Nitroxide-Mediated Controlled Radical Copolymerization of α-Trifluoromethylstyrenes with Styrenes
Abstract
1. Introduction
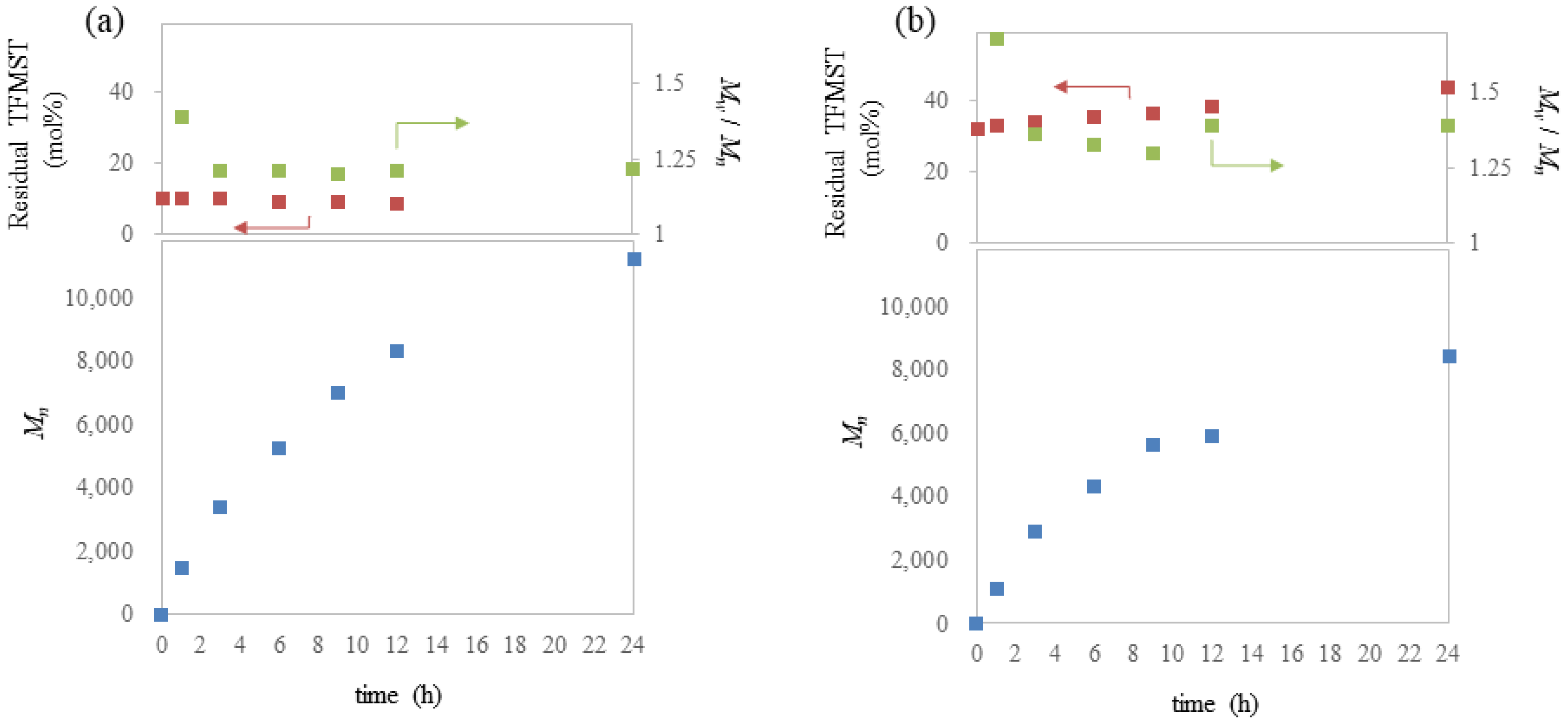
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.1.1. Materials
3.1.2. General Measurements
3.1.3. Thermal Measurements
3.1.4. Water and Oil Repellency Measurements
3.2. General Procedure for Synthesis of Substituted TFMST
3.3. General Procedure for Copolymerization
3.3.1. ATRP Polymerization
3.3.2. RAFT Polymerization
3.3.3. NMP Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teng, H. Overview of the development of the fluoropolymer industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- Ameduri, B. Frontispiece: Fluoropolymers: The right material for the right applications. Chem. Eur. J. 2018, 23, 18830–18841. [Google Scholar] [CrossRef] [PubMed]
- Puts, G.J.; Crouse, P.; Ameduri, B. Polytetrafluoroethylene: Synthesis and characterization of the original extreme polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef] [PubMed]
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers: Synthesis, Properties and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Hercules, D.A.; Parrish, C.A.; Sayler, T.S.; Tice, K.T.; Williams, S.M.; Lowery, L.E.; Brady, M.E.; Coward, R.B.; Murphy, J.A.; Hey, T.A.; et al. Preparation of tetrafluoroethylene from the pyrolysis of pentafluoropropionate salts. J. Fluor. Chem. 2017, 196, 107–116. [Google Scholar] [CrossRef]
- Ferrero, F.; Meyer, R.; Kluge, M.; Schroeder, V.; Spoormaker, T. Study of the spontaneous ignition of stoichiometric tetrafluoroethylene-air mixtures at elevated pressures. J. Loss Prev. Proc. Ind. 2013, 26, 759–765. [Google Scholar] [CrossRef]
- Tervoort, T.A.; Visjager, J.F.; Smith, P. Melt-processable poly(tetrafluoroethylene)—Compounding, fillers and dyes. J. Fluor. Chem. 2002, 114, 133–137. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Wang, C.; Wang, H. Melt processability of polytetrafluoroethylene: Effect of melt treatment on tensile deformation mechanism. J. Appl. Polym. Sci. 2012, 123, 1667–1674. [Google Scholar] [CrossRef]
- Jaye, J.A.; Sletten, E.M. Recent advances in the preparation of semifluorinated polymers. Polym. Chem. 2021, 12, 6515–6526. [Google Scholar] [CrossRef]
- Friesen, C.M.; Ameduri, B. Outstanding telechelic perfluoropolyalkylethers and applications therefrom. Prog. Polym. Sci. 2018, 81, 238–280. [Google Scholar] [CrossRef]
- He, E.; Tum, K.; Cheng, J.; Wang, Y.; Lu, H.; Zhang, L.; Cheng, Z. Synthesis and phase behavior of (semifluorinated alkane)-based side-chain liquid crystalline copolymers. Macromol. Rapid Commun. 2022, 43, 2200266. [Google Scholar] [CrossRef]
- Kanbara, T.; Arase, M.; Tanaka, M.; Yamaguchi, A.; Tagami, K.; Yajima, T. Amine-catalyzed synthesis of fluorine-containing polymers through halogen bonding. Chem. Asian J. 2023, 8, e202300035. [Google Scholar] [CrossRef] [PubMed]
