Neurotensin (8-13) and Neuromedin N Neuropeptides Radiolabelling with Copper-64 Produced on Solid or Liquid Targets
Abstract
1. Introduction
2. Results
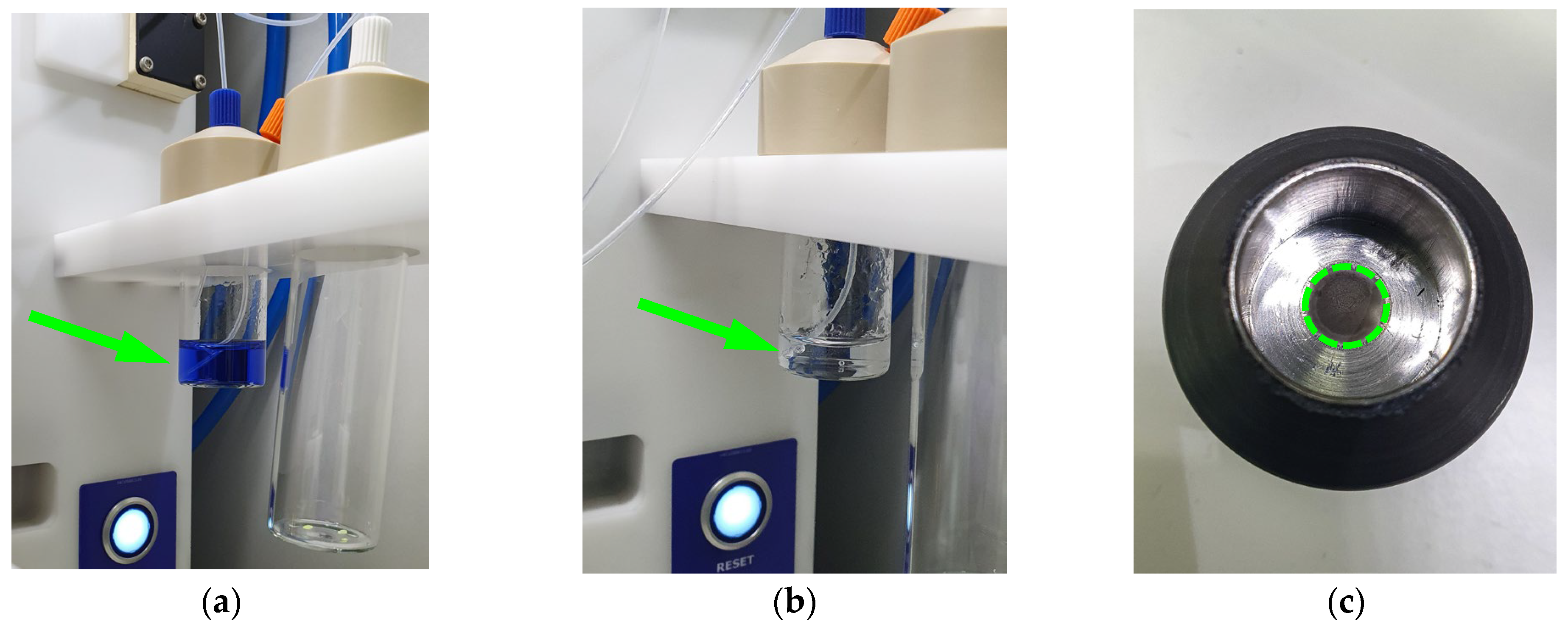
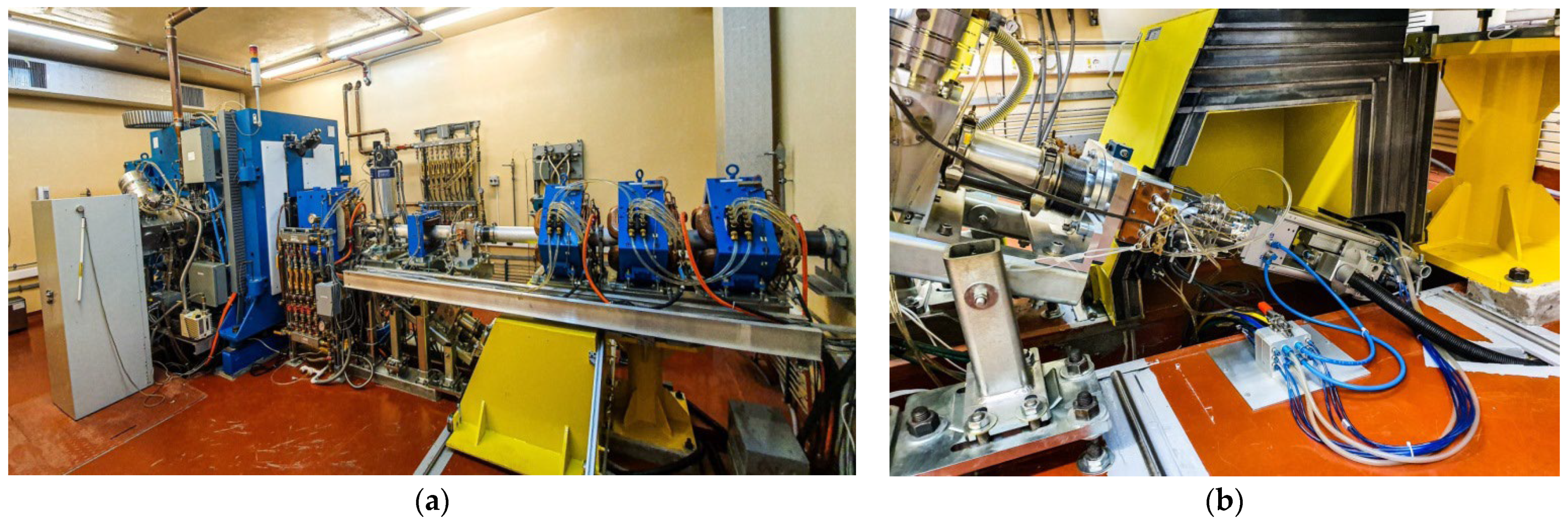
2.1. Copper-64 Production and Post-Irradiation Processing via Solid Target (ST) System
