Solubilization of Phospholipid by Surfactin Leading to Lipid Nanodisc and Fibrous Architecture Formation
Abstract
1. Introduction
2. Results
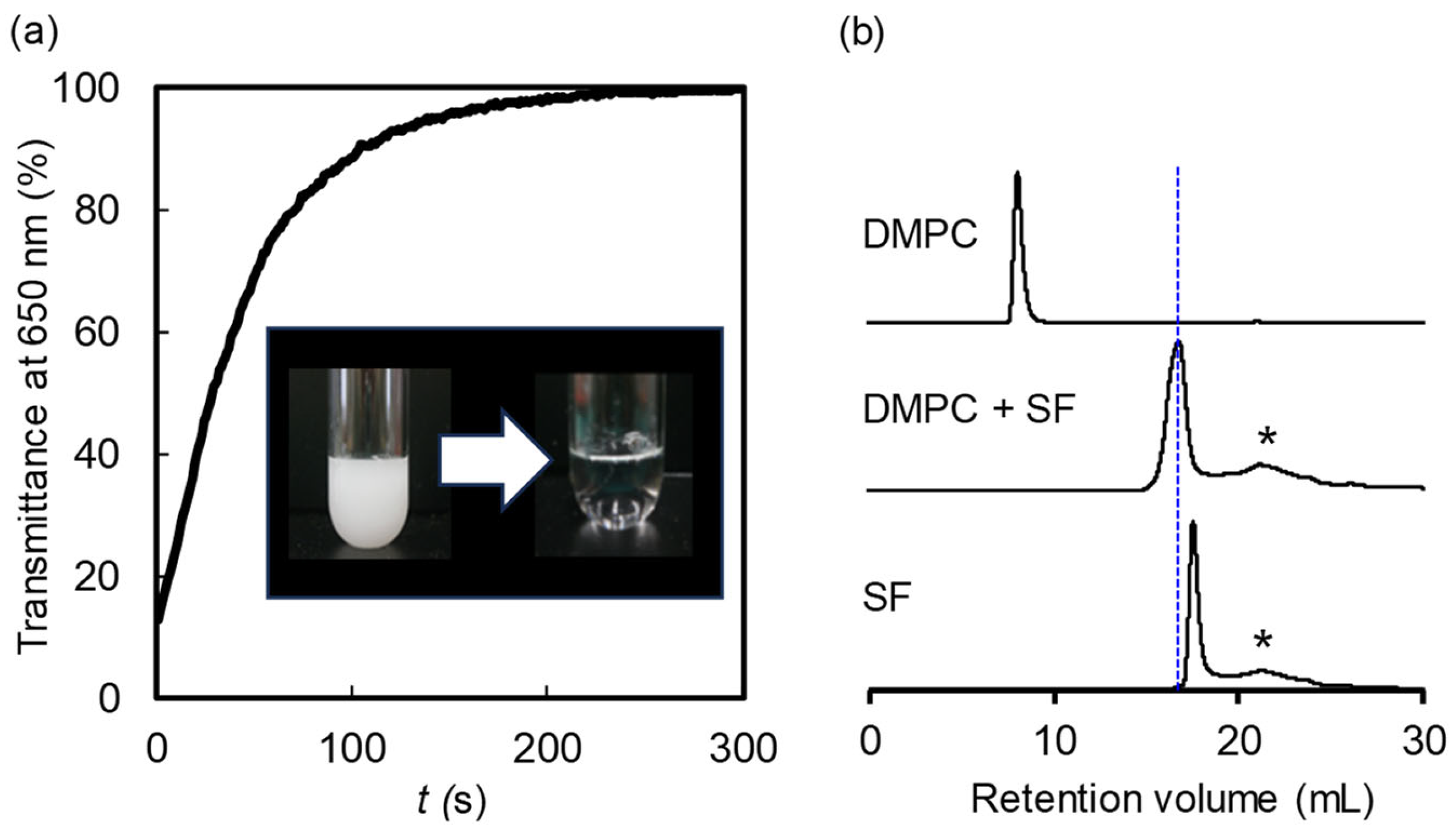
2.1. Ability of SF to Solubilize DMPC
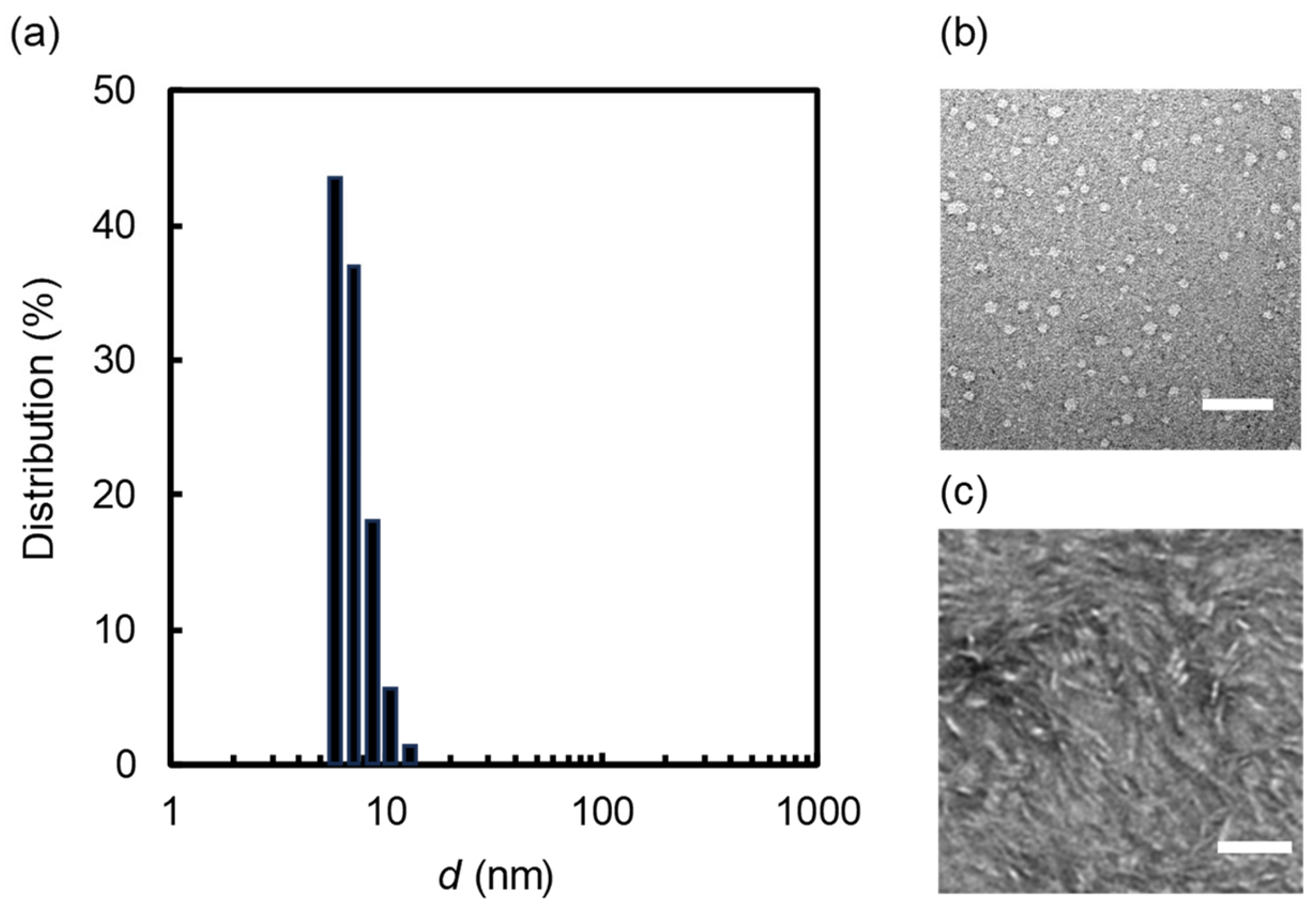
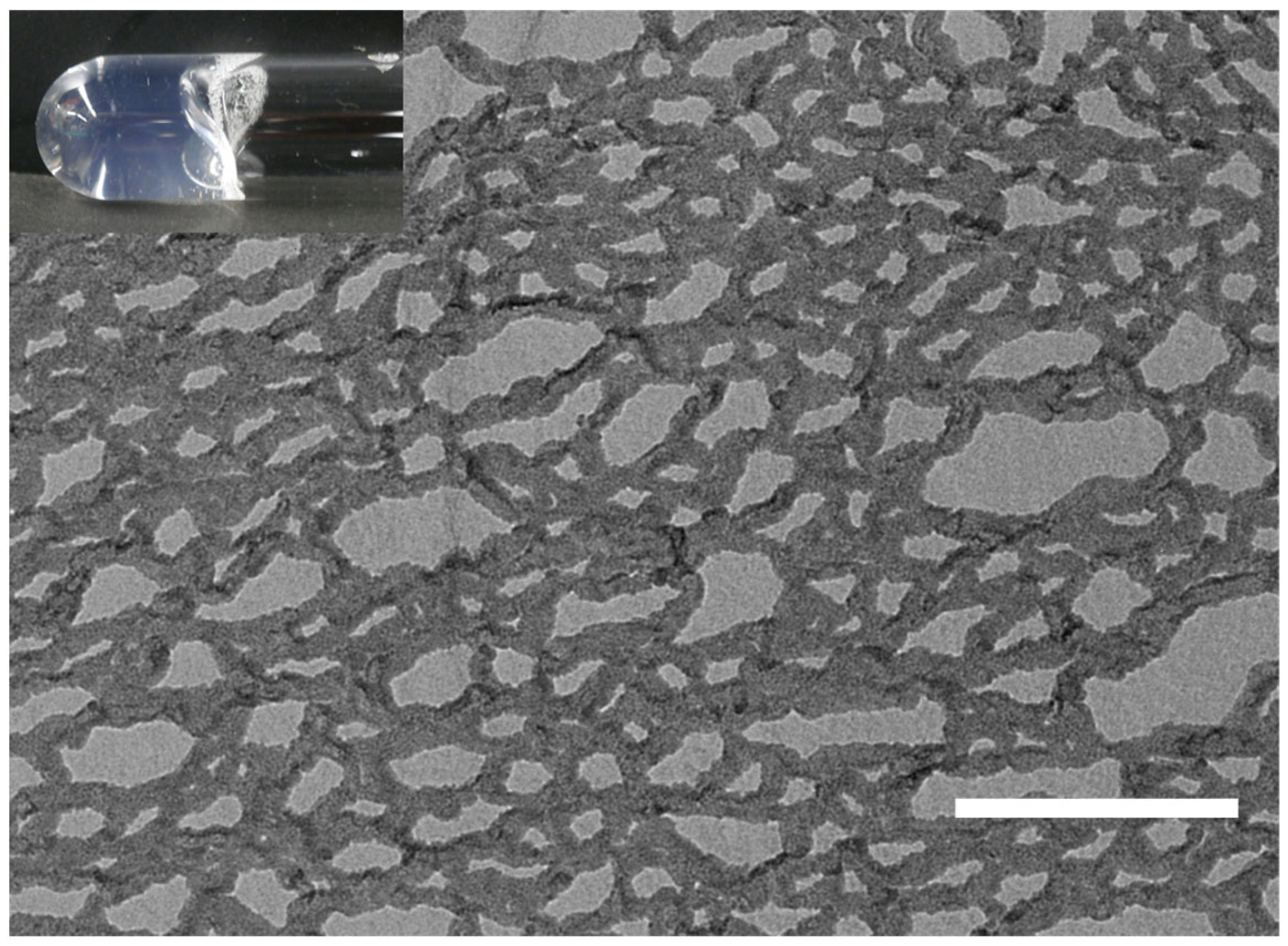
2.2. Nanodisc Formation
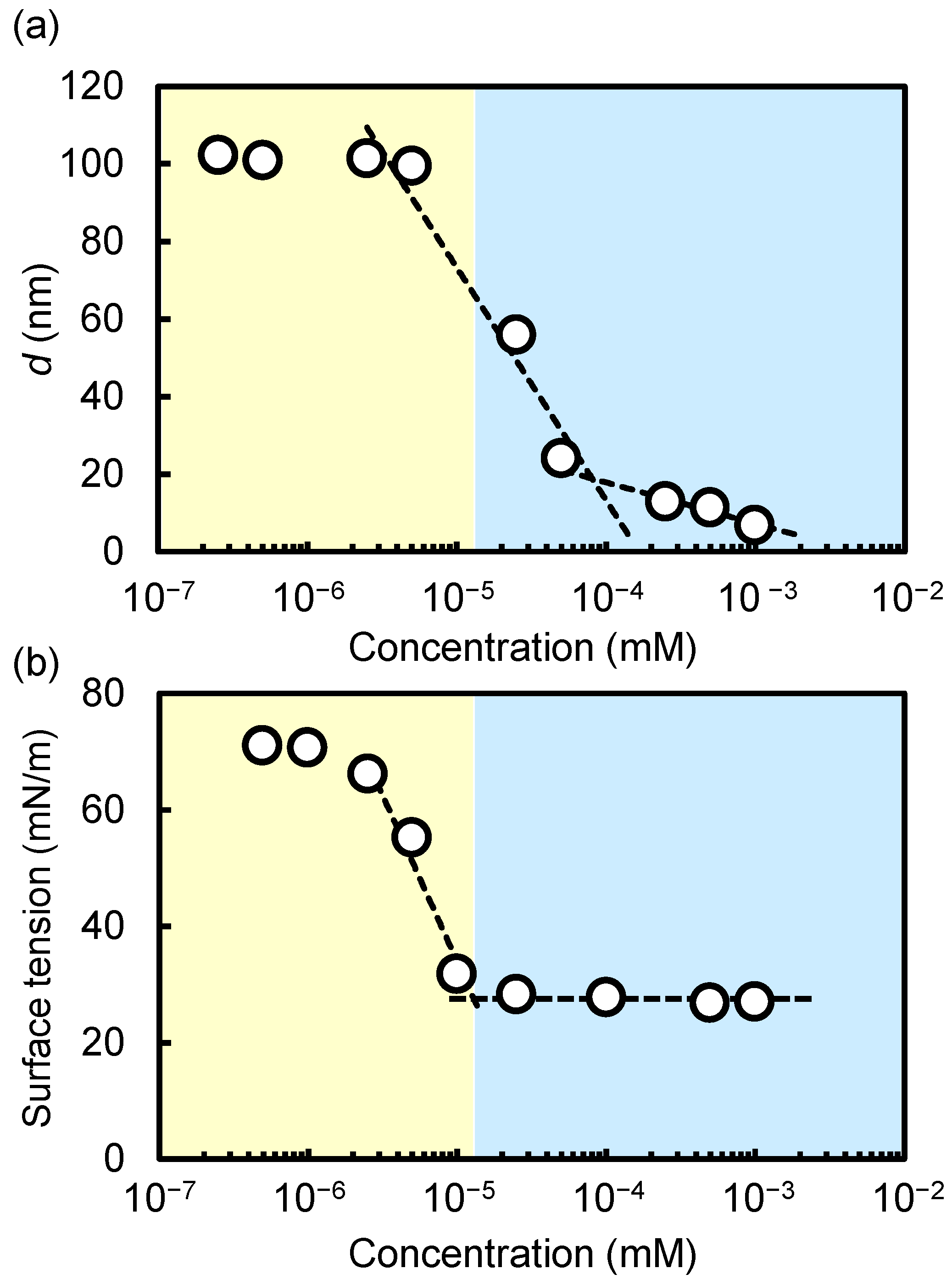
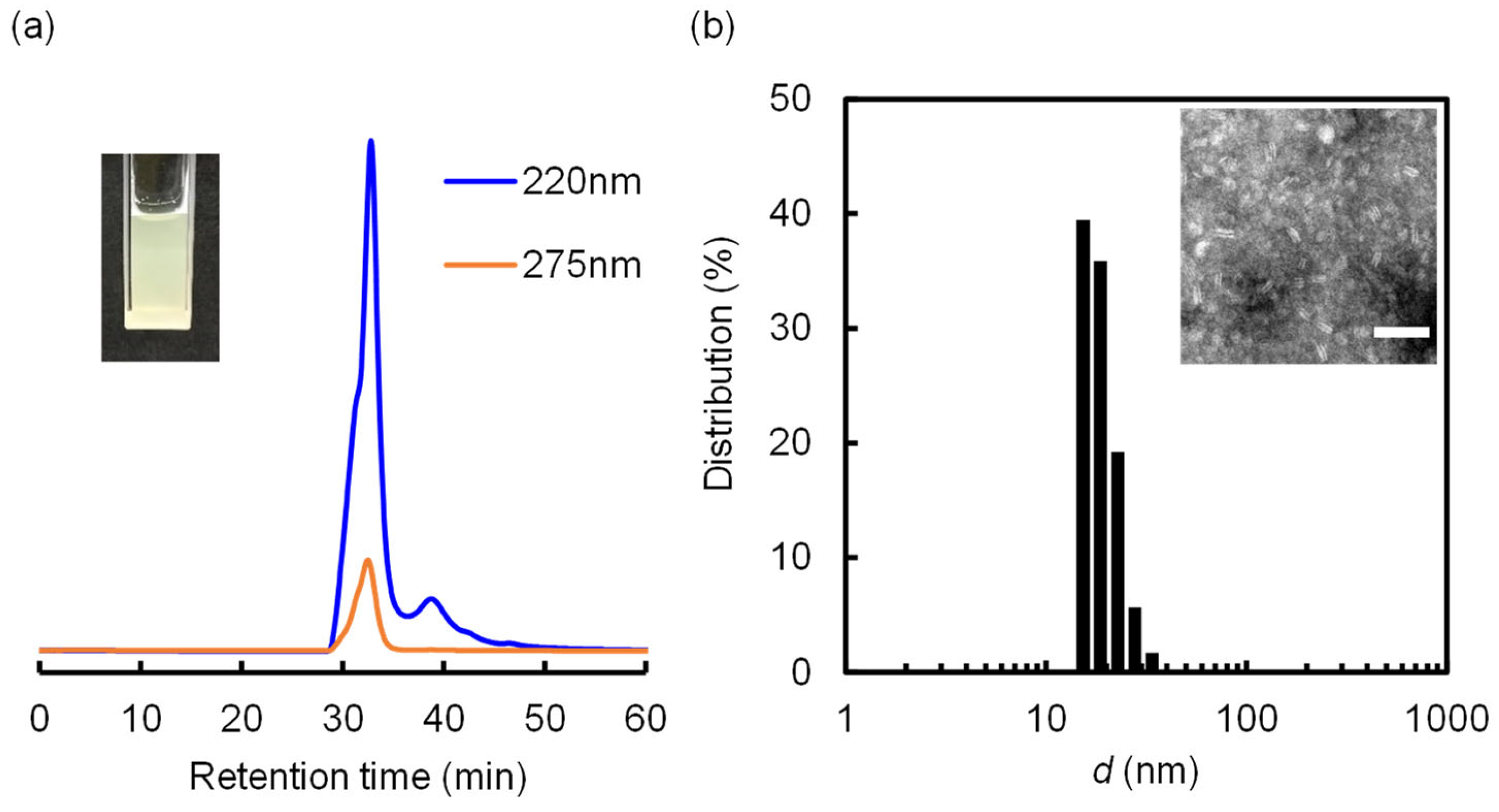
2.3. Phase Diagram
3. Materials and Methods
3.1. Materials
3.2. SF−DMPC Nanodisc Preparation [16]
3.3. Surface Tension Measurement
3.4. Size-Exclusion Chromatography (SEC)
3.5. Dynamic Light Scattering (DLS)
3.6. Transmission Electron Microscopy (TEM)
3.6.1. Negative Staining
3.6.2. Freeze Etching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and Liposomes: A Self-Assembly Principle Beyond Lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Deamer, D.W. Membrane Self-Assembly Processes: Steps Toward the First Cellular Life. Anat. Rec. 2002, 268, 196–207. [Google Scholar] [CrossRef]
- Yildirim, M.A.; Goh, K.-I.; Cusick, M.E.; Barabási, A.-L.; Vidal, M. Drug-target network. Nat. Biotechnol. 2007, 25, 1119–1126. [Google Scholar] [CrossRef]
