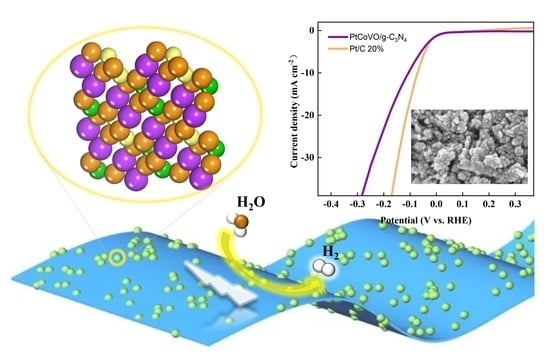
Intercalated PtCo Electrocatalyst of Vanadium Metal Oxide Increases Charge Density to Facilitate Hydrogen Evolution
Abstract
1. Introduction
2. Results and Discussion
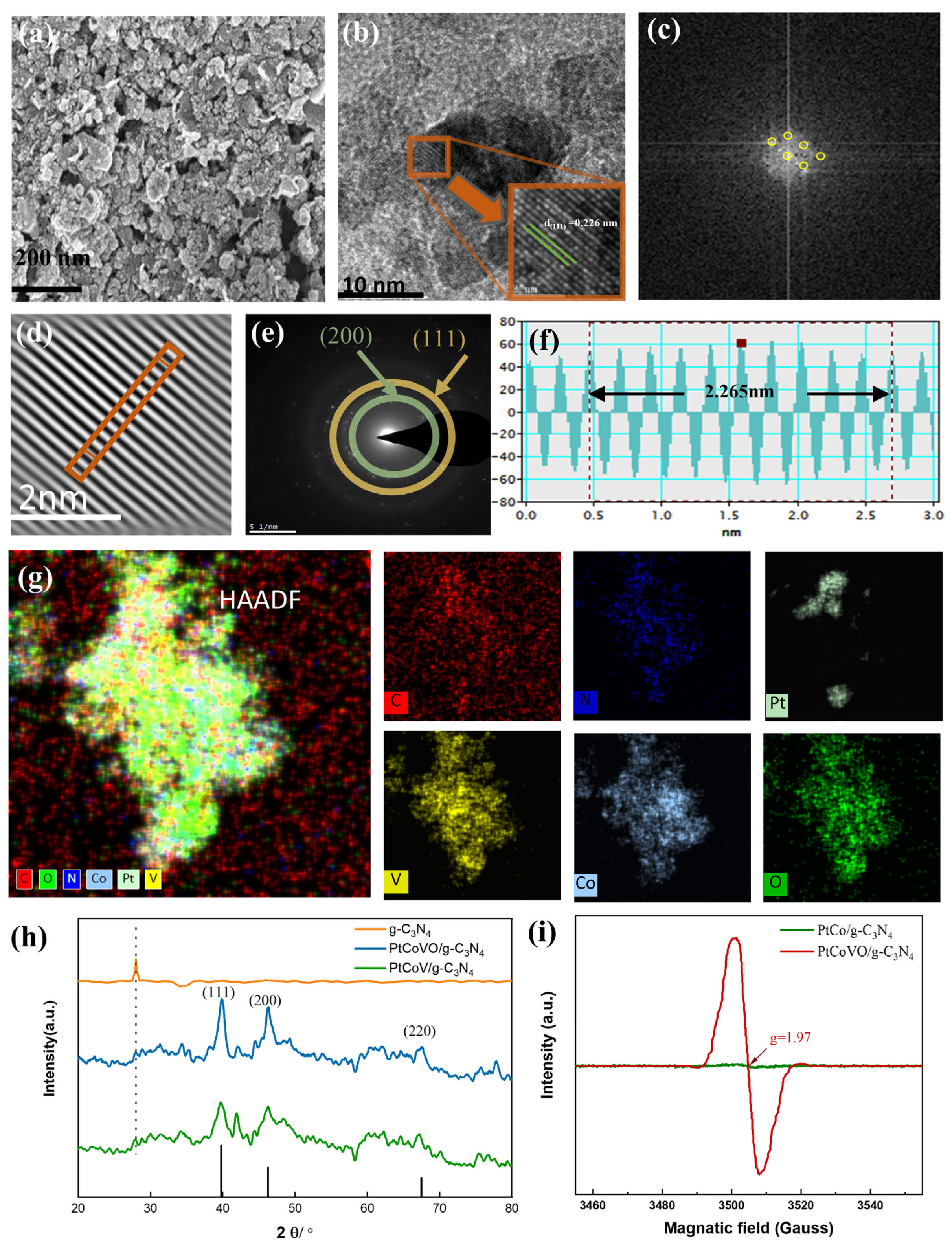
2.1. Morphologies and Structures
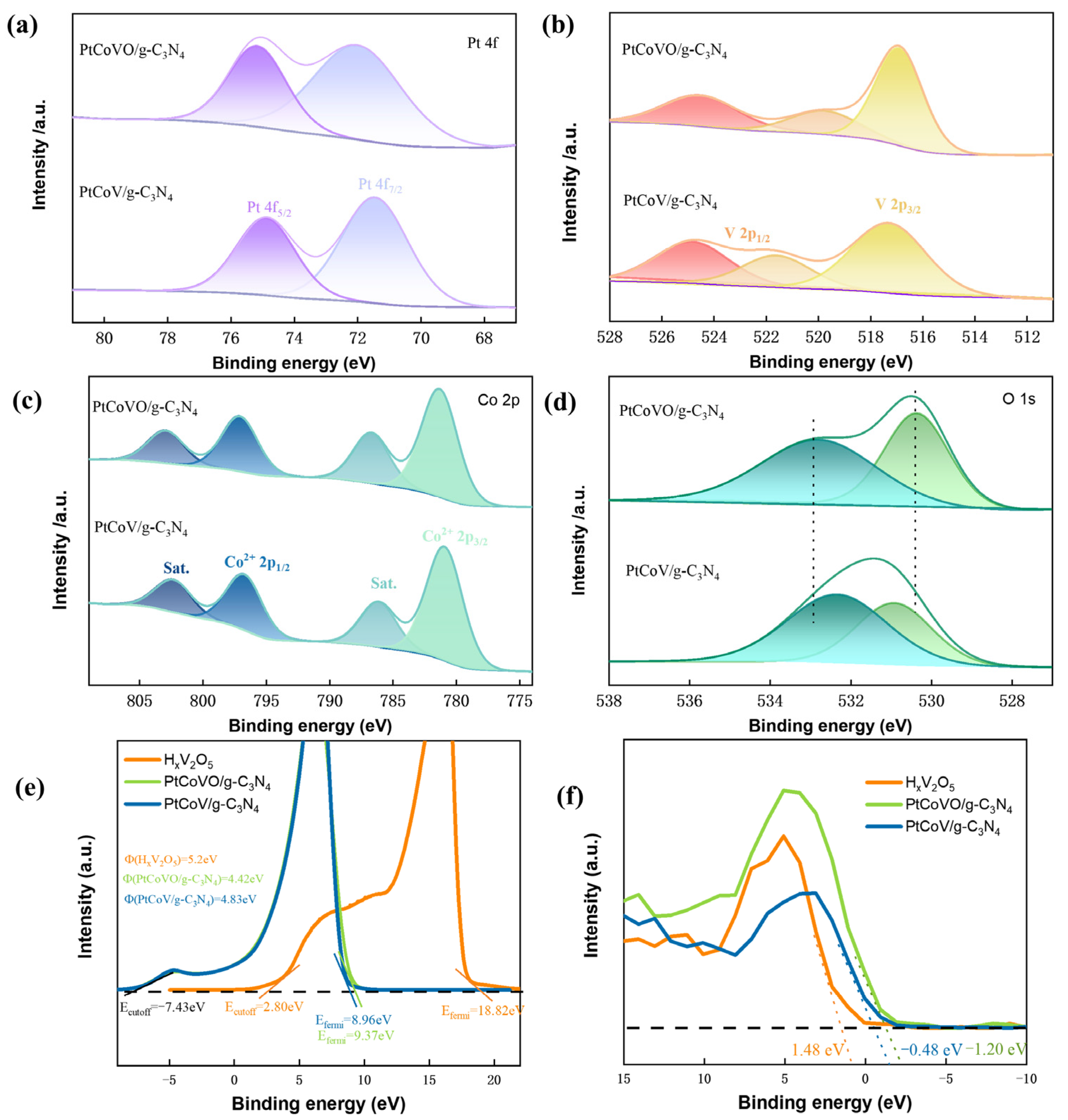
2.2. Electrocatalytic Enhancement Mechanism
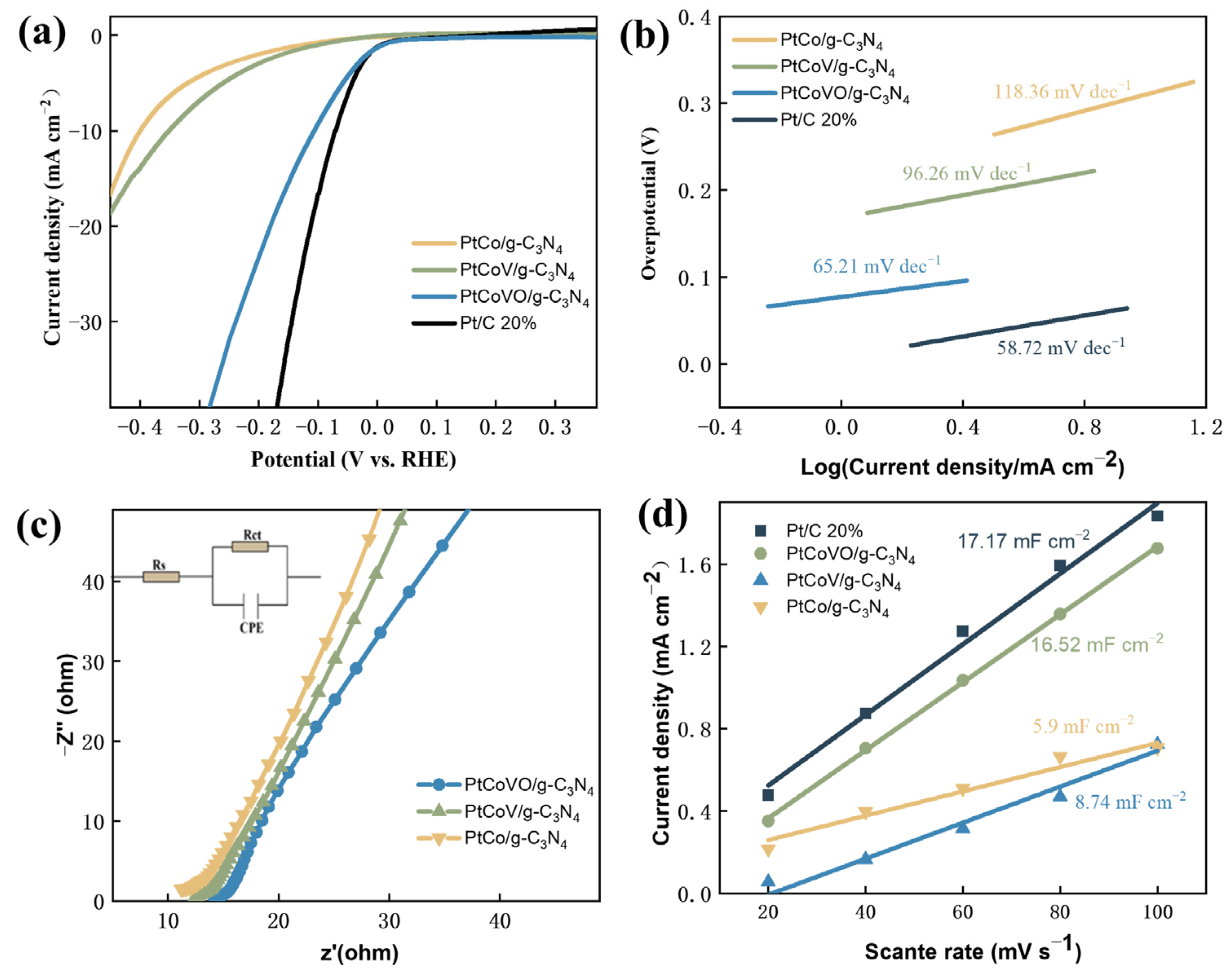
2.3. HER Electrocatalytic Performance Analyses
3. Experimental Methods
3.1. Materials
3.2. Methods
3.2.1. Fabrication Process for g-C3N4 Nanosheets
3.2.2. Synthesis of HxV2O5 HMOs
3.2.3. Fabrication of PtCo/g-C3N4, PtCoV/g-C3N4, and PtCoVO/g-C3N4 Catalysts
- (1)
- Preparation process for PtCo/g-C3N4 materials
- (2)
- Preparation of PtCoVO/g-C3N4 materials
- (3)
- Preparation of PtCoV/g-C3N4 materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seif, R.; Salem, F.Z.; Allam, N.K. E-waste recycled materials as efficient catalysts for renewable energy technologies and better environmental sustainability. Environ. Dev. Sustain. 2023, 26, 5473–5508. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xie, W.S.; Li, D.; Gai, Y.P.; Chen, Z.D.; Yu, J.; Yang, R.Q.; Bao, X.C.; Jiang, F. Controllable tuning of polymetallic Co-Ni-Ru-S-Se ultrathin nanosheets to boost electrocatalytic oxygen evolution. NPG Asia Mater. 2022, 14, 25. [Google Scholar] [CrossRef]
- Sangtani, R.; Nogueira, R.; Yadav, A.K.; Kiran, B. Systematizing Microbial Bioplastic Production for Developing Sustainable Bioeconomy: Metabolic Nexus Modeling, Economic and Environmental Technologies Assessment. J. Polym. Environ. 2023, 31, 2741–2760. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, C.; Qin, M.; Li, B.; Lin, B.; Mao, Q.; Yang, H.; Liu, B.; Wang, Y. Reversible hydrogen spillover in Ru-WO(3-x) enhances hydrogen evolution activity in neutral pH water splitting. Nat. Commun. 2022, 13, 5382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Choy, W.C.H.; Li, X.C.; Zhang, D.; Cheng, J.Q. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Hanan, A.; Lakhan, M.N.; Shu, D.; Hussain, A.; Ahmed, M.; Soomro, I.A.; Kumar, V.; Cao, D. An efficient and durable bifunctional electrocatalyst based on PdO and Co2FeO4 for HER and OER. Int. J. Hydrogen Energy 2023, 48, 19494–19508. [Google Scholar] [CrossRef]
- Ganci, F.; Cusumano, V.; Livreri, P.; Aiello, G.; Sunseri, C.; Inguanta, R. Nanostructured Ni-Co alloy electrodes for both hydrogen and oxygen evolution reaction in alkaline electrolyzer. Int. J. Hydrogen Energy 2021, 46, 10082–10092. [Google Scholar] [CrossRef]
