Discovery of Acyl-Surugamide A2 from Marine Streptomyces albidoflavus RKJM-0023—A New Cyclic Nonribosomal Peptide Containing an N-ε-acetyl-L-lysine Residue
Abstract
1. Introduction
2. Results and Discussion
2.1. Targeted Isolation of Acyl-Surugamide A2 from S. albidoflavus RKJM-0023 Isolated from a Marine Tunicate
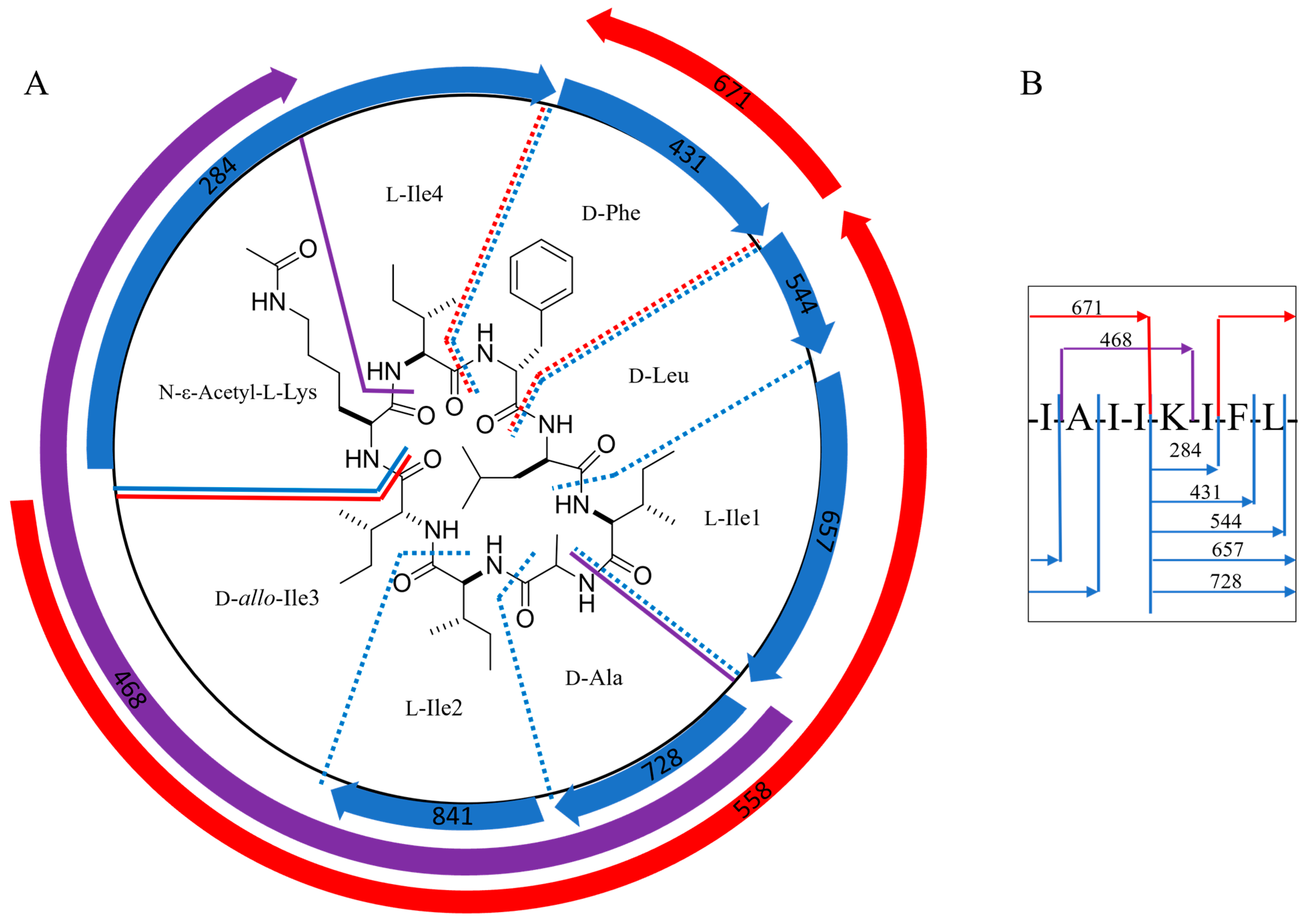
2.2. Structural Characterization of Acyl-Surugamide A2 via NMR and UPLC-HR-ESI-MS/MS Fragment Annoatation
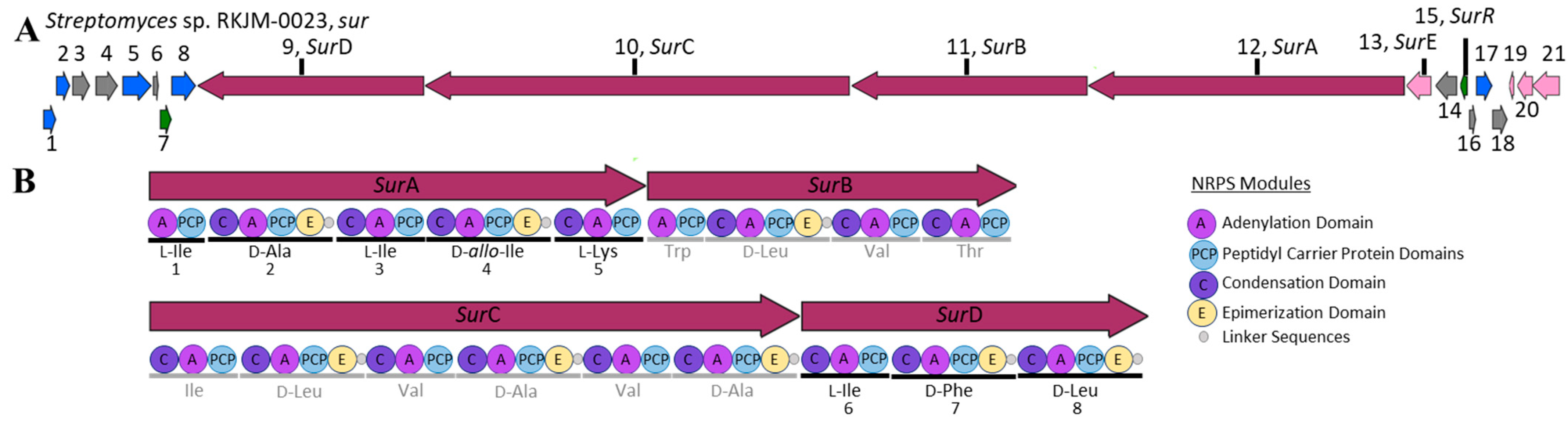
2.3. S. albidoflavus RKJM-0023 Surugamide Biosynthetic Gene Cluster Analysis (sur)
3. Methods and Materials
3.1. General Experimental for MS Analysis
3.2. Isolation of RKJM-0023
3.3. Fermentations and Extraction
3.4. Global Natural Product Social Networking (GNPS) Analysis of Family Members
3.5. Chromatographic Purification
3.6. DNA Isolation, Genome Sequencing, and Biosynthetic Gene Cluster Analysis of RKJM-0023
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takada, K.; Ninomiya, A.; Naruse, M.; Sun, Y.; Miyazaki, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Surugamides A–E, Cyclic Octapeptides with Four d-Amino Acid Residues, from a Marine Streptomyces sp.: LC–MS-Aided Inspection of Partial Hydrolysates for the Distinction of d- and l-Amino Acid Residues in the Sequence. J. Org. Chem. 2013, 78, 6746–6750. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kuranaga, T.; Sano, A.; Ninomiya, A.; Takada, K.; Wakimoto, T. The Revised Structure of the Cyclic Octapeptide Surugamide A. Chem. Pharm. Bull. 2019, 67, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Nazari, B.; Moon, K.; Bushin, L.B.; Seyedsayamdost, M.R. Discovery of a Cryptic Antifungal Compound from Streptomyces albus J1074 Using High-Throughput Elicitor Screens. J. Am. Chem. Soc. 2017, 139, 9203–9212. [Google Scholar] [CrossRef]
- Matsuda, K.; Kobayashi, M.; Kuranaga, T.; Takada, K.; Ikeda, H.; Matsunaga, S.; Wakimoto, T. SurE Is a Trans -Acting Thioesterase Cyclizing Two Distinct Non-Ribosomal Peptides. Org. Biomol. Chem. 2019, 17, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Kuranaga, T.; Matsuda, K.; Sano, A.; Kobayashi, M.; Ninomiya, A.; Takada, K.; Matsunaga, S.; Wakimoto, T. Total Synthesis of the Nonribosomal Peptide Surugamide B and Identification of a New Offloading Cyclase Family. Angew. Chem. 2018, 130, 9591–9595. [Google Scholar] [CrossRef]
