An Evaluation of the Edible Value of Salvia miltiorrhiza Seeds: Proximate Composition, Phytochemical Components and Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Characteristics of SMS
2.2. Characteristics of SMS Oil
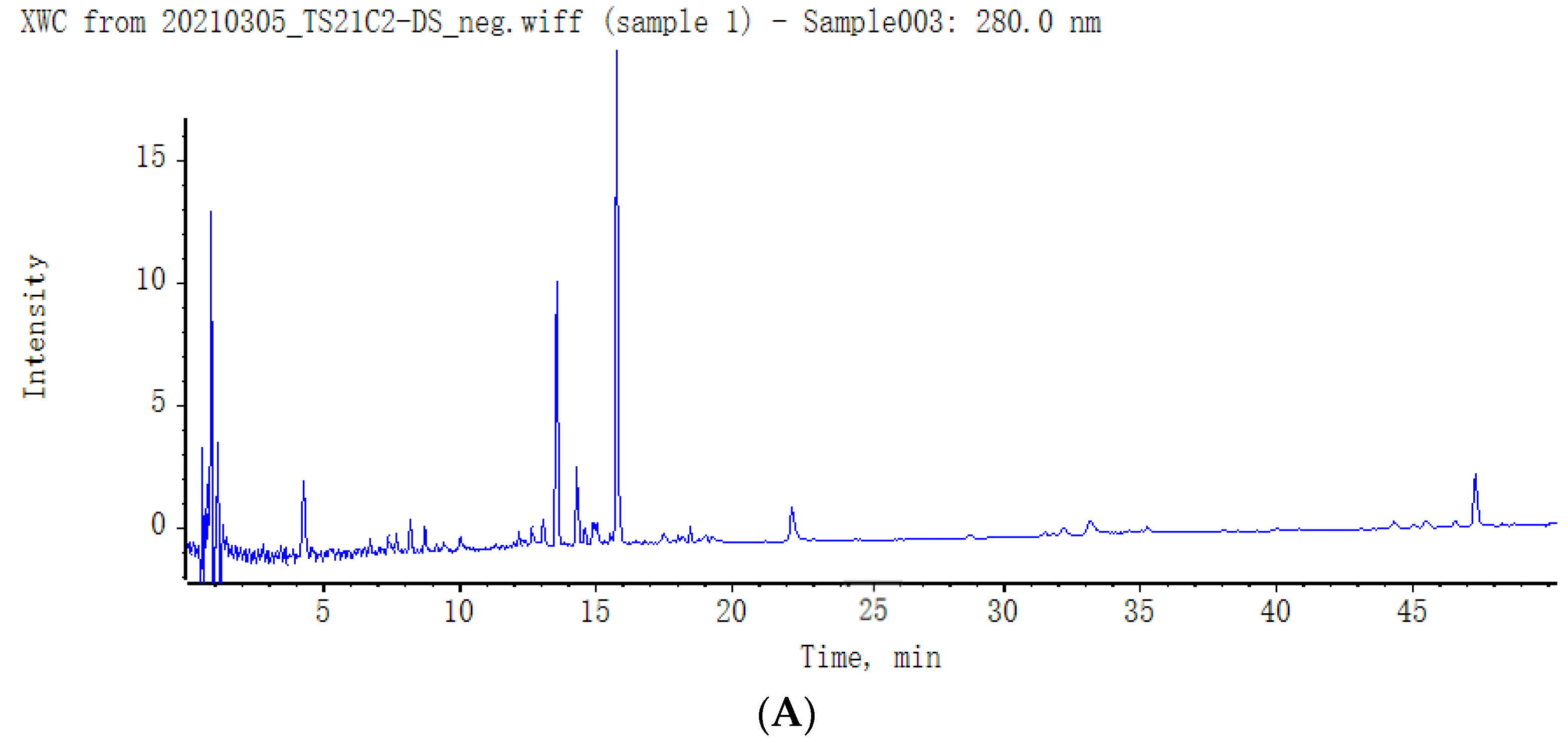
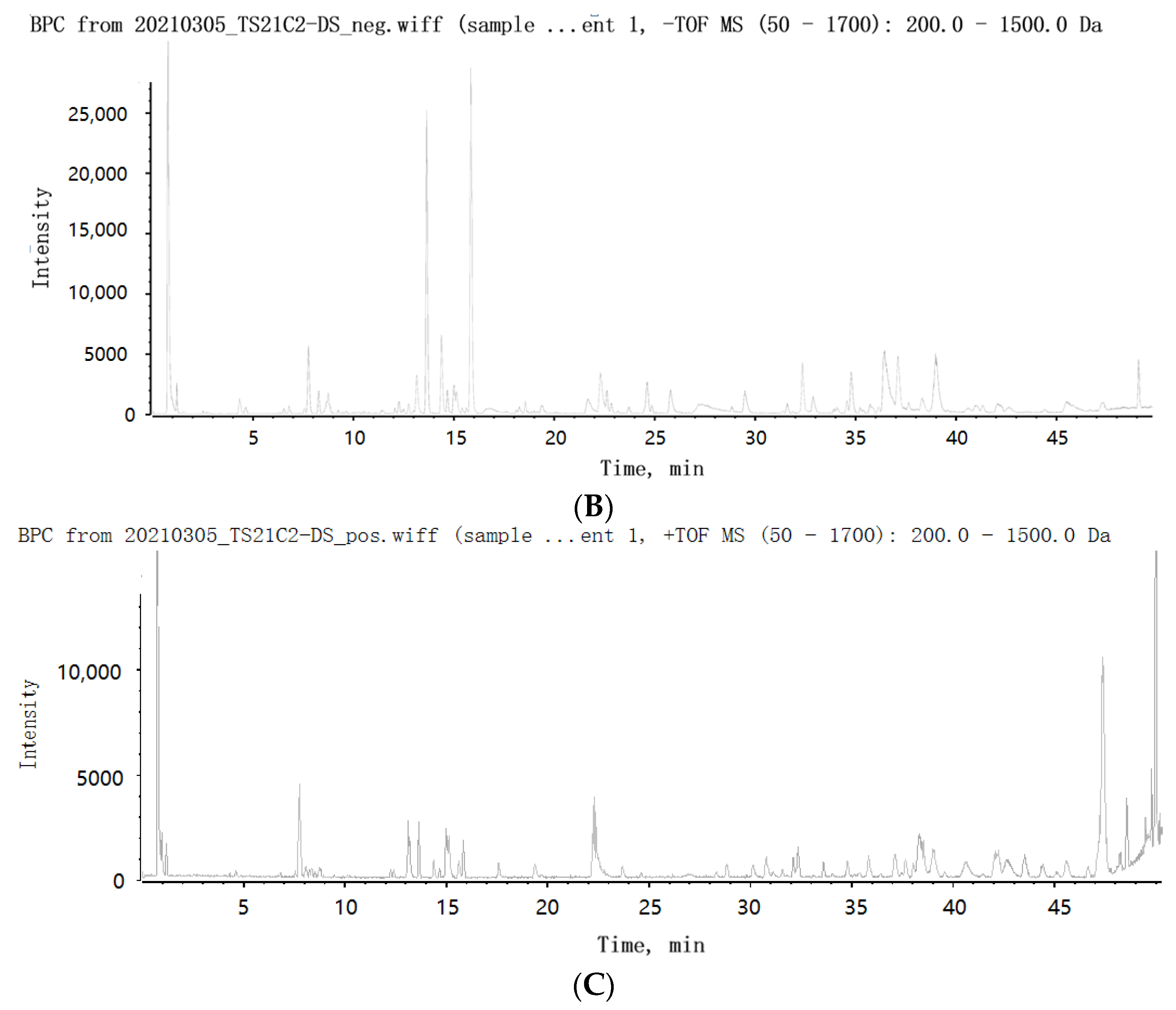
2.3. Identification of Secondary Metabolites in SMS
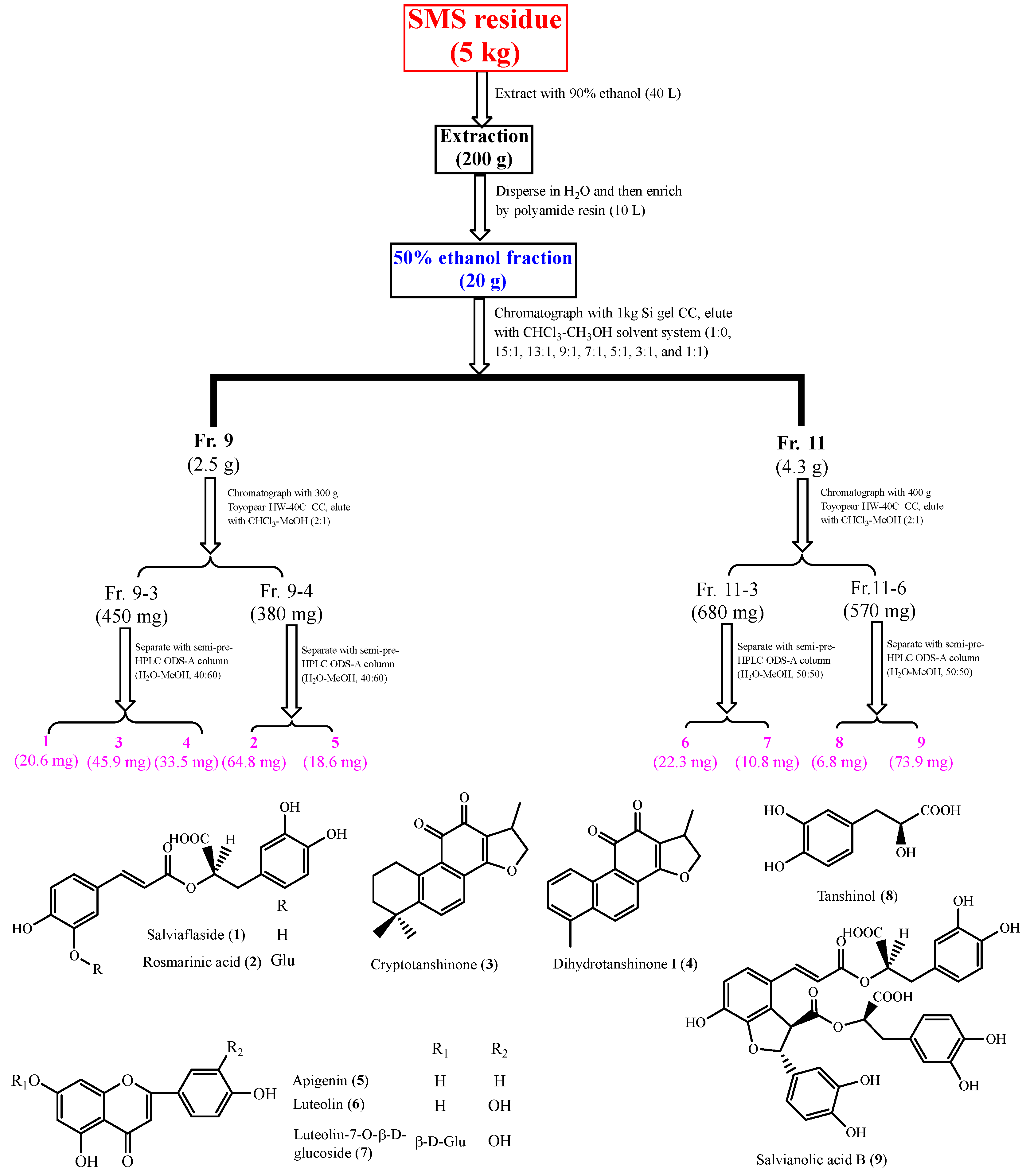
2.4. Structure Identification of the Isolated Compounds
2.5. Antioxidant Activity of TPE
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals
4.3. Characterization of SMS
4.4. Characterization of SMS Oil
4.5. The Investigation of the Secondary Metabolites in SMS
4.5.1. The Sample Preparation
4.5.2. UPLC-Q-TOF/MS Analysis
4.6. The Extraction and Isolation of Phenolic Compounds from TPE
4.7. Antioxidant Capacity
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMS | Salvia miltiorrhiza seeds |
| FA | fatty acid |
| TSW | thousand seed weight |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| GAE | gallic acid equivalent |
| RE | rutin equivalent |
| LA | linoleic acid |
| OA | oleic acid |
| ALA | α-linolenic acid |
| PA | palmitic acid |
| SA | stearic acid |
| USFA | unsaturated fatty acid |
| TUFA | total unsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| TPE | total phenolic extraction |
| POV | peroxide value |
| AV | acid value |
| SV | saponification value |
| IV | iodine value |
References
- Zhou, J.; Zhang, L.; Zheng, B.; Zhang, L.H.; Qin, Y.; Zhang, X.H.; Yang, Z.; Nie, Z.Y.; Yang, G.S.; Yu, J.; et al. Salvia miltiorrhiza Bunge exerts anti-oxidative effects through inhibiting KLF10 expression in vascular smooth muscle cells exposed to high glucose. J. Ethnopharmacol. 2020, 262, 113208. [Google Scholar] [CrossRef] [PubMed]
- Gören, A.C.; Kiliç, T.; Dirmenci, T.; Bilsel, G. Chemotaxonomic evaluation of Turkish species of Salvia: Fatty acid compositions of seed oils. Biochem. Syst. Ecol. 2006, 34, 160–164. [Google Scholar] [CrossRef]