- Saifi, S.; Dey, G.; Karthikeyan, J.; Sinha, A.S.K.; Aijaz, A. MoS2 and WS2 Nanosheets Decorated on Metal–Organic Framework-Derived Cobalt/Carbon Nanostructures as Electrocatalysts for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 10696–10703. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, Y.; Xing, Z.; Wu, Z.; Cheng, C.; Ma, T.; Li, S. Interfacial electron-engineered tungsten oxynitride interconnected rhodium layer for highly efficient all-pH-value hydrogen production. J. Mater. Chem. A 2024, 12, 4484–4491. [Google Scholar] [CrossRef]
- Shen, H.; Liang, S.; Adimi, S.; Guo, X.; Zhu, Y.; Guo, H.; Thomas, T.; Attfield, J.P.; Yang, M. Supporting nickel on vanadium nitride for comparable hydrogen evolution performance to platinum in alkaline solution. J. Mater. Chem. A 2021, 9, 19669–19674. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, C.; Ye, F.; Pang, R.; Liu, Y.; Wu, Z.; Shao, Z.; Sun, Z.; Hu, L. Selenic Acid Etching Assisted Vacancy Engineering for Designing Highly Active Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2007523. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.S.; Lee, J.; Kim, J.; Joo, S.H. Metastable Phase-Controlled Synthesis of Mesoporous Molybdenum Carbides for Efficient Alkaline Hydrogen Evolution. ACS Catal. 2022, 12, 7415–7426. [Google Scholar] [CrossRef]
- Griesser, C.; Li, H.; Wernig, E.-M.; Winkler, D.; Nia, N.S.; Mairegger, T.; Götsch, T.; Schachinger, T.; Steiger-Thirsfeld, A.; Penner, S.; et al. True Nature of the Transition-Metal Carbide/Liquid Interface Determines Its Reactivity. ACS Catal. 2021, 11, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-based electrocatalysts for cost-effective ultrahigh-current-density water splitting in anion exchange membrane electrolyzer cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Sun, H.; Xu, X.; Li, Y.; Ran, R.; Zhou, W.; Shao, Z. Understanding the bifunctional catalytic ability of electrocatalysts for oxygen evolution reaction and urea oxidation reaction: Recent advances and perspectives. Chem. Eng. J. 2023, 471, 144660. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Lei, H.; Wan, Q.; Tan, S.; Wang, Z.; Mai, W. Pt-Quantum-Dot-Modified Sulfur-Doped NiFe Layered Double Hydroxide for High-Current-Density Alkaline Water Splitting at Industrial Temperature. Adv. Mater. 2023, 35, 2208209. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, K.; Cao, X.; Zhang, L.; Wang, T.; Peng, D.; Zhang, B.; Liu, Z.; Wu, J.; Zhang, Y.; et al. Atomically Dispersed Intrinsic Hollow Sites of M-M1-M (M1 = Pt, Ir; M = Fe, Co, Ni, Cu, Pt, Ir) on FeCoNiCuPtIr Nanocrystals Enabling Rapid Water Redox. Adv. Funct. Mater. 2022, 32, 2110645. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Wan, T.; Shi, H.; Lv, A.; Xiao, W.; Jiao, S. Novel (Pt-Ox)-(Co-Oy) Nonbonding Active Structures on Defective Carbon from Oxygen-Rich Coal Tar Pitch for Efficient HER and ORR. Adv. Mater. 2022, 34, e2206960. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Wang, K.; Wang, X.; Lu, Z.; Xie, J.; Cao, Y. Hollow porous nitrogen-doped carbon embedded with ultrafine Co nanoparticles boosting lithium-ion storage. CrystEngComm 2021, 23, 2006–2015. [Google Scholar] [CrossRef]
