Unified Synthesis and Biological Evaluation of Makaluvamine J and Its Analogs
Abstract
1. Introduction
2. Results and Discussions
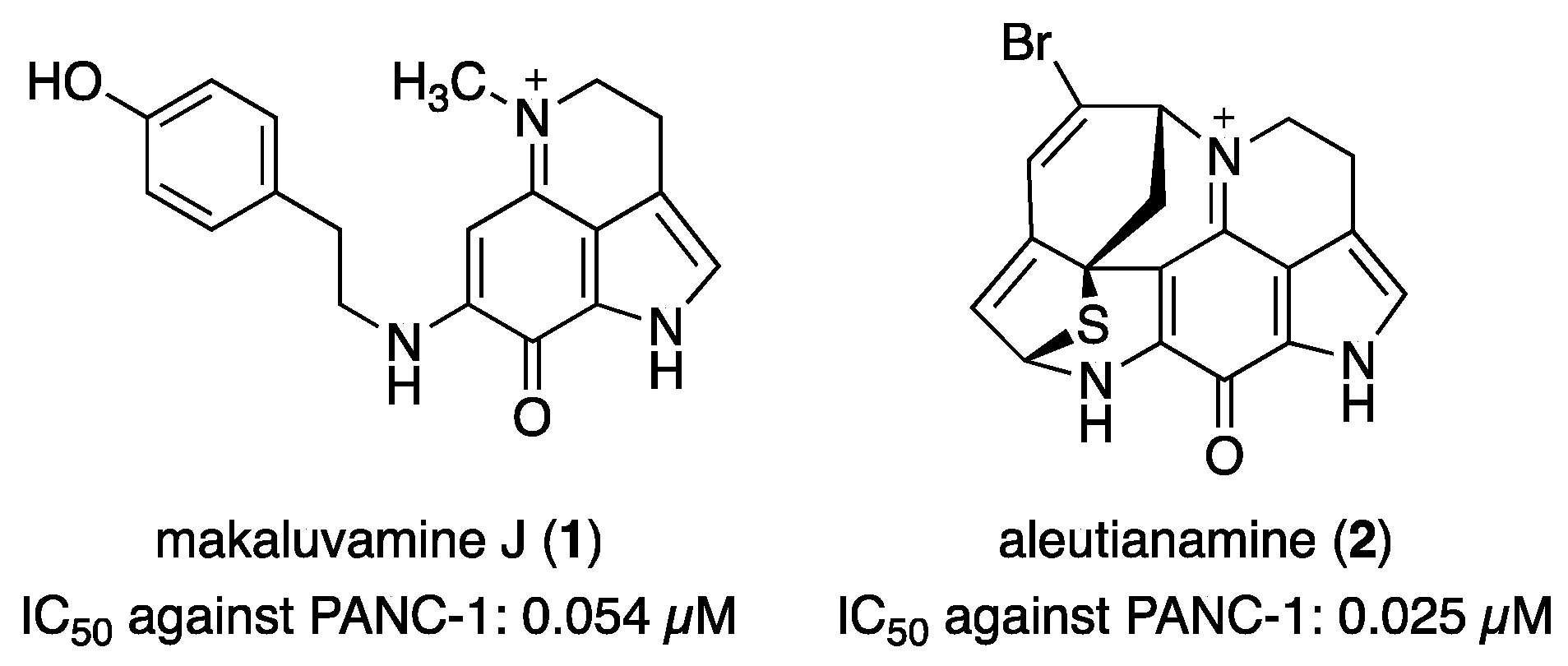
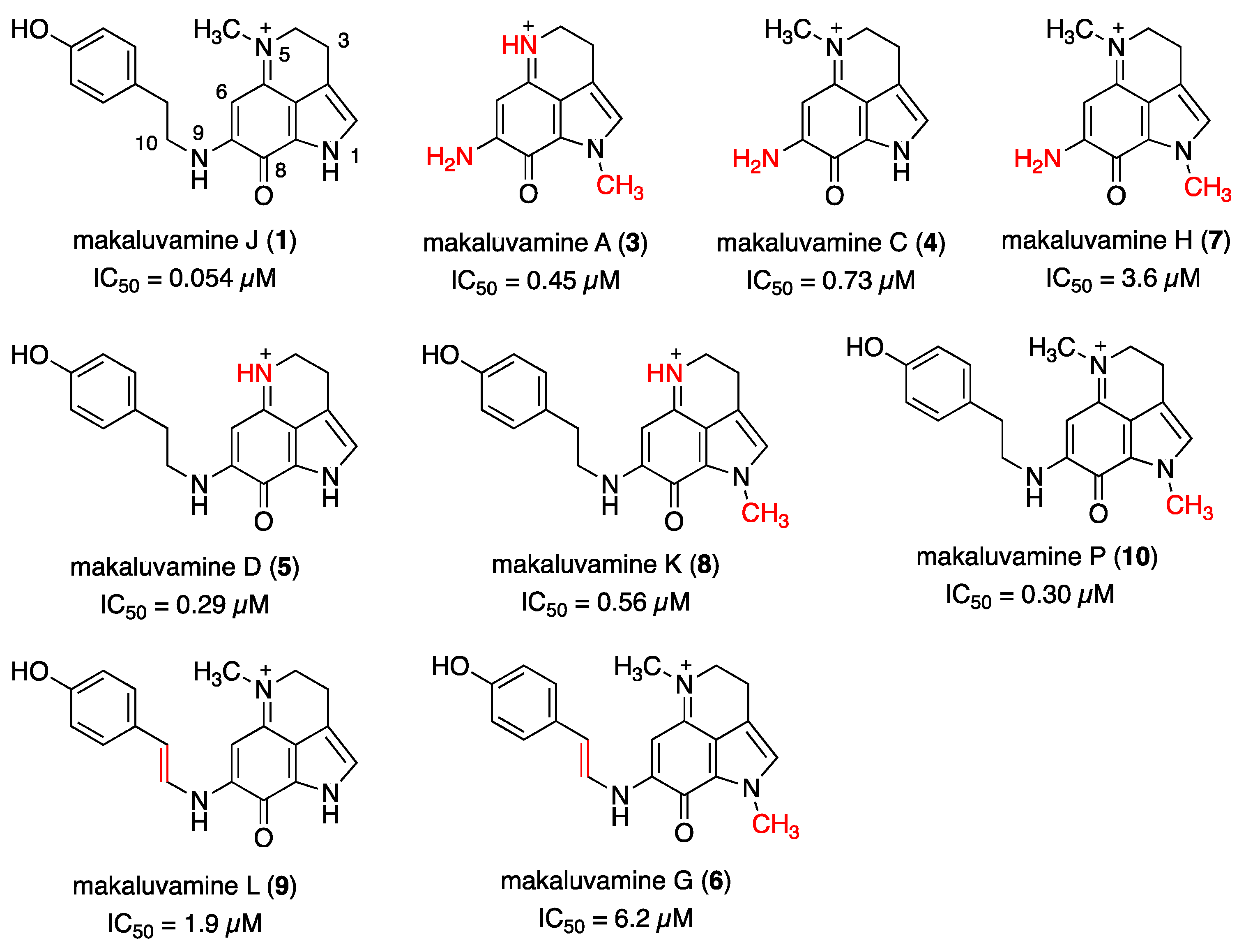
2.1. Reported Bioactivity and SAR of Makaluvamines against Pancreatic Cancer Cells
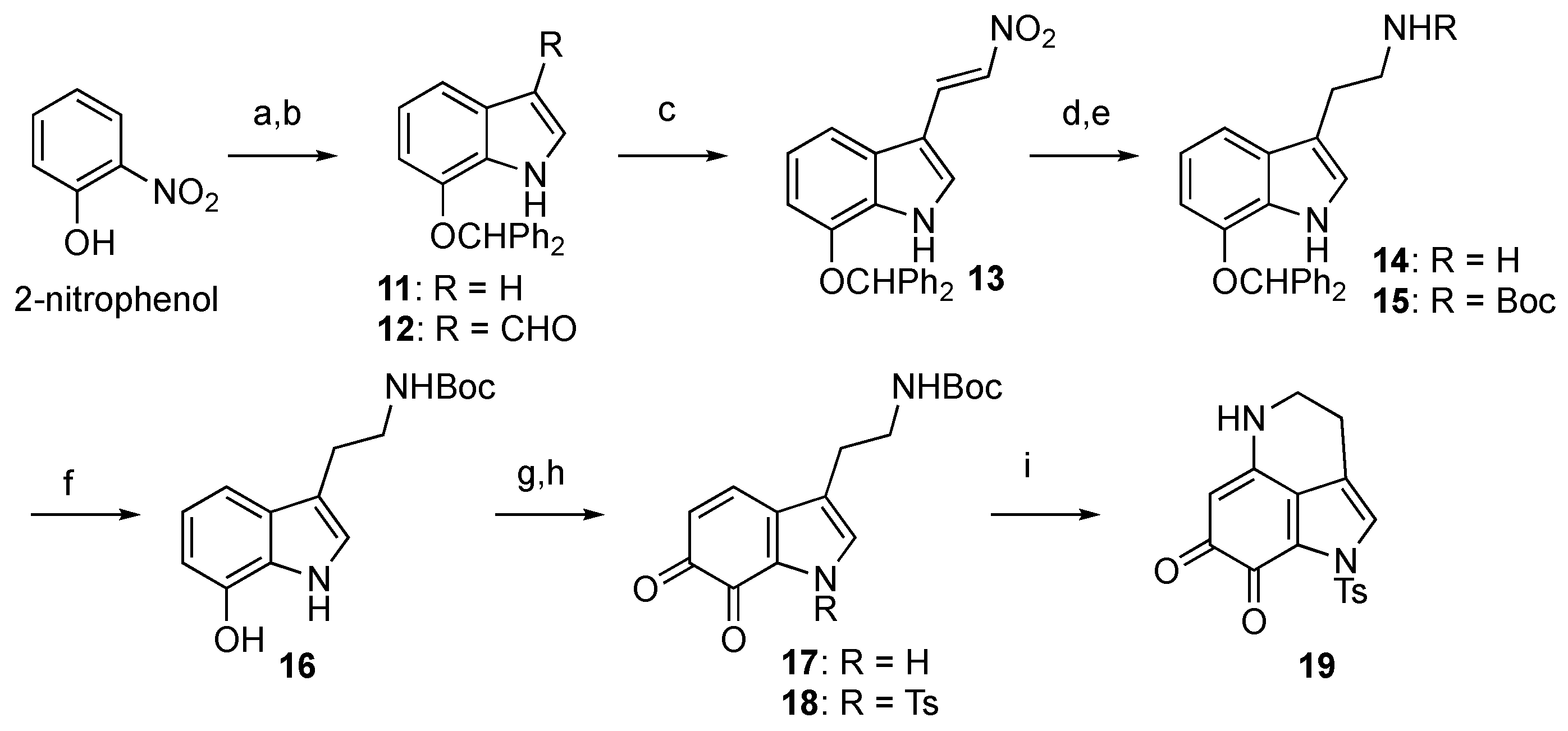
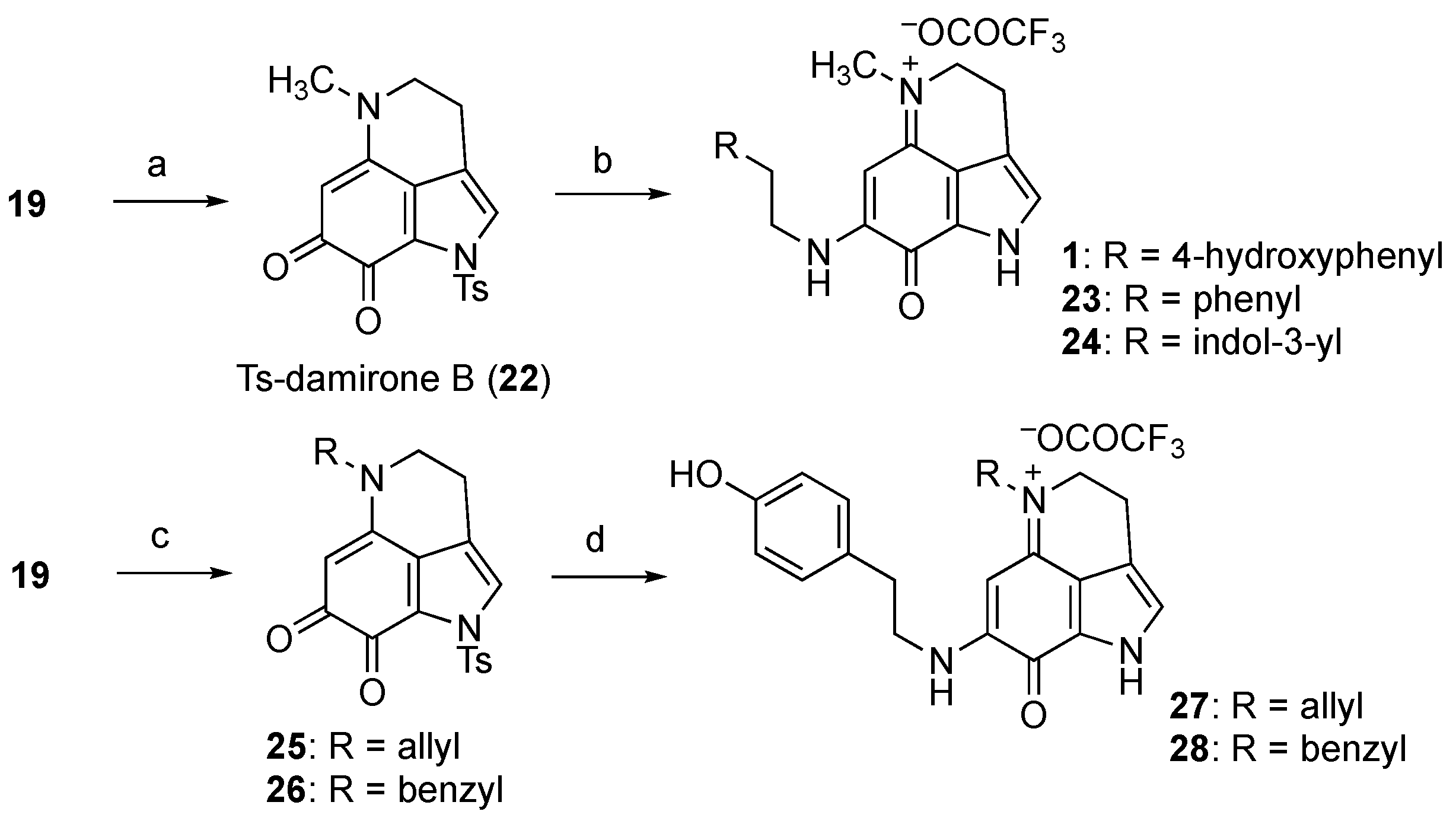
2.2. Unified Divergent Synthesis of Makaluvamines and Analogs
3. Materials and Methods
3.1. General
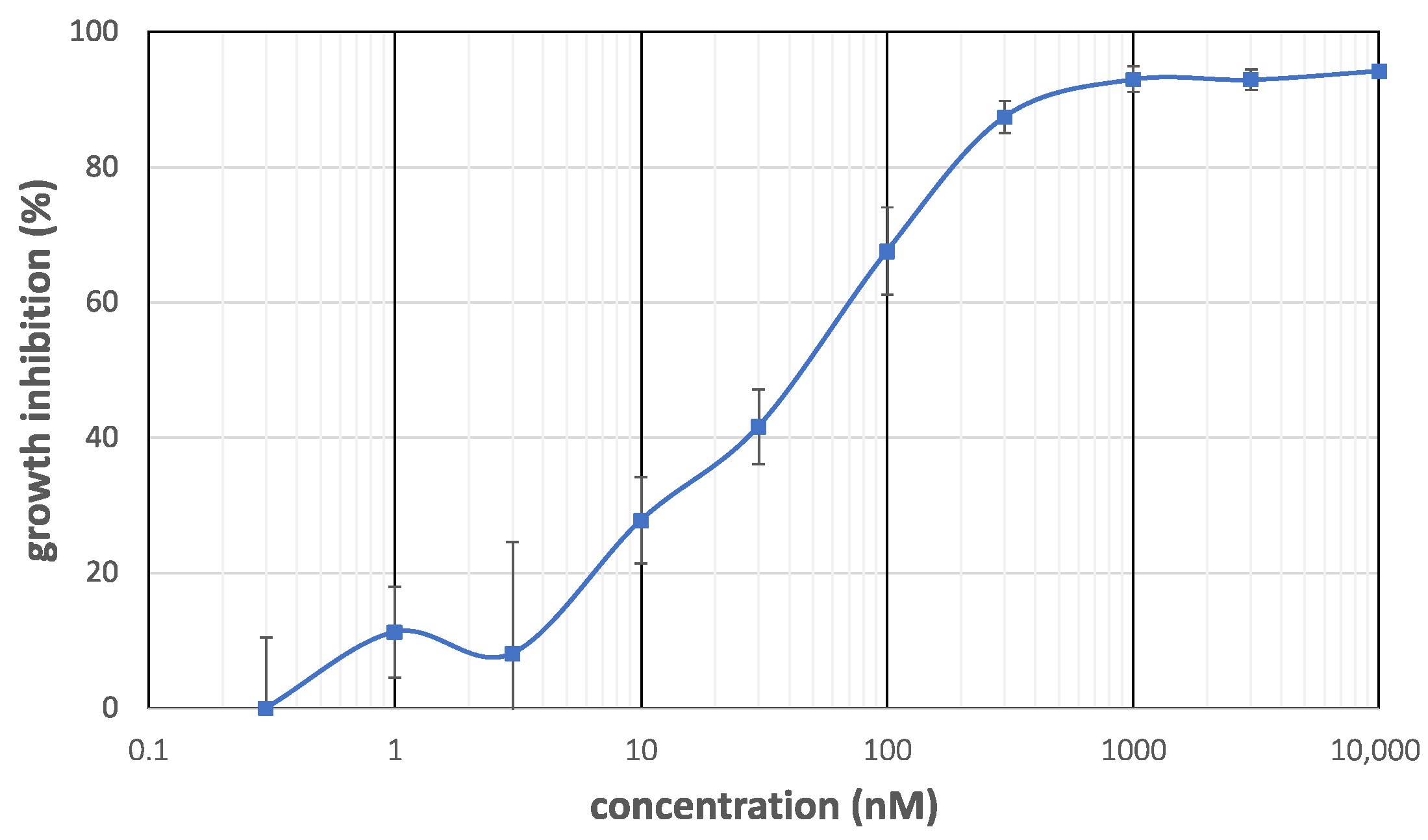
3.2. Antiproliferative Activity of the Compounds against Cancer Cells
3.3. Synthesis
3.3.1. 7-(Benzhydryloxy)-1H-indole (11)
3.3.2. 7-(Benzhydryloxy)-1H-indole-3-carbaldehyde (12)
3.3.3. (E)-7-(Benzhydryloxy)-3-(2-nitrovinyl)-1H-indole (13)
3.3.4. 2-(7-(Benzhydryloxy)-1H-indol-3-yl)ethan-1-amine (14)
3.3.5. tert-Butyl (2-(7-(benzhydryloxy)-1H-indol-3-yl)ethyl)carbamate (15)
3.3.6. tert-Butyl (2-(7-hydroxy-1H-indol-3-yl)ethyl)carbamate (16)
3.3.7. tert-Butyl (2-(6,7-dioxo-6,7-dihydro-1H-indol-3-yl)ethyl)carbamate (17)
3.3.8. tert-Butyl (2-(6,7-dioxo-1-tosyl-6,7-dihydro-1H-indol-3-yl)ethyl)carbamate (18)
3.3.9. 1-Tosyl-1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline-7,8-dione (Ts-damirone C, 19)
3.3.10. Ts-makaluvamine D (20)
3.3.11. Makaluvamine D (5)
3.3.12. Makaluvamine P (10)