- Ameduri, B. Controlled radical (co)polymerization of fluoromonomers. Macromolecules 2010, 43, 10163–10184. [Google Scholar] [CrossRef]
- Gong, H.; Gu, Y.; Chen, M. Controlled/living radical polymerization of semifluorinated (meth)acrylates. Synlett 2018, 29, 1543–1551. [Google Scholar]
- Yao, W.; Li, Y.; Huang, X. Fluorinated poly(meth)acrylate: Synthesis and properties. Polymer 2014, 55, 6197–6211. [Google Scholar] [CrossRef]
- Borkar, S.; Jankova, K.; Siesler, H.W.; Hvilsted, S. New highly fluorinated styrene-based materials with low surface energy prepared by ATRP. Macromolecules 2004, 37, 788–794. [Google Scholar] [CrossRef]
- Ceretta, F.; Zaggia, A.; Conte, L.; Ameduri, B. Conventional radical polymerization and iodine-transfer polymerization of 4′-nonafluorobutyl styrene: Surface and thermal characterizations of the resulting poly(fluorostyrene)s. J. Ploym. Sci. A Polym. Chem. 2013, 51, 3203–3212. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Itoh, T.; Iida, T. Absolute rate constants for radical homopolymerization of methyl α-(trifluoromethyl) acrylate. J. Polym. Sci. Part A Poym. Chem. 1994, 32, 1389–1392. [Google Scholar] [CrossRef]
- Agliettot, M.; Passaglia, E.; Mirabello, L.M. Synthesis of new polymers containing α-(trifluoromethy1) acrylate units. Macromol. Chem. Phys. 1995, 196, 2843–2853. [Google Scholar] [CrossRef]
- Ueda, M.; Ito, H. Radical reactivity of α-trifluoromethylstyrene. Polym. Chem. 1998, 26, 89–98. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Szwajca, A.; Boschet, F.; Gouverneur, V.; Ameduri, B. Poly(vinylidene fluoride)-b-poly(styrene) block copolymers by Iodine transfer polymerization (ITP): Synthesis, characterization, and kinetics of ITP. Macromolecules 2014, 47, 8634–8644. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Szwajca, A.; Gouverneur, V.; Ameduri, B. Synthesis, characterization, and hermal and surface properties of co- and terpolymers based on fluorinated α-methylstyrenes and styrene. Polym. Chem. 2017, 8, 6558–6569. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3731. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J.H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2987. [Google Scholar] [CrossRef] [PubMed]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: From process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, H. RAFT agent design and synthesis. Macromolecules 2012, 45, 5321–5343. [Google Scholar] [CrossRef]
- Favier, A.; Charreyre, M.T. Experimental requirements for an efficient control of free-radical polymerizations via the reversible addition-fragmentation chain transfer (RAFT) process. Macromol. Rapid Commun. 2006, 27, 665–689. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, H. Living radical polymerization by the RAFT process—Second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide-mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3698. [Google Scholar] [CrossRef]