- Xie, F.; Choy, W.C.; Wang, C.; Li, X.; Zhang, S.; Hou, J. Low-temperature solution-processed hydrogen molybdenum and vanadium bronzes for an efficient hole-transport layer in organic electronics. Adv. Mater. 2013, 25, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, W.; Weng, Y.; Li, X.; Mao, H.; Zhang, W.; Lu, T.; Long, D.; Jiang, F. Theoretical Revelation and Experimental Verification Synergistic Electronic Interaction of V-Doped RuNi as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Sustain. Chem. Eng. 2023, 11, 16288–16299. [Google Scholar] [CrossRef]
- Shinde, P.V.; Mane, P.; Chakraborty, B.; Rout, C.S. Spinel NiFe2O4 nanoparticles decorated 2D Ti3C2 MXene sheets for efficient water splitting: Experiments and theories. J. Colloid Interface Sci. 2021, 602, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Li, Z.; Zhao, P.; Tao, C.; Cheng, G.; Luo, W. Enhanced catalytic activity of Ru through N modification toward alkaline hydrogen electrocatalysis. Chin. Chem. Lett. 2022, 33, 1065–1069. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Truong, L.; Akbar, K.; Rabani, I.; Kim, H.-S.; Chun, S.-H.; Jung, J. Facile and cost-effective growth of MoS2 on 3D porous graphene-coated Ni foam for robust and stable hydrogen evolution reaction. J. Alloys Compd. 2019, 788, 267–276. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Ni, L.; Zhao, Z.L.; Li, H. Scalable synthesis of hcp ruthenium-molybdenum nanoalloy as a robust bifunctional electrocatalyst for hydrogen evolution/oxidation. J. Energy Chem. 2022, 72, 176–185. [Google Scholar] [CrossRef]
- Li, X.; Deng, W.; Weng, Y.; Zhang, J.; Mao, H.; Zhang, W.; Lu, T.; Long, D.; Jiang, F. Controllable tuning graphene composited PtCoYOx nanocomposites to promote electrocatalytic hydrogen evolution. Appl. Catal. A Gen. 2023, 665, 119359. [Google Scholar] [CrossRef]
- Martínez-Alonso, C.; Guevara-Vela, J.M.; Llorca, J. The effect of elastic strains on the adsorption energy of H, O, and OH in transition metals. Phys. Chem. Chem. Phys. 2021, 23, 21295–21306. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, M.; An, K.; Liu, D.; Chen, Y.; Zhou, P.; Li, J.; Feng, J.; Ke, Y.; Liu, D.; et al. In situ surface reconstruction on LaCoO3−δ leads to enhanced hydrogen evolution reaction. J. Alloys Compd. 2022, 891, 161754. [Google Scholar] [CrossRef]
- Su, X.; Meng, F.; Tan, H.; Chen, G. Unravelling the CO2 methanation mechanisms on a Ni-BaTiO3 catalyst: A theoretical investigation. J. CO2 Util. 2022, 64, 102170. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Zhang, Y.; Hou, R.; Zhang, X.; Xue, C.; Wang, S.; Zhu, B.; Li, N.; Shao, G. Photogenerated electron transfer process in heterojunctions: In situ irradiation XPS. Small Methods 2020, 4, 2000214. [Google Scholar] [CrossRef]
