Ionic Route to Atmospheric Relevant HO2 and Protonated Formaldehyde from Methanol Cation and O2
Abstract
1. Introduction
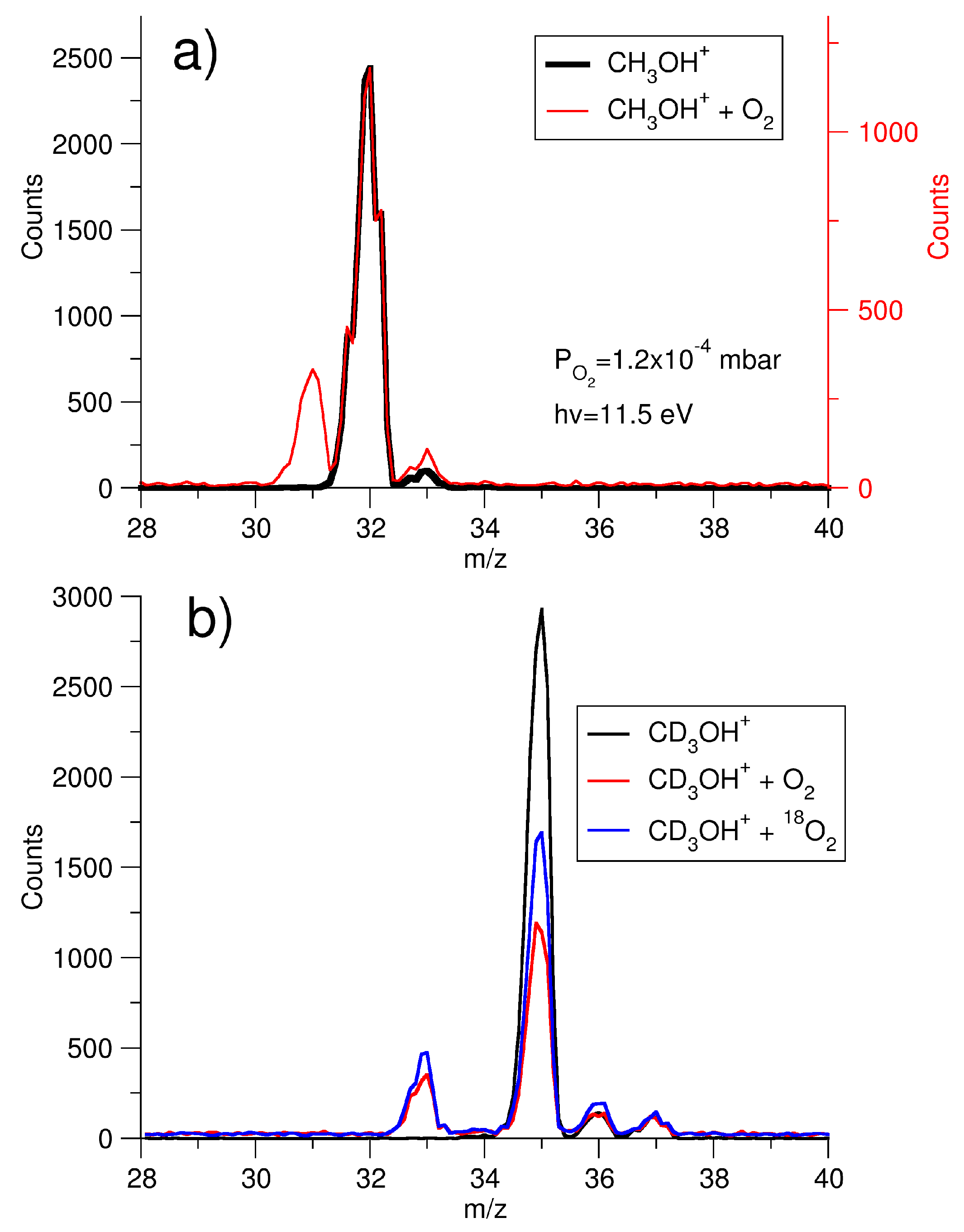
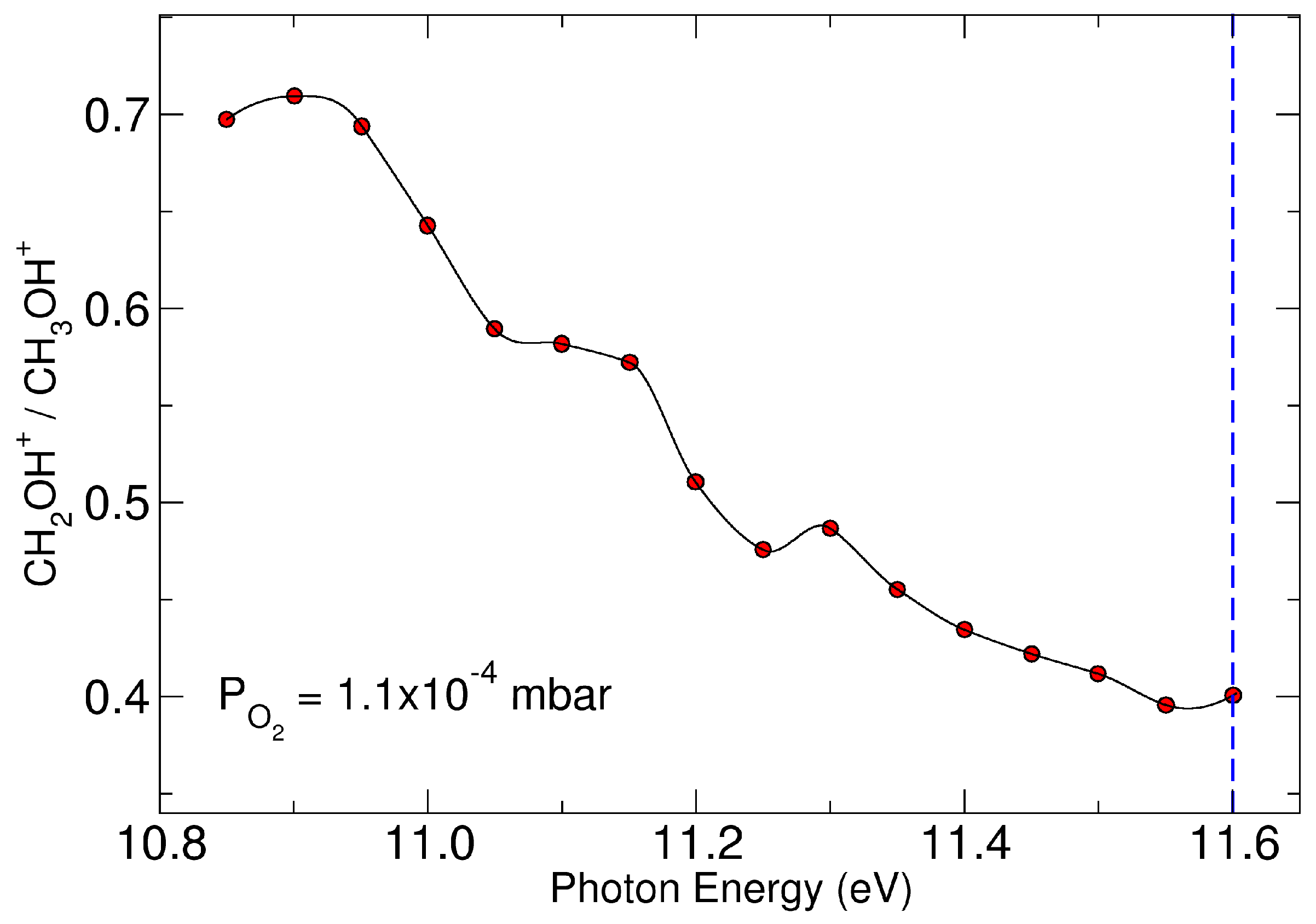
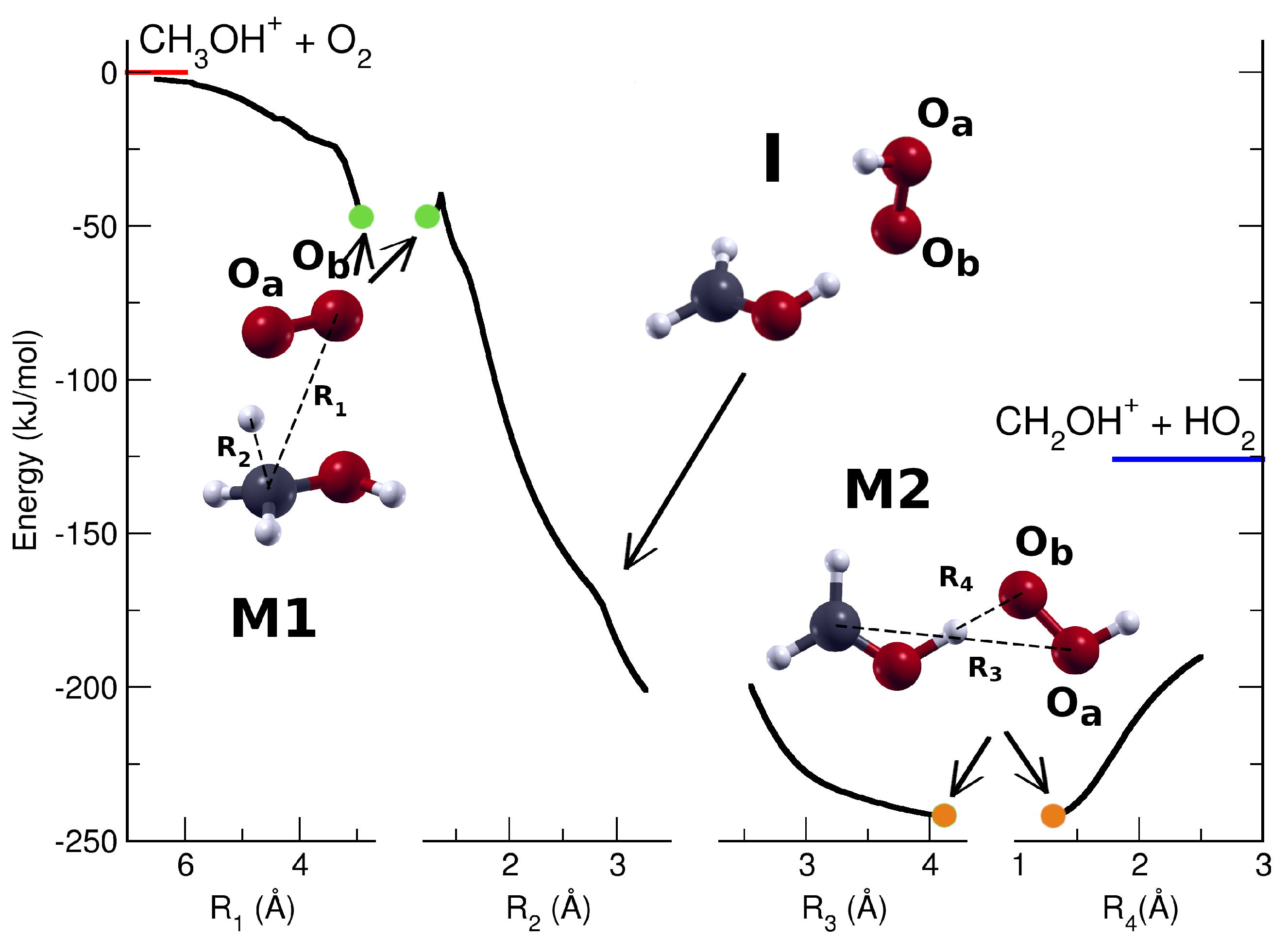
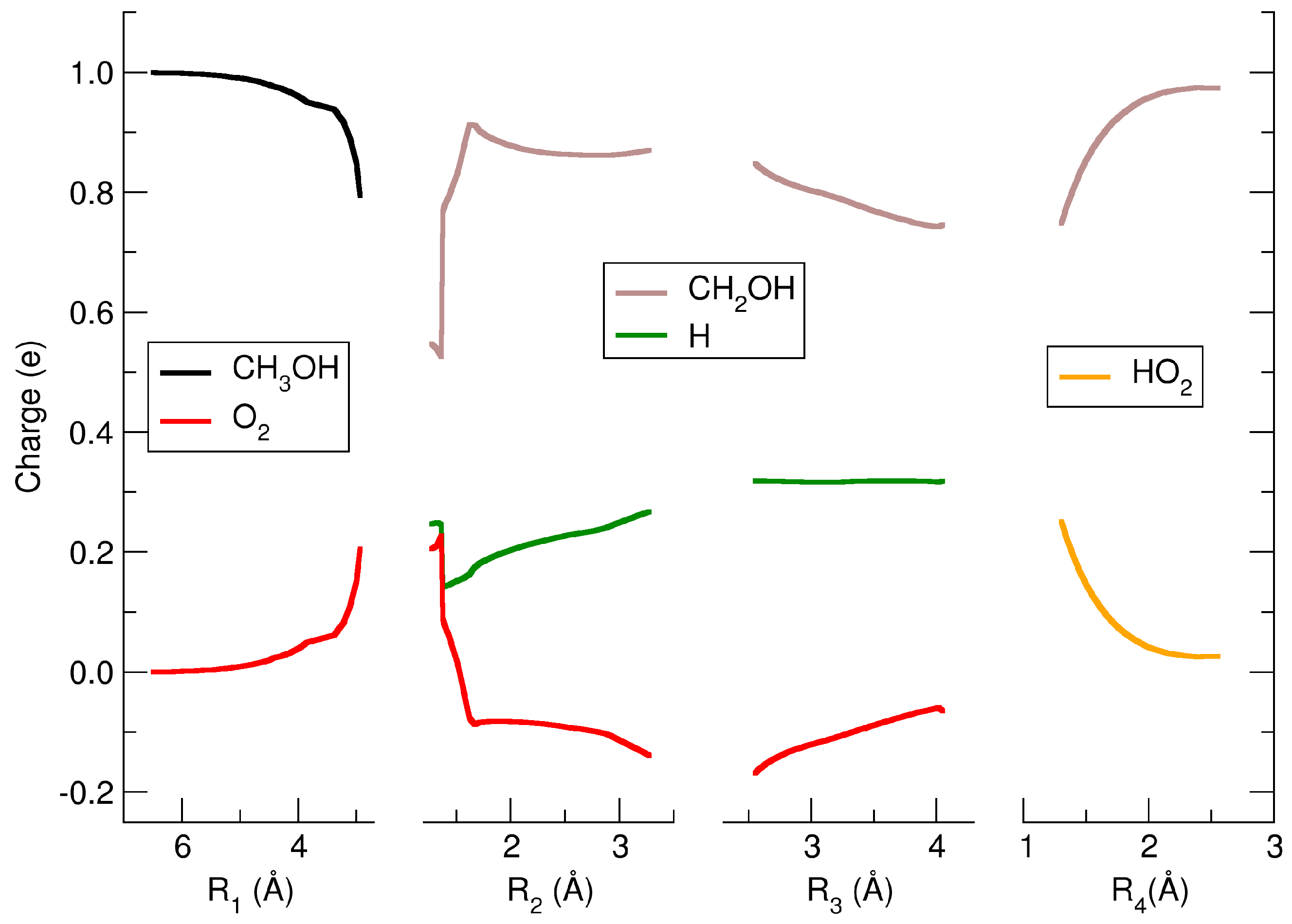
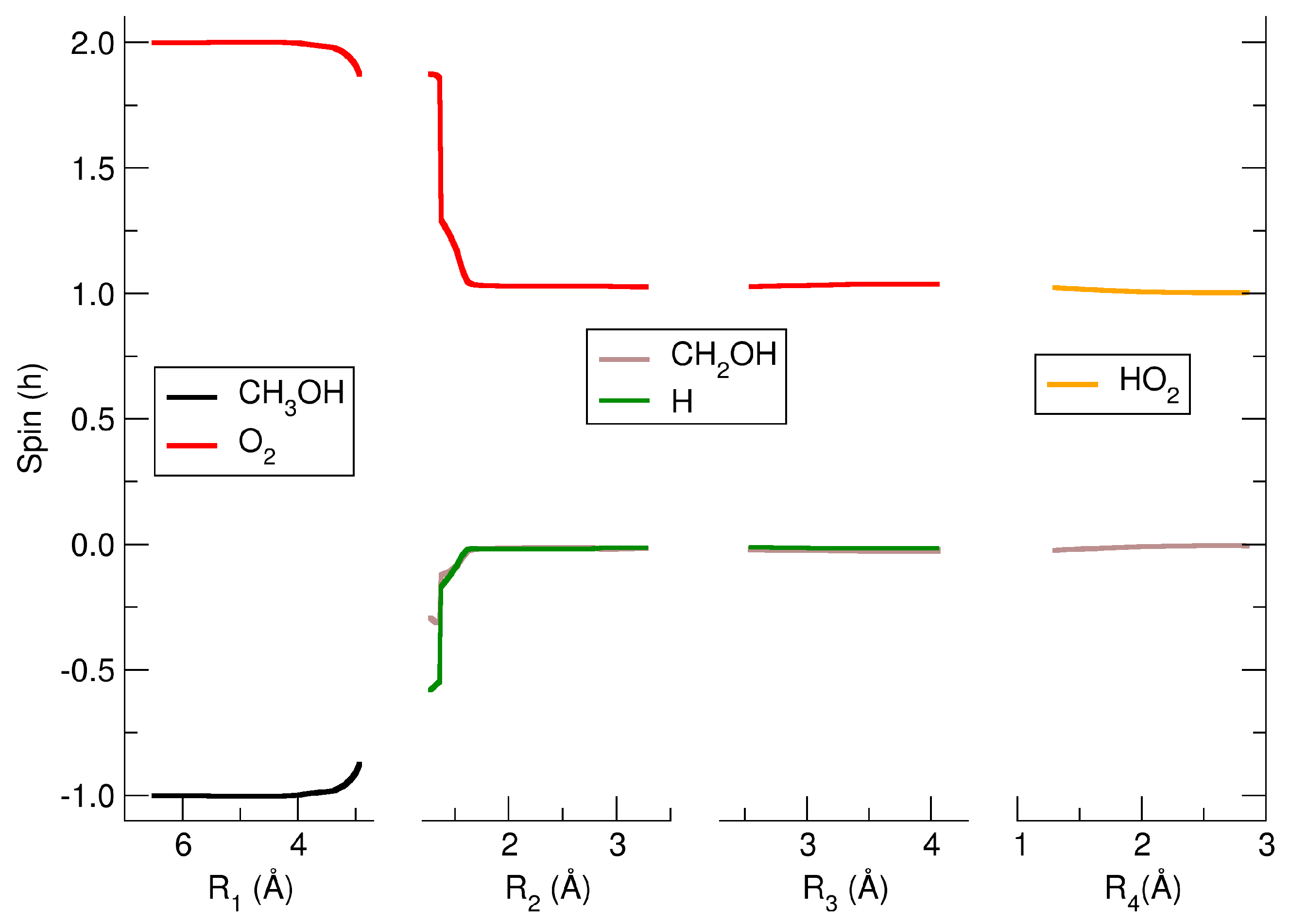
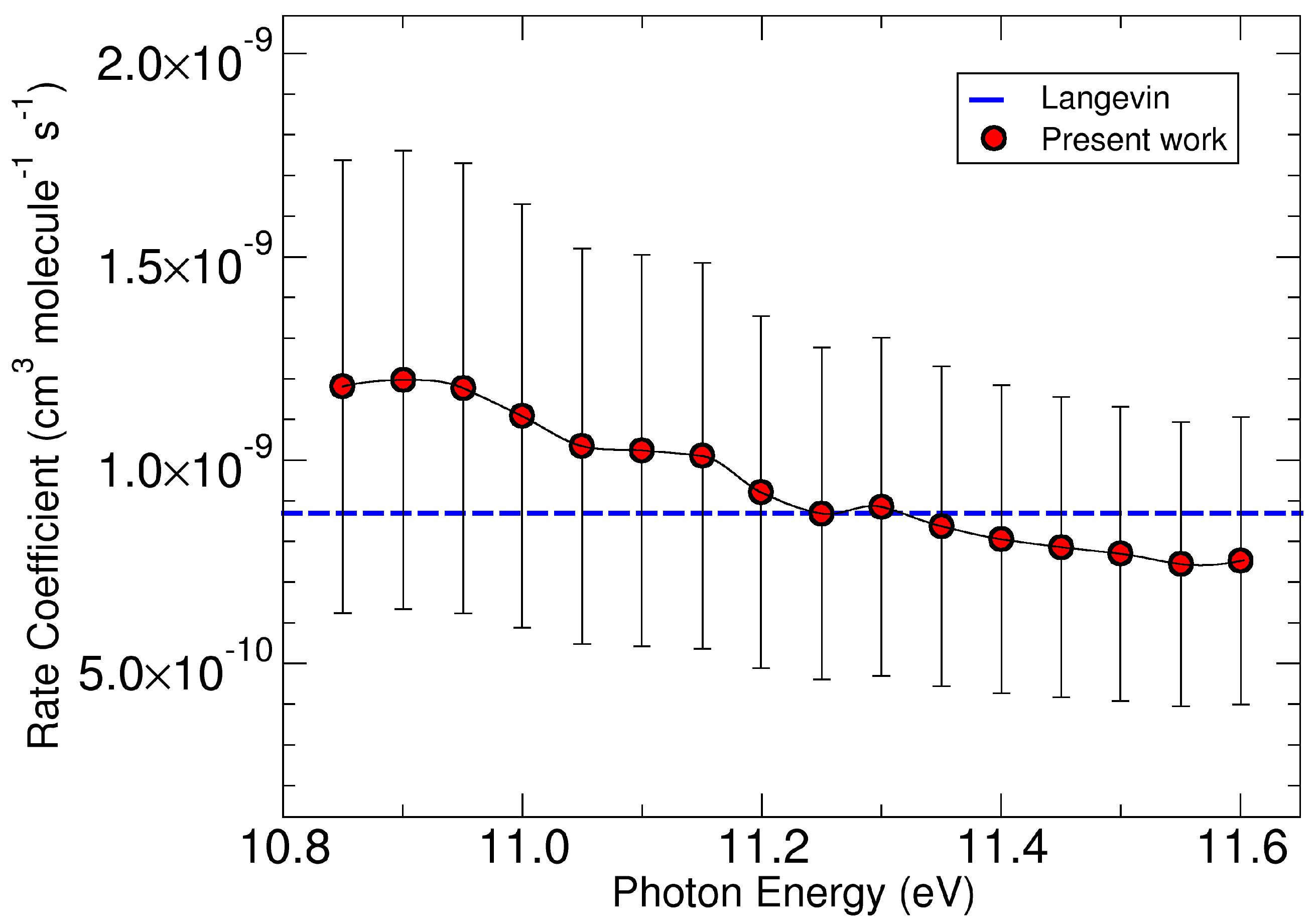
2. Results and Discussions
3. Material and Methods
3.1. Synchrotron Experiments
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anicich, G. An Index of the Literature for Bimolecular Gas Phase Cation-Molecule Reaction Kinetics; Technical Report JPL Publication 03-19; Jet Propulsion Laboratory: Pasadena, CA, USA, 2003. [Google Scholar]
- Svensmark, H.; Svensmark, J.; Bodker Enghoff, M.; Shaviv, N.J. Cosmic ray decreases affect atmospheric aerosols and clouds. Sci. Rep. 2021, 11, 19668. [Google Scholar] [CrossRef] [PubMed]
- Bazilevskaya, G.A.; Usoskin, I.G.; Fluckiger, E.O.; Harrison, R.G.; Desorgher, L.; Butikofer, R.; Krainev, M.B.; Makhmutov, V.S.; Stozhkov, Y.I.; Svirzhevskaya, A.K.; et al. Cosmic Ray Induced Ion Production in the Atmosphere. Space Sci. Rev. 2008, 137, 149–173. [Google Scholar] [CrossRef]
- Dunne, E.M.; Gordon, H.; Kürten, A.; Almeida, J.; Duplissy, J.; Williamson, C.; Ortega, I.K.; Pringle, K.J.; Adamov, A.; Baltensperger, U.; et al. Global atmospheric particle formation from CERN CLOUD measurements. Science 2016, 354, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S. Stratospheric ozone depletion: A review of concepts and history. Rev. Geophys. 1999, 37, 275–316. [Google Scholar] [CrossRef]
