Temperature Dependence of the Number of Defect-Structures in Poly(vinylidene fluoride)
Abstract
1. Introduction
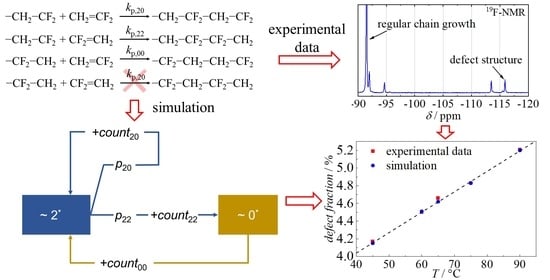
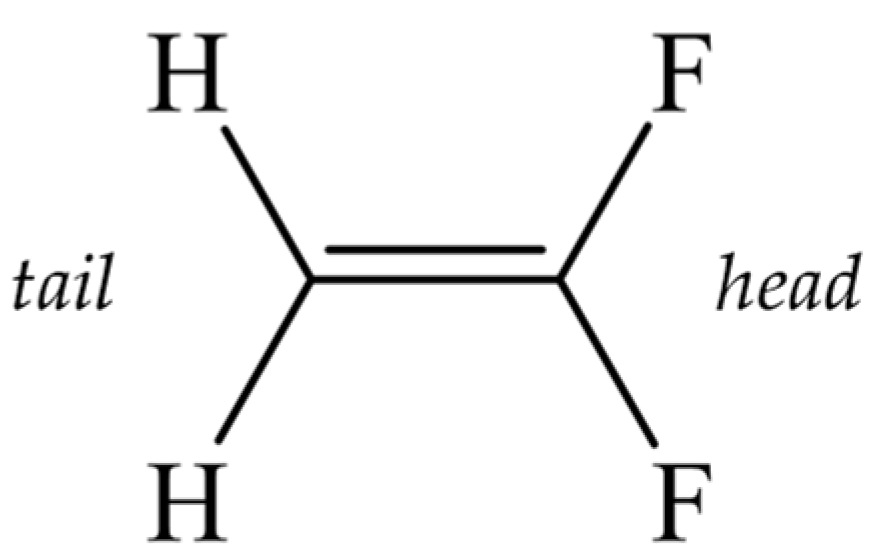
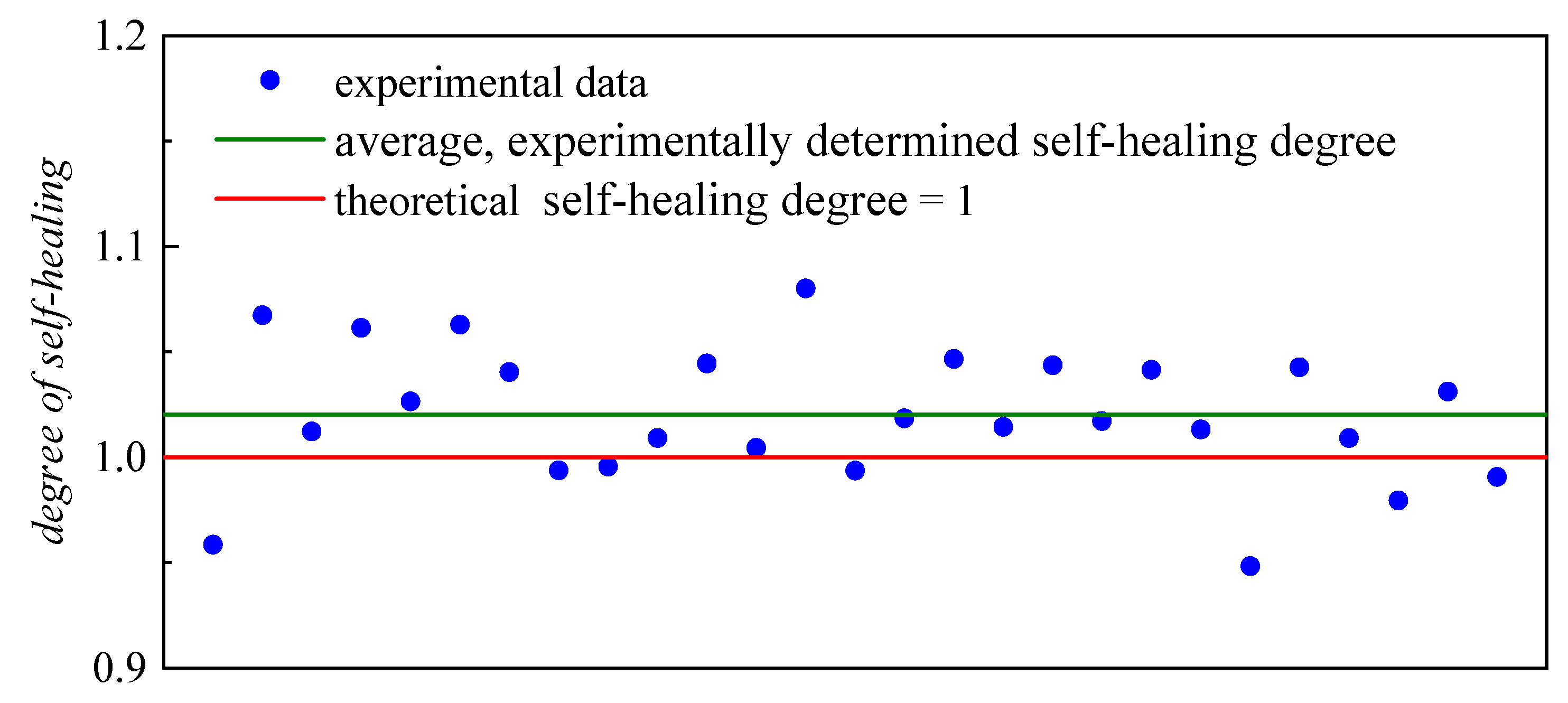
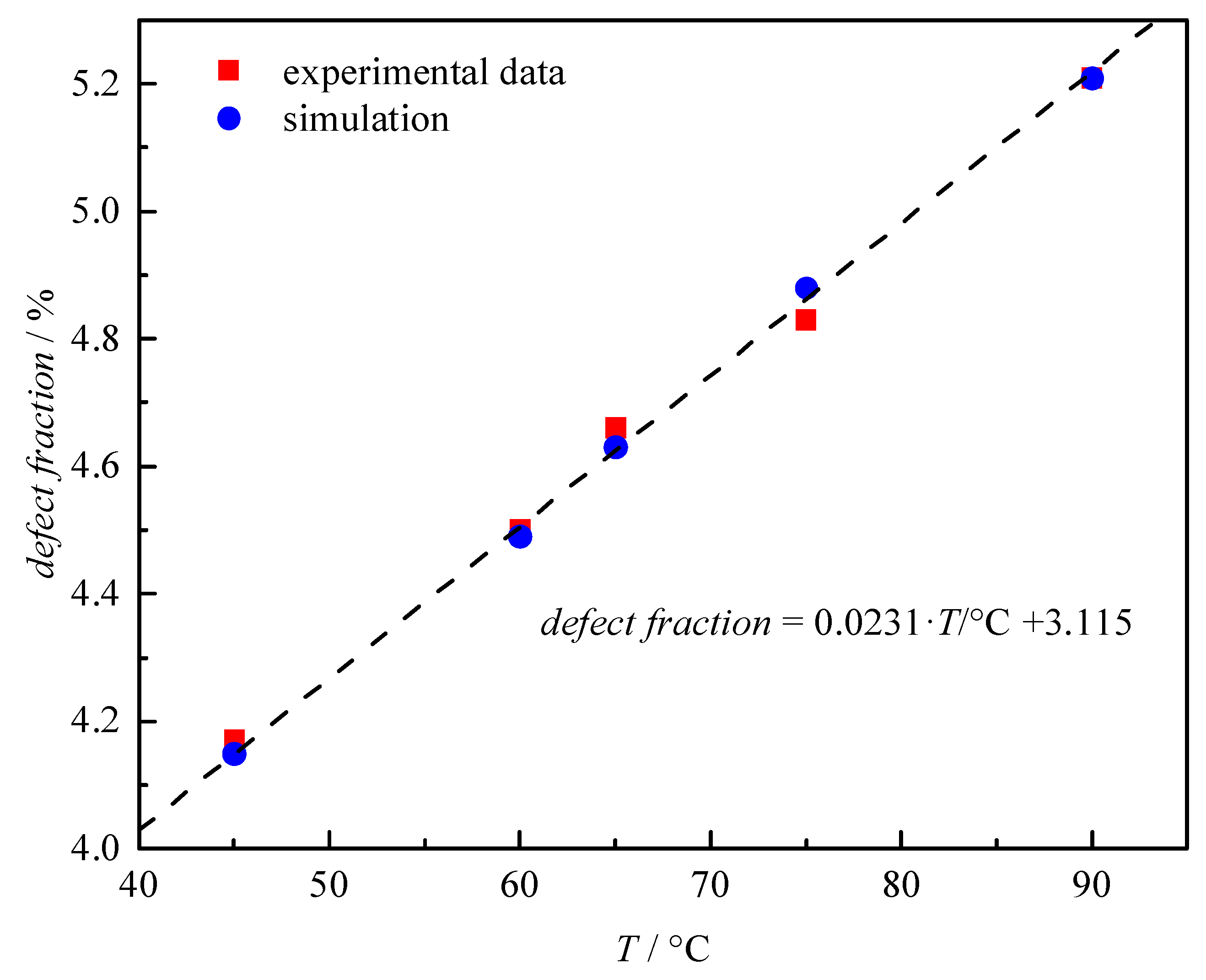
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2015, 68, 13–22. [Google Scholar] [CrossRef]
- Ameduri, B. Fluoropolymers: The right material for the right application. Chem. Eur. J. 2018, 24, 18830–18841. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Marcus, M.A. Ferroelectric polymers and their applications. Ferroelectrics 1982, 40, 29–41. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Dillon, D.R.; Tenneti, K.K.; Li, C.Y.; Ko, F.K.; Sics, I.; Hsiao, B.S. On the structure and morphology of polyvinylidene fluoride-nanoclay nanocomposites. Polymer 2006, 47, 1678–1688. [Google Scholar] [CrossRef]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef]
- Soulestin, T.; Ladmiral, V.; Dos Santos, F.D.; Ameduri, B. Vinylidene fluoride- and trifluoroethylene-containing fluorinated electroactive copolymers. How does chemistry impact properties? Prog. Polym. Sci. 2017, 72, 16–60. [Google Scholar] [CrossRef]
- Pladis, P.; Alexopoulos, A.H.; Kiparissides, C. Mathematical Modeling and Simulation of Vinylidene Fluoride Emulsion Polymerization. Ind. Eng. Chem. Res. 2014, 53, 7352–7364. [Google Scholar] [CrossRef]
- Brandl, F.; Beuermann, S. Semibatch Emulsion Polymerization of Vinylidene fluoride. Chem. Ing. Technol. 2018, 90, 372–379. [Google Scholar] [CrossRef]
- Boyer, C.; Valade, D.; Sauguet, L.; Ameduri, B.; Boutevin, B. Iodine Transfer Polymerization (IPT) of Vinylidene Fluoride (VDF). Influence of the Defect of VDF Chaining on the Control of ITP. Macromolecules 2005, 38, 10353–10362. [Google Scholar] [CrossRef]