- Almén, M.S.; Nordström, K.J.; Fredriksson, R.; Schiöth, H.B. Mapping the human membrane proteome: A majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009, 7, 50. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Bilayer Nanoparticles with Membrane Scaffold Proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Shih, A.Y.; Freddolino, P.L.; Sligar, S.G.; Schulten, K. Disassembly of Nanodiscs with Cholate. Nano Lett. 2007, 7, 1692–1696. [Google Scholar] [CrossRef]
- Denisov, I.G.; Sligar, S.G. Nanodiscs in Membrane Biochemistry and Biophysics. Chem. Rev. 2017, 117, 4669–4713. [Google Scholar] [CrossRef]
- Günsel, U.; Hagn, F. Lipid Nanodiscs for High-Resolution NMR Studies of Membrane Proteins. Chem. Rev. 2022, 122, 9395–9421. [Google Scholar] [CrossRef]
- Anantharamaiah, G.M.; Jones, J.L.; Brouillette, C.G.; Schmidt, C.F.; Chung, B.H.; Hughes, T.A.; Bhown, A.S.; Segrest, J.P. Studies of Synthetic Peptide Analogs of the Amphipathic Helix. J. Biol. Chem. 1985, 260, 10248–10255. [Google Scholar] [CrossRef]
- Zhao, Y.; Imura, T.; Leman, L.J.; Curtiss, L.K.; Maryanoff, B.E.; Ghadiri, M.R. Mimicry High-Density Lipoprotein: Functional Peptide-Lipid Nanoparticles Based on Multivalent Peptide Constructs. J. Am. Chem. Soc. 2013, 135, 13414–13424. [Google Scholar] [CrossRef]
- Larsen, A.N.; Sørensen, K.K.; Johansen, N.T.; Martel, A.; Kirkensgaard, J.J.K.; Jensen, K.J.; Arleth, L.; Midtgaard, R. Dimeric peptides with three different linkers self-assemble with phospholipids to form peptide nanodiscs that stabilize membrane proteins. Soft Matter 2016, 12, 5937–5949. [Google Scholar] [CrossRef]
- Kariyazono, H.; Nadai, R.; Miyajima, R.; Takechi-Haraya, Y.; Baba, T.; Shigenaga, A.; Okuhira, K.; Otaka, A.; Saito, H. Formation of stable nanodiscs by bihelical apolipoprotein A-I mimetic peptide. J. Pept. Sci. 2016, 22, 116–122. [Google Scholar] [CrossRef]
- Anada, C.; Ikeda, K.; Nakao, H.; Nakano, M. Improvement of Thermal Stability of Amphipathic Peptide-Phospholipid Nanodiscs via Lateral Association of α-Helices by Disulfide Cross-Linking. Langmuir 2022, 38, 6977–6983. [Google Scholar] [CrossRef]