- Miao, S.; Anstee, M.R.; LaMarco, K.; Matthew, J.; Huang, L.H.T.; Brasseur, M.M. Inhibition of Bacterial RNA Polymerases. Peptide Metabolites from the Cultures of Streptomyces sp. J. Nat. Prod. 1997, 60, 858–861. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Liu, X.; Chen, Y.; Zhang, Y.; Sun, A.; Zhang, W.; Zhang, J.; Ju, J. Cyclic Hexapeptides from the Deep South China Sea-Derived Streptomyces scopuliridis SCSIO ZJ46 Active Against Pathogenic Gram-Positive Bacteria. J. Nat. Prod. 2014, 77, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.G.; Salim, A.A.; Lacey, E.; Blumenthal, A.; Capon, R.J. Wollamides: Antimycobacterial Cyclic Hexapeptides from an Australian Soil Streptomyces. Org. Lett. 2014, 16, 5120–5123. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Hong, Y.-S.; Jang, M.; Heo, K.T.; Lee, B.; Jang, J.-P.; Kim, J.-W.; Ryoo, I.-J.; Kim, W.-G.; Ko, S.-K.; et al. Genomics-Driven Discovery of Chlorinated Cyclic Hexapeptides Ulleungmycins A and B from a Streptomyces Species. J. Nat. Prod. 2017, 80, 3025–3031. [Google Scholar] [CrossRef]
- Mudalungu, C.M.; von Törne, W.J.; Voigt, K.; Rückert, C.; Schmitz, S.; Sekurova, O.N.; Zotchev, S.B.; Süssmuth, R.D. Noursamycins, Chlorinated Cyclohexapeptides Identified from Molecular Networking of Streptomyces noursei NTR-SR4. J. Nat. Prod. 2019, 82, 1478–1486. [Google Scholar] [CrossRef]
- Kaweewan, I.; Komaki, H.; Hemmi, H.; Kodani, S. Isolation and Structure Determination of New Antibacterial Peptide Curacomycin Based on Genome Mining. Asian J. Org. Chem. 2017, 6, 1838–1844. [Google Scholar] [CrossRef]
- Fazal, A.; Webb, M.E.; Seipke, R.F. The Desotamide Family of Antibiotics. Antibiotics 2020, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, A.; Katsuyama, Y.; Kuranaga, T.; Miyazaki, M.; Nogi, Y.; Okada, S.; Wakimoto, T.; Ohnishi, Y.; Matsunaga, S.; Takada, K. Biosynthetic Gene Cluster for Surugamide A Encompasses an Unrelated Decapeptide, Surugamide F. ChemBioChem 2016, 17, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Joo, S.H. Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2020, 28, 18–24. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Shepherd, N.E.; Xu, W.; Lucke, A.J.; Stoermer, M.J.; Fairlie, D.P. Orally Absorbed Cyclic Peptides. Chem. Rev. 2017, 117, 8094–8128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Northfield, S.E.; Colless, B.; Chaousis, S.; Hamernig, I.; Lohman, R.