- Modena, S.; Pianca, M.; Tato, M.; Moggi, G. Radical Telomerization of Vinylidene Fluoride in the Presence of 1,2-Dibromotetrafluoroethane. J. Fluor. Chem. 1989, 43, 15–25. [Google Scholar] [CrossRef]
- Maiti, P.; Nandi, A.K. Influence of Chain Structure on the Miscibility of Poly(vinylidene fluoride) with Poly(methyl acrylate). Macromolecules 1995, 28, 8511–8516. [Google Scholar] [CrossRef]
- Durand, N.; Ameduri, B.; Takashima, K.; Ishida, K.; Horie, S.; Ueda, Y. Vinylidene fluoride telomers for piezoelectric devices. Polym. J. 2011, 43, 171–179. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Davis, D.D.; Cais, R.E.; Kometani, J.M. The role of molecular defects on the structure and phase transitions of poly(vinylidene fluoride). Polymer 1987, 28, 617–626. [Google Scholar] [CrossRef]
- Vohlídal, J.; Graeff, C.F.O.; Hiorns, R.C.; Jones, R.G.; Luscombe, C.; Schué, F.; Stingelin, N.; Walter, M.G. Glossary of terms relating to elevtronic, photonic and magnetic properties of polymers (IUPAC Recommendations 2021). Pure Appl. Chem. 2022, 94, 15–69. [Google Scholar] [CrossRef]
- Guerre, M.; Rahaman, S.M.W.; Ameduri, B.; Poli, R.; Ladmiral, V. Limits of Vinylidene Fluoride RAFT Polymerization. Macromolecules 2016, 49, 5386–5396. [Google Scholar] [CrossRef]
- Balague, J.; Ameduri, B.; Boutevin, B.; Caporiccio, G. Synthesis of fluorinated telomers. Part 1. Telomerization of vinylidene fluoride with perfluoroalkyl iodides. J. Fluor. Chem. 1995, 70, 215–223. [Google Scholar] [CrossRef]
- Russo, S.; Behari, K.; Chengji, S.; Pianca, M.; Barchiesi, E.; Moggi, G. Synthesis and microstructural characterization of low-molar-mass poly(vinylidene fluoride). Polymer 1993, 34, 4777–4781. [Google Scholar] [CrossRef]