- Du, J.; Qin, Y.; Dou, T.; Ge, J.; Wang, Y.; Zhao, X.; Zhang, F.; Lei, X. Copper Nanoparticles Dotted on Copper Sulfide Nanosheets for Selective Electrocatalytic Oxidation of Glycerol to Formate. ACS Appl. Nano Mater. 2022, 5, 10174–10182. [Google Scholar] [CrossRef]
- Dawn, R.; Zzaman, M.; Faizal, F.; Kiran, C.; Kumari, A.; Shahid, R.; Panatarani, C.; Joni, I.M.; Verma, V.K.; Sahoo, S.K.; et al. Origin of Magnetization in Silica-coated Fe3O4 Nanoparticles Revealed by Soft X-ray Magnetic Circular Dichroism. Braz. J. Phys. 2022, 52, 99. [Google Scholar] [CrossRef]
- Li, X.; Deng, W.; Weng, Y.; Zhang, J.; Mao, H.; Lu, T.; Zhang, W.; Yang, R.; Jiang, F. Implanting HxYO2-x sites into Ru-doped graphene and oxygen vacancies for low-overpotential alkaline hydrogen evolution. NPG Asia Mater. 2023, 15, 55. [Google Scholar] [CrossRef]
- Kitta, M.; Taguchi, N.; Sudrajat, H.; Onishi, H. Direct confirmation of the dopant site in indium-doped SrTiO3 photocatalyst via atomic-scale analytical transmission electron microscopy imaging. Appl. Phys. Lett. 2021, 118, 153901. [Google Scholar] [CrossRef]
- Zheng, H.-B.; Li, Y.-L.; Wang, Y.-L.; Ma, F.; Gao, P.-Z.; Guo, W.-M.; Qin, H.; Liu, X.-P.; Xiao, H.-N. Fabrication of Co(PO3)2@NPC/MoS2 heterostructures for enhanced electrocatalytic hydrogen evolution. J. Alloys Compd. 2022, 894, 162411. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Zong, R.; Yao, W.; Wang, J.; Zhu, Y. Controlled synthesis of a highly dispersed BiPO4 photocatalyst with surface oxygen vacancies. Nanoscale 2015, 7, 13943–13950. [Google Scholar] [CrossRef]
- Li, J.-J.; Weng, B.; Cai, S.-C.; Chen, J.; Jia, H.-P.; Xu, Y.-J. Efficient promotion of charge transfer and separation in hydrogenated TiO2/WO3 with rich surface-oxygen-vacancies for photodecomposition of gaseous toluene. J. Hazard. Mater. 2018, 342, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Besley, N.A. Density functional theory based methods for the calculation of X-ray spectroscopy. Acc. Chem. Res. 2020, 53, 1306–1315. [Google Scholar] [CrossRef]
- Baronia, R.; Goel, J.; Tiwari, S.; Singh, P.; Singh, D.; Singh, S.P.; Singhal, S.K. Efficient electro-oxidation of methanol using PtCo nanocatalysts supported reduced graphene oxide matrix as anode for DMFC. Int. J. Hydrogen Energy 2017, 42, 10238–10247. [Google Scholar] [CrossRef]