-J.; Nielsen, D.S.; Schroeder, C.I.; Liras, S.; Price, D.A.; et al. Rational Design and Synthesis of an Orally Bioavailable Peptide Guided by NMR Amide Temperature Coefficients. Proc. Natl. Acad. Sci. USA 2014, 111, 17504–17509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shaaban, K.A.; Elshahawi, S.I.; Ponomareva, L.V.; Sunkara, M.; Copley, G.C.; Hower, J.C.; Morris, A.J.; Kharel, M.K.; Thorson, J.S. Mullinamides A and B, New Cyclopeptides Produced by the Ruth Mullins Coal Mine Fire Isolate Streptomyces sp. RM-27-46. J. Antibiot. 2014, 67, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Hight, S.K.; Clark, T.N.; Kurita, K.L.; McMillan, E.A.; Bray, W.; Shaikh, A.F.; Khadilkar, A.; Haeckl, F.P.J.; Carnevale-Neto, F.; La, S.; et al. High-Throughput Functional Annotation of Natural Products by Integrated Activity Profiling. Proc. Natl. Acad. Sci. USA 2022, 119, e2208458119. [Google Scholar] [CrossRef]
- Mohimani, H.; Yang, Y.-L.; Liu, W.-T.; Hsieh, P.-W.; Dorrestein, P.C.; Pevzner, P.A. Sequencing Cyclic Peptides by Multistage Mass Spectrometry. Proteomics 2011, 11, 3642–3650. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jarmusch, A.K.; Vargas, F.; Aksenov, A.A.; Gauglitz, J.M.; Weldon, K.; Petras, D.; da Silva, R.; Quinn, R.; Melnik, A.V.; et al. Mass Spectrometry Searches Using MASST. Nat. Biotechnol. 2020, 38, 23–26. [Google Scholar] [CrossRef] [PubMed]
- LeClair, M.M.; Maw, Z.A.; Grunwald, A.L.; Kelly, J.R.; Haltli, B.A.; Kerr, R.G.; Cartmell, C. Discovery of Levesquamide B through Global Natural Product Social Molecular Networking. Molecules 2022, 27, 7794. [Google Scholar] [CrossRef] [PubMed]
- Allfrey, V.G.; Faulkner, R.; Mirsky, A.E. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc. Natl. Acad. Sci. USA 1964, 51, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S rRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Behsaz, B.; Bode, E.; Gurevich, A.; Shi, Y.-N.; Grundmann, F.; Acharya, D.; Caraballo-Rodríguez, A.M.; Bouslimani, A.; Panitchpakdi, M.; Linck, A.; et al. Integrating Genomics and Metabolomics for Scalable Non-Ribosomal Peptide Discovery. Nat. Commun. 2021, 12, 3225. [Google Scholar] [CrossRef] [PubMed]
- Mohimani, H.; Liu, W.-T.; Yang, Y.-L.; Gaudêncio, S.P.; Fenical, W.; Dorrestein, P.C.; Pevzner, P.A. Multiplex De Novo Sequencing of Peptide Antibiotics. J. Comput. Biol. 2011, 18, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Kitani, S.; Yoshida, M.; Boonlucksanawong, O.; Panbangred, W.; Anuegoonpipat, A.; Kurosu, T.; Ikuta, K.; Igarashi, Y.; Nihira, T. Cystargamide B, a Cyclic Lipodepsipeptide with Protease Inhibitory Activity from Streptomyces sp. J. Antibiot. 2018, 71, 662–666. [Google Scholar] [CrossRef]