- Guiot, J.; Ameduri, B.; Boutevin, B. Radical Homopolymerization of Vinylidene Fluoride Initiated by tert-Butyl Peroxypivalate. Investigation of the Microstructure by 19F and 1H NMR Spectroscopies and Mechanisms. Macromolecules 2002, 35, 8694–8707. [Google Scholar] [CrossRef]
- Ameduri, B.; Ladaviere, C.; Delolme, F.; Boutevin, B. First MALDI-TOF Mass Spectrometry of Vinylidene Fluoride Telomers Endowed with Low Defect Chaining. Macromolecules 2004, 37, 7602–7609. [Google Scholar] [CrossRef]
- Patil, Y.; Zhao, J.; Ameduri, B.; Rastogi, S. Tailoring Electroactive β- and γ-Phases via Synthesis in the Nascent Poly(vinylidene fluoride) Homopolymers. Macromolecules 2024, 57, 616–627. [Google Scholar] [CrossRef]
- Giannetti, E. Semi-crystalline fluorinated polymers. Polym. Int. 2001, 50, 10–26. [Google Scholar] [CrossRef]
- Timmerman, R. The Predominant Reaction of Some Fluorinated Polymers to Ionizing Radiation. J. Appl. Polym. Sci. 1962, 22, 456–460. [Google Scholar] [CrossRef]
- Siegmann, R.; Drache, M.; Beuermann, S. Propagation Rate Coefficients for Vinylidene Fluoride Homopolymerizations. Macromolecules 2013, 46, 9507–9514. [Google Scholar] [CrossRef]
- Schäfer, T. Kinetische Untersuchungen und PREDICI Modellierung der Pulslaserinduzierten Hochdruckpolymerisation von Vinylidenfluorid in Überkritischem Kohlendioxid. Ph.D. Thesis, TU Clausthal, Clausthal-Zellerfeld, Germany, 2017. [Google Scholar]
