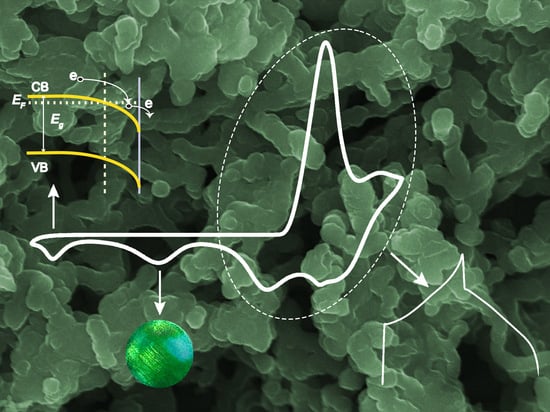
Electrodeposition of Polyaniline on Tantalum: Redox Behavior, Morphology and Capacitive Properties
Abstract
:1. Introduction
2. Results
2.1. Cyclic Voltammetric Deposition of PANI on the Ta|Ta2O5 Electrode
- We aimed to prevent the formation of PANI hydrolysis products, which might occur due to possible overoxidation of PANI at relatively high potential values (>0.8 VSCE).
- We aimed to detect any possible cathodic peaks that could emerge during the successive potential sweeps.
2.2. Electrochemical Response of the Ta|Ta2O5-PANI|PANI Electrode
- When Eupper ≤ 0.3 VSCE, peaks C3 and C4 disappeared concomitantly with the anodic peaks A1 and A2, as can be clearly seen in Figure 4c.
- When Eupper ranged between 0.35 and 0.9 VSCE, cathodic peaks C1 and C2 remained almost constant and started to gradually diminish at Eupper ≤ 0.6 VSCE (Figure 4a). Peaks C3 and C4 remained almost unchanged within the whole potential region.
- When Eupper > 0.9 VSCE, peaks C1 and C2 gradually merged into a single peak that shifted toward lower potentials. Eventually, at Eupper = 1.5 VSCE, the single cathodic peak originating from the combination of peaks C1 and C2 merged with C3, whereas peak C4 remained unaffected (Figure 4b).
2.3. Effect of the Potential Scan Rate on the Synthesis of PANI
2.4. Morphology of PANI Films Electrodeposited on Ta
2.5. Galvanostatic Charge/Discharge Curves
3. Discussion
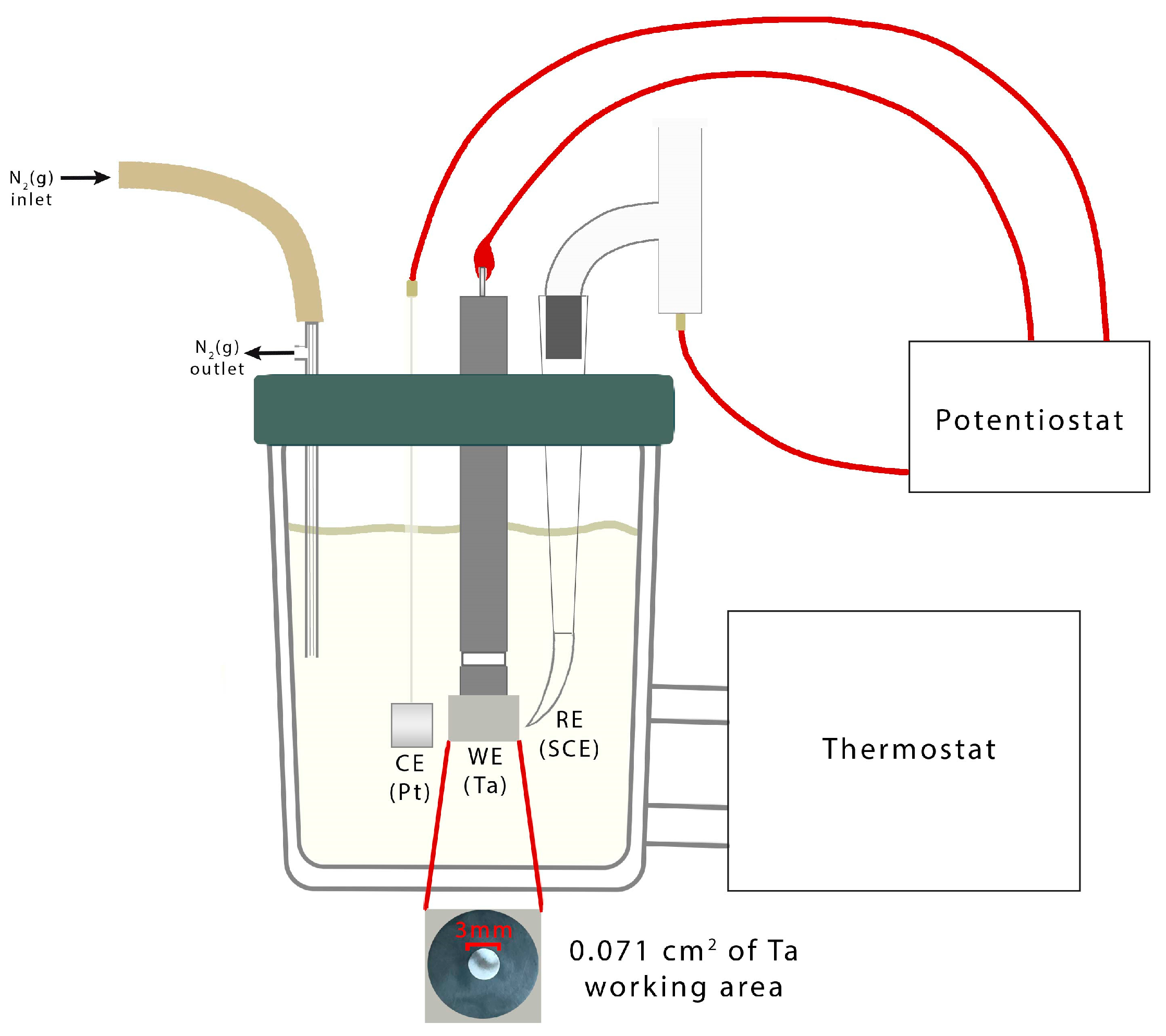
4. Materials and Methods
5. Conclusions
