Simple and Safe Synthesis of Yolk-Shell-Structured Silicon/Carbon Composites with Enhanced Electrochemical Properties
Abstract
1. Introduction
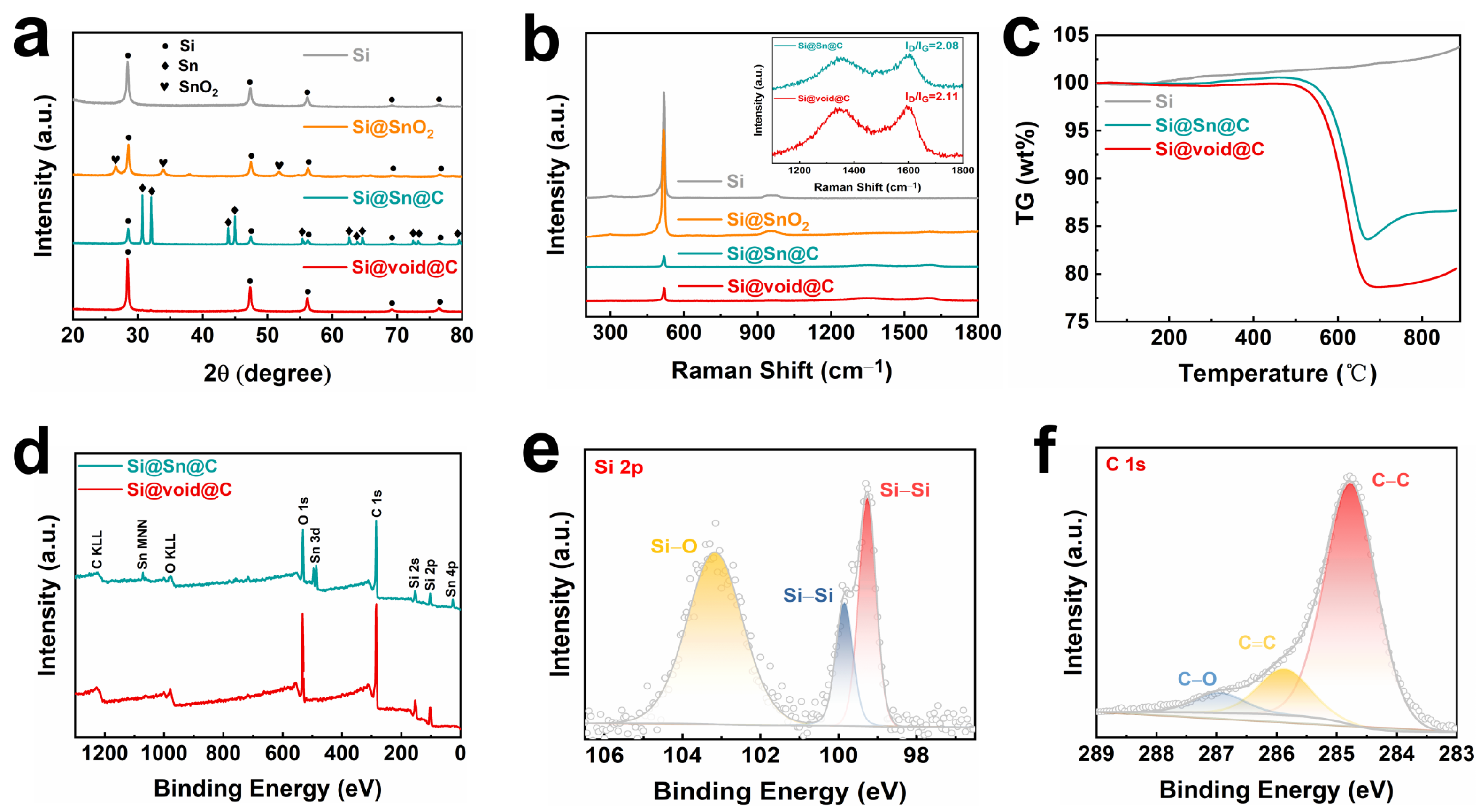
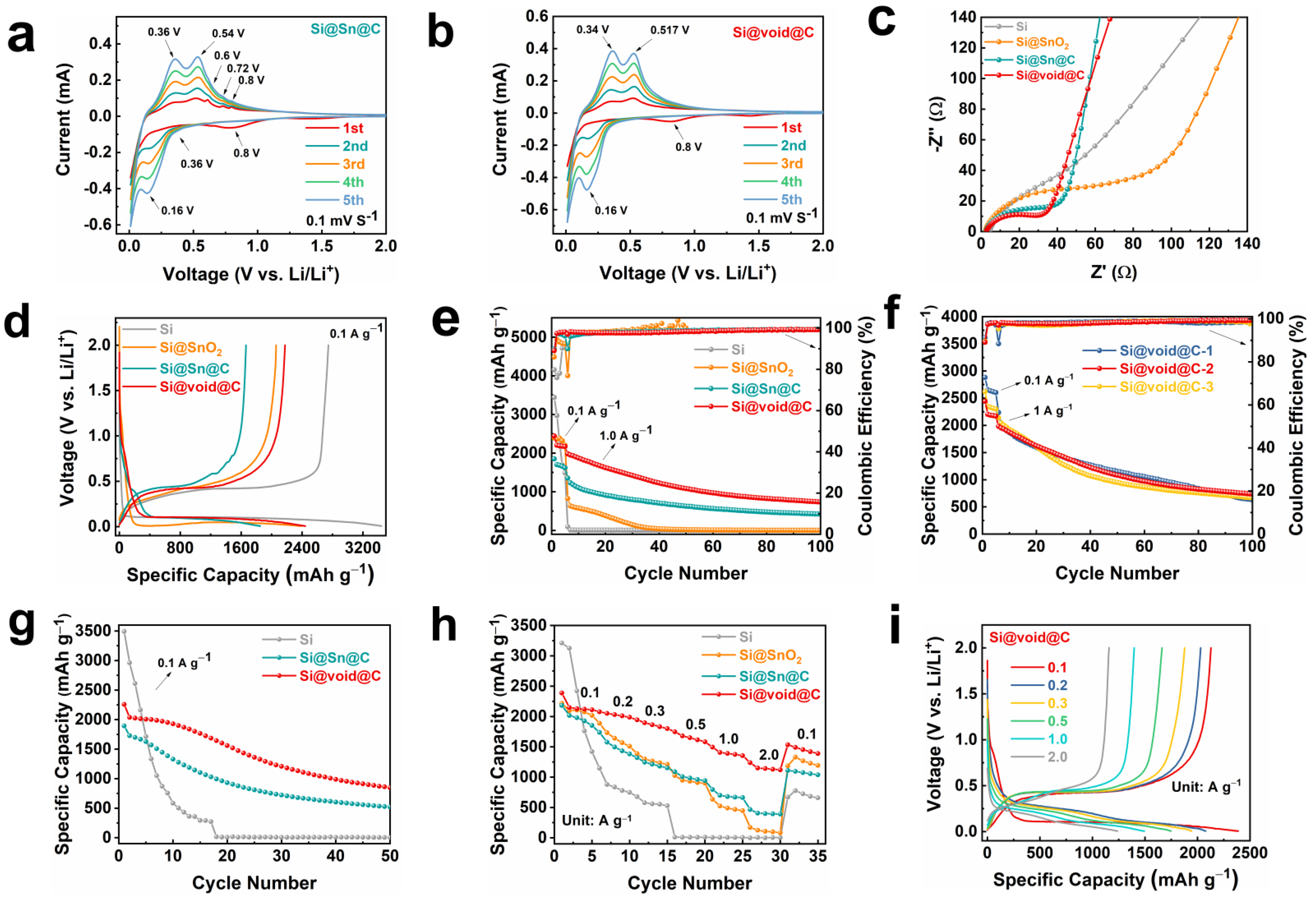
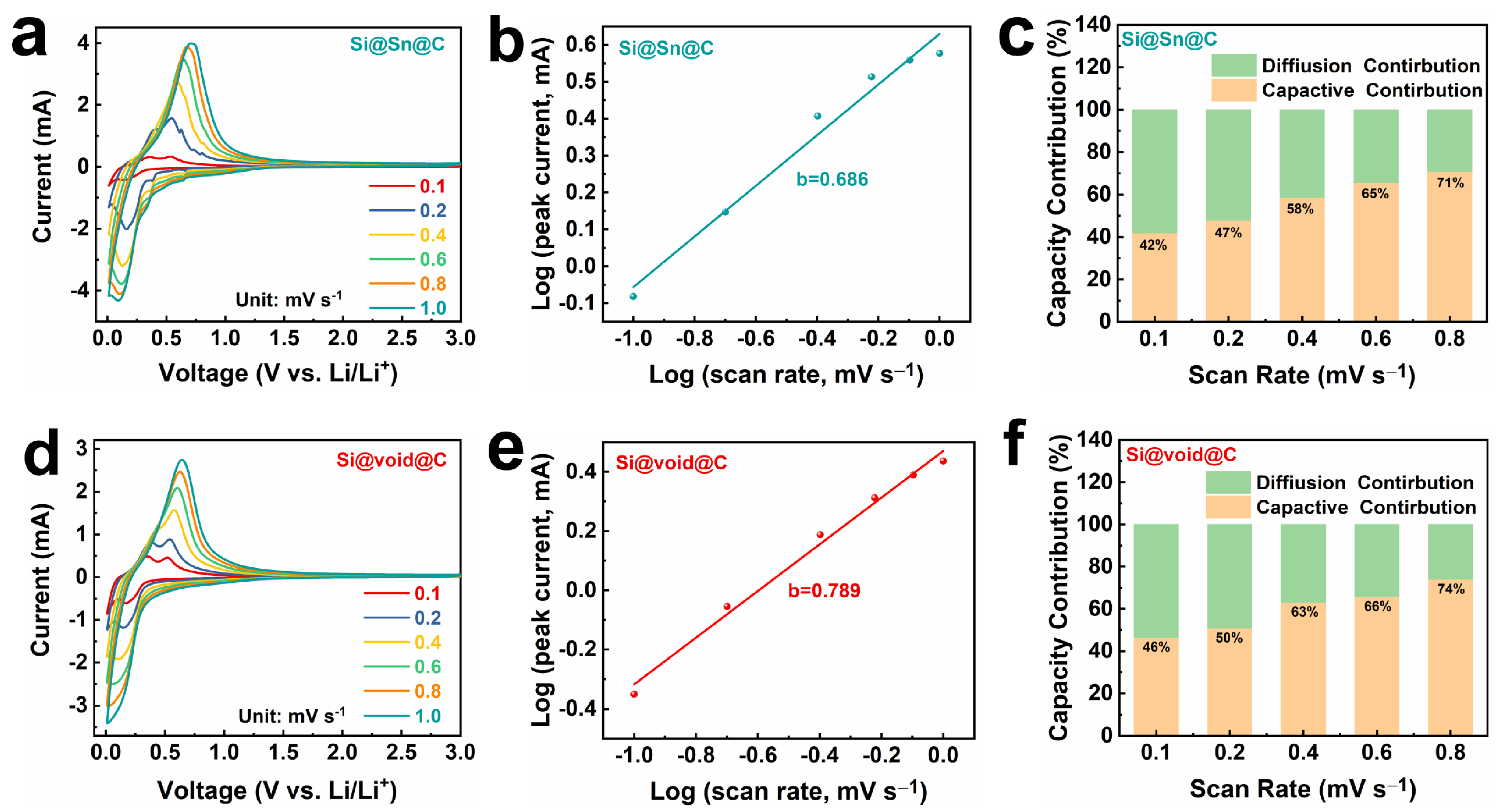
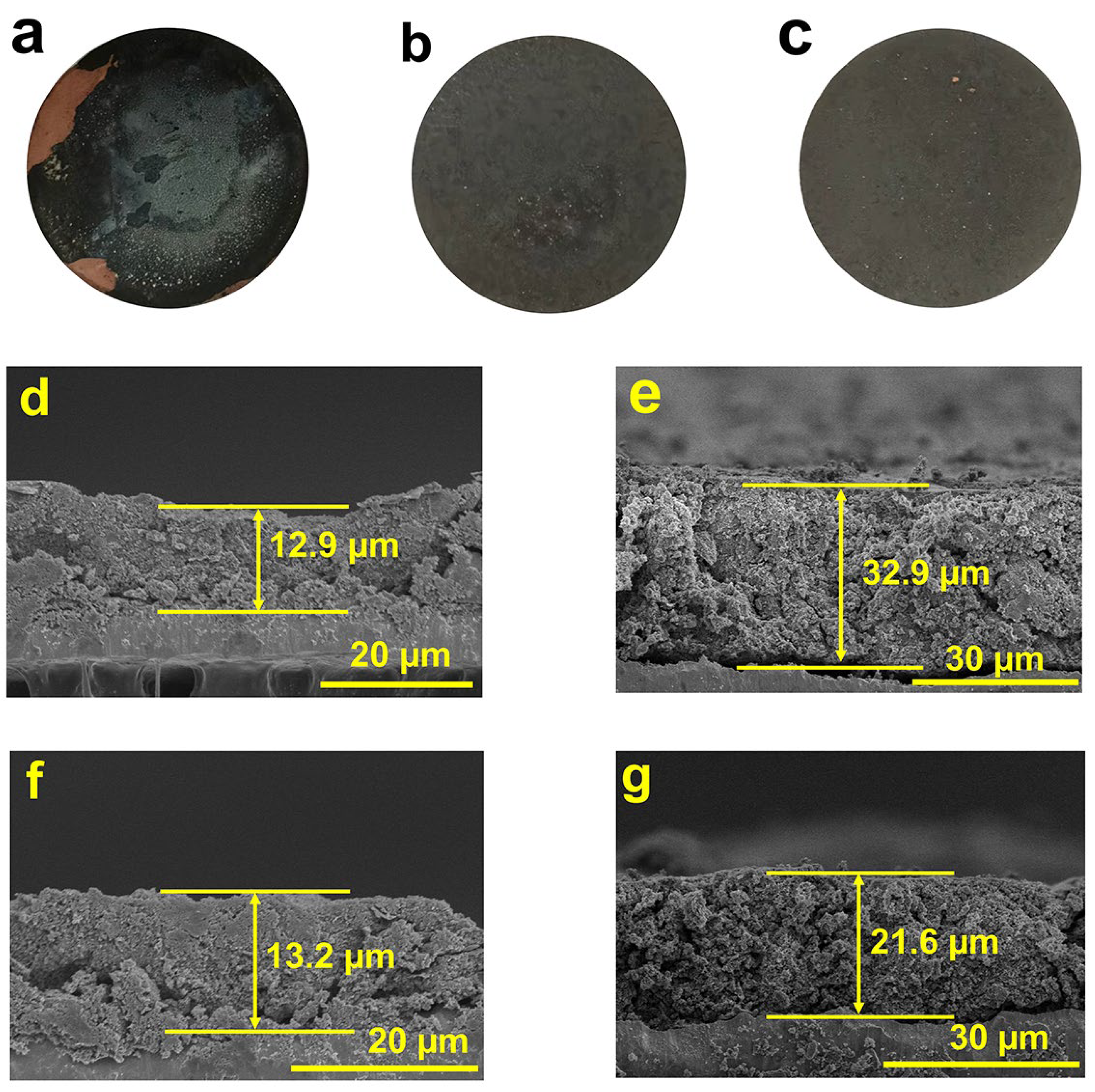
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M.A. Supercapacitors as next Generation Energy Storage Devices: Properties and Applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liu, T.; Yang, X.-G.; Ge, S.; Stanley, N.V.; Rountree, E.S.; Leng, Y.; McCarthy, B.D. Fast Charging of Energy-Dense Lithium-Ion Batteries. Nature 2022, 611, 485–490. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.; Ashirov, T.; Kazzi, M.E.; Cancellieri, C.; Jeurgens, L.P.H.; Choi, J.W.; Coskun, A. Fluorinated Ether Electrolyte with Controlled Solvation Structure for High Voltage Lithium Metal Batteries. Nat. Commun. 2022, 13, 2575. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Peng, D.; Kimura, H.; Zhang, X.; Sun, X.; Pashameah, R.A.; Alzahrani, E.; Wang, B.; Guo, Z.; Du, W.; et al. Honeycomb-like Nitrogen-Doped Porous Carbon Decorated with Co3O4 Nanoparticles for Superior Electrochemical Performance Pseudo-Capacitive Lithium Storage and Supercapacitors. Adv. Compos. Hybrid. Mater. 2022, 5, 3146–3157. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, M.; Chen, G.; Dudko, N.; Li, Y.; Liu, H.; Shi, L.; Wu, G.; Zhang, D. High-Performance Microsized Si Anodes for Lithium-Ion Batteries: Insights into the Polymer Configuration Conversion Mechanism. Adv. Mater. 2022, 34, 2109658. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Han, J.; Chen, M.; Zhou, W.; Wang, X.; Xu, M.; Lin, H.; Liu, H.; Chen, H.; Chen, J.; et al. Enabling Robust Structural and Interfacial Stability of Micron-Si Anode toward High-Performance Liquid and Solid-State Lithium-Ion Batteries. Energy Storage Mater. 2022, 52, 547–561. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. One-Step, Vacuum-Assisted Construction of Micrometer-Sized Nanoporous Silicon Confined by Uniform Two-Dimensional N-Doped Carbon toward Advanced Li Ion and MXene-Based Li Metal Batteries. ACS Nano 2022, 16, 4560–4577. [Google Scholar] [CrossRef]