2.2. Copper-64 Production and Post-Irradiation Processing via Liquid Target (LT) System
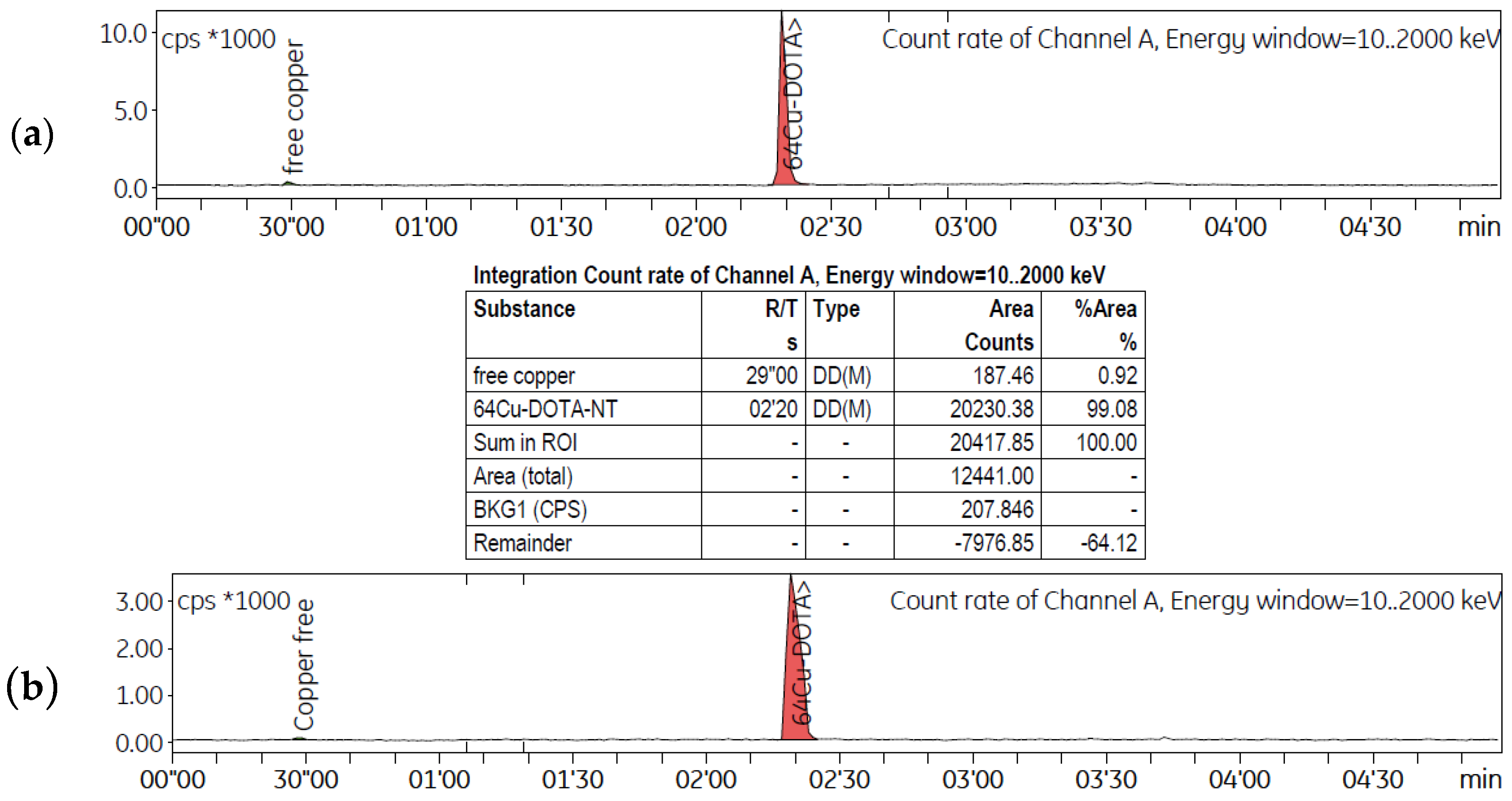
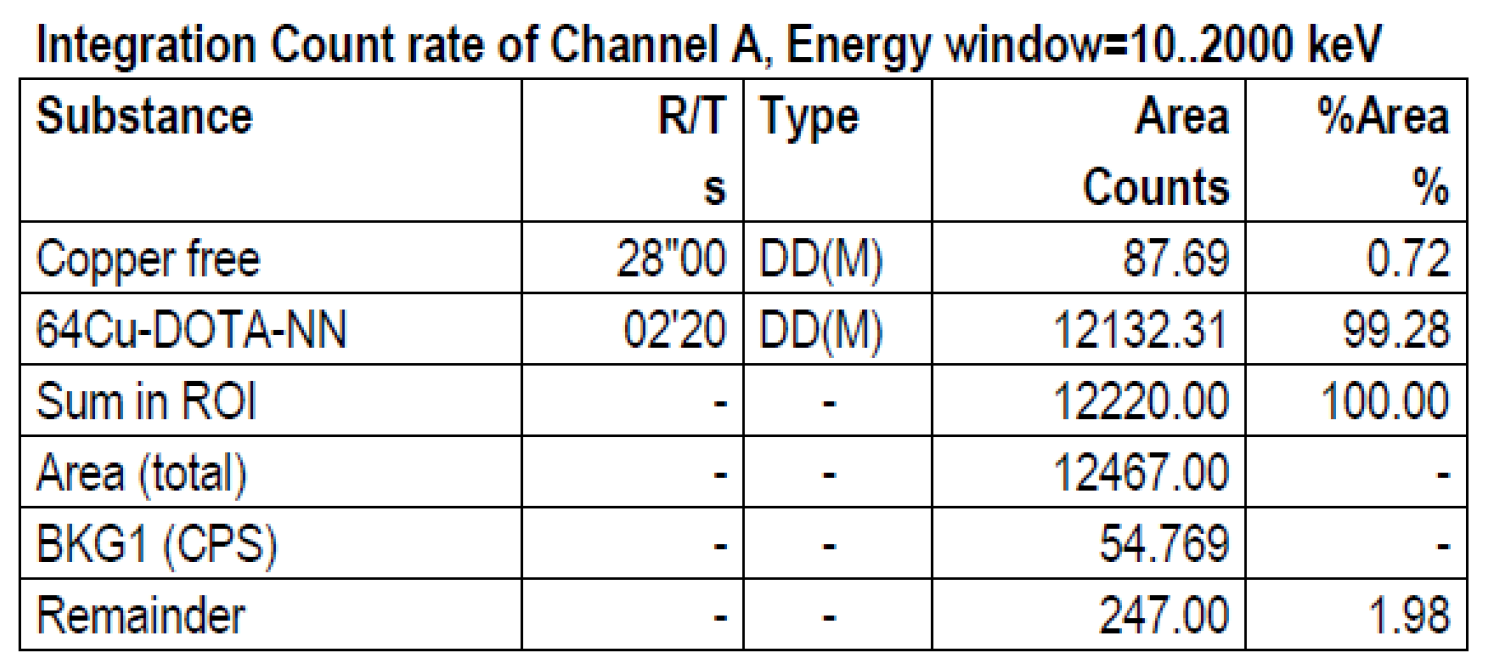
2.3. Comparative Evaluation of the Neuropeptides Radiolabelling Using [64Cu]CuCl2 Prepared via ST and LT