- Yi, M.; Hu, S.; Lu, B.; Li, N.; Zhu, Z.; Huang, X.; Wang, M.; Zhang, J. Multicomponent Pt/PtTe2/NiCoTe2 embedded in ternary heteroatoms-doped carbon for efficient and pH-universal hydrogen evolution reaction. J. Alloys Compd. 2021, 884, 161042. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, W.; Weng, Y.; Li, X.; Mao, H.; Lu, T.; Zhang, W.; Long, D.; Jiang, F. Experimentally revealed and theoretically certified synergistic electronic interaction of V-doped CoS for facilitating the oxygen evolution reaction. Phys. Chem. Chem. Phys. 2023, 25, 21661–21672. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Gai, Y.; Li, D.; Chen, Z.; Xie, W.; Yu, J.; Yang, R.; Bao, X.; Jiang, F. Bifunctional doped transition metal CoSSeNi–Pt/C for efficient electrochemical water splitting. Int. J. Hydrogen Energy 2022, 47, 16862–16872. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, W.; Li, D.; Gai, Y.; Xie, W.; Hu, X.; Han, S.; Xu, N.; Qiao, S.; Yu, J.; et al. Construction of CoNiSSe-g-C3N4 nanosheets with high exposed conductive interface for boosting oxygen evolution reaction. J. Alloys Compd. 2021, 887, 161346. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, T.; Doi, T.; Kurokawa, S.; Seshimo, K.; Ye, D.; Cai, J. Effect of Mn-Based Slurries on Chemical Mechanical Polishing of SiC Substrates. ECS J. Solid State Sci. Technol. 2022, 11, 074002. [Google Scholar] [CrossRef]
- Béchu, S.; Ralaiarisoa, M.; Etcheberry, A.; Schulz, P. Photoemission spectroscopy characterization of halide perovskites. Adv. Energy Mater. 2020, 10, 1904007. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, R.; Shen, D.; Liu, Q.; Luo, K.H.; Wu, C.; Gu, S. Performance of intrinsic heteroatoms in cobalt phosphide loaded ginkgo leave-based carbon material on promoting the electrocatalytic activity during hydrogen evolution reaction and oxygen evolution reaction. Fuel 2023, 333, 126368. [Google Scholar] [CrossRef]
- Alli, Y.A.; Oladoye, P.O.; Ejeromedoghene, O.; Bankole, O.M.; Alimi, O.A.; Omotola, E.O.; Olanrewaju, C.A.; Philippot, K.; Adeleye, A.S.; Ogunlaja, A.S. Nanomaterials as catalysts for CO2 transformation into value-added products: A review. Sci. Total Environ. 2023, 868, 161547. [Google Scholar] [CrossRef] [PubMed]
- Reynosa-Martínez, A.C.; Gómez-Chayres, E.; Villaurrutia, R.; López-Honorato, E. Controlled reduction of graphene oxide using sulfuric acid. Materials 2020, 14, 59. [Google Scholar] [CrossRef]
- Kumar, P.S.; Prakash, P. Metal free nanocomposite of graphitic carbon nitride, boron nitride and chitosan for efficient evolution of hydrogen: A strategic approach to achieving sustainable and effective electrocatalysis. J. Environ. Chem. Eng. 2023, 11, 109045. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, L.; Yu, G.; Zhang, X.; Jin, H.; He, D. Visual and colorimetric determination of mercury (II) based on lignosulfonate-capped silver nanoparticles. Green Chem. Lett. Rev. 2023, 16, 2169590. [Google Scholar] [CrossRef]