- He, Z.; Xiao, Z.; Yue, H.; Jiang, Y.; Zhao, M.; Zhu, Y.; Yu, C.; Zhu, Z.; Lu, F.; Jiang, H.; et al. Single-Walled Carbon Nanotube Film as an Efficient Conductive Network for Si-Based Anodes. Adv. Funct. Mater. 2023, 33, 2300094. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, E.; Xiao, Z.; Shao, H.; Liu, Y.; Wang, J. Photo-Initiated in Situ Synthesis of Polypyrrole Fe-Coated Porous Silicon Microspheres for High-Performance Lithium-Ion Battery Anodes. Chem. Eng. J. 2023, 459, 141543. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, Z.; Lin, Z.; Yan, X.; He, Z.; Jiang, H.; Yang, Z.; Jia, X.; Wei, F. Unraveling the Fundamental Mechanism of Interface Conductive Network Influence on the Fast-Charging Performance of SiO-Based Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2023, 16, 43. [Google Scholar] [CrossRef]
- Han, D.; Xiang, S.; Cunha, J.; Xie, Y.; Zhou, M.; Hou, Z.; Yin, H. Pre-Lithiated Silicon/Carbon Nanosphere Anode with Enhanced Cycling Ability and Coulombic Efficiency for Lithium-Ion Batteries. J. Energy Storage 2024, 79, 110183. [Google Scholar] [CrossRef]
- Dai, X.; Liu, H.; Liu, X.; Liu, Z.; Liu, Y.; Cao, Y.; Tao, J.; Shan, Z. Silicon Nanoparticles Encapsulated in Multifunctional Crosslinked Nano-Silica/Carbon Hybrid Matrix as a High-Performance Anode for Li-Ion Batteries. Chem. Eng. J. 2021, 418, 129468. [Google Scholar] [CrossRef]
- Imtiaz, S.; Amiinu, I.S.; Storan, D.; Kapuria, N.; Geaney, H.; Kennedy, T.; Ryan, K.M. Dense Silicon Nanowire Networks Grown on a Stainless-Steel Fiber Cloth: A Flexible and Robust Anode for Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2105917. [Google Scholar] [CrossRef]
- Jing, S.; Xiao, J.; Shen, Y.; Hong, B.; Gu, D.; Xiao, W. Silicate-Mediated Electrolytic Silicon Nanotube from Silica in Molten Salts. Small 2022, 18, 2203251. [Google Scholar] [CrossRef]