- The redox behavior of PANI exhibits two additional cathodic peaks, apart from the expected redox behavior comprising transitions between different oxidation states. This behavior was not previously observed for the Ta electrode. It is suggested that the cathodic peak close to −1.2 VSCE is related to the n-type semiconducting properties of the Ta2O5, as it appears close to its EFB, and the other one, located at less negative potentials, is associated with the reduction of phenazine species formed during the initiation step of polymerization.
- Polymerization occurs simultaneously with the oxide layer, and PANI seems to develop from the adsorbed oligomer nucleates comprising hydrophobic phenazine-containing species fixed toward the hydrophobic Ta2O5 surface. The growth of the PANI continues, presumably from the hydrophilic protonated part of nucleates with enhanced polymerization rates due to the gradual increase in the conductivity in the inner Ta2O5-PANI composite layer, affecting the PANI morphology.
- The morphology of PANI films depends on the synthesis potential scan rate. It comprises nano- or microspheres that can be joined, leading to nano- or microrods/fibers with a cluster networking structure.
- Preliminary galvanostatic charge-discharge experiments indicate that the prepared Ta|Ta2O5-PANI|PANI electrodes display sufficient capacitive properties, being dependent on the potential scan rate used for the synthesis of PANI. The obtained specific capacitance varies between ~700 and ~1200 F g−1 at current density values ranging from ~75 to ~2 mA cm−2 in an operating potential window of −0.35–0.8 VSCE. Certainly, further studies are required to characterize the capacitive responses and cycling stability values of these electrodes.