- Olaj, O.F.; Bitai, I.; Hinkelmann, F. The laser-flash-initiated polymerization as a tool of evaluating (individual) kinetic constants of free-radical polymerization, The direct determination of the rate constant of chain propagation. Makromol. Chem. 1987, 188, 1689–1702. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M. Rate coefficients of free-radical polymerization deduced from pulsed laser experiments. Prog. Polym. Sci. 2002, 27, 191–254. [Google Scholar] [CrossRef]
- Twum, E.B.; Li, X.; McCord, E.F.; Fox, P.A.; Lyons, D.F.; Rinaldi, P.L. 2D-NMR Studies of Polyvinylidene Fluoride. In Fluorine Containing Polymers: Advances in Fluorine-Containing Polymers; Smith, D.W., Iacono, S.T., Kettwich, S.C., Boday, D.J., Eds.; American Chemical Society: Washington, DC, USA, 2012; pp. 187–213. [Google Scholar] [CrossRef]
- Monyatsi, O.; Nikitin, A.N.; Hutchinson, R.A. Effect of Head-To-Head Addition on Vinyl Acetate Propagation Kinetics in Radical Polymerization. Macromolecules 2014, 47, 8145–8153. [Google Scholar] [CrossRef]
- Feuerpfeil, A.; Drache, M.; Jantke, L.-A.; Melchin, T.; Rodriguez-Fernandez, J.; Beuermann, S. Modeling Semi-Batch Vinyl Acetate Polymerization Processes. Ind. Eng. Chem. Res. 2021, 60, 18256–18267. [Google Scholar] [CrossRef]
- Drache, M.; Stehle, M.; Mätzig, J.; Brandl, K.; Jungbluth, M.; Namyslo, J.C.; Schmidt, A.; Beuermann, S. Identification of β scission products from free radical polymerizations of butyl acrylate at high temperature. Polym. Chem. 2019, 10, 1956. [Google Scholar] [CrossRef]
- van Herk, A.M.; Manders, L.G.; Canelas, D.A.; Quadir, M.A.; DeSimone, J.M. Propagation rate coefficients of styrene and methyl methacrylate in supercritical CO2. Macromolecules 1997, 30, 4780–4782. [Google Scholar] [CrossRef][Green Version]