- Kumar, V.; Dhaka, S.K.; Hitchman, M.H.; Yoden, S. The influence of solar-modulated regional circulations and galactic cosmic rays on global cloud distribution. Sci. Rep. 2023, 13, 3707. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, A.; Catone, D.; Bolognesi, P.; Satta, M.; Markus, P.; Avaldi, L. HSO2+ Formation from Ion-Molecule Reactions of SO2+ with Water and Methane: Two Fast Reactions with Reverse Temperature-Dependent Kinetic Trend. Chem.-A Eur. J. 2017, 23, 6772–6780. [Google Scholar] [CrossRef] [PubMed]
- Catone, D.; Satta, M.; Cartoni, A.; Castrovilli, M.C.; Bolognesi, P.; Turchini, S.; Avaldi, L. Gas phase oxidation of carbon monoxide by sulfur dioxide radical cation: Reaction dynamics and kinetic trend with the temperature. Front. Chem. 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Satta, M.; Cartoni, A.; Catone, D.; Castrovilli, M.C.; Bolognesi, P.; Zema, N.; Avaldi, L. The reaction of sulfur dioxide radical cation with hydrogen and its relevance in solar geoengineering models. ChemPhysChem 2020, 21, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Catone, D.; Satta, M.; Castrovilli, M.C.; Bolognesi, P.; Avaldi, L.; Cartoni, A. Photoionization of methanol: A molecular source for the prebiotic chemistry. Chem. Phys. Lett. 2021, 771, 138467. [Google Scholar] [CrossRef]