- Takeuchi, A.; Hirata, A.; Teshima, A.; Ueki, M.; Satoh, T.; Matsuda, K.; Wakimoto, T.; Arakawa, K.; Ishikawa, M.; Suzuki, T. Characterization of the Surugamide Biosynthetic Gene Cluster of TUA-NKU25, a Streptomyces diastaticus Strain Isolated from Kusaya, and Its Effects on Salt-Dependent Growth. Biosci. Biotechnol. Biochem. 2023, 87, 320–329. [Google Scholar] [CrossRef]
- Shin, S.C.; Ahn, D.H.; Kim, S.J.; Lee, H.; Oh, T.-J.; Lee, J.E.; Park, H. Advantages of Single-Molecule Real-Time Sequencing in High-GC Content Genomes. PLoS ONE 2013, 8, e68824. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, B.R.; Blin, K.; Navarro-Muñoz, J.C.; Avalon, N.E.; Chevrette, M.G.; Egbert, S.; Lee, S.; Meijer, D.; Recchia, M.J.J.; Reitz, Z.L.; et al. MIBiG 3.0: A Community-Driven Effort to Annotate Experimentally Validated Biosynthetic Gene Clusters. Nucleic Acids Res. 2023, 51, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, X.; Xu, C.; Shen, Y.; Wang, S.-P.; Liao, H.; Li, L.; Deng, H.; Lin, H.-W. Investigation of Penicillin Binding Protein (PBP)-like Peptide Cyclase and Hydrolase in Surugamide Non-Ribosomal Peptide Biosynthesis. Cell Chem. Biol. 2019, 26, 737–744.e4. [Google Scholar] [CrossRef]
- Matsuda, K.; Zhai, R.; Mori, T.; Kobayashi, M.; Sano, A.; Abe, I.; Wakimoto, T. Heterochiral Coupling in Non-Ribosomal Peptide Macrolactamization. Nat. Catal. 2020, 3, 507–515. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Almeida, E.L.; Kaur, N.; Jennings, L.K.; Carrillo Rincón, A.F.; Jackson, S.A.; Thomas, O.P.; Dobson, A.D.W. Genome Mining Coupled with OSMAC-Based Cultivation Reveal Differential Production of Surugamide A by the Marine Sponge Isolate Streptomyces sp. SM17 When Compared to Its Terrestrial Relative S. albidoflavus J1074. Microorganisms 2019, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Marfey, P. Determination ofD-Amino Acids. II. Use of a Bifunctional Reagent, 1,5-Difluoro-2,4-Dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591. [Google Scholar] [CrossRef]
- Stachelhaus, T.; Walsh, C.T. Mutational Analysis of the Epimerization Domain in the Initiation Module PheATE of Gramicidin S Synthetase. Biochemistry 2000, 39, 5775–5787. [Google Scholar] [CrossRef]