- An, W.; He, P.; Che, Z.; Xiao, C.; Guo, E.; Pang, C.; He, X.; Ren, J.; Yuan, G.; Du, N.; et al. Scalable Synthesis of Pore-Rich Si/C@C Core–Shell-Structured Microspheres for Practical Long-Life Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2022, 14, 10308–10318. [Google Scholar] [CrossRef]
- Peng, J.; Li, W.; Wu, Z.; Li, H.; Zeng, P.; Chen, G.; Chang, B.; Zhang, X.; Wang, X. Si/C Composite Embedded Nano-Si in 3D Porous Carbon Matrix and Enwound by Conductive CNTs as Anode of Lithium-Ion Batteries. Sustain. Mater. Technol. 2022, 32, e00410. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Li, S.; Xi, F.; Tong, Z.; Chen, X.; Wan, X.; Ma, W.; Deng, R. High-Performance Silicon Carbon Anodes Based on Value-Added Recycling Strategy of End-of-Life Photovoltaic Modules. Energy 2023, 281, 128345. [Google Scholar] [CrossRef]
- Zhu, T.; Sternlicht, H.; Ha, Y.; Fang, C.; Liu, D.; Savitzky, B.H.; Zhao, X.; Lu, Y.; Fu, Y.; Ophus, C.; et al. Formation of Hierarchically Ordered Structures in Conductive Polymers to Enhance the Performances of Lithium-Ion Batteries. Nat. Energy 2023, 8, 129–137. [Google Scholar] [CrossRef]
- Xu, C.; Shen, L.; Zhang, W.; Huang, Y.; Sun, Z.; Zhao, G.; Lin, Y.; Zhang, Q.; Huang, Z.; Li, J. Efficient Implementation of Kilogram-Scale, High-Capacity and Long-Life Si-C/TiO2 Anodes. Energy Storage Mater. 2023, 56, 319–330. [Google Scholar] [CrossRef]
- Li, Z.; Han, M.; Yu, P.; Lin, J.; Yu, J. Macroporous Directed and Interconnected Carbon Architectures Endow Amorphous Silicon Nanodots as Low-Strain and Fast-Charging Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2024, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Huang, Y.; Liu, N.; Wang, X.; Zhang, Y.; Guo, Y.; Wu, H.-H.; Chen, H.; Tang, X.; Zhang, Q. N-Doped Porous Carbon Nanofibers Sheathed Pumpkin-like Si/C Composites as Free-Standing Anodes for Lithium-Ion Batteries. J. Energy Chem. 2021, 54, 727–735. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yang, H.-S.; Lee, K.H.; Han, S.; Yu, W.-R. Rational Design of a Si–Sn–C Ternary Anode Having Exceptional Rate Performance. Energy Storage Mater. 2019, 17, 62–69. [Google Scholar] [CrossRef]