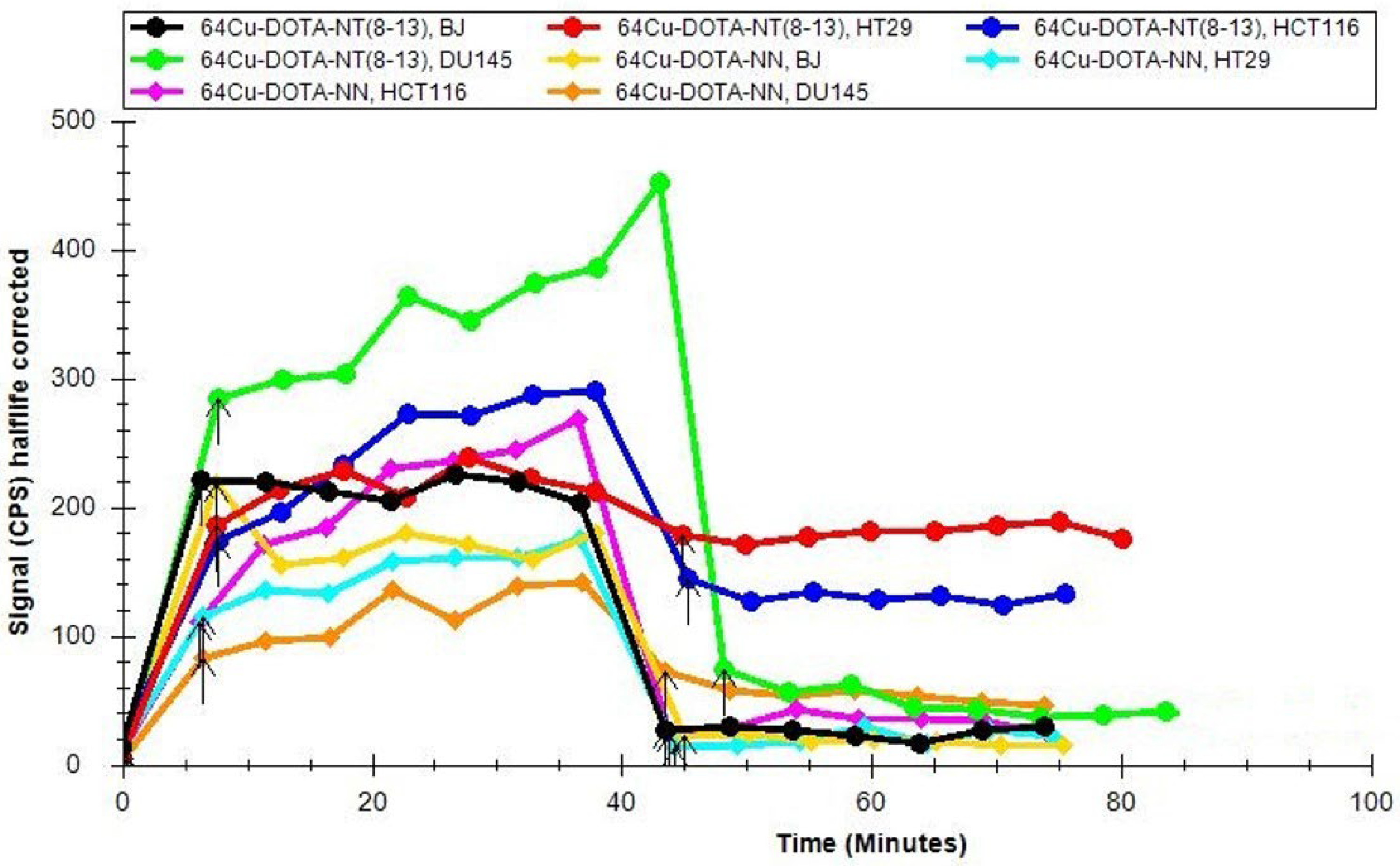
2.4. In Vitro Study for Peptide Radiolabelling
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Target Preparation of Solid and Liquid Targets
4.2.1. Preparation of Solid Target
4.2.2. Preparation of Liquid Target Solution
4.3. Cyclotrons and Irradiation