- Finally, it should be mentioned that the results of this study can give valuable leads to control the formation of PANI films with a desired morphology and properties on other valve metals, extending their application range.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Skotheim, T.A.; Reynolds, J.R. Conjugated Polymers: Theory, Synthesis, Properties, and Characterization, 3rd ed.; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Eftekhari, A. (Ed.) Nanostructured Conductive Polymers; John Wiley & Sons Ltd.: Chichester, UK, 2010; pp. 631–672. [Google Scholar]
- Guo, Y.P.; Zhou, Y. Polyaniline nanofibers fabricated by electrochemical polymerization: A mechanistic study. Eur. Polym. J. 2007, 43, 2292–2297. [Google Scholar] [CrossRef]
- Huang, J. Syntheses and applications of conducting polymer polyaniline nanofibers. Pure Appl. Chem. 2006, 78, 15–27. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Nanostructured polyaniline sensors. Chem. Eur. J. 2004, 10, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Sazou, D. Corrosion Protection of Metals by Intrinsically Conducting Polymers; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Dwivedi, G.; Munjal, G.; Bhaskarwar, A.N.; Chaudhary, A. Dye-sensitized solar cells with polyaniline: A review. Inorg. Chem. Commun. 2022, 135, 109087. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Kumar, N.; Ghosh, S.; Thakur, D.; Lee, C.-P.; Sahoo, P.K. Recent advancements in zero- to three-dimensional carbon networks with a two-dimensional electrode material for high-performance supercapacitors. Nanoscale Adv. 2023, 5, 3146–3176. [Google Scholar] [CrossRef]
- Jabeen, N.; Hussain, A.; Elsaeedy, H.I.; Rahman, A.U.; Tarique, M. Unique hierarchical architecture of SnO2 hexagonal interconnected nanolayered arrays as negative electrode for high performance asymmetric supercapacitors. Mater. Chem. Phys. 2023, 303, 127796. [Google Scholar] [CrossRef]
- Jabeen, N.; Ul Hassan, N.; Bokhari, A.; Khan, M.F.; Eldin, S.M.; Ul Arifeen, W.; Hussain, A.; Bahajjaj, A.A.A. High performance δ-Bi2O3 nanosheets transformed Bi2S3 nanoflakes interconnected nanosheets as negative electrode for supercapacitor applications. Fuel 2023, 347, 128392. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Yu, J.; Tian, F.; Luo, X.; Hao, Y.; Huang, J.; Wang, F.; Zhou, W.; Xu, J.; et al. Flexible Supercapacitors Based on Stretchable Conducting Polymer Electrodes. Polymers 2023, 15, 1856. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenović, M.M.; Jugović, B.; Stevanović, J.S.; Trišović, T.; Grgur, B. Electrochemical Polymerization of Aniline. In Electropolymerization; Schab-Balcerzak, E., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Sazou, D.; Kourouzidou, M.; Pavlidou, E. Potentiodynamic and potentiostatic deposition of polyaniline on stainless steel: Electrochemical and structural studies for a potential application to corrosion control. Electrochim. Acta 2007, 52, 4385–4397. [Google Scholar] [CrossRef]
- Sazou, D.; Georgolios, C. Formation of conducting polyaniline coatings on iron surfaces by electropolymerization of aniline in aqueous solutions. J. Electroanal. Chem. 1997, 429, 81–93. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Abalyaeva, V.V.; Kogan, I.L. Initiating agents for electrochemical polymerization of aniline on titanium electrodes. Synth. Met. 1994, 63, 109–113. [Google Scholar] [CrossRef]
- Arsov, L.D. Electrochemical study of polyaniline deposited on a titanium surface. J. Solid. State Electrochem. 1998, 2, 266–272. [Google Scholar] [CrossRef]