- Liu, H.Y.; Niu, M.; Zhu, S.; Zhang, F.; Liu, Q.; Liu, Y.; Liu, R.H.; Zhang, Y.Q. Effect study of continuous monoculture on the quality of Salvia miltiorrhiza Bge roots. Biomed. Res. Int. 2020, 2020, 4284385. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and the rapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Lin, H.W.; Lin, Y.L.; Yang, D.J.; Yu, Y.S.; Chen, J.W.; Wang, S.Y.; Chen, Y.C. Nutritional composition in the chia seed its processing properties on restructured ham-like products. J. Food Drug Anal. 2018, 26, 124–134. [Google Scholar] [CrossRef]
- Munoz, L.A.; Cobos, A.; Diaz, O.; Agilera, J.M. Chia seeds: Microstructure mucilage extraction hydration. J. Food Eng. 2012, 108, 216–224. [Google Scholar] [CrossRef]
- Marineli, R.S.; Moraes, É.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Maróstica, M.R., Jr. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT-Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, X.D.; Chen, Z.; Sang, J.Y.; Xie, W.J.; Song, S.W.; Zhang, P.; Zhu, S. Analysis of nutrition components in brown rice, germinated rice, and polished rice. Food Sci. Technol. 2016, 41, 156–159. [Google Scholar] [CrossRef]
- Zhang, T.W.; She, Y.X.; Wu, X.L.; Liu, Q.H.; Li, Y.; Hao, Z.H.; Qiu, C. Analysis on the differences in the contents of nutrients and mineral elementsin hulless barley varieties grown in different ecological environments. Barley Cereal Sci. 2020, 37, 6–9. [Google Scholar]
- Liu, Y.; Liu Xi, Q.; Liang, Y.H.; Feng, W.H.; Yang, L.X.; Li, C.; Wang, Z.M. Comparison of fatty acid compositions and antioxidant activities of eleven vegetable oils. China Oils Fats 2020, 45, 52–57. [Google Scholar]
- Tian, Z.F.; Liang, X.; Meng, T.T.; Shi, L.; Zhou, B.L. Analysis of nutrition and quality between different oat varieties. Farm Prod. Process. 2019, 4, 57–59. [Google Scholar]
- Li, H.L.; Yin, F.P.; Sun, X.Y.; Tang, X.Z. Extraction process of sorghum protein by alkaline method the nutritional value evaluation. J. Anhui Agri. Univ. 2019, 46, 589–594. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, L.N.; Wang, X.S.; Gao, J.Y.; Yi, J.P.; Deng, R.X. Characterization of Paeonia ostii seed and oil sourced from different cultivation areas in China. Ind. Crop. Prod. 2019, 133, 63–71. [Google Scholar] [CrossRef]
- Liu, P.; Xu, Y.F.; Gao, X.D.; Zhu, X.Y.; Du, M.Z.; Wang, Y.X.; Deng, R.X.; Gao, J.Y. Optimization of ultrasonic-assisted extraction of oil from the seed kernels and isolation of monoterpene glycosides from the oil residue of Paeonia lactiflora Pall. Ind. Crop. Prod. 2017, 107, 260–270. [Google Scholar] [CrossRef]
- Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Martínez, M.L. Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants. LWT-Food Sci. Technol. 2017, 75, 107–113. [Google Scholar] [CrossRef]
- Deng, R.X.; Gao, J.Y.; Yi, J.P.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oil? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.L.; Qin, F.M.; Zhou, G.X. Studies on chemical constituents of Ranunculus muricatus Linn. Nat. Prod. Res. Dev. 2013, 25, 736–741. [Google Scholar]
- Šimko, I.; Omer, E.A.; Ewing, E.E.; McMurry, S.; Koch, J.L.; Davies, P.J. Tuberonic (12-OH-jasmonic) acid glucoside and its methyl ester in potato. Phytochemistry 1996, 43, 727–730. [Google Scholar] [CrossRef]
- Çaliş, İ.; Ersöz, T.; Saracoǧlu, İ.; Sticher, O. Scalbidoside and albidoside, two iridoid glycosides from Scutellaria albida subsp. Colchica. Phytochemistry 1993, 32, 1213–1217. [Google Scholar] [CrossRef]
- Deng, F.; Tang, N.; Chang, J.; Wang, Y.P.; Zhang, J.S. A new depside glucosides from Salvia miltiorrhiza f. alba. Asian J. Chem. 2008, 20, 6129–6133. [Google Scholar]
- Quirantes-Piné, R.; Zurek, G.; Barrajón-Catalán, E.; Bäßmann, C.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A metabolite-profiling approach to assess the uptake and metabolism of phenolic compounds from olive leaves in SKBR3 cells by HPLC-ESI-QTOF-MS. J. Pharmaceut. Biomed. 2013, 72, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Qiu, J.; Zhang, S.; Zhang, Y.; Zhang, Y.; Sun, M.; Zhang, J.H.; Liu, B.; Cheng, F.F.; Jiang, Y. Screening out the anti-insomnia components from Prunella vulgaris Lbased on plasma pharmacochemistry combined with pharmacodynamic experiments and UPLC-MS/MS analysis. J. Ethnopharmacol. 2021, 279, 114373. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Xu, M.; Guo, D.A. Detection characterization identification of phenolic acids in Danshen using high-performance liquid chromatography with diode array detection electrospray ionization mass spectrometry. J. Chromatogr. A 2007, 1161, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.W.; Lin, Y.P.; Wang, L.; Huo, Y.; Zhang, Y.Y.; Guo, J.D.; Han, L.F.; Chang, Y.X.; Gao, X.M. Simultaneous determination of pinoresinol di-glucopyranoside and pinoresinol glucoside in rat plasma by HPLC-tandem MS/MS for pharmacokinetic study. Chin. Herb. Med. 2016, 8, 337–343. [Google Scholar] [CrossRef]