4.3.1. Solid Target Irradiation
4.3.2. Liquid Target Irradiation
4.4. Post-Irradiation Processing
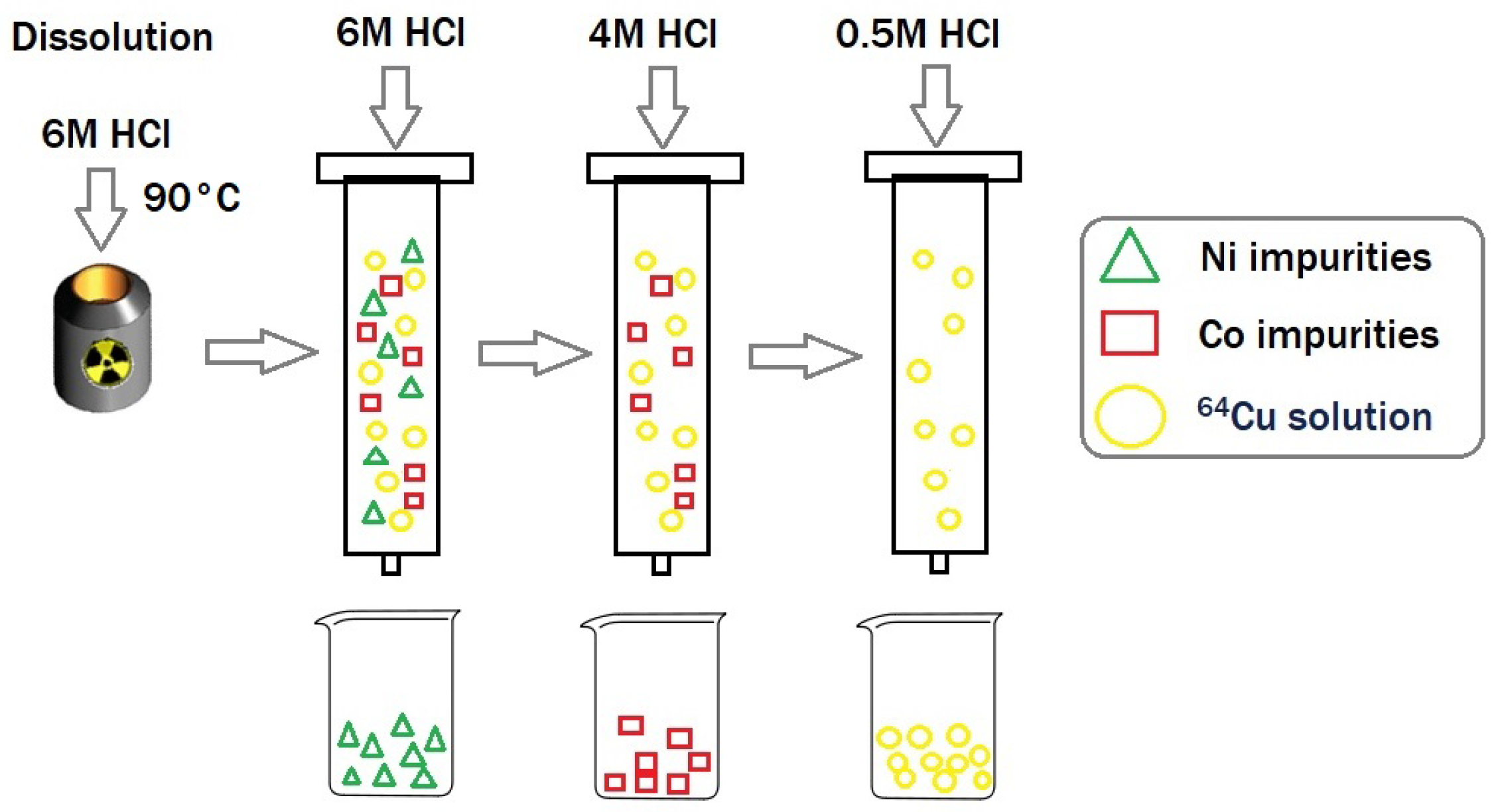
4.4.1. Solid Target Dissolution and Purification
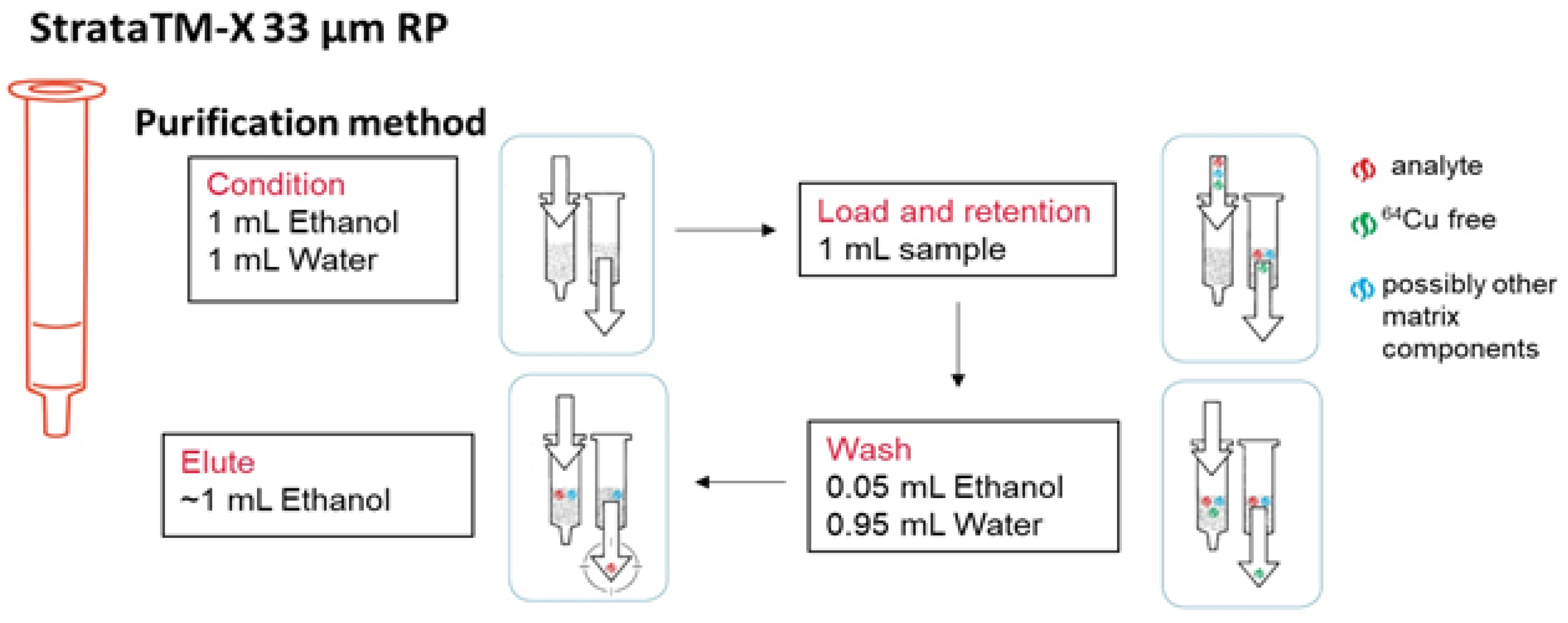
4.4.2. Liquid Target Purification
4.5. Peptide Radiolabelling
4.6. In Vitro Uptake-Retention Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, B.; Chen, J. Nuclear Data Sheets for A=64. Nucl. Data Sheets 2021, 178, 41–537. [Google Scholar] [CrossRef]
- Xie, Q.; Zhu, H.; Wang, F.; Meng, X.; Ren, Q.; Xia, C.; Yang, Z. Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging. Molecules 2017, 22, 641. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- National Nuclear Data Center, Brookhaven National Laboratory. (18 March 2008). NuDat (Nuclear Structure and Decay Data). Available online: https://www.nndc.bnl.gov/nudat2/ (accessed on 7 March 2024).