- Keating, T.A.; Marshall, C.G.; Walsh, C.T.; Keating, A.E. The Structure of VibH Represents Nonribosomal Peptide Synthetase Condensation, Cyclization and Epimerization Domains. Nat. Struct. Mol. Biol. 2002, 9, 522–526. [Google Scholar] [CrossRef]
- Chen, W.-H.; Li, K.; Guntaka, N.S.; Bruner, S.D. Interdomain and Intermodule Organization in Epimerization Domain Containing Nonribosomal Peptide Synthetases. ACS Chem. Biol. 2016, 11, 2293–2303. [Google Scholar] [CrossRef]
- Takahashi-Íñiguez, T.; Flores, M.E. Acetyl Phosphate Acetylates Proteins of Streptomyces coelicolor M-145. Appl. Biochem. Microbiol. 2023, 59, 450–455. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Xie, L.; Li, X.; Cheng, Z.; Xie, J. Unexpected Extensive Lysine Acetylation in the Trump-Card Antibiotic Producer Streptomyces roseosporus Revealed by Proteome-Wide Profiling. J. Proteom. 2014, 106, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.; Zeng, L.; Zhou, M.-M. Structure and Acetyl-Lysine Recognition of the Bromodomain. Oncogene 2007, 26, 5521–5527. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Weber, T.; Medema, M.H. BiG-FAM: The Biosynthetic Gene Cluster Families Database. Nucleic Acids Res. 2021, 49, D490–D497. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.A.; Fragoso-Yáñez, D.; Pérez-García, A.; Rosellón-Druker, J.; Quintana, E.T. Actinobacterial Diversity from Marine Sediments Collected in Mexico. Antonie Van Leeuwenhoek 2009, 95, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine Sediment-Derived Streptomyces Bacteria from British Columbia, Canada Are a Promising Microbiota Resource for the Discovery of Antimicrobial Natural Products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Duncan, K.R.; Haltli, B.; Gill, K.A.; Correa, H.; Berrué, F.; Kerr, R.G. Exploring the Diversity and Metabolic Potential of Actinomycetes from Temperate Marine Sediments from Newfoundland, Canada. J. Ind. Microbiol. Biotechnol. 2015, 42, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Zazopoulos, E.; Huang, K.; Staffa, A.; Liu, W.; Bachmann, B.O.; Nonaka, K.; Ahlert, J.; Thorson, J.S.; Shen, B.; Farnet, C.M. A Genomics-Guided Approach for Discovering and Expressing Cryptic Metabolic Pathways. Nat. Biotechnol. 2003, 21, 187–190. [Google Scholar] [CrossRef]