- Sciannamea, V.; Jerome, C.; Detrembleur, C. In-situ nitroxide-mediated radical polymerization (NMP) processes: Their understanding and optimization. Chem. Rev. 2008, 108, 1104–1126. [Google Scholar] [CrossRef]
- Xia, J.; Matyjaszewski, K. Controlled/”living” radical polymerization. Atom transfer radical polymerization using multidentate amine ligands. Macromolecules 1997, 30, 7697–7700. [Google Scholar] [CrossRef]
- Barlow, K.J.; Hao, X.; Hughes, T.C.; Hutt, O.E.; Polyzos, A.; Turner, K.A.; Moad, G. Porous, functional, poly(styrene-co-divinylbenzene) monoliths by RAFT polymerization. Polym. Chem. 2014, 5, 722–732. [Google Scholar] [CrossRef]
- Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C.J. Development of a universal alkoxyamine for “living” free radical polymerizations. J. Am. Chem. Soc. 1999, 121, 3904–3920. [Google Scholar] [CrossRef]
- Banerjee, S.; Domenichelli, I.; Ameduri, B. Nitroxide-mediated alternating copolymerization of vinyl acetate with tert-butyl-2-trifluoromethacrylate using a SG1-based alkoxyamine. ACS Macro Lett. 2016, 5, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Langlais, M.; Coutelier, O.; Destarac, M. Thiolacetone-functional reversible deactivation radical polymerization agents for advanced macromolecular engineering. Macromolecules 2018, 51, 4315–4324. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Zhao, Y.; Qu, J. Synthesis of gem-difluoroallylboronates via FeCl2-catalyzed boration/β-fluorine elimination of trifluoromethyl alkenes. Org. Lett. 2017, 19, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak-Kulikowska, J.; Boschet, F.; Kostov, G.; Gouverneur, V.; Ameduri, B. On the reactivity of α-trifluoromethylstyrene in radical copolymerizations with various fluoroalkenes. Eur. Polym. J. 2016, 84, 612–621. [Google Scholar] [CrossRef]
- Lin, J.W.P.; Dudek, L.P.; Majumdar, D. Wetting properties of homopolymers and copolymers of pentafluorostyrene and methacrylate and homopolymer blends. J. Appl. Polym. Sci. 1987, 33, 657–667. [Google Scholar] [CrossRef]
- Pan, R.; Liu, X.; Deng, M. A novel and convenient synthetic method for producing α-(trifluoromethyl)styrenes. J. Fluor. Chem. 1999, 95, 167–170. [Google Scholar] [CrossRef]
- Miura, T.; Ito, Y.; Murakami, M. Synthesis of gem-difluoroalkenes via β-fluoride elimination of organorhodium(I). Chem. Lett. 2008, 37, 1006–1007. [Google Scholar] [CrossRef]
- Nader, B.S.; Cordova, J.A.; Reese, K.E.; Powell, C.L. A novel fluoride mediated olefination of electron-deficient aryl ketones by alkanesulfonyl halides. J. Org. Chem. 1994, 59, 2898–2901. [Google Scholar] [CrossRef]