- Tian, M.; Ben, L.; Jin, Z.; Ji, H.; Yu, H.; Zhao, W.; Huang, X. Excellent Low-Temperature Electrochemical Cycling of an Anode Consisting of Si Nanoparticles Seeded in Sn Nanowires for Lithium-Ion Batteries. Electrochim. Acta 2021, 396, 139224. [Google Scholar] [CrossRef]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A Yolk-Shell Design for Stabilized and Scalable Li-Ion Battery Alloy Anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Kim, J.-C.; Kim, D.-W. Waste Glass Microfiber Filter-Derived Fabrication of Fibrous Yolk-Shell Structured Silicon/Carbon Composite Freestanding Electrodes for Lithium-Ion Battery Anodes. J. Power Sources 2020, 468, 228407. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, F.; Zhang, Y.; Dang, M.; Li, B.; Ma, J.; Du, X. Ni/NiO/SiO2/C Nanofibers with Strong Wideband Microwave Absorption and Robust Hydrophobicity. Appl. Surf. Sci. 2022, 588, 152964. [Google Scholar] [CrossRef]
- Su, H.; Li, X.; Liu, C.; Shang, Y.; Liu, H. Scalable Synthesis of Micrometer-Sized Porous Silicon/Carbon Composites for High-Stability Lithium-Ion Battery Anodes. Chem. Eng. J. 2023, 451, 138394. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Huang, W.; Tao, J.; Lv, F.; Ye, R.; Lin, Y.; Li, Y.Y.; Huang, Z.; Lu, J. Simple Designed Micro–Nano Si–Graphite Hybrids for Lithium Storage. Small 2021, 17, 2006373. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Yang, Z.; Zhang, M.; Li, Z.; Zhao, H. Carbon-Coated Mesoporous Silicon Shell-Encapsulated Silicon Nano-Grains for High Performance Lithium-Ion Batteries Anode. Carbon 2022, 192, 277–284. [Google Scholar] [CrossRef]