- R.Tormo, J.; García, J.B.; DeAntonio, M.; Feliz, J.; Mira, A.; Díez, M.T.; Hernández, P.; Peláez, F. A Method for the Selection of Production Media for Actinomycete Strains Based on Their Metabolite HPLC Profiles. J. Ind. Microbiol. Biotechnol. 2003, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.; Bollin, C.J. NCBI Genome Workbench: Desktop Software for Comparative Genomics, Visualization, and GenBank Data Submission. In Multiple Sequence Alignment: Methods and Protocols; Katoh, K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; pp. 261–295. ISBN 978-1-07-161036-7. [Google Scholar]
- Gilchrist, C.L.M.; Chooi, Y.-H. Clinker & Clustermap.Js: Automatic Generation of Gene Cluster Comparison Figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Udwary, D.W.; Zeigler, L.; Asolkar, R.N.; Singan, V.; Lapidus, A.; Fenical, W.; Jensen, P.R.; Moore, B.S. Genome Sequencing Reveals Complex Secondary Metabolome in the Marine Actinomycete Salinispora Tropica. Proc. Natl. Acad. Sci. USA 2007, 104, 10376–10381. [Google Scholar] [CrossRef]
- Kopp, F.; Marahiel, M.A. Macrocyclization Strategies in Polyketide and Nonribosomal Peptide Biosynthesis. Nat. Prod. Rep. 2007, 24, 735–749. [Google Scholar] [CrossRef]
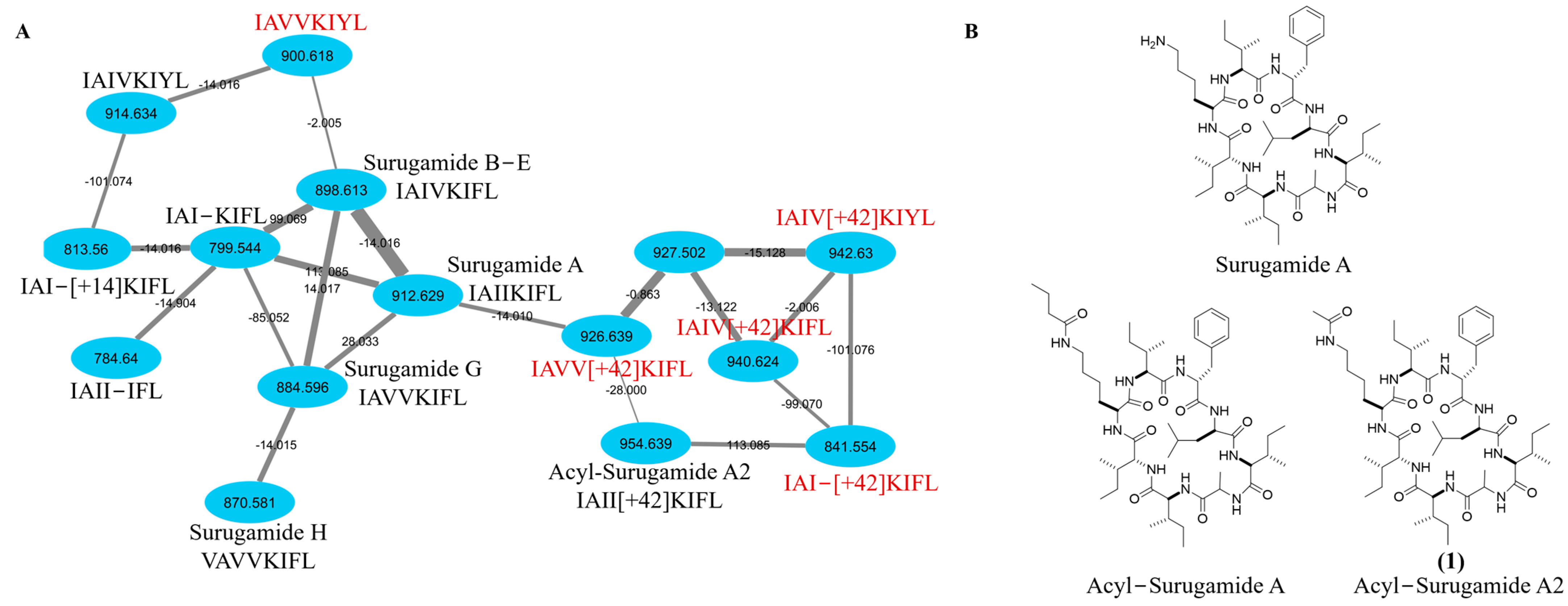
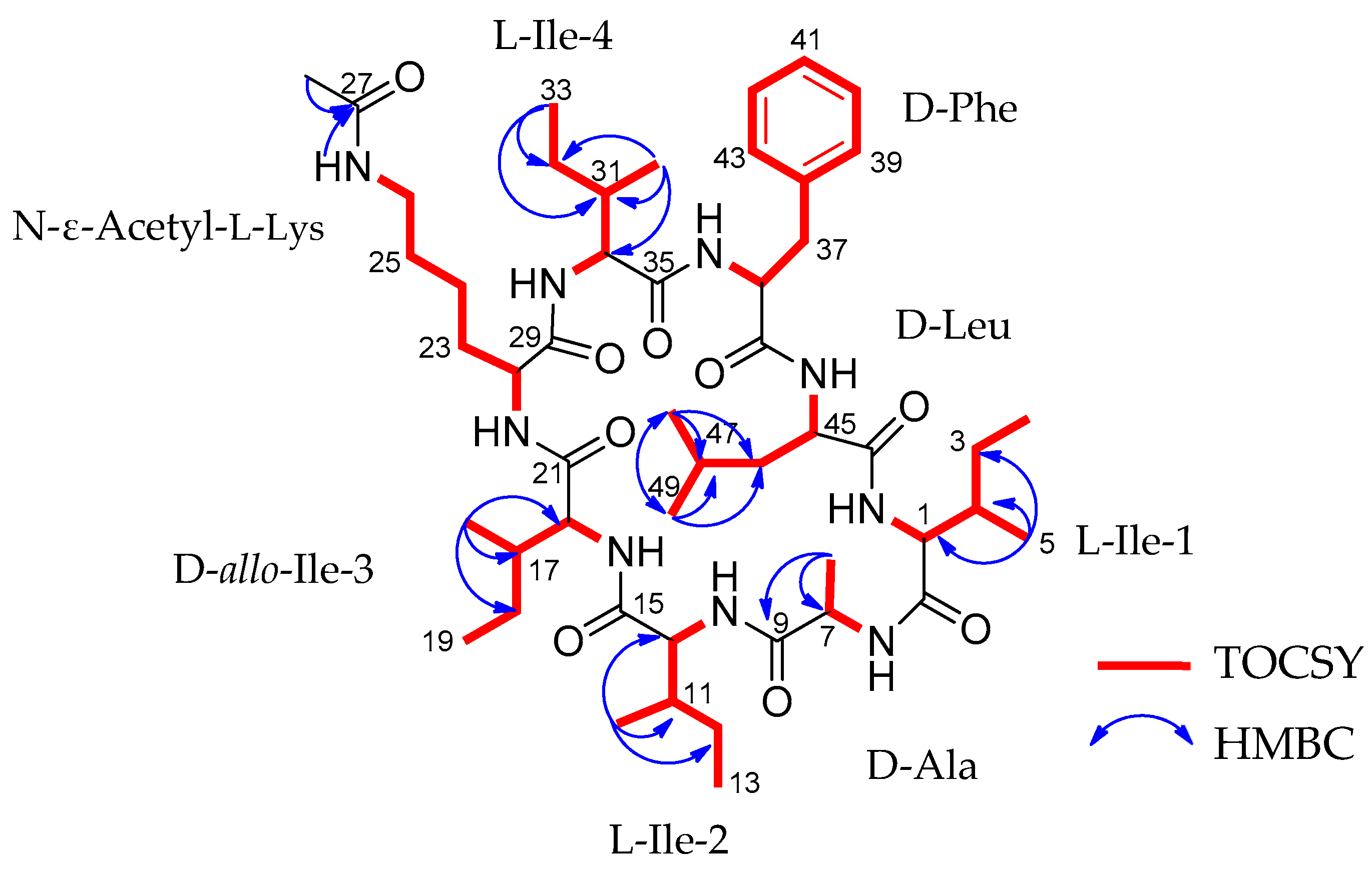

| Acyl-Surugamide A2 (1) | |||||
|---|---|---|---|---|---|
| Residue | Position | a δC type | δH (J in Hz) | TOCSY | HMBC |
| L-Ile-1 | 50-NH | 7.13, m | 1, 2, 4, 5 | ||
| 1 | 57.7, CH | 4.07, t (7.0) | 50-NH, 2, 3, 4, 5 | ||
| 2 | 36.0, CH | 1.77, m | 50-NH, 1, 3, 4, 5 | ||
| 3 | 24.5, CH2 | 1.26, 1.11, m | 1, 2, 4, 5 | ||
| 4 | 11.7, CH3 | 0.79, m | 50-NH, 2, 3 | ||
| 5 | 15.6, CH3 | 0.79, m | 50-NH, 2, 3 | 3, 2, 1 | |
| 6 | ND, C | ||||
| D-Ala | 6-NH | 7.82, m | 7, 8 | ||
| 7 | 48.45, CH | 4.22, m | 6-NH, 8 | ||
| 8 | 19.3, CH3 | 1.21, d (6.7) | 9, 7 | ||
| 9 | b 173.0, C | ||||
| L-Ile-2 | 9-NH | 8.29, brd (7.44) | 10, 11, 12, 13, 14 | ||
| 10 | 58.0, CH | 4.16, m | 9-NH, 11, 12, 13, 14 | ||
| 11 | 35.8, CH | 1.75, m | 9-NH, 10, 12, 13, 14 | ||
| 12 | 24.9, CH2 | 1.46, 1.12, m | 9-NH, 10, 11, 13, 14 | ||
| 13 | 11.1, CH3 | 0.82, m | 9-NH, 10, 11, 12, 14 | ||
| 14 | 14.9, CH3 | 0.82, m | 9-NH, 10, 11, 12, 13 | 12, 11, 10 | |
| 15 | ND, C | ||||
| D-allo-Ile-3 | 15-NH | 7.95, m | 16, 17, 18, 19, 20 | ||
| 16 | 56.9, CH | 4.18, m | 15-NH, 17, 18, 19, 20 | ||
| 17 | 36.7, CH | 1.81, m | 15-NH, 16, 18, 19, 20 | ||
| 18 | 26.2, CH2 | 1.30, 1.21, m | 15-NH, 16, 17, 19, 20 | ||