- Tian, J.F.; Yan, H.; Wang, R.J.; Li, W.; Yue, H.S.; Luo, X.J.; He, Y. Isolation and identification of chemical constituents from extract of salvia polyphenolic acids. Chin. Tradit. Herbal Drugs 2018, 49, 5024–5028. [Google Scholar] [CrossRef]
- Yang, N.; Huang, W.; Dan, L.L.; Duan, J.Y.; Yu, H.X.; Yang, K.K.; Li, Y.B. Rapid classification and identification of Salvia miltiorrhiza BgeBy UPLC-Q-TOF/MS combined with data post-processing. Lishizhen Med. Mater. Medica Res. 2019, 30, 2408–2412. [Google Scholar]
- Zhou, W.; Xie, H.; Xu, X.; Liang, Y.; Wei, X. Phenolic constituents from Isodon lophanthoides vargraciliflorus and their antioxidant and antibacterial activities. J. Funct. Foods 2014, 6, 492–498. [Google Scholar] [CrossRef]
- Hu, F.; Liao, X.; Guo, Y.; Yamaki, S.; Li, X.; Hamada, N.; Hashi, K.; Chen, Z. Fast determination of isomeric triterpenic acids in Osmanthus fragrans (Thunb.) Lourfruits by UHPLC coupled with triple quadrupole mass spectrometry. Food Chem. 2020, 322, 126781. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.P.; Xiang, C.; Zhuang, W.T.; He, J.; Li, P.; Li, B.C. Study on the chemical constituents of Salvia przewalskii Maxim. Nat. Prod. Res. Dev. 2013, 25, 785–788, 801. [Google Scholar] [CrossRef]
- Zhang, L.G.; Hu, T.T.; Zhang, F.F.; Luan, S.R.; Li, W.; Deng, H.X.; Lan, Z.H. Analysis of lipophilic components of Salvia miltiorrhiza roots and Syunnanensis roots by UPLC and LC-MS /MS. China J. Chin. Mater. Med. 2019, 44, 1208–1215. [Google Scholar]
- Guo, Y.S.; Wang, G.C.; Wang, C.H.; Huang, X.J.; Li, Y.L.; Ye, W.C. Chemical constituents from Origanum vulgare. Chin. Pharm. J. 2012, 47, 1109–1113. [Google Scholar]
- Wu, J.P.; Song, Z.; Liu, Y.L.; Li, X.R.; Xu, Q.M.; Yang, S.L. Chemical constituents from Coriandrum sativum. Chin. Tradit. Pat. Med. 2018, 40, 1543–1546. [Google Scholar]
- Rahman, M.J.; Camargo, A.C.; Shahidi, F. Phenolic polyphenolic profiles of chia seeds their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- Kibui, A.N.; Owaga, E.; Mburu, M. Proximate composition and nutritional characterization of Chia enriched yoghurt. Afr. J. Food Agric. Nutr. Dev. 2018, 18, 13239–13253. [Google Scholar] [CrossRef]