- Niculae, D.; Dusman, R.; Leonte, R.A.; Chilug, L.E.; Dragoi, C.M.; Nicolae, A.; Dumitrescu, I.B.; Draganescu, D. Biological pathways as substantiation of the use of copper radioisotopes in cancer theranostics. Front. Phys. 2021, 8, 568296. [Google Scholar] [CrossRef]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J.H. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am. J. Physiol.-Cell Physiol. 2013, 304, C768–C779. [Google Scholar] [CrossRef]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Gutfilen, B.; Souza, S.A.; Valentini, G. Copper-64: A real theranostic agent. Drug Des. Dev. Ther. 2018, 12, 3235–3245. [Google Scholar] [CrossRef]
- Leonte, R.A.; Cocioabă, D.; Chilug, L.E.; Băruță, S.I.; Eșanu, T.R.; Burghelea, B.; Chiriacescu, A.; Craciun, L.S.; Niculae, D. Process validation for production of copper radioisotopes in a TR-19 variable energy cyclotron. In American Institute of Physics (AIP) Conference Proceedings; AIP Publishing: New York, NY, USA, 2020; Volume 2295, p. 020022. [Google Scholar] [CrossRef]
- Alves, F.; Alves, V.H.P.; Do Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Hrynchak, I.; Cocioabă, D.; Fonseca, A.I.; Leonte, R.; do Carmo, S.J.C.; Cornoiu, R.; Falcão, A.; Niculae, D.; Abrunhosa, A.J. Antibody and Nanobody Radiolabeling with Copper-64: Solid vs. Liquid Target Approach. Molecules 2023, 28, 4670. [Google Scholar] [CrossRef]
- Liu, G.; Grant, W.M.; Persky, D.; Latham, V.M., Jr.; Singer, R.H.; Condeelis, J. Interactions of elongation factor 1α with F-actin and β-actin mRNA: Implications for anchoring mRNA in cell protrusions. Mol. Biol. Cell 2002, 13, 579–592. [Google Scholar] [CrossRef]
- Dobner, P.R.; Barber, D.L.; Villa-Komaroff, L.; McKiernan, C. Cloning and sequence analysis of cDNA for the canine neurotensin/neuromedin N precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 3516–3520. [Google Scholar] [CrossRef] [PubMed]
- Bozou, J.C.; Amar, S.; Vincent, J.P.; Kitabgi, P. Neurotensin-mediated inhibition of cyclic AMP formation in neuroblastoma NIE115 cells: Involvement of the inhibitory GTP-binding component of adenylate cyclase. Mol. Pharmacol. 1986, 29, 489–496. [Google Scholar] [PubMed]
- Camby, I.; Salmon, I.; Danguy, A.; Pasteels, J.L.; Brotchi, J.; Martinez, J.; Kiss, R. Influence of gastrin on human astrocytic tumor cell proliferation. J. Nat. Cancer Inst. 1996, 88, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Iwase, K.; Evers, B.M.; Hellmich, M.R.; Kim, H.J.; Higashide, S.; Gully, D.; Thompson, J.C.; Townsend, C.M., Jr. Inhibition of neurotensin-induced pancreatic carcinoma growth by a nonpeptide neurotensin receptor antagonist, SR48692. Cancer 1997, 79, 1787–1793. [Google Scholar] [CrossRef]
- Seethalakshmi, L.; Mitra, S.P.; Dobner, P.R.; Menon, M.; Carraway, R.E. Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate 1997, 31, 183–192. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Neurotensin receptors in human neoplasms: High incidence in Ewing’s sarcomas. Int. J. Cancer 1999, 82, 213–218. [Google Scholar] [CrossRef]
- Sarret, P.; Cavelier, F. Neurotensin and Its Receptors q. In Neuroscience and Biobehavioral Psychology; HAL Open Sciencehal-03150277; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Rovère, C.; Barbero, P.; Maoret, J.J.; Laburthe, M.; Kitabgi, P. Pro-neurotensin/neuromedin N expression and processing in human colon cancer cell lines. Biochem. Biophys. Res. Commun. 1998, 246, 155–159. [Google Scholar] [CrossRef]
- Baruta, S.; Leonte, R.; Cocioaba, D.; Craciun, L.; Ur, C.A.; Niculae, D. Cyclotron production of 64Cu by proton irradiation of enriched 64Ni target: Validation of Geant4 simulation parameters through experimental data. Front. Phys. 2022, 10, 1038014. [Google Scholar] [CrossRef]