- Yang, X.; Kong, W.; Du, G.; Li, S.; Tang, Y.; Cao, J.; Lu, X.; Tan, R.; Qian, G. Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries. Batteries 2023, 9, 446. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Cao, Y.; Wu, X.; Shan, Z. Silicon Nanoparticles Embedded in Chemical-Expanded Graphite through Electrostatic Attraction for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 9457–9464. [Google Scholar] [CrossRef]
- Lei, Y.; Li, S.; Du, M.; Mi, J.; Gao, D.-C.; Hao, L.; Jiang, L.-J.; Luo, M.; Jiang, W.-Q.; Li, F.; et al. Preparation of Double-Shell Si@SnO2@C Nanocomposite as Anode for Lithium-Ion Batteries by Hydrothermal Method. Rare Met. 2023, 42, 2972–2981. [Google Scholar] [CrossRef]
- Yang, D.; Shi, J.; Shi, J.; Yang, H. Simple Synthesis of Si/Sn@C-G Anodes with Enhanced Electrochemical Properties for Li-Ion Batteries. Electrochim. Acta 2018, 259, 1081–1088. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, F.; Wang, J.; Wang, S.; Tong, H.; An, T.; Yu, W.-J. Facile Synthesis of N-C/Si@G Nanocomposite as a High-Performance Anode Material for Li-Ion Batteries. J. Alloys Compd. 2021, 872, 159716. [Google Scholar] [CrossRef]
- Nyamtara, K.J.; Song, J.K.; Karima, N.C.; Kim, S.H.; Nguyen, M.C.; Duong, T.P.M.; Lee, K.J.; Ahn, W. Two Step Pyrolysis Synthesis Method of Graphite-Enhanced Nano-Si/Pitch Composite as Long Cycle Life Anode for Lithium-Ion Batteries. J. Alloys Compd. 2024, 976, 173229. [Google Scholar] [CrossRef]
- Ai, W.; Kirkaldy, N.; Jiang, Y.; Offer, G.; Wang, H.; Wu, B. A Composite Electrode Model for Lithium-Ion Batteries with Silicon/Graphite Negative Electrodes. J. Power Sources 2022, 527, 231142. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Lin, R. Improving the Electrochemical Performance of Silicon Materials by SnO2 through Structural Design and Conductivity. Appl. Surf. Sci. 2022, 581, 152230. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Zhao, X.; Chai, D.; Ding, N.; Zhang, Q.; Li, X. Turning Complexity into Simplicity: In Situ Synthesis of High-Performance Si@C Anode in Battery Manufacturing Process by Partially Carbonizing the Slurry of Si Nanoparticles and Dual Polymers. Molecules 2024, 29, 175. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yin, X.; Xiao, R.; Mu, T.; Huo, H.; Zuo, P.; Ma, Y.; Cheng, X.; Gao, Y.; Yin, G.; et al. Layered Porous Silicon Encapsulated in Carbon Nanotube Cage as Ultra-Stable Anode for Lithium-Ion Batteries. Chem. Eng. J. 2022, 431, 133982. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Park, K.; Kim, S.; Nam, K.W.; Char, K.; Choi, J.W. Host–Guest Interlocked Complex Binder for Silicon–Graphite Composite Electrodes in Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103718. [Google Scholar] [CrossRef]
- Liang, B.; Tan, W.; Chen, M.; Yi, M.; Hu, J.; Zeng, K.; Wang, Y.; Li, Y.; Yang, G. Facile Synthesis of Two-Dimensional Carbon/Si Composite Assembled by Ultrasonic Atomization-Assisted-Ice Template Technology as Electrode for Lithium-Ion Battery. J. Alloys Compd. 2024, 976, 173030. [Google Scholar] [CrossRef]
- Hao, Q.; Hou, J.; Ye, J.; Yang, H.; Du, J.; Xu, C. Hierarchical Macroporous Si/Sn Composite: Easy Preparation and Optimized Performances towards Lithium Storage. Electrochim. Acta 2019, 306, 427–436. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, M.; Shi, W.; Liu, X.; Wu, L.; Hu, Z.-Y.; Chen, L.; Li, Y.; Su, B.-L. Chelation-Assisted Formation of Carbon Nanotubes Interconnected Yolk-Shell Silicon/Carbon Anodes for High-Performance Lithium-Ion Batteries. J. Colloid. Interface Sci. 2023, 641, 747–757. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, K.; Zhang, H.; Wang, X.; Zhou, Y.; He, W.; Cui, J.; Sun, J. Constructing Biomass-Based Ultrahigh-Rate Performance SnOy@C/SiOx Anode for LIBs via Disproportionation Effect. Small 2023, 19, 2204867. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, R.; Xu, J.; Tang, Y.; Huang, F. Long-Life and High Volumetric Capacity Bi2Sn2O7 Anode with Interpenetrating Bi–O and Sn–O Networks. Cell Rep. Phys. Sci. 2022, 3, 101109. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Zhang, K.; Ozawa, K.; Qin, L.-C. A Sandwich-like Silicon–Carbon Composite Prepared by Surface-Polymerization for Rapid Lithium-Ion Storage. Nano Energy 2020, 78, 105341. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, J.; Zhang, Y.; Yang, D.; Du, N. Functionalization-Assistant Ball Milling towards Si/Graphene Anodes in High Performance Li-Ion Batteries. Mater. Today Nano 2022, 18, 100175. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Hu, Q.; Li, H.; Jiao, F.; Wang, W.; Zhang, S.; Du, H. In Situ Copper Coating on Silicon Particles Enables Long Durable Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 5058–5066. [Google Scholar] [CrossRef]
- Wang, X.; Wen, K.; Chen, T.; Chen, S.; Zhang, S. Supercritical Fluid-Assisted Preparation of Si/CNTs@FG Composites with Hierarchical Conductive Networks as a High-Performance Anode Material. Appl. Surf. Sci. 2020, 522, 146507. [Google Scholar] [CrossRef]
- He, Y.; Ye, Z.; Chamas, M.; Sougrati, M.T.; Lippens, P.-E. Si/Cu-Zn(Ox)/C Composite as Anode Material for Li-Ion Batteries. Solid State Ion. 2021, 372, 115774. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhong, W.; Li, C.; Li, L.; Zhang, H. Facile Synthesis of Ultrasmall Si Particles Embedded in Carbon Framework Using Si-Carbon Integration Strategy with Superior Lithium Ion Storage Performance. Chem. Eng. J. 2017, 319, 1–8. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, W.; Sun, H.; Wang, Y.; Dong, A.; Hu, J.; Yang, D. Ionic Liquid Assist to Prepare Si@N-Doped Carbon Nanoparticles and Its High Performance in Lithium Ion Batteries. J. Alloys Compd. 2017, 691, 178–184. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Liu, Y.; Zhang, K.; Emani, S.; Sawicki, M.S.; Shamie, J.S.; Shaw, L.L. Hollow Silicon Nanospheres Encapsulated with a Thin Carbon Shell: An Electrochemical Study. Electrochim. Acta 2016, 215, 126–141. [Google Scholar] [CrossRef]
- Dong, H.; Fu, X.; Wang, J.; Wang, P.; Ding, H.; Song, R.; Wang, S.; Li, R.; Li, S. In-Situ Construction of Porous Si@C Composites with LiCl Template to Provide Silicon Anode Expansion Buffer. Carbon 2021, 173, 687–695. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Liu, C.; Li, D.; Kim, H.-K.; Harris, C.; Lao, C.; Abdelkader, A.; Xi, K. Facile Mechanochemical Synthesis of Non-Stoichiometric Silica-Carbon Composite for Enhanced Lithium Storage Properties. J. Alloys Compd. 2019, 801, 658–665. [Google Scholar] [CrossRef]