- Beuermann, S.; Buback, M.; Isemer, C.; Lacik, I.; Wahl, A. Pressure and Temperature Dependence of the Propagation Rate Coefficient of Free-Radical Styrene Polymerization in Supercritical Carbon Dioxide. Macromolecules 2002, 35, 3866–3869. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M.; Schmaltz, C.; Kutcha, F.-D. Determination of free-radical propagation rate coefficients for methyl methacrylate and butyl acrylate homopolymerizations in fluid CO2. Macromol. Chem. Phys. 1998, 199, 1209–1216. [Google Scholar] [CrossRef]




| A20 | A22 | A00 | Ea,20 | Ea,22 | Ea,00 |
|---|---|---|---|---|---|
| L∙mol−1∙s−1 | kJ∙mol−1 | ||||
| 1.99 × 108 | 6.50 × 107 | 3.52 × 109 | 25.5 | 30.7 | 35.4 |
| T/°C | defect fractionexp/% | defect fractionsim/% | kp,app,exp /L·mol−1·s−1 | kp,app,sim /L·mol−1·s−1 |
|---|---|---|---|---|
| 45 | 4.2 | 4.2 | 11,200 | 12,700 |
| 60 | 4.5 | 4.5 | 20,000 | 19,900 |
| 65 | 4.7 | 4.6 | 23,300 | 23,000 |
| 75 | 4.8 | 4.8 | 30,900 | 30,100 |
| 90 | 5.2 | 5.2 | 46,000 | 43,800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwaderer, J.; Drache, M.; Beuermann, S. Temperature Dependence of the Number of Defect-Structures in Poly(vinylidene fluoride). Molecules 2024, 29, 1551. https://doi.org/10.3390/molecules29071551
Schwaderer J, Drache M, Beuermann S. Temperature Dependence of the Number of Defect-Structures in Poly(vinylidene fluoride). Molecules. 2024; 29(7):1551. https://doi.org/10.3390/molecules29071551
Chicago/Turabian StyleSchwaderer, Jan, Marco Drache, and Sabine Beuermann. 2024. "Temperature Dependence of the Number of Defect-Structures in Poly(vinylidene fluoride)" Molecules 29, no. 7: 1551. https://doi.org/10.3390/molecules29071551
APA StyleSchwaderer, J., Drache, M., & Beuermann, S. (2024). Temperature Dependence of the Number of Defect-Structures in Poly(vinylidene fluoride). Molecules, 29(7), 1551. https://doi.org/10.3390/molecules29071551