3.3.13. 5-Methyl-1-tosyl-1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline-7,8-dione (Ts-damirone B, 22)
3.3.14. Makaluvamine J (1)
3.3.15. 5-Methyl-8-oxo-7-(phenethylamino)-1,3,4,8-tetrahydropyrrolo[4,3,2-de]quinolin-5-ium trifluoroacetate (23)
3.3.16. 7-((2-(1H-Indol-3-yl)ethyl)amino)-5-methyl-8-oxo-1,3,4,8-tetrahydropyrrolo[4,3,2-de]quinolin-5-ium trifluoroacetate (24)
3.3.17. 1-Tosyl-5-vinyl-1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline-7,8-dione (25)
3.3.18. 5-Benzyl-1-tosyl-1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline-7,8-dione (26)
3.3.19. 5-Allyl-7-((4-hydroxyphenethyl)amino)-8-oxo-1,3,4,8-tetrahydropyrrolo[4,3,2-de]quinolin-5-ium trifluoroacetate (27)
3.3.20. 5-Benzyl-7-((4-hydroxyphenethyl)amino)-8-oxo-1,3,4,8-tetrahydropyrrolo[4,3,2-de]quinolin-5-ium trifluoroacetate (28)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; the International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Kotoku, N. Marine Natural Products Targeting Tumor Microenvironment. In New Tide of Natural Product Chemistry; Ishikawa, H., Takayama, H., Eds.; Springer: Singapore, 2023; Volume 20, Chapter 3; pp. 35–58. [Google Scholar] [CrossRef]
- Kotoku, N.; Ishida, R.; Matsumoto, H.; Arai, M.; Toda, K.; Setiawan, A.; Muraoka, O.; Kobayashi, M. Biakamides A-D, Unique Polyketides from a Marine Sponge, Act as Selective Growth Inhibitors of Tumor Cells Adapted to Nutrient Starvation. J. Org. Chem. 2017, 82, 1705–1718. [Google Scholar] [CrossRef]