- Li, G.; Huang, L.-B.; Yan, M.-Y.; Li, J.-Y.; Jiang, K.-C.; Yin, Y.-X.; Xin, S.; Xu, Q.; Guo, Y.-G. An Integral Interface with Dynamically Stable Evolution on Micron-Sized SiOx Particle Anode. Nano Energy 2020, 74, 104890. [Google Scholar] [CrossRef]
- Yuan, T.; Tang, R.; Xiao, F.; Zuo, S.; Wang, Y.; Liu, J. Modifying SiO as a Ternary Composite Anode Material((SiOx/G/SnO2)@C) for Lithium Battery with High Li-Ion Diffusion and Lower Volume Expansion. Electrochim. Acta 2023, 439, 141655. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Terabe, K.; Yu, X.; Sasaki, T.; Hashimoto, A.; Asano, K.; Suzuki, M.; Nakura, K. Preparation of Layered Si Materials as Anode for Lithium-Ion Batteries. Chem. Phys. Lett. 2019, 730, 198–205. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wu, M.; Du, Q.; Zhai, G.; He, H. Simple and Safe Synthesis of Yolk-Shell-Structured Silicon/Carbon Composites with Enhanced Electrochemical Properties. Molecules 2024, 29, 1301. https://doi.org/10.3390/molecules29061301
Li J, Wu M, Du Q, Zhai G, He H. Simple and Safe Synthesis of Yolk-Shell-Structured Silicon/Carbon Composites with Enhanced Electrochemical Properties. Molecules. 2024; 29(6):1301. https://doi.org/10.3390/molecules29061301
Chicago/Turabian StyleLi, Jinhuan, Min Wu, Quan Du, Gangpeng Zhai, and Haiyong He. 2024. "Simple and Safe Synthesis of Yolk-Shell-Structured Silicon/Carbon Composites with Enhanced Electrochemical Properties" Molecules 29, no. 6: 1301. https://doi.org/10.3390/molecules29061301
APA StyleLi, J., Wu, M., Du, Q., Zhai, G., & He, H. (2024). Simple and Safe Synthesis of Yolk-Shell-Structured Silicon/Carbon Composites with Enhanced Electrochemical Properties. Molecules, 29(6), 1301. https://doi.org/10.3390/molecules29061301