- Imura, T.; Tsukui, Y.; Taira, T.; Aburai, K.; Sakai, K.; Sakai, H.; Abe, M.; Kitamoto, D. Surfactant-like Properties of an Amphiphilic α-Helical Peptide Leading to Lipid Nanodisc Formation. Langmuir 2014, 30, 4752–4759. [Google Scholar] [CrossRef]
- Imura, T.; Tsukui, Y.; Sakai, K.; Sakai, H.; Taira, T.; Kitamoto, D. Minimum Amino Acid Residues of an α-Helical Peptide Leading to Lipid Nanodisc Formation. J. Oleo Sci. 2014, 63, 1203–1208. [Google Scholar] [CrossRef][Green Version]
- Ikeda, Y.; Taira, T.; Sakai, K.; Sakai, H.; Shigeri, Y.; Imura, T. Lipid Nanodisc Formation using Pxt-5 Peptide Isolated from Amphibian (Xenopus tropicalis) Skin, and its Altered Form, Modify-Pxt-5. J. Oleo Sci. 2018, 67, 1035–1041. [Google Scholar] [CrossRef]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–494. [Google Scholar] [CrossRef]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. Food Technol. Biotechnol. 2008, 3, 123–133. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Denisov, I.G.; McLean, M.A.; Shaw, A.W.; Grinkova, Y.V.; Sligar, S.G. Thermotropic Phase Transition in Soluble Nanoscale Lipid Bilayers. J. Phys. Chem. B 2005, 109, 15580–15588. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, K.; Arakida, J.; Ravula, T.; Ramadugu, S.K.; Sahoo, B.; Kikuchi, J.; Ramamoorthy, A. Spontaneous Lipid Nanodisc Formation by Amphiphilic Polymethacrylate Copolymers. J. Am. Chem. Soc. 2017, 139, 18657–18663. [Google Scholar] [CrossRef] [PubMed]
- Taira, T.; Ikeda, S.; Kawamura, D.; Sakai, H.; Abe, M.; Kitamoto, D.; Imura, T. Monolayer Behavior of Cyclic and Linear Forms of Surfactins: Thermodynamic Analysis of Langmuir Monolayers and AFM Study of Langmuir-Blodgett Monolayers. J. Oleo Sci. 2014, 63, 407–412. [Google Scholar] [CrossRef][Green Version]
- Eeman, M.; Berquand, A.; Dufrěne, Y.F.; Paquot, M.; Dufour, S.; Deleu, M. Penetration of Surfactin into Phospholipid Monolayers: Nanoscale Interfacial Organization. Langmuir 2006, 22, 11337–11345. [Google Scholar] [CrossRef] [PubMed]
- Gilliard, G.; Furlan, A.L.; Smeralda, W.; Pršić, J.; Deleu, M. Added Value of Biophysics to Study Lipid-Driven Biological Processes: The Case of Surfactins, a Class of Natural Amphiphile Molecules. Int. J. Mol. Sci. 2022, 23, 13831. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Shin, J.