- Ishida, R.; Matsumoto, H.; Ichii, S.; Kobayashi, M.; Arai, M.; Kotoku, N. Structure-Activity Relationship of Biakamide, Selective Growth Inhibitors under Nutrient-Starved Condition from Marine Sponge. Chem. Pharm. Bull. 2019, 67, 210–223. [Google Scholar] [CrossRef]
- Lu, J.; Kunimoto, S.; Yamazaki, Y.; Kaminishi, M.; Esumi, H. Kigamicin D, A Novel Anticancer Agent Based on A New Anti-Austerity Strategy Targeting Cancer Cells’ Tolerance to Nutrient Starvation. Cancer Sci. 2004, 95, 547–552. [Google Scholar] [CrossRef]
- Awale, S.; Lu, J.; Kalauni, S.K.; Kurashima, Y.; Tezuka, Y.; Kadota, S.; Esumi, H. Identification of Arctigenin as an Antitumor Agent Having the Ability to Eliminate the Tolerance of Cancer Cells to Nutrient Starvation. Cancer Res. 2006, 66, 1751–1757. [Google Scholar]
- Arai, M.; Kamiya, K.; Shin, D.; Matsumoto, H.; Hisa, T.; Setiawan, A.; Kotoku, N.; Kobayashi, M. N-Methylniphatyne A, a New 3-Alkylpyridine Alkaloid as an Inhibitor of the Cancer Cells Adapted to Nutrient Starvation, from an Indonesian Marine Sponge of Xestospongia sp. Chem. Pharm. Bull. 2016, 64, 766–771. [Google Scholar] [CrossRef]