- Shan, X.X.; Hong, B.Z.; Liu, J.; Wang, G.K.; Chen, W.D.; Yu, N.J.; Peng, D.Y.; Wang, L.; Zhang, C.Y. Review of chemical composition, pharmacological effects, and clinical application of Salviae Miltiorrhizae Radix et Rhizoma and prediction of its Q-markers. China J. Chin. Mater. Med. 2021, 46, 5496–5511. [Google Scholar]
- Chen, Y.H.; Zhang, X.R.; Guo, Q.S.; Liu, L.; Li, C.; Cao, L.P.; Qin, Q.; Zhao, M.; Wang, W.M. Effects of UV-B radiation on the content of bioactive components and the antioxidant activity of Prunella vulgaris L. Spica during development. Molecules 2018, 23, 989. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lei, Y.Q.; Wei, X.Z.; Lv, S.Z.; Luo, F.J. Study on the stability of scavenging DPPH free radical by Viola philippica apigenin. J. Food Saf. Qual. 2018, 9, 2775–2779. [Google Scholar] [CrossRef]
- Su, X.H.; Liang, D.Q.; Wang, X.M. The effect of danshensu of the injury of oxygen free radicals in myocardial mitochondria from rat myocardium Chin. J. Pathophysiol. 1992, 8, 122–124. [Google Scholar]
- Tian, Y.; He, K.J.; Zhu, J.B. Antioxidative activity of salvianolic acid, B.J. Dalian Polytochnic Univ. 2008, 27, 304–308. [Google Scholar]
- GB/T 5519; Determination of the Mass of 1000 Grains. Chinese Specification Press: Beijing, China, 2018.
- Zhang, Y.; Liu, P.; Gao, J.Y.; Wang, X.S.; Yan, M.; Xue, N.C.; Qu, C.X.; Deng, R.X. Paeonia veitchii seeds as a promising high potential by-product: Proximate composition, phytochemical components, bioactivity evaluation and potential applications. Ind. Crop. Prod. 2018, 125, 248–260. [Google Scholar] [CrossRef]
- ISO 771:1977; Determination of Moisture and Volatile Matter Content. Chinese Specification Press: Beijing, China, 1997.
- ISO 9184:1994; Determination of Fibre Coarseness. Chinese Specification Press: Beijing, China, 2010.
- ISO 20483: 2013; Determination of the Nitrogen Content and Calculation of the Crude Protein Content. Chinese Specification Press: Beijing, China, 2013.
- ISO 749: 1977; Determination of Total Ash. Chinese Specification Press: Beijing, China, 1977.