- Satta, M.; Catone, D.; Castrovilli, M.C.; Bolognesi, P.; Avaldi, L.; Zema, N.; Cartoni, A. Ion Chemistry of Carbon Dioxide in Nonthermal Reaction with Molecular Hydrogen. J. Phys. Chem. A 2022, 126, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, G.; Amelynck, C.; Ammann, C.; Arneth, A.; Bamberger, I.; Goldstein, A.H.; Gu, L.; Guenther, A.; Hansel, A.; Heinesch, B.; et al. An ecosystem-scale perspective of the net land methanol flux: Synthesis of micrometeorological flux measurements. Atmos. Chem. Phys. 2015, 15, 7413–7427. [Google Scholar] [CrossRef]
- Ashworth, K.; Chung, S.H.; McKinney, K.A.; Liu, Y.; Munger, J.W.; Martin, S.T.; Steiner, A.L. Modelling bidirectional fluxes of methanol and acetaldehyde with the FORCAsT canopy exchange model. Atmos. Chem. Phys. 2016, 16, 15461–15484. [Google Scholar] [CrossRef]
- Tie, X.; Guenther, A.; Holland, E. Biogenic methanol and its impacts on tropospheric oxidants. Geophys. Res. Lett. 2003, 30, 1881. [Google Scholar] [CrossRef]
- Guenther, A. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Karl, T.; Harley, P.; Guenther, A.; Rasmussen, R.; Baker, B.; Jardine, K.; Nemitz, E. The bi-directional exchange of oxygenated VOCs between a loblolly pine. Pinus taeda plantation and the atmosphere. Atmos. Chem. Phys. 2005, 5, 3015–3031. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Beale, R.; Liss, P.; Johnson, M.; Blomquist, B.; Nightingale, P. Air-sea fluxes of oxygenated volatile organic compounds across the Atlantic Ocean. Atmos. Chem. Phys. 2014, 14, 7499–7517. [Google Scholar] [CrossRef]