- Arai, M.; Shin, D.; Kamiya, K.; Ishida, R.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Marine Spongean Polybrominated Diphenyl Ethers, Selective Growth Inhibitors against the Cancer Cells Adapted to Glucose Starvation, Inhibits Mitochondrial Complex II. J. Nat. Med. 2017, 71, 44–49. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hisa, T.; Toda, K.; Ishida, R.; Setiawan, A.; Arai, M.; Kotoku, N. Fasciospyrinadinone and Fasciospyrinadinol, Novel 3-Alkylpyridine Sesquiterpenoids from an Indonesian Marine Sponge, as Selective Growth Inhibitors of the Cancer Cells under Nutrient Starvation. Heterocycles 2021, 103, 827–838. [Google Scholar] [CrossRef]
- Ikeda, M.; Sato, A.; Mochizuki, N.; Toyosaki, K.; Miyoshi, C.; Fujioka, R.; Mitsunaga, S.; Ohno, I.; Hashimoto, Y.; Takahashi, H.; et al. Phase I Trial of GBS-01 for Advanced Pancreatic Cancer Refractory to Gemcitabine. Cancer Sci. 2016, 107, 1818–1824. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Lin, S.; McCauley, E.P.; Lorig-Roach, N.; Tenney, K.; Naphen, C.N.; Yang, A.-M.; Johnson, T.A.; Hernadez, T.; Rattan, R.; Valeriote, F.A.; et al. Another Look at Pyrroloiminoquinone Alkaloids—Perspectives on Their Therapeutic Potential from Known Structures and Semisynthetic Analogues. Mar. Drugs 2017, 15, 98. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Sims, J.; Wang, B.; Pandey, P.; Welsh, C.L.; Stone, R.P.; Avery, M.A.; Doerksen, R.J.; Ferreira, D.; et al. Computationally Assisted Discovery and Assignment of a Highly Strained and PANC-1 Selective Alkaloid from Alaska’s Deep Ocean. J. Am. Chem. Soc. 2019, 141, 4338–4344. [Google Scholar] [CrossRef]
- Wang, B.; Shen, C.; Li, Y.; Zhang, T.; Huang, H.; Ren, J.; Hu, Z.; Xu, J.; Xu, B. Oridonin overcomes the gemcitabine resistant PANC-1/Gem cells by regulating GST pi and LRP/1 ERK/JNK signaling. Onco Targets Ther. 2019, 12, 5751–5765. [Google Scholar] [CrossRef]
- Fujioka, H.; Kita, Y. Marine Pyrroloiminoquinone Alkaloids, Makaluvamines and Discorhabdins, and Marine Pyrrole-Imidazole Alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 251–283. [Google Scholar]
- Hu, J.; Fan, H.; Xiong, J.; Wu, S. Discorhabdins and Pyrroloiminoquinone-Related Alkaloids. Chem. Rev. 2011, 111, 5465–5491. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Yager, K.M.; Yakura, T. Synthetic Studies of the Pyrroloquinoline Nucleus of the Makaluvamine Alkaloids. Synthesis of the Topoisomerase II Inhibitor Makaluvamine D. J. Am. Chem. Soc. 1994, 116, 1831–1838. [Google Scholar] [CrossRef]
- Izawa, T.; Nishiyama, S.; Yamamura, S. Total syntheses of makaluvamines A, B, C, D and E, cytotoxic pyrroloiminoquinone alkaloids isolated from marine sponge bearing inhibitory activities against topoisomerase II. Tetrahedron 1994, 50, 13593–13600. [Google Scholar] [CrossRef]