- Zhang, H.F.; Li, X.F.; Wu, K.; Wang, M.K.; Liu, P.; Wang, X.S.; Deng, R.X. Antioxidant activities and chemical constituents from the flower of Paeonia ostia. Molecules 2017, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17376-2008; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. Chinese Specification Press: Beijing, China, 2008.
- GB/T 17377-2008; Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids. Chinese Specification Press: Beijing, China, 2008.
- ISO 660: 1996; Determination of Acid Value and Acidity. Chinese Specification Press: Beijing, China, 1996.
- ISO 3960: 2001; Determination of Peroxide Value. Chinese Specification Press: Beijing, China, 2001.
- ISO 3657: 2002; Determination of Saponification Value. Chinese Specification Press: Beijing, China, 2002.
- ISO 3961: 1996; Determination of Iodine Value. Chinese Specification Press: Beijing, China, 1996.
- LS/T 6119-2017; Determination of Polyphenols in Vegetable Oil. Chinese Specification Press: Beijing, China, 2017.
- LS/T 6120-2017; Determination of Squalene in Vegetable Oil. Chinese Specification Press: Beijing, China, 2017.
- GB 5009.82-2016; Determination of Retinol and Tocopherol in Foods. Chinese Specification Press: Beijing, China, 2016.
- GB/T 25223-2010; Animal and Vegetable Fats and Oils—Determination of Individual and Total Sterols Contents—Gas Chromatographic Method. Chinese Specification Press: Beijing, China, 2010.
- Ammar, R.B.; Bhouri, W.; Sghaier, M.B.; Boubaker, J.; Skandrani, I.; Neffat, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.M.; Leila, C.G.; et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae): A structure-activity relationship study. Food Chem. 2009, 116, 258–264. [Google Scholar] [CrossRef]
- Chen, Q.H.; Xing, L. Fragmentation pathways of cryptotanshinone revealed by Ion Trap Mass Spectrometry in positive mod. Chin. Med. J. Res. Prac. 2016, 30, 9–12. [Google Scholar] [CrossRef]
- Ding, J.H.; Wang, X.X.; Zhang, H.; Pan, S.S.; Luo, M.B.; Li, J.Q.; Chen, H.W. Extrative electrospray ionization Tandem Mass Spectrometry of apigenin. Chem. J. Chin. U. 2011, 32, 1714–1719. [Google Scholar]
- Gan, G.S.; Cao, C.; Gan, Z.Y.; Zuo, Q. Effect of oil refining process and decolorization conditions on Soybean oil quality. J. Anhui Sci. Technol. Univ. 2018, 32, 50–55. [Google Scholar] [CrossRef]
- Gao, L.; Hu, B.R.; Qi, Y.L.; Zhu, Y.N. Grape seed oil: Organic solvent extraction with the assistance of cellulase or ultrasonic and evaluation of physico-chemical properties. Food Sci. 2009, 30, 81–83. [Google Scholar]
- Fu, R.; Zhang, Y.T.; Guo, Y.R.; Liu, F.X.; Chen, F. Determination of phenolic contents and antioxidant activities of extracts of Jatropha curcas L. seed shell, a by-product, a new source of natural antioxidant. Ind. Crop. Prod. 2014, 58, 265–270. [Google Scholar] [CrossRef]
- Hui, R.H.; Hou, D.Y.; Li, X.C.; Liu, X.Y.; Han, Y. Preparation of corn oil and determination of fatty acids in corn oil. Food Sci. 2006, 27, 418–450. [Google Scholar]
- Li, Y.; Li, Y.J.; Zhang, G.Q.; Li, H.Y.; Jing, M. Analysis of chemical constituents in Biebersteinia heterstemon Maxim by HPLC-Q-TOF-MS/MS. Chin. J. Ethnomedi. Ethnopharm. 2022, 31, 45–52. [Google Scholar]
- Liu, Y.J.; Chen, Y.H.; Yang, X.S.; Ren, G.X. Research progress on nutrition and functional components of millet. Cereals Oils 2020, 33, 1–3. [Google Scholar]