- Sander, S.P.; Finlayson-Pitts, B.J.; Friedl, R.R.; Golden, D.M.; Huie, R.E.; Keller-Rudek, H.; Kolb, C.E.; Kurylo, M.J.; Molina, M.J.; Moortgat, G.K.; et al. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies: Evaluation Number 15; Technical Report JPL Publication 02-25; Jet Propulsion Laboratory: Pasadena, CA, USA, 2006. [Google Scholar]
- Bates, K.H.; Jacob, D.J.; Wang, S.; Hornbrook, R.S.; Apel, E.C.; Kim, M.J.; Millet, D.; Wells, K.C.; Chen, X.; Brewer, J.; et al. The global budget of atmospheric methanol: New constraints on secondary, oceanic, and terrestrial sources. J. Geophys. Res. Atmos. 2021, 126, e2020JD033439. [Google Scholar] [CrossRef]
- Liszt, H.S.; Pety, J.; Lucas, R. Limits on chemical complexity in diffuse clouds: Search for CH3OH and HC5N absorption. Astron. Astrophys. 2008, 486, 493. [Google Scholar] [CrossRef]
- Bizzocchi, L.; Caselli, P.; Spezzano, S.; Leonardo, E. Deuterated methanol in the pre-stellar core L1544. Astron. Astrophys. 2014, 569, A27. [Google Scholar] [CrossRef]
- Spezzano, S.; Bizzocchi, L.; Caselli, P.; Harju, J.; Brunken, S. Chemical differentiation in a prestellar core traces non-uniform illumination. Astron. Astrophys. 2016, 592, L11. [Google Scholar] [CrossRef]