- Sadanandan, E.V.; Pillai, S.K.; Lakshmikantham, M.V.; Billimoria, A.D.; Culpepper, J.S.; Cava, M.P. Efficient Syntheses of the Marine Alkaloids Makaluvamine D and Discorhabdin C: The 4,6,7-Trimethoxyindole Approach. J. Org. Chem. 1995, 60, 1800–1805. [Google Scholar] [CrossRef]
- Iwao, M.; Motoi, O.; Fukuda, T.; Ishibashi, F. New synthetic approach to pyrroloiminoquinone marine alkaloids. Total synthesis of makaluvamines A, D, I, and K. Tetrahedron 1998, 54, 8999–9010. [Google Scholar] [CrossRef]
- An, J.; Jackson, R.K., III; Tuccinardi, J.P.; Wood, J.L. Pyrroloiminoquinone Alkaloids: Total Synthesis of Makaluvamines A and K. Org. Lett. 2023, 25, 1868–1871. [Google Scholar] [CrossRef]
- Dobson, D.; Todd, A.; Gilmore, J. The synthesis of 7-Alkoxyindoles. Synth. Commun. 1991, 21, 611–617. [Google Scholar] [CrossRef]
- Magdziak, D.; Rodriguez, A.A.; Van De Water, R.W.; Pettus, T.R.R. Regioselective Oxidation of Phenols to o-Quinones with o-Iodoxybenzoic Acid (IBX). Org. Lett. 2002, 4, 285–288. [Google Scholar] [CrossRef]
- Smith, M.W.; Falk, I.D.; Ikemoto, H.; Burns, N.Z. A convenient C–H functionalization platform for pyrroloiminoquinone alkaloid synthesis. Tetrahedron 2019, 75, 3366–3370. [Google Scholar] [CrossRef]