- Tai, Z.G.; Chen, A.Y.; Qin, B.D.; Cai, L.; Xu, Y.Q. Chemical constituents and antioxidant activity of the Musa basjoo flower. Eur. Food Res. Technol. 2014, 239, 501–508. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M.; Assadpoor, E.; Nowrouzieh, S.; Alishah, O. Optimization of microwave-assisted extraction of cottonseed oil and evaluation of its oxidative stability and physicochemical properties. Food Chem. 2014, 160, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhou, Y.T.; Ma, J.H.; Shi, T.Y.; Lu, M.; Fu, X.; Zhang, L.C. Study on the physicochemical properties and antioxidant activity of two varieties cold-pressed peanut oil. Food Res. Dev. 2019, 40, 38–43. [Google Scholar]
- Zhang, Y.N.; Guo, L.; Su, Y.; Li, X.D. The Process of ultrasound and freeze-microwave assisted aqueous enzymatic extraction of sesame oil. Food Res. Dev. 2019, 40, 102–109. [Google Scholar]
- Zhang, Y.Y.; Chen, F.X.; Gu, B.; Liang, A.Y. Physicochemical properties and chemical composition of high oleic sunflower oil. Cereals Oils Process. 2015, 6, 34–36. [Google Scholar]
| Seeds | S. miltiorrhiza |
|---|---|
| Crude protein | 26.65 ± 2.51 |
| Crude fibre | 28.68 ± 4.66 |
| Oil content | 28.45 ± 3.44 |
| Moisture | 4.26 ± 0.65 |
| Crude ash | 3.69 ± 0.37 |
| Carbohydrate | 34.62 ± 3.64 |
| Amino Acid | S. miltiorrhiza | Amino Acid | S. miltiorrhiza |
|---|---|---|---|
| Aspartic acid | 1.72 ± 0.15 | Isoleucine | 0.74 ± 0.08 |
| Threonine | 0.81 ± 0.10 | Leucine | 1.24 ± 0.15 |
| Serine | 1.14 ± 0.11 | Tyrosine | 0.97 ± 0.12 |
| Glutamic acid | 3.14 ± 0.45 | Phenylalanine | 0.89 ± 0.12 |
| Glycine | 1.05 ± 0.13 | Lysine | 1.05 ± 0.08 |
| Alanine | 0.87 ± 0.08 | Histidine | 0.46 ± 0.04 |
| Cysteine | 0.35 ± 0.04 | Arginine | 2.08 ± 0.22 |
| Valine | 0.93 ± 0.09 | Proline | 0.68 ± 0.05 |
| Methionine | 0.52 ± 0.05 | Tryptophan | 0.46 ± 0.03 |
| Fatty Acid Composition | Content (%) | Fatty Acid Composition | Content (%) |
|---|---|---|---|
| Dodecanoic acid (C12:0) | 0.01 ± 0.01 | Linoleic acid (C18:2) (ω-6) | 33.774 ± 4.68 |
| Myristic acid (C14:0) | 0.113 ± 0.01 | Linolenic acid (C18:3) (ω-3) | 25.968 ± 2.96 |
| Pentadecanoic acid (C15:0) | 0.037 ± 0.01 | 18-Methylnonadecanoic acid (C19:0) | 0.478 ± 0.01 |
| Cis-5-dodecenoic acid (C12:1) | 0.012 ± 0.01 | Cis-13-Eicosenoic acid (C20:1) | 0.511 ± 0.11 |
| Palmitic acid (C16:0) | 8.634 ± 0.67 | 9,11,13,15-Octadecatetraenoic acid (C18:4) (ω-3) | 0.013 ± 0.01 |
| E-9-Hexadecenoic acid (C16:1) | 0.148 ± 0.06 | ω-3 fatty acid | 26.098 ± 2.97 |
| Heptadecanoic acid (C17:0) | 0.145 ± 0.08 | ω-6 fatty acid | 33.774 ± 4.68 |
| Cis-10-Heptadecenoic acid (C17:1) | 0.064 ± 0.01 | (ω-3)/(ω-6) | 0.773 |
| 16-methyl-Heptadecanoic acid (C18:0) | 0.094 ± 0.03 | Total fatty acid | 95.879 |
| Stearic acid (C18:0) | 5.884 ± 0.86 | TUFAs a | 80.62 |
| Oleic acid (C18:1) | 20.139 ± 2.67 |
| No. | RT (min) | Compound | Adduct Ions | Class | Found m/z | Expected m/z | Error (ppm) | Formula | Major Fragments | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.86 | Tanshinol | [M − H]− | Phenolic acids | 197.0454 | 197.0455 | −0.7 | C9H10O5 | 179.0350, 162.8392, 135.0472, 123.0465, 72.9933 | [17] |
| 2 | 8.27 | p-Hydroxycinnamic acid sophoroside | [M − H]− | Phenolic acids | 487.1458 | 487.1457 | 0.2 | C21H28O13 | 487.1515, 265.0692, 205.0507, 163.0405, 145.0300 | GNPS; TCM |
| 3 | 8.74 | Tuberonic acid glucoside | [M − H]− | Other | 387.1659 | 387.1661 | −0.4 | C18H28O9 | 387.1625, 207.0936, 163.1129 | [18] |
| 4 | 11.42 | Salvianolic acid H | [M − H]− | Phenolic acids | 537.1047 | 537.1039 | 1.6 | C27H22O12 | 339.0464, 295.0487, 267.0722, 229.0140 | GNPS |
| 5 | 12.27 | Albidoside | [M − H]− | Iridoid glycosides | 521.1681 | 521.1665 | −0.5 | C25H30O12 | 521.1694, 503.1556, 325.0907, 265.0707, 205.0491 | [19] |
| 6 | 12.75 | Salvinoside | [M − H]− | Phenolic acids | 879.1983 | 879.1989 | −0.7 | C42H40O21 | 879.1907, 717.1536, 699.1454, 519.0918, 475.0770, 399.0467 | [20] |
