Investigation of the Performance of Hastelloy X as Potential Bipolar Plate Materials in Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase
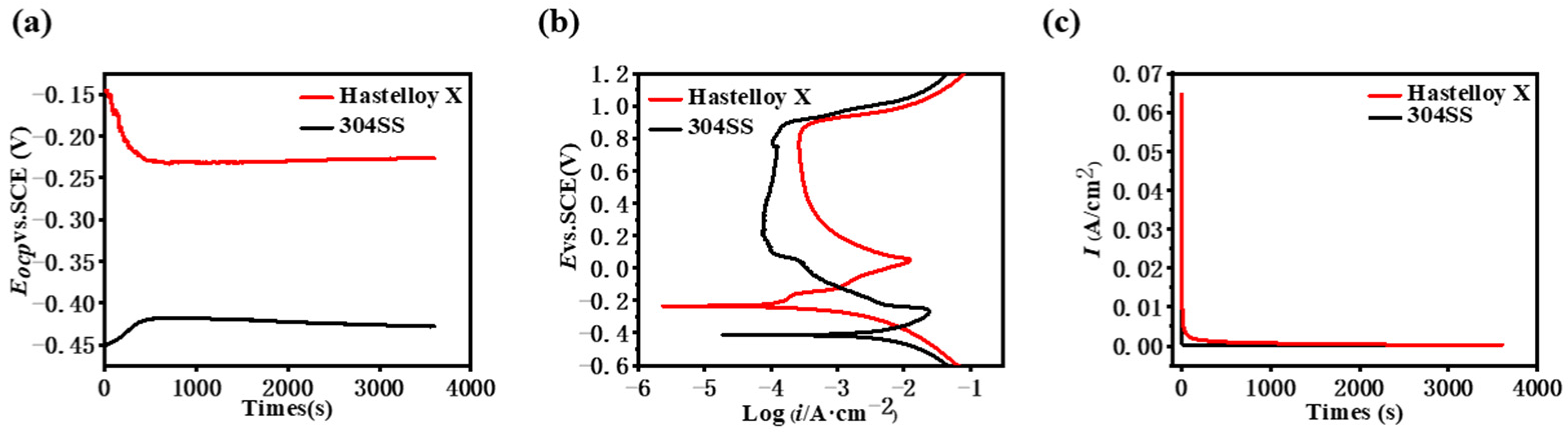
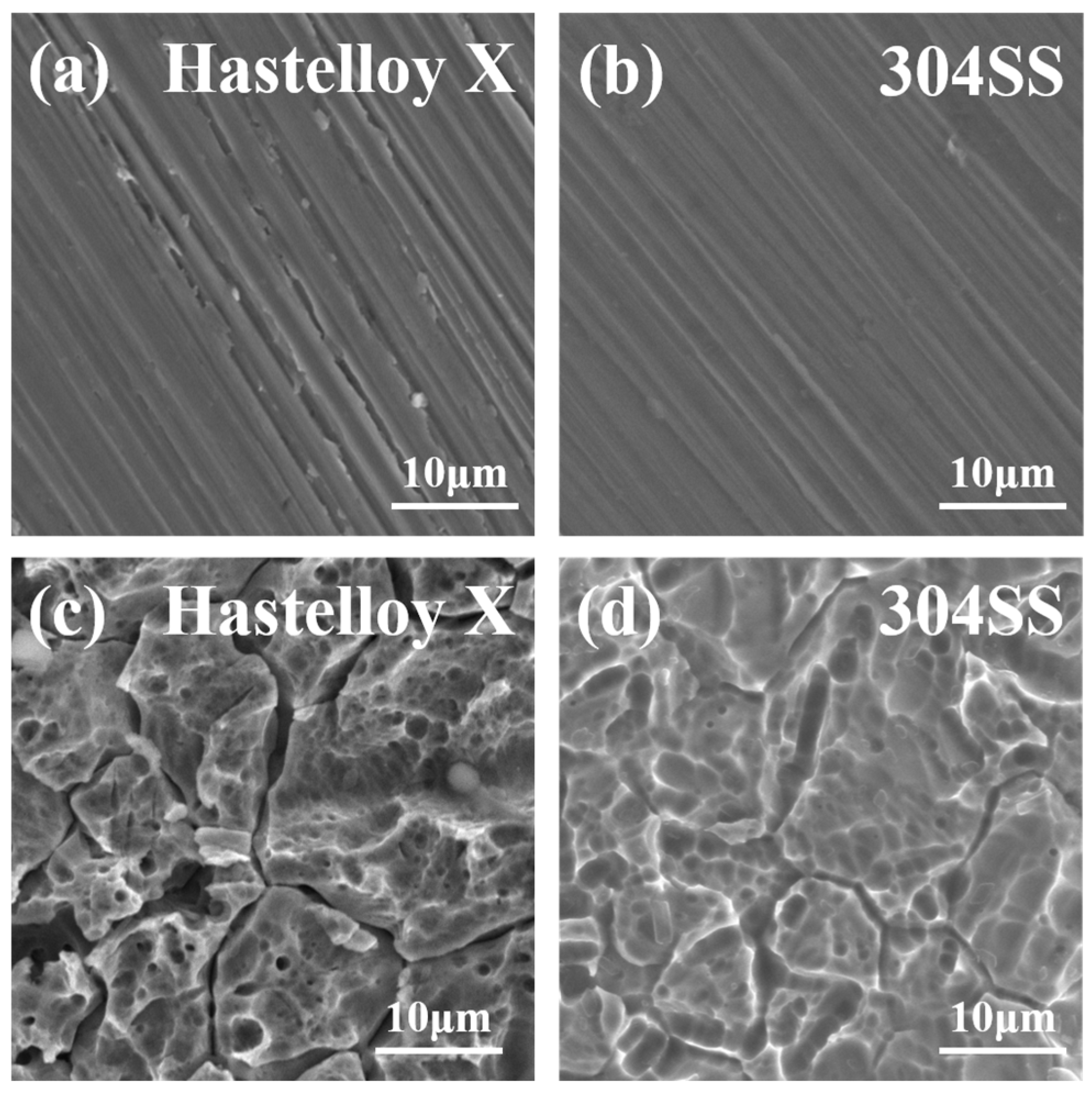
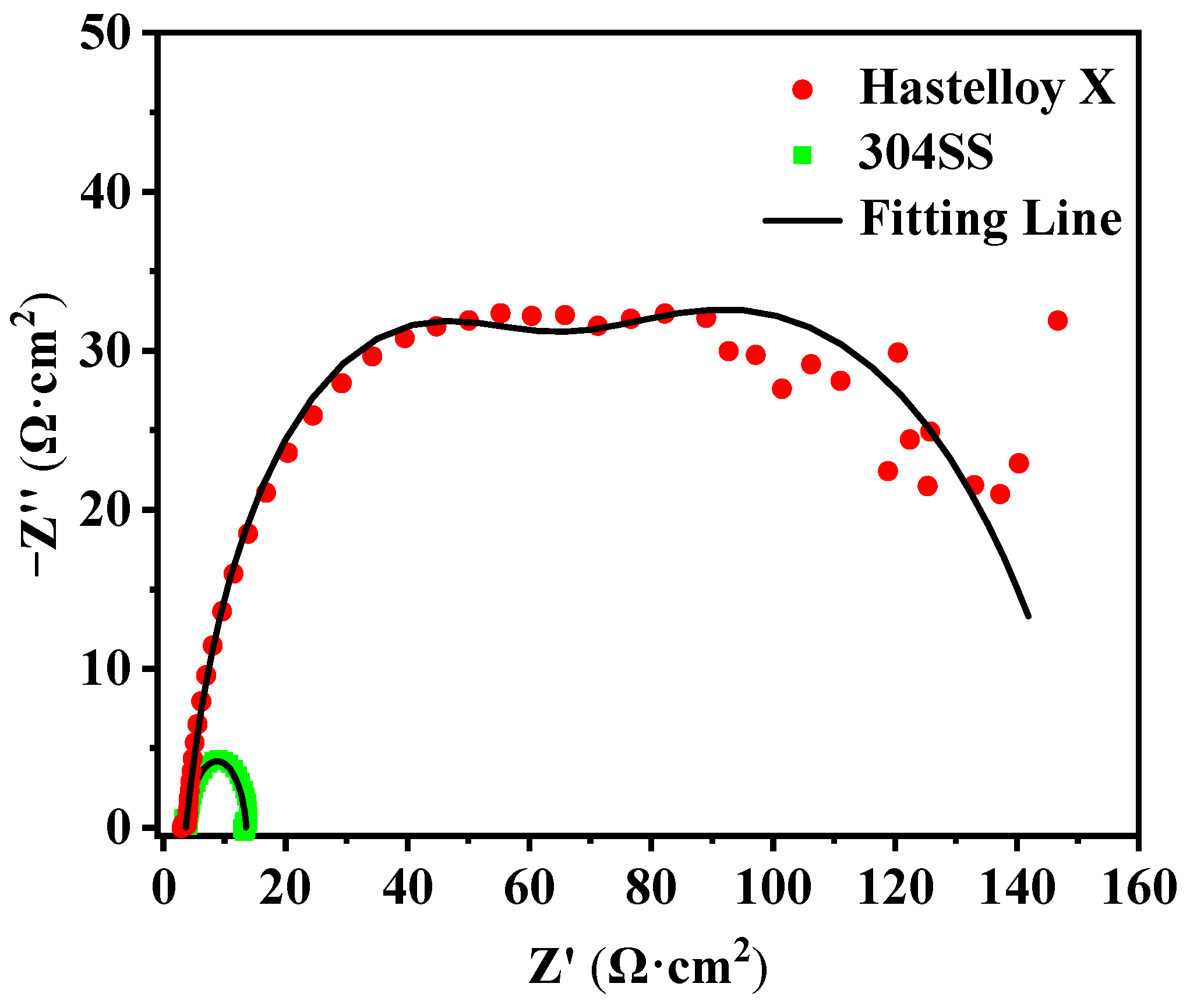