- Purnawati, D.; Regonia, P.R.; Bermundo, J.P.; Ikeda, K.; Uraoka, Y. Machine-Learned Fermi Level Prediction of Solution-Processed Ultrawide-Bandgap Amorphous Gallium Oxide (a-Ga2Ox). ACS Appl. Electron. Mater. 2022, 4, 5838–5846. [Google Scholar] [CrossRef]
- Stanca, M.; Gaidau, C.; Alexe, C.-A.; Stanculescu, I.; Vasilca, S.; Matei, A.; Simion, D.; Constantinescu, R.-R. Multifunctional leather surface design by using carbon nanotube-based composites. Materials 2021, 14, 3003. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, S.; Cao, Y.; Chen, R.; Lu, Z.; Hu, J.; Xie, J.; Hao, A. High-performance bifunctional electrocatalysts of CoFe-LDH/NiCo2O4 heterostructure supported on nickel foam for effective overall water splitting. J. Alloys Compd. 2022, 926, 166846. [Google Scholar] [CrossRef]
- Li, C.; Pan, J.; Zhang, L.; Fang, J. Colloidal synthesis of monodisperse trimetallic Pt-Fe-Ni nanocrystals and their enhanced electrochemical performances. Nanotechnology 2022, 34, 075401. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Y.; Chen, Z.; Feng, F.; Ma, Z.; Zhang, S.; An, Q. Elemental diversity-enhanced HER and OER photoelectrochemical catalytic performance in FeCo-AuNP/nitrogen-carbon composite catalysts. Appl. Surf. Sci. 2021, 568, 151005. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, P.; Sharma, L.; Mao, B.; Kakkar, R.; Meng, T.; Zheng, L.; Cao, M. Further insights into bifunctional mechanism in alkaline hydrogen evolution for hybridized nanocatalysts and general route toward mechanism-oriented synthesis. Nano Energy 2021, 81, 105645. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Liu, H.; Wu, X.; Jiang, H.; Yang, L.; Qi, Z.; Li, G.; Shan, L.; Lin, Y.; et al. Achieving high-efficient urea oxidation via regulating the rate-determining step over a V single atom incorporated Co hydroxide electrocatalyst. Chem. Eng. J. 2022, 439, 135768. [Google Scholar] [CrossRef]
- Anantharaj, S. Hydrogen evolution reaction on Pt and Ru in alkali with volmer-step promotors and electronic structure modulators. Curr. Opin. Electrochem. 2022, 33, 100961. [Google Scholar] [CrossRef]
- Díaz-Coello, S.; García, G.; Arévalo, M.C.; Pastor, E. Precise determination of Tafel slopes by DEMS. Hydrogen evolution on tungsten-based catalysts in alkaline solution. Int. J. Hydrogen Energy 2019, 44, 12576–12582. [Google Scholar] [CrossRef]
- Sarac, B.; Karazehir, T.; Mühlbacher, M.; Sarac, A.S.; Eckert, J. Electrocatalytic behavior of hydrogenated Pd-metallic glass nanofilms: Butler-Volmer, Tafel, and impedance analyses. Electrocatalysis 2020, 11, 94–109. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Liu, Y.; Li, W.; Wang, Y.; Qin, G.; Lv, Z. Electrocatalytic HER Enhancement of C3N4 in NiCo2O4. Chem.—Asian J. 2022, 17, e202200377. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-S.; Zhang, L.