- Beyerman, H.C.; Hirt, J.; Kranenburg, P.; Syrier, J.L.M.; van Zon, A. Excess mixed anhydride peptide synthesis with histidine derivatives. Recl. Trav. Chim. Pays-Bas 1974, 93, 256–257. [Google Scholar] [CrossRef]
- Bajwa, J.S.; Chen, G.P.; Prasad, K.; Oljan, R.; Blacklock, T.J. Deprotection of N-tosylated indoles and related structures using cesium carbonate. Tetrahedron Lett. 2006, 47, 6425–6427. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Hu, Y.; Geng, H.; Jiang, Y.; Zhou, Y.; Zhu, W.; Hu, M.; Hu, H.; Wang, X.; et al. A NaH-promoted N-detosylation reaction of diverse p-toluenesulfonamides. Tetrahedron Lett. 2020, 61, 152442. [Google Scholar] [CrossRef]
- Kitagawa, K.; Kitade, K.; Kiso, Y.; Akita, T.; Funakoshi, S.; Fujii, N.; Yajima, H. Studies on Peptides. LXXXV. A New Deprotecting Procedure for p-Toluenesulfonyl and p-Methoxybenzenesulfonyl Groups from the Nim-Function of Histidine. Chem. Pharm. Bull. 1980, 28, 926–931. [Google Scholar] [CrossRef]
- Casapullo, A.; Cutignano, A.; Bruno, I.; Bifulco, G.; Debitus, C.; Gomez-Paloma, L.; Riccio, R. Makaluvamine P, a New Cytotoxic Pyrroloiminoquinone from Zyzzya cf. fuliginosa. J. Nat. Prod. 2001, 64, 1354–1356. [Google Scholar] [CrossRef]
- Inoue, K.; Ishikawa, Y.; Nishiyama, S. Synthesis of Tetrahydropyrroloiminoquinone Alkaloids Based on Electrochemically Generated Hypervalent Iodine Oxidative Cyclization. Org. Lett. 2010, 12, 436–439. [Google Scholar] [CrossRef]