- Chilug, L.E.; Niculae, D.; Leonte, R.A.; Nan, A.; Turcu, R.; Mustaciosu, C.; Serban, R.M.; Lavric, V.; Manda, G. Preclinical evaluation of NHS-activated gold nanoparticles functionalized with bombesin or neurotensin-like peptides for targeting colon and prostate tumours. Molecules 2020, 25, 3363. [Google Scholar] [CrossRef]
- Didier, E.S.; Rogers, L.B.; Orenstein, J.M.; Baker, M.D.; Vossbrinck, C.R.; Van Gool, T.O.M.; Hartskeerl, R.; Soave, R.; Beaudet, L.M. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J. Eukaryot. Microbiol. 1996, 43, 34–43. [Google Scholar] [CrossRef]
- Brattain, M.G.; Fine, W.D.; Khaled, F.M.; Thompson, J.; Brattain, D.E. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981, 41, 1751–1756. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Tesmer, V.M.; Savre-Train, I.; Shay, J.W.; Wright, W.E. Both transcriptional and posttranscriptional mechanisms regulate human telomerase template RNA levels. Mol. Cell Biol. 1999, 19, 3989–3997. [Google Scholar] [CrossRef] [PubMed]
- Leonte, R.A.; Niculae, D.; Craciun, L.S.; Cata-Danil, G. Medical radioisotopes production at TR-19 cyclotron from IFIN-HH. UPB Sci. Bull. Ser. A 2017, 79, 223–236. [Google Scholar]
- Bjorke, H.; Andersson, K. Automated, high-resolution cellular retention and uptake studies in vitro. Appl. Radiat. Isot. 2006, 64, 901–905. [Google Scholar] [CrossRef]



| Parameters | Previous Settings [21] | New Settings | |
|---|---|---|---|
| Proton energy (MeV) extracted/on target | 14/11.6 | 14.2/11.8 | |
| Beam current (μA) | 20 | 25 | |
| Beam time (hours) | 4 | 6 | |
| Integrated beam current (μA·h) | 80 | 150 | |
| Activity 1 (GBq) | Simulation | 10.3 ± 0.5 | 21.6 ± 0.8 |
| Experiment | 8.7 ± 0.7 | 17.6 ± 2.1 | |
| ST | LT | |||
|---|---|---|---|---|
| Peptides | DOTA-NT(8-13) | DOTA-NN | DOTA-NT(8-13) | DOTA-NN |
| Quantity (nmol) | 20 | 20 | ||
| Radiolabelling pH | 3.9 ± 0.1 | 3.9 ± 0.1 | ||
| [64Cu]CuCl2 volume (mL) | 1.00 ± 0.2 | 1.75 ± 0.25 | ||
| [64Cu]CuCl2 activity (MBq) | 1340.4 ± 70.1 | 861.8 ± 17.3 | ||
| Temperature (°C) | 95 | 95 | ||
| Reaction time (min) | 25 | 25 | ||
| Radiolabelling yield (%) | 67.04 ± 2.68 | 78.15 ± 3.12 | 63.24 ± 2.53 | 61.22 ± 2.45 |
| Radiochemical purity (%) | 99.99% | 99.99% | 99.08% | 99.28% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocioabă, D.; Fonseca, A.I.; Leonte, R.; Hrynchak, I.; Tudoroiu-Cornoiu, R.; do Carmo, S.J.C.; Burghelea, B.; Băruță, S.; Almeida, A.R.; Șerban, R.; et al. Neurotensin (8-13) and Neuromedin N Neuropeptides Radiolabelling with Copper-64 Produced on Solid or Liquid Targets. Molecules 2024, 29, 1390. https://doi.org/10.3390/molecules29061390
Cocioabă D, Fonseca AI, Leonte R, Hrynchak I, Tudoroiu-Cornoiu R, do Carmo SJC, Burghelea B, Băruță S, Almeida AR, Șerban R, et al. Neurotensin (8-13) and Neuromedin N Neuropeptides Radiolabelling with Copper-64 Produced on Solid or Liquid Targets. Molecules. 2024; 29(6):1390. https://doi.org/10.3390/molecules29061390
Chicago/Turabian StyleCocioabă, Diana, Alexandra I. Fonseca, Radu Leonte, Ivanna Hrynchak, Roxana Tudoroiu-Cornoiu, Sergio J. C. do Carmo, Bogdan Burghelea, Simona Băruță, Ana Rita Almeida, Radu Șerban, and et al. 2024. "Neurotensin (8-13) and Neuromedin N Neuropeptides Radiolabelling with Copper-64 Produced on Solid or Liquid Targets" Molecules 29, no. 6: 1390. https://doi.org/10.3390/molecules29061390
APA StyleCocioabă, D., Fonseca, A. I., Leonte, R., Hrynchak, I., Tudoroiu-Cornoiu, R., do Carmo, S. J. C., Burghelea, B., Băruță, S., Almeida, A. R., Șerban, R., Dinischiotu, A., Abrunhosa, A. J., & Niculae, D. (2024). Neurotensin (8-13) and Neuromedin N Neuropeptides Radiolabelling with Copper-64 Produced on Solid or Liquid Targets. Molecules, 29(6), 1390. https://doi.org/10.3390/molecules29061390