-I.; Kim, J.-S.; Yang, Y.-S.; Hwang, Y.; Yang, J.-S.; Shin, D.; Seo, J.-H.; Jin, Y.-S.; Park, Y.-C.; et al. Assembly of Coenzyme Q10 nanostructure resembling nascent discoidal high density lipoprotein particle. Biochem. Biophys. Res. Commun. 2009, 388, 217–221. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H. Recent Progress on Cyclic Peptides’ Assembly and Biomedical Applications. ChemBioChem 2023, 24, e202300018. [Google Scholar] [CrossRef]
- Kanazawa, S.; Morimoto, K.; Tabata, E.; Okura, A.; Ikemoto, Y.; Yamamoto, K.; de Campo, L.; Akiba, I. Self-Assembly of Surfactin into Nanofibers with Hydrophilic Channels in Nonpolar Organic Media. Langmuir 2020, 36, 7622–7633. [Google Scholar] [CrossRef]
- Ikeda, K.; Horiuchi, A.; Yoshino, M.; Shimizu, C.; Nakao, H.; Nakao, M. Amphipathic Peptide-Phospholipid Nanofibers: Phospholipid Specificity and Dependence on Concentration and Temperature. Langmuir 2021, 37, 713–721. [Google Scholar] [CrossRef]
- Wallin, M.; Altskär, A.; Nordstierna, L.; Andersson, M. Meso-Ordered PEG-Based Particles. Langmuir 2015, 31, 13–16. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imura, T.; Yanagisawa, S.; Ikeda, Y.; Moriyama, R.; Sakai, K.; Sakai, H.; Taira, T. Solubilization of Phospholipid by Surfactin Leading to Lipid Nanodisc and Fibrous Architecture Formation. Molecules 2024, 29, 1300. https://doi.org/10.3390/molecules29061300
Imura T, Yanagisawa S, Ikeda Y, Moriyama R, Sakai K, Sakai H, Taira T. Solubilization of Phospholipid by Surfactin Leading to Lipid Nanodisc and Fibrous Architecture Formation. Molecules. 2024; 29(6):1300. https://doi.org/10.3390/molecules29061300
Chicago/Turabian StyleImura, Tomohiro, Satohiro Yanagisawa, Yuri Ikeda, Ryodai Moriyama, Kenichi Sakai, Hideki Sakai, and Toshiaki Taira. 2024. "Solubilization of Phospholipid by Surfactin Leading to Lipid Nanodisc and Fibrous Architecture Formation" Molecules 29, no. 6: 1300. https://doi.org/10.3390/molecules29061300
APA StyleImura, T., Yanagisawa, S., Ikeda, Y., Moriyama, R., Sakai, K., Sakai, H., & Taira, T. (2024). Solubilization of Phospholipid by Surfactin Leading to Lipid Nanodisc and Fibrous Architecture Formation. Molecules, 29(6), 1300. https://doi.org/10.3390/molecules29061300