- Jensen, S.S.; Spezzano, S.; Caselli, P.; Grassi, T.; Haugbolle, T. 3D physico-chemical model of a pre-stellar core I. Environmental and structural impact on the distribution of CH3OH and c-C3H2. Astron. Astrophys. 2023, 675, A34. [Google Scholar] [CrossRef]
- Leroux, K.; Krim, L. Thermal and photochemical study of CH3OH and CH3OH-O2 astrophysical ices. Mon. Not. R. Astron. Soc. 2021, 500, 1188–1200. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook. NIST Standard Reference Database Number 69. 2005. Available online: https://webbook.nist.gov/chemistry/ (accessed on 20 September 2023).
- Dovrou, E.; Bates, K.H.; Moch, J.M.; Mickley, L.J.; Jacob, D.J.; Keutsch, F.N. Catalytic role of formaldehyde in particulate matter formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2113265119. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, C.M.; Crowley, J.N.; Schuladen, J.; Williams, J.; Hafermann, S.; Reiffs, A.; Axinte, R.; Harder, H.; Ernest, C.; Novelli, A.; et al. Measurement report: Photochemical production and loss rates of formaldehyde and ozone across Europe. Atmos. Chem. Phys. 2021, 21, 18413–18432. [Google Scholar] [CrossRef]
- Franco, B.; Blumenstock, T.; Cho, C.; Clarisse, L.; Clerbaux, C.; Coheur, P.F.; De Maziere, M.; De Smedt, I.; Dorn, H.P.; Emmerichs, T.; et al. Ubiquitous atmospheric production of organic acids mediated by cloud droplets. Nature 2021, 593, 233–237. [Google Scholar] [CrossRef]
- Li, B.; Kumar, M.; Zhou, C.; Li, L.; Francisco, J.S. Mechanistic Insights into Criegee Intermediate-Hydroperoxyl Radical Chemistry. J. Am. Chem. Soc. 2022, 144, 14740–14747. [Google Scholar] [CrossRef] [PubMed]