 | ||||||||
| Entry | Reagents | Temp. [°C] | Time [h] | Yield (a) [%] | Ratio of ST (b) [%] | Ratio of TFMST (b) [%] | Mn (c) | Mw/Mn (c) |
| 1 (e) | 3a (1 eq.), Cu-TMEDA (1 eq.) | 110 | 12 | 62 | 91 | 9 | 10,000 | 1.30 |
| 2 (f) | 3b (1 eq.), AIBN (0.25 eq.) 1,4-dioxane | 75 | 48 | 28 (d) | 90 | 10 | 2200 | 1.27 |
| 3 (f) | 3b (1 eq.), AIBN (0.25 eq.) 1,4-dioxane | 75 | 96 | 21 | 87 | 13 | 4500 | 1.10 |
| 4 (g) | 3c (1 eq.) | 110 | 6 | 16 | 90 | 10 | 2800 | 1.18 |
| 5 (g) | 3d (1 eq.) | 110 | 6 | 56 | 91 | 9 | 7300 | 1.14 |
 | |||||||||
| Entry | ST (1a) [eq.] | 2a or 4 [eq.] | Yield (a) [%] | Ratio of ST [%] | Ratio of (TF)MST [%] | Mn (b) | Mw/Mn (b) | Td5% [°C] | Tg [°C] |
| 1 | 100 | - | 65 | 100 | - | 8300 | 1.07 | 275 | 71 |
| 2 | 90 | 2a (10) | 56 | 91 | 9 (c) | 7300 | 1.14 | 285 | 103 |
| 3 | 70 | 2a (30) | 54 | 82 | 18 (c) | 8300 | 1.31 | 279 | 107 |
| 4 | 50 | 2a (50) | 32 | 66 | 34 (c) | 7400 | 1.30 | 261 | 80 |
| 5 | 30 | 2a (70) | 20 | 60 | 40 (c) | 6100 | 1.47 | 261 | 91 |
| 6 (d) | 90 | 4 (10) | 47 | 100 | 0 | 7100 | 1.11 | - | - |
| 7 (e) | 50 | 4 (50) | 27 | 71 | 29 (f) | 7500 | 1.34 | 278 | 65 |
 | |||||||||
| Entry | ST | Yield (b) [%] | Copolymer | Ratio of ST (c) [%] | Ratio of TFMST (c) [%] | Mn (d) | Mw/Mn | Td5% [°C] | Tg [°C] |
| 1 | 1b | 26 | 5ba | 69 | 31 | 5800 | 1.50 | 252 | 100 |
| 2 | 1c | 19 | 5ca | 77 | 23 | 4700 | 1.18 | 238 | 94 |
| 3 | 1d | 35 | 5da | 70 | 30 | 9300 | 1.49 | 293 | 130 |
 | |||||||||
| Entry | TFMST | Yield (b) [%] | Copolymer | Ratio of ST (c) [%] | Ratio of TFMST (c) [%] | Mn (d) | Mw/Mn (d) | Td5% [°C] | Tg [°C] |
| 1 | 2b | 20 | 5ab | 73 | 27 | 8600 | 1.24 | 251 | 107 |
| 2 | 2c | 33 | 5ac | 68 | 32 | 6100 | 1.47 | 255 | 93 |
| 3 | 2d | 46 | 5ad | 70 | 30 | 10,000 | 1.37 | 278 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanbara, T.; Ito, Y.; Yamaguchi, A.; Yajima, T. Nitroxide-Mediated Controlled Radical Copolymerization of α-Trifluoromethylstyrenes with Styrenes. Molecules 2024, 29, 1214. https://doi.org/10.3390/molecules29061214
Kanbara T, Ito Y, Yamaguchi A, Yajima T. Nitroxide-Mediated Controlled Radical Copolymerization of α-Trifluoromethylstyrenes with Styrenes. Molecules. 2024; 29(6):1214. https://doi.org/10.3390/molecules29061214
Chicago/Turabian StyleKanbara, Tadashi, Yuriko Ito, Airi Yamaguchi, and Tomoko Yajima. 2024. "Nitroxide-Mediated Controlled Radical Copolymerization of α-Trifluoromethylstyrenes with Styrenes" Molecules 29, no. 6: 1214. https://doi.org/10.3390/molecules29061214
APA StyleKanbara, T., Ito, Y., Yamaguchi, A., & Yajima, T. (2024). Nitroxide-Mediated Controlled Radical Copolymerization of α-Trifluoromethylstyrenes with Styrenes. Molecules, 29(6), 1214. https://doi.org/10.3390/molecules29061214