-M.; Fan, K.; Xie, J.-Y.; Shang, X.; Zhang, J.-Q.; Chi, J.-Q.; Yang, X.-L.; Wang, L.; Chai, Y.-M.; et al. In Situ formation of ultrathin C3N4 layers on metallic WO2 nanorods for efficient hydrogen evolution. Appl. Surf. Sci. 2019, 487, 945–950. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, H.; Zhu, Y.; Luo, S.; Ma, J. Interfacial N-Cu-S coordination mode of CuSCN/C3N4 with enhanced electrocatalytic activity for hydrogen evolution. Nanoscale 2019, 11, 12938–12945. [Google Scholar] [CrossRef] [PubMed]
- Moradighadi, N.; Nesic, S.; Tribollet, B. Identifying the dominant electrochemical reaction in electrochemical impedance spectroscopy. Electrochim. Acta 2021, 400, 139460. [Google Scholar] [CrossRef]
- Sengur-Tasdemir, R.; Guler-Gokce, Z.; Sezai Sarac, A.; Koyuncu, I. Determination of membrane protein fouling by UV spectroscopy and electrochemical impedance spectroscopy. Polym.-Plast. Technol. Eng. 2018, 57, 59–69. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhang, J.; Yan, H.; Wang, C.; Wu, F.; Tian, A.; Hong, X.; Dong, W.; Yang, S. Effect of average interlayer spacing on capacitance of NiMn layered double hydroxide. Chem. Eng. J. 2020, 398, 125618. [Google Scholar] [CrossRef]
- Fang, Q.; Sun, M.; Ren, X.; Sun, Y.; Yan, Y.; Gan, Z.; Huang, J.; Cao, B.; Shen, W.; Li, Z.; et al. MnCo2O4/Ni3S4 nanocomposite for hybrid supercapacitor with superior energy density and long-term cycling stability. J. Colloid Interface Sci. 2022, 611, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xue, Q.; Zhao, Y.; Jiang, J.X.; Zeng, J.H.; Yin, S.B.; Chen, Y. Component-dependent electrocatalytic activity of ultrathin PdRh alloy nanocrystals for the formate oxidation reaction. ACS Sustain. Chem. Eng. 2018, 7, 2830–2836. [Google Scholar] [CrossRef]
- Rao, F.; Ding, K.; Zhou, Y.; Zheng, Y.; Xia, M.; Lv, S.; Song, Z.; Feng, S.; Ronneberger, I.; Mazzarello, R.; et al. Reducing the stochasticity of crystal nucleation to enable subnanosecond memory writing. Science 2017, 358, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Nissimagoudar, A.S.; Umer, M.; Ha, M.; Zafari, M.; Umer, S.; Lee, G.; Kim, K.S. Late transition metal doped MXenes showing superb bifunctional electrocatalytic activities for water splitting via distinctive mechanistic pathways. Adv. Energy Mater. 2021, 11, 2102388. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Y.; Yao, N.; Yang, F.; Cheng, G.; Luo, W. IrMo nanocatalysts for efficient alkaline hydrogen electrocatalysis. ACS Catal. 2020, 10, 7322–7327. [Google Scholar] [CrossRef]
- Wang, P.; Shao, Q.; Guo, J.; Bu, L.; Huang, X. Promoting alkaline hydrogen evolution catalysis on P-decorated, Ni-segregated Pt–Ni–P nanowires via a synergetic cascade route. Chem. Mater. 2020, 32, 3144–3149. [Google Scholar] [CrossRef]