- Brune, W.H.; McFarland, P.J.; Bruning, E.; Waugh, S.; MacGorman, D.; Miller, D.O.; Jenkins, J.M.; Ren, X.; Mao, J.; Peischl, J. Extreme oxidant amounts produced by lightning in storm clouds. Science 2021, 372, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.E.; Okumura, M.; Sander, S.P.; Salawitch, R.J.; Toon, G.C.; Sen, B.; Blavier, J.F.; Jucks, K.W. Kinetics of HO2 + HO2 -> H2O2 + O2: Implications for stratospheric H2O2. Geophys. Res. Lett. 2002, 29, 1299. [Google Scholar] [CrossRef]
- Zheng, J.; Springston, S.R.; Weinstein-Lloyd, J. Quantitative Analysis of Hydroperoxyl Radical Using Flow Injection Analysis with Chemiluminescence Detection. Anal. Chem. 2003, 75, 4696–4700. [Google Scholar] [CrossRef]
- He, X.C.; Simon, M.; Iyer, S.; Xie, H.B.; Rorup, B.; Shen, J.; Finkenzeller, H.; Stolzenburg, D.; Zhang, R.; Baccarini, A.; et al. Iodine oxoacids enhance nucleation of sulfuric acid particles in the atmosphere. Science 2023, 382, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Ruscic, B.; Berkowitz, J. Heat of formation of hydroxymethyl and methanol D0(H-CH2OH). J. Phys. Chem. 1993, 97, 11451–11455. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Derossi, A.; Lama, F.; Piacentini, M.; Prosperi, T.; Zema, N. High flux and High Resolution Beamline for Elliptically Polarized Radiation in the Vacuum Ultraviolet and Soft X-ray Regions. Rev. Sci. Instrum. 1995, 66, 1718–1720. [Google Scholar] [CrossRef]