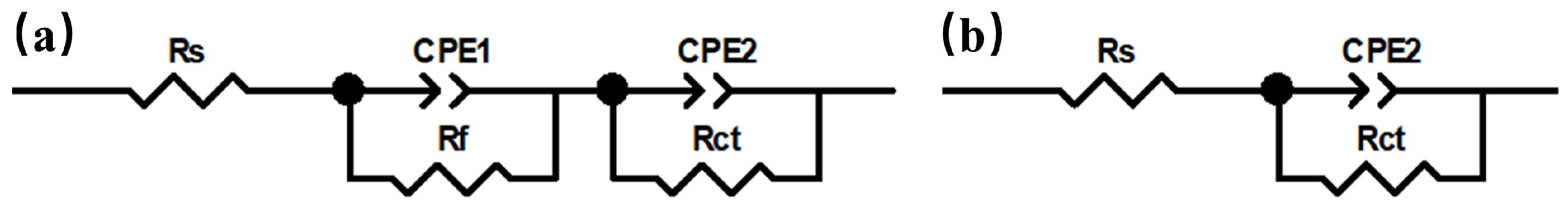
2.2. Corrosion Resistance
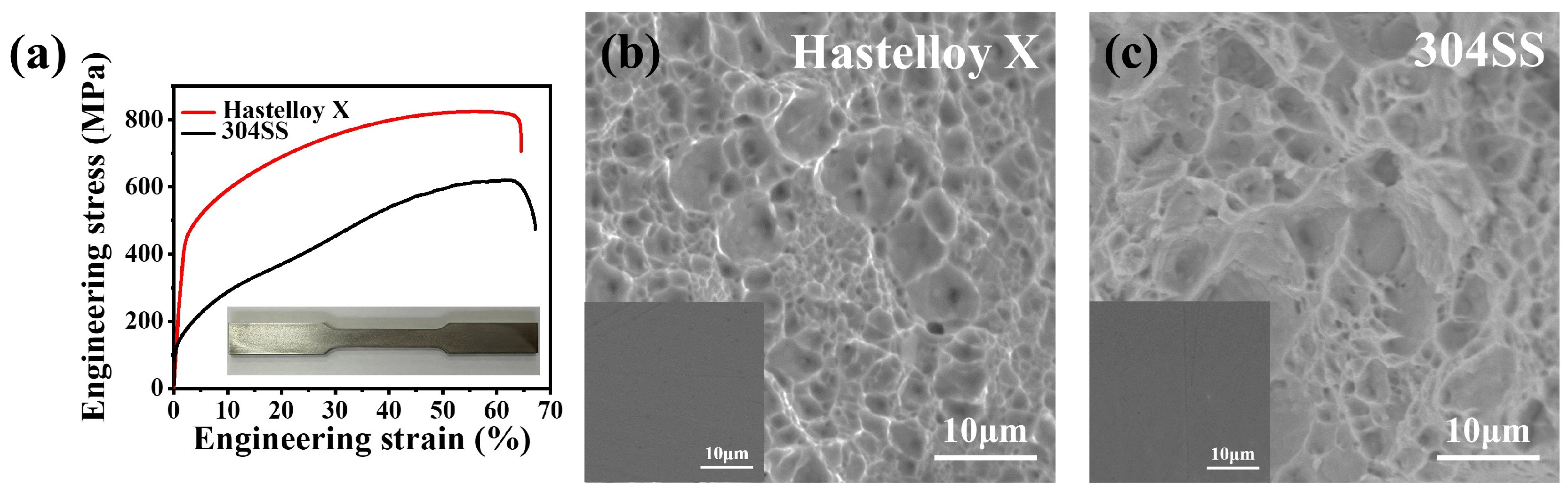
2.3. Mechanical Properties