- Rakovska, B.; Valiūnienė, A.; Malinauskas, A.; Kubilius, V.; Valiūnas, R. Electrochemical formation of polyaniline on Ti and electrochemically oxidized Ti electrodes. Chemija 2012, 23, 12–17. [Google Scholar] [CrossRef]
- Saltidou, K.; Pavlidou, E.; Sazou, D. The effect of ionic and electronic properties of titanium oxide on the electrochemical growth and redox behavior of polyaniline on titanium surfaces. J. Solid. State Electrochem. 2017, 21, 2055–2069. [Google Scholar] [CrossRef]
- Kellenberger, A.; Plesu, N.; Tara-Lunga Mihali, M.; Vaszilcsin, N. Synthesis of polyaniline nanostructures by electrochemical deposition on niobium. Polymer 2013, 54, 3166–3174. [Google Scholar] [CrossRef]
- Tara-Lunga-Mihali, M.; Plesu, N.; Kellenberger, A.; Ilia, G. Adsorption of an Azo Dye on Polyaniline/ Niobium Substrate. Int. J. Electrochem. Sci. 2015, 10, 7643–7659. [Google Scholar] [CrossRef]
- Abalyaeva, V.V.; Efimov, O.N. Electrocatalytic synthesis of polyaniline on non-noble metal electrodes. Polym. Adv. Technol. 1997, 8, 517–524. [Google Scholar] [CrossRef]
- Kogan, I.L.; Abalyaeva, V.V.; Gedrovich, G. Electrochemical Synthesis of Polyaniline on Tantalum and Stainless-Steel Electrodes. Synth. Met. 1994, 63, 153–156. [Google Scholar] [CrossRef]
- Cavigliasso, G.E.; Esplandiu, M.J.; Macagno, V.A. Influence of the forming electrolyte on the electrical properties of tantalum and niobium oxide films: An EIS comparative study. J. Appl. Electrochem. 1998, 28, 1213–1219. [Google Scholar] [CrossRef]
- Sulyma, C.M.; Roy, D. Voltammetric current oscillations due to general and pitting corrosion of tantalum: Implications for electrochemical–mechanical planarization. Corros. Sci. 2010, 52, 3086–3098. [Google Scholar] [CrossRef]
- Stilwell, D.E.; Park, S.M. Electrochemistry of conductive polymers. 2. Electrochemical studies on growth properties of polyaniline. J. Electrochem. Soc. 1988, 135, 2254–2262. [Google Scholar] [CrossRef]
- Puskás, Z.; Inzelt, G. Electrochemical microgravimetric study on microcrystalline particles of phenazine attached to gold electrodes. J. Solid. State Electrochem. 2004, 8, 828–841. [Google Scholar] [CrossRef]
- Scholz, F.; Lovrić, M.; Stojek, Z. The role of redox mixed phases {oxx(Cnred)1−x} in solid state electrochemical reactions and the effect of miscibility gaps in voltammetry. J. Solid. State Electr. 1997, 1, 134–142. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Soriaga, M.P. Electron-Transfer-Induced Molecular Reorientations: The Benzoquinone/Hydroquinone Reaction at Pd(111)-(□3×□3)R30°-I Studied by EC-STM. J. Colloid Interface Sci. 2001, 236, 197–199. [Google Scholar] [CrossRef]
- Aggadi, S.; Loudiyi, N.; Chadil, A.; Zoubida, E.-A.; Hourch, A. Electropolymerization of aniline monomer and effects of synthesis conditions on the characteristics of synthesized polyaniline thin films. Mediterr. J. Chem. 2020, 10, 138–145. [Google Scholar] [CrossRef]
- Kellenberger, A.; Ambros, D.; Plesu, N. Scan Rate Dependent Morphology of Polyaniline Films Electrochemically Deposited on Nickel. Int. J. Electrochem. Sci. 2014, 9, 6821–6833. [Google Scholar] [CrossRef]
- Putri, N.P.; Suaebah, E.; Rohmawati, L.; Santjojo, D.J.D.H.; Masruroh, M.; Sakti, S.P. Implications of the Electrodeposition Scan Rate on the Morphology of Polyaniline Layer and the Impedance of a QCM Sensor. Trends Sci. 2023, 20, 6411. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, H.; Zhu, X.; Li, D.; Zhang, S.; Chen, X.; Song, Y. Electropolymerization of Aniline onto Anodic WO3 Film: An Approach to Extend Polyaniline Electroactivity Beyond pH 7. J. Phys. Chem. C 2014, 118, 27449–27458. [Google Scholar] [CrossRef]