- Satta, M.; Casavola, A.R.; Cartoni, A.; Castrovilli, M.C.; Catone, D.; Chiarinelli, J.; Borocci, S.; Avaldi, L.; Bolognesi, P. Ionization of 2- and 4(5)-Nitroimidazoles Radiosensitizers: A “Kinetic Competition” between NO2 and NO Losses. ChemPhysChem 2021, 22, 2387–2391. [Google Scholar] [CrossRef]
- Casavola, A.R.; Cartoni, A.; Castrovilli, M.C.; Borocci, S.; Bolognesi, P.; Chiarinelli, J.; Catone, D.; Avaldi, L. VUV Photofragmentation of Chloroiodomethane: The Iso-CH2I-Cl and Iso-CH2Cl-I Radical Cation Formation. J. Phys. Chem. A 2020, 124, 7491–7499. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. Direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Vanduijneveldt, F.; Vanduijneveldtvanderijdt, J.; Vanlenthe, J. State-of-the-art in counterpoise theory. Chem. Rev. 1994, 94, 1873–1885. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Usoskin, I.G.; Desorgher, L.; Velinov, P.; Storini, M.; Flückiger, E.O.; Bütikofer, R.; Kovaltsov, G.A. Ionization of the earth’s atmosphere by solar and galactic cosmic rays. Acta Geophys. 2009, 57, 88–101. [Google Scholar] [CrossRef]
- Desorgher, L.; Flückiger, E.O.; Gurtner, M.; Moser, M.R.; Bütikofer, R. Atmocosmics: A Geant 4 Code For Computing The Interaction Of Cosmic Rays With The Earth’s Atmosphere. Int. J. Mod. Phys. A 2005, 20, 6802–6804. [Google Scholar] [CrossRef]