| 7 | 13.15 | Luteolin 7-O-β-d-glucoside | [M − H]− | Flavonoids | 447.0933 | 447.0933 | −0.4 | C21H20O11 | 447.0932, 285.0379 | [21] |
| 8 | 13.65 | Salviaflaside | [M − H]− | Phenolic acids | 521.1300 | 521.1301 | −0.1 | C24H26O13 | 521.1264, 359.0740, 323.0750, 161.0238, 135.0439 | [22] |
| 9 | 14.39 | Salvianolic acid E | [M − H]− | Phenolic acids | 717.1467 | 717.1461 | 0.8 | C36H30O16 | 717.1377, 519.0900, 475.0994, 339.0475, 321.0375, 243.0286, 197.0465, 109.0299 | [23] |
| 10 | 14.68 | Pinoresinol glucoside | [M − H]− | Lignin | 535.1821 | 535.1815 | 0.6 | C26H32O12 | 535.1818, 373.1288, 355.1191, 295.1067, 179.0550 | [24] |
| 11 | 15.85 | Rosmarinic acid | [M − H]− | Phenolic acids | 359.0773 | 359.0772 | 0.2 | C18H16O8 | 359.0742, 197.0441, 179.0343, 161.0238, 135.0446, 123.0442, 72.9927 | [25] |
| 12 | 16.60 | Salvianolic acid B | [M − H]− | Phenolic acids | 717.1469 | 717.1461 | 1.1 | C36H30O16 | 717.1342, 519.1010, 475.0997, 321.0416 | [23] |
| 13 | 17.59 | 7‴,8‴-Didehydro-salvianolic acid B | [M − H]− | Phenolic acids | 715.1301 | 715.1305 | −0.5 | C36H28O16 | 715.1297 | GNPS; TCM |
| 14 | 17.68 | Salvianolic acid L | [M − H]− | Phenolic acids | 717.1470 | 717.1461 | 1.2 | C36H30O16 | 519.1012, 321.0432 | [23] |
| 15 | 18.26 | Clinopodic acid A | [M − H]− | Phenolic acids | 343.0826 | 343.0823 | 0.8 | C18H16O7 | 191.0428, 181.0517, 161.0238, 135.0452, 119.0515 | [26] |
| 16 | 18.86 | Methyl rosmarinate | [M − H]− | Phenolic acids | 373.0928 | 373.0929 | −0.2 | C19H18O8 | 197.0423, 179.0397, 175.0398, 160.0170, 135.0448, 123.0456 | [27] |
| 17 | 18.99 | Salvianolic acid C | [M − H]− | Phenolic acids | 491.0991 | 491.0989 | 1.1 | C26H20O10 | 491.1027, 311.0567, 267.0635, 135.0465 | [23] |
| 18 | 19.38 | Luteolin | [M − H]− | Flavonoids | 285.0399 | 285.0405 | −0.6 | C15H10O6 | 285.0382, 175.0397, 149.0249, 133.0297 | [21] |
| 19 | 22.31 | Apigenin | [M − H]− | Flavonoids | 269.0454 | 269.0455 | −0.5 | C15H10O5 | 269.0425, 225.0518, 183.0548, 161.0242, 149.0240, 117.0348 | [21] |
| 20 | 34.08 | Asiatic acid | [M − H]− | Triterpenoids | 487.3424 | 487.3429 | −1 | C30H48O5 | 487.3437, 469.3293 | [28] |
| 21 | 35.54 | Dihydrotanshinone I | [M + H]+ | Diterpenoids | 279.1014 | 279.1016 | −0.6 | C18H14O3 | 279.0966, 203.0817, 189.0682, 149.0217, 121.0254 | [29] |
| 22 | 40.72 | Cryptotanshinone | [M + H]+ | Diterpenoids | 297.1486 | 297.1485 | 0.3 | C19H20O3 | 297.1516, 255.0994, 236.0855 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, R.; Ren, X.; Liu, D.; Lu, Z.; Liu, P. An Evaluation of the Edible Value of Salvia miltiorrhiza Seeds: Proximate Composition, Phytochemical Components and Antioxidant Activity. Molecules 2024, 29, 1483. https://doi.org/10.3390/molecules29071483
Deng R, Ren X, Liu D, Lu Z, Liu P. An Evaluation of the Edible Value of Salvia miltiorrhiza Seeds: Proximate Composition, Phytochemical Components and Antioxidant Activity. Molecules. 2024; 29(7):1483. https://doi.org/10.3390/molecules29071483
Chicago/Turabian StyleDeng, Ruixue, Xueli Ren, Dongjie Liu, Zongyuan Lu, and Pu Liu. 2024. "An Evaluation of the Edible Value of Salvia miltiorrhiza Seeds: Proximate Composition, Phytochemical Components and Antioxidant Activity" Molecules 29, no. 7: 1483. https://doi.org/10.3390/molecules29071483
APA StyleDeng, R., Ren, X., Liu, D., Lu, Z., & Liu, P. (2024). An Evaluation of the Edible Value of Salvia miltiorrhiza Seeds: Proximate Composition, Phytochemical Components and Antioxidant Activity. Molecules, 29(7), 1483. https://doi.org/10.3390/molecules29071483