| Compound | PANC-1 (µM) 1 | KB3-1 (µM) 1 | S.I. 2 |
|---|---|---|---|
| 1 | 0.046 | 0.2 | 4.3 |
| 5 | 4.4 | 3.2 | 0.7 |
| 10 | 0.2 | 0.51 | 2.6 |
| 23 | 0.042 | 0.41 | 9.8 |
| 24 | 0.029 | 0.38 | 13.1 |
| 25 | 2.9 | >10 | >3.4 |
| 26 | 1.9 | 6.7 | 3.5 |
| 27 | 0.038 | 0.078 | 2.1 |
| 28 | 0.027 | 0.047 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiichi, Y.; Fukuoka, K.; Kitano, A.; Ishino, K.; Kotoku, N. Unified Synthesis and Biological Evaluation of Makaluvamine J and Its Analogs. Molecules 2024, 29, 1389. https://doi.org/10.3390/molecules29061389
Kiichi Y, Fukuoka K, Kitano A, Ishino K, Kotoku N. Unified Synthesis and Biological Evaluation of Makaluvamine J and Its Analogs. Molecules. 2024; 29(6):1389. https://doi.org/10.3390/molecules29061389
Chicago/Turabian StyleKiichi, Yo, Koshiro Fukuoka, Anna Kitano, Koya Ishino, and Naoyuki Kotoku. 2024. "Unified Synthesis and Biological Evaluation of Makaluvamine J and Its Analogs" Molecules 29, no. 6: 1389. https://doi.org/10.3390/molecules29061389
APA StyleKiichi, Y., Fukuoka, K., Kitano, A., Ishino, K., & Kotoku, N. (2024). Unified Synthesis and Biological Evaluation of Makaluvamine J and Its Analogs. Molecules, 29(6), 1389. https://doi.org/10.3390/molecules29061389






