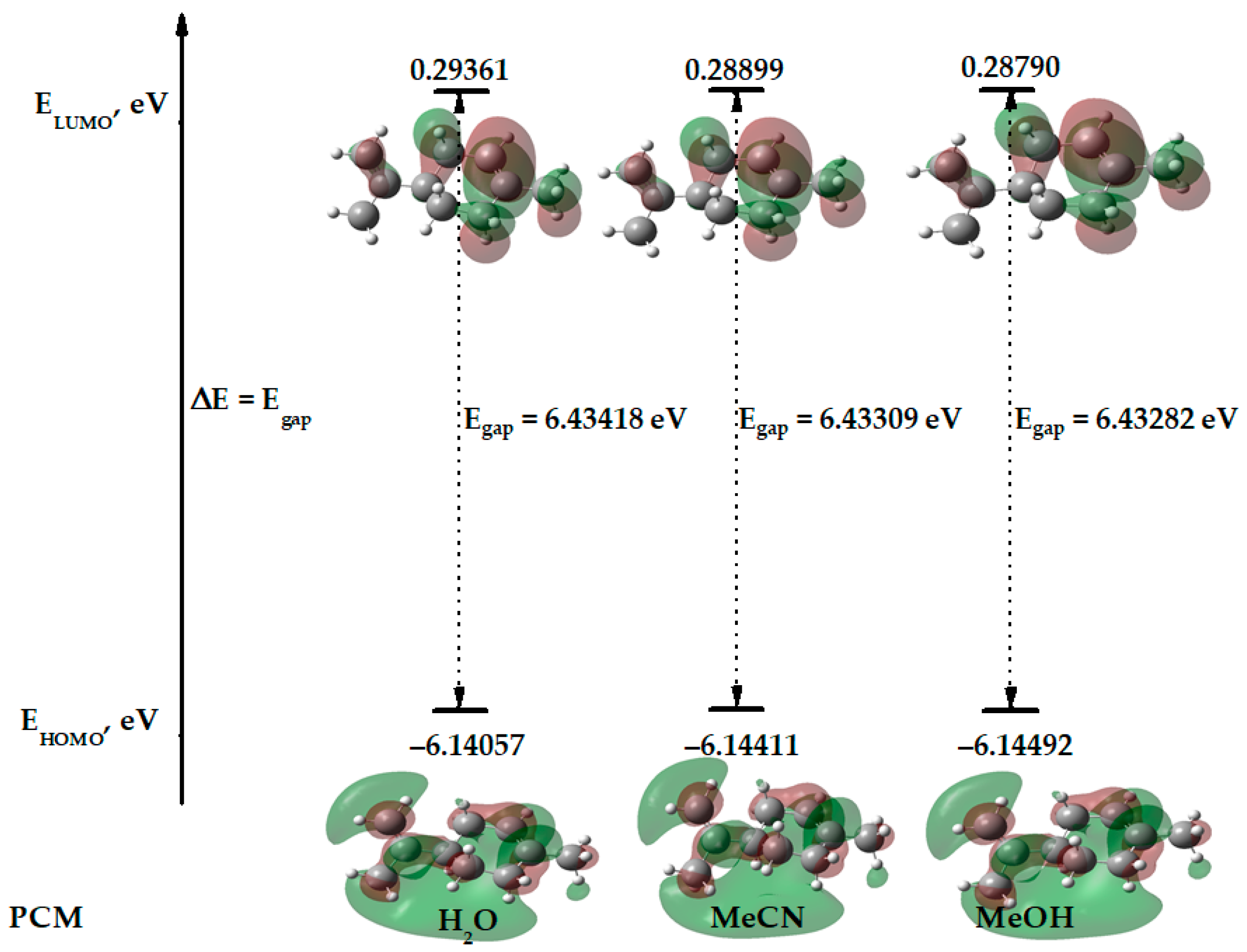
| limonene | −6.41867 | −0.02367 | 6.39500 | −6.14057 | 0.29361 | 6.43418 | −6.14411 | 0.28890 | 6.43309 | −6.14492 | 0.28790 | 6.43282 |
| cymene | −6.45105 | −0.32817 | 6.12288 | −6.22846 | −0.11647 | 6.11199 | −6.23010 | −0.11783 | 6.11227 | −6.23037 | −0.11810 | 6.11227 |
| pinene | −6.20724 | 0.02748 | 6.23472 | −5.91145 | 0.33198 | 6.24343 | −5.91553 | 0.32708 | 6.24261 | −5.91635 | 0.32627 | 6.24262 |
| thymol | −6.08669 | −0.39212 | 5.69457 | −5.83036 | −0.11211 | 5.71825 | −5.83362 | −0.11701 | 5.71661 | −5.84614 | −0.12789 | 5.71825 |
| menthol | −7.27230 | −0.03837 | 7.23393 | −7.03801 | 0.37089 | 7.40890 | −7.04209 | 0.363554 | 7.40564 | −7.04263 | 0.36219 | 7.40482 |
| Terpene | E0 [a.u.] | I [eV] | A [eV] | X [eV] | η [eV] | S [eV] | ω [eV] | μ [eV] | |
|---|---|---|---|---|---|---|---|---|---|
| limonene | −390.76016952 | 6.419 | 0.024 | 3.221 | 3.197 | 0.156 | 1.623 | −3.221 | |
| cymene | −389.59360262 | 6.451 | 0.328 | 3.390 | 3.061 | 0.163 | 1.876 | −3.390 | |
| pinene | −390.74018343 | 6.207 | −0.027 | 3.090 | 3.117 | 0.160 | 1.531 | −3.090 | |
| thymol | −464.83540277 | 6.087 | 0.392 | 3.239 | 2.847 | 0.176 | 1.843 | −3.239 | |
| menthol | −468.44884783 | 7.272 | 0.038 | 3.655 | 3.617 | 0.138 | 1.847 | −3.655 | |
| MeCN | limonene | −390.76198136 | 6.144 | −0.289 | 2.928 | 3.217 | 0.155 | 1.332 | −2.928 |
| cymene | −389.59597035 | 6.230 | 0.118 | 3.174 | 3.056 | 0.164 | 1.648 | −3.174 | |
| pinene | −390.74104612 | 5.916 | −0.327 | 2.794 | 3.121 | 0.160 | 1.251 | −2.794 | |
| thymol | −464.84156521 | 5.834 | 0.117 | 2.975 | 2.858 | 0.175 | 1.549 | −2.975 | |