- Molina, J.; del Río, A.I.; Bonastre, J.; Cases, F. Influence of the scan rate on the morphology of polyaniline grown on conducting fabrics. Centipede-like morphology. Synth. Met. 2010, 160, 99–107. [Google Scholar] [CrossRef]
- Shah, A.-u.-H.A.; Khan, M.O.; Bilal, S.; Rahman, G.; Hoang, H.V. Electrochemical co-deposition and characterization of polyaniline and manganese oxide nanofibrous composites for energy storage properties. Adv. Polym. Technol. 2018, 37, 2230–2237. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled Synthesis and Energy Applications of One-Dimensional Conducting Polymer Nanostructures: An Overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Cabrera-Sierra, R.; Vazquez-Arenas, J.; Cardoso, S.; Luna-Sanchez, R.M.; Trejo, M.A.; Marin-Cruz, J.; Hallen, J.M. Analysis of the formation of Ta2O5 passive films in acid media through mechanistic modeling. Electrochim. Acta 2011, 56, 8040–8047. [Google Scholar] [CrossRef]
- Pligovka, A. Reflectant Photonic Crystals Produced via Porous-Alumina-Assisted-Anodizing of Al/Nb and Al/Ta Systems. Surf. Rev. Lett. 2021, 28, 2150055. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Turavets, U.; Hoha, A.; Salerno, M. Two-Level 3D Column-like Nanofilms with Hexagonally—Packed Tantalum Fabricated via Anodizing of Al/Nb and Al/Ta Layers—A Potential Nano-Optical Biosensor. Materials 2023, 16, 993. [Google Scholar] [CrossRef]
- Zaffora, A.; Cho, D.-Y.; Lee, K.-S.; Di Quarto, F.; Waser, R.; Santamaria, M.; Valov, I. Electrochemical Tantalum Oxide for Resistive Switching Memories. Adv. Mater. 2017, 29, 1703357. [Google Scholar] [CrossRef]
- Xia, S.; Ni, J.; Savilov, S.V.; Li, L. Oxygen-deficient Ta2O5 nanoporous films as self-supported electrodes for lithium microbatteries. Nano Energy 2018, 45, 407–412. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers-Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Di Quarto, F.; Gentile, C.; Piazza, S.; Sunseri, C. A photoelectrochemical study on anodic tantalum oxide films. Corros. Sci. 1993, 35, 801–808. [Google Scholar] [CrossRef]
- Schultze, J.W.; Macagno, V.A. Electron transfer reaction on passive tantalum electrodes. Electrochim. Acta 1986, 31, 355–363. [Google Scholar] [CrossRef]
- Sato, N. Electrochemistry at Metal and Semiconductor Electrodes; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Mozaffari, S.A.; Mahmoudi Najafi, S.H.; Norouzi, Z. Hierarchical NiO@Ni(OH)2 nanoarrays as high-performance supercapacitor electrode material. Electrochim. Acta 2021, 368, 137633. [Google Scholar] [CrossRef]












| υ (mV s−1) for PANI Synthesis | 1 | 1 |
|---|---|---|
| 20 | 9.11 | 152.54 |
| 50 | 3.48 | 18.78 1 |
| 75 | 3.46 | 10.82 |
| 100 | 2.56 | 5.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkili, C.; Deligiannakis, K.; Lappa, E.; Papoulia, C.; Sazou, D. Electrodeposition of Polyaniline on Tantalum: Redox Behavior, Morphology and Capacitive Properties. Molecules 2023, 28, 7286. https://doi.org/10.3390/molecules28217286
Gkili C, Deligiannakis K, Lappa E, Papoulia C, Sazou D. Electrodeposition of Polyaniline on Tantalum: Redox Behavior, Morphology and Capacitive Properties. Molecules. 2023; 28(21):7286. https://doi.org/10.3390/molecules28217286
Chicago/Turabian StyleGkili, Chrysanthi, Konstantinos Deligiannakis, Eirini Lappa, Chrysanthi Papoulia, and Dimitra Sazou. 2023. "Electrodeposition of Polyaniline on Tantalum: Redox Behavior, Morphology and Capacitive Properties" Molecules 28, no. 21: 7286. https://doi.org/10.3390/molecules28217286
APA StyleGkili, C., Deligiannakis, K., Lappa, E., Papoulia, C., & Sazou, D. (2023). Electrodeposition of Polyaniline on Tantalum: Redox Behavior, Morphology and Capacitive Properties. Molecules, 28(21), 7286. https://doi.org/10.3390/molecules28217286