| 19 | 11.9, CH3 | 0.81, m | 15-NH, 16, 17, 18, 20 | ||
| 20 | 15.1, CH3 | 0.81, m | 15-NH, 16, 17, 18, 19 | 18, 17, 16 | |
| 21 | ND, C | ||||
| N-ε-Acetyl-L-Lys | 21-NH | 7.61, m | 22, 23, 24 | ||
| 22 | 52.43, CH | 4.27, m | 21-NH, 23, 24, 25, 26 | ||
| 23 | 32.1, CH2 | 1.54, 1.41, m | 21-NH, 22, 26, 26-NH | ||
| 24 | 22.7, CH2 | 1.20, 1.13, m | 21-NH, 22, 26, 26-NH | ||
| 25 | 28.8, CH2 | 1.27, m | 26, 22, 26-NH | ||
| 26 | 38.93, CH2 | 3.01, 2.87, m | 23, 24, 25, 26-NH | ||
| 26-NH | 7.75, m | 22, 23, 24, 25, 26 | 27 | ||
| 27 | b 169.4, C | ||||
| 28 | 23.1, CH3 | 1.77, s | 27 | ||
| 29 | ND, C | ||||
| L-Ile-4 | 29-NH | 7.81, m | 30, 31, 32, 33, 34 | ||
| 30 | 58.3, CH | 3.85, m | 29-NH, 31, 32, 33, 34 | ||
| 31 | 36.2, CH2 | 1.43, m | 29-NH, 30, 32, 33, 34 | ||
| 32 | 25.1, CH2 | 1.14, 0.81, m | 29-NH, 30, 32, 33, 34 | ||
| 33 | 11.5, CH3 | 0.68, t (7.55) | 29-NH, 30, 31, 32, 34 | 32, 31 | |
| 34 | 15.2, CH3 | 0.44, d (6.75) | 29-NH, 30, 31, 32, 33 | 32, 31, 30 | |
| 35 | ND, C | ||||
| D-Phe | 35-NH | 8.44, d (8.24) | 36, 37 | ||
| 36 | 55.0, CH | 4.38, m | 35-NH, 37 | ||
| 37 | 36.8, CH2 | 2.68, t (12.57), 3.24, m | 35-NH | ||
| 38 | b 138.5, C | ||||
| 39, 43 | 128.6, CH | 7.24, m | 37 | ||
| 40, 42 | 129.6, CH | 7.22, m | 37 | ||
| 41 | 126.7, CH | 7.17, m | |||
| 44 | ND, C | ||||
| D-Leu | 44-NH | 7.73, m | 45, 46, 47, 48, 49 | ||
| 45 | 52.6, CH | 4.23, m | 44-NH, 46, 47, 48, 49 | ||
| 46 | 40.8, CH2 | 1.85, 1.47, m | 44-NH, 45, 47, 48, 49 | ||
| 47 | 24.8, CH | 1.68, m | 44-NH, 45, 46, 48, 49 | ||
| 48 | 23.7, CH3 | 0.92, d (6.7) | 44-NH,45, 46, 47, 49 | 49, 47, 46 | |
| 49 | 21.9, CH3 | 0.85, d (6.6) | 44-NH, 45, 46, 47, 48 | 48, 47, 46 | |
| 50 | ND, C | ||||
| MS2 Fragments of Acyl-Surugamide A2, m/z | Fragment Amino Acid Sequence | Equivalent MS2 Fragments Surugamide A, m/z | Mass Difference, m/z |
|---|---|---|---|
| 841 | KIFLIAI- | 799 | 42 |
| 728 | KIFLIA-- | 686 | 42 |
| 657 | KIFLI--- | 615 | 42 |
| 544 | KIFL---- | 502 | 42 |
| 431 | KIF----- | ND | |
| 284 | KI------ | ND | |
| 397 | K-----II | 373 | 42 |
| 671 | --FLIAII | 671 | 0 |
| 581 | KI---AII | 539 | 42 |
| 558 | --FLIAI- | 558 | 0 |
| 468 | K----AII | 426 | 42 |
| 374 | --FLI--- | 374 | 0 |
| 298 | -----AII | 298 | 0 |
| 261 | --FL---- | 261 | 0 |
| 185 | -----IA- | 185 | 0 |
| Function | Predicted Function | sur Homolog | |
|---|---|---|---|
| 1 | transport | ABC transporter permease | |