| menthol | −468.45328899 | 7.042 | −0.364 | 3.339 | 3.703 | 0.135 | 1.506 | −3.339 | |
| H2O | limonene | −390.76203510 | 6.141 | −0.293 | 2.923 | 3.217 | 0.155 | 1.328 | −2.923 |
| cymene | −389.59604600 | 6.228 | 0.116 | 3.172 | 3.056 | 0.164 | 1.647 | −3.172 | |
| pinene | −390.74107098 | 5.911 | −0.332 | 2.790 | 3.122 | 0.160 | 1.247 | −2.790 | |
| thymol | −464.84176291 | 5.830 | 0.112 | 2.971 | 2.859 | 0.175 | 1.544 | −2.971 | |
| menthol | −468.45341693 | 7.038 | −0.371 | 3.334 | 3.705 | 0.135 | 1.500 | −3.334 | |
| MeOH | limonene | −390.76197221 | 6.145 | −0.288 | 2.929 | 3.216 | 0.155 | 1.333 | −2.929 |
| cymene | −389.59595751 | 6.230 | 0.118 | 3.174 | 3.056 | 0.164 | 1.648 | −3.174 | |
| pinene | −390.74104188 | 5.916 | −0.326 | 2.795 | 3.121 | 0.160 | 1.251 | −2.795 | |
| thymol | −464.84153739 | 5.846 | 0.128 | 2.987 | 2.859 | 0.175 | 1.560 | −2.987 | |
| menthol | −468.45326721 | 7.043 | −0.362 | 3.340 | 3.702 | 0.135 | 1.507 | −3.340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydel-Ciszek, K. DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes. Molecules 2024, 29, 1579. https://doi.org/10.3390/molecules29071579
Rydel-Ciszek K. DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes. Molecules. 2024; 29(7):1579. https://doi.org/10.3390/molecules29071579
Chicago/Turabian StyleRydel-Ciszek, Katarzyna. 2024. "DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes" Molecules 29, no. 7: 1579. https://doi.org/10.3390/molecules29071579
APA StyleRydel-Ciszek, K. (2024). DFT Studies of the Activity and Reactivity of Limonene in Comparison with Selected Monoterpenes. Molecules, 29(7), 1579. https://doi.org/10.3390/molecules29071579