- Banjac, S.; Herbst, K.; Heber, B. The Atmospheric Radiation Interaction Simulator (AtRIS): Description and Validation. J. Geophys. Res.-Space Phys. 2019, 124, 50–67. [Google Scholar] [CrossRef]
- Nicolanti, F.; Caccia, B.; Cartoni, A.; Emfietzoglou, D.; Faccini, R.; Incerti, S.; Kyriakou, I.; Satta, M.; Tran, H.N.; Mancini-Terracciano, C. Calculation of electron interaction models in N2 and O2. Phys. Medica-Eur. J. Med Phys. 2023, 114, 102661. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satta, M.; Catone, D.; Castrovilli, M.C.; Nicolanti, F.; Cartoni, A. Ionic Route to Atmospheric Relevant HO2 and Protonated Formaldehyde from Methanol Cation and O2. Molecules 2024, 29, 1484. https://doi.org/10.3390/molecules29071484
Satta M, Catone D, Castrovilli MC, Nicolanti F, Cartoni A. Ionic Route to Atmospheric Relevant HO2 and Protonated Formaldehyde from Methanol Cation and O2. Molecules. 2024; 29(7):1484. https://doi.org/10.3390/molecules29071484
Chicago/Turabian StyleSatta, Mauro, Daniele Catone, Mattea Carmen Castrovilli, Francesca Nicolanti, and Antonella Cartoni. 2024. "Ionic Route to Atmospheric Relevant HO2 and Protonated Formaldehyde from Methanol Cation and O2" Molecules 29, no. 7: 1484. https://doi.org/10.3390/molecules29071484
APA StyleSatta, M., Catone, D., Castrovilli, M. C., Nicolanti, F., & Cartoni, A. (2024). Ionic Route to Atmospheric Relevant HO2 and Protonated Formaldehyde from Methanol Cation and O2. Molecules, 29(7), 1484. https://doi.org/10.3390/molecules29071484