- Creus, J.; Drouet, S.; Surinach, S.; Lecante, P.; Collière, V.; Poteau, R.; Philippot, K.; García-Antón, J.; Sala, X. Ligand-capped Ru nanoparticles as efficient electrocatalyst for the hydrogen evolution reaction. ACS Catal. 2018, 8, 11094–11102. [Google Scholar] [CrossRef]
- Zhuang, M.; Ou, X.; Dou, Y.; Zhang, L.; Zhang, Q.; Wu, R.; Ding, Y.; Shao, M.; Luo, Z. Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N, P-doped graphene for hydrogen generation. Nano Lett. 2016, 16, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pei, J.; He, C.T.; Wan, J.; Ren, H.; Zhu, Y.; Wang, Y.; Dong, J.; Tian, S.; Cheong, W.; et al. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew. Chem. 2017, 129, 16302–16306. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.X.; Jiang, H.L. Metal–organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y. In situ cobalt–cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Huang, J.; Xi, Y.; Gao, R.; Su, G.; Wang, W.; Cao, L.; Dong, B. Vertical growth of 2D amorphous FePO4 nanosheet on Ni foam: Outer and inner structural design for superior water splitting. Adv. Mater. 2017, 29, 1704574. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey reduced graphene oxide coupled with an Mo2N–Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 2018, 30, 1704156. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Li, Y.; Li, Z.; Lv, F.; Lang, Z.; Zhao, K.; Zhou, L.; Moskaleva, L.; Guo, S.; Mai, L. MoB/g-C3N4 interface materials as a schottky catalyst to boost hydrogen evolution. Angew. Chem. 2018, 130, 505–509. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Deng, W.; Weng, Y.; Jiang, J.; Mao, H.; Zhang, W.; Lu, T.; Long, D.; Jiang, F. Intercalated PtCo Electrocatalyst of Vanadium Metal Oxide Increases Charge Density to Facilitate Hydrogen Evolution. Molecules 2024, 29, 1518. https://doi.org/10.3390/molecules29071518
Zhang J, Deng W, Weng Y, Jiang J, Mao H, Zhang W, Lu T, Long D, Jiang F. Intercalated PtCo Electrocatalyst of Vanadium Metal Oxide Increases Charge Density to Facilitate Hydrogen Evolution. Molecules. 2024; 29(7):1518. https://doi.org/10.3390/molecules29071518
Chicago/Turabian StyleZhang, Jingjing, Wei Deng, Yun Weng, Jingxian Jiang, Haifang Mao, Wenqian Zhang, Tiandong Lu, Dewu Long, and Fei Jiang. 2024. "Intercalated PtCo Electrocatalyst of Vanadium Metal Oxide Increases Charge Density to Facilitate Hydrogen Evolution" Molecules 29, no. 7: 1518. https://doi.org/10.3390/molecules29071518
APA StyleZhang, J., Deng, W., Weng, Y., Jiang, J., Mao, H., Zhang, W., Lu, T., Long, D., & Jiang, F. (2024). Intercalated PtCo Electrocatalyst of Vanadium Metal Oxide Increases Charge Density to Facilitate Hydrogen Evolution. Molecules, 29(7), 1518. https://doi.org/10.3390/molecules29071518