| 2 | transport | ABC transporter permease | |
| 3 | other | ABC transporter substrate-binding protein | |
| 4 | other | Secreted protein | |
| 5 | transport | MFS transporter | |
| 6 | other | hypothetical protein | |
| 7 | regulatory | TetR/AcrR family transcriptional regulator | |
| 8 | transport | MFS transporter | |
| 9 | biosynthetic | non-ribosomal peptide synthase | surA |
| 10 | biosynthetic | non-ribosomal peptide synthase | surB |
| 11 | biosynthetic | non-ribosomal peptide synthase | surC |
| 12 | biosynthetic | non-ribosomal peptide synthase | surD |
| 13 | biosynthetic-additional | serine hydrolase domain-containing protein | surE |
| 14 | other | membrane protein | |
| 15 | regulatory | GntR family transcriptional regulator | surR |
| 16 | other | hypothetical protein | |
| 17 | transport | ATP-binding cassette domain-containing protein | |
| 18 | other | ABC transporter permease | |
| 19 | biosynthetic-additional | MbtH family protein | |
| 20 | biosynthetic-additional | alpha/beta hydrolase | |
| 21 | biosynthetic-additional | aldehyde dehydrogenase family protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maw, Z.A.; Haltli, B.; Guo, J.J.; Baldisseri, D.M.; Cartmell, C.; Kerr, R.G. Discovery of Acyl-Surugamide A2 from Marine Streptomyces albidoflavus RKJM-0023—A New Cyclic Nonribosomal Peptide Containing an N-ε-acetyl-L-lysine Residue. Molecules 2024, 29, 1482. https://doi.org/10.3390/molecules29071482
Maw ZA, Haltli B, Guo JJ, Baldisseri DM, Cartmell C, Kerr RG. Discovery of Acyl-Surugamide A2 from Marine Streptomyces albidoflavus RKJM-0023—A New Cyclic Nonribosomal Peptide Containing an N-ε-acetyl-L-lysine Residue. Molecules. 2024; 29(7):1482. https://doi.org/10.3390/molecules29071482
Chicago/Turabian StyleMaw, Zacharie A., Bradley Haltli, Jason J. Guo, Donna M. Baldisseri, Christopher Cartmell, and Russell G. Kerr. 2024. "Discovery of Acyl-Surugamide A2 from Marine Streptomyces albidoflavus RKJM-0023—A New Cyclic Nonribosomal Peptide Containing an N-ε-acetyl-L-lysine Residue" Molecules 29, no. 7: 1482. https://doi.org/10.3390/molecules29071482
APA StyleMaw, Z. A., Haltli, B., Guo, J. J., Baldisseri, D. M., Cartmell, C., & Kerr, R. G. (2024). Discovery of Acyl-Surugamide A2 from Marine Streptomyces albidoflavus RKJM-0023—A New Cyclic Nonribosomal Peptide Containing an N-ε-acetyl-L-lysine Residue. Molecules, 29(7), 1482. https://doi.org/10.3390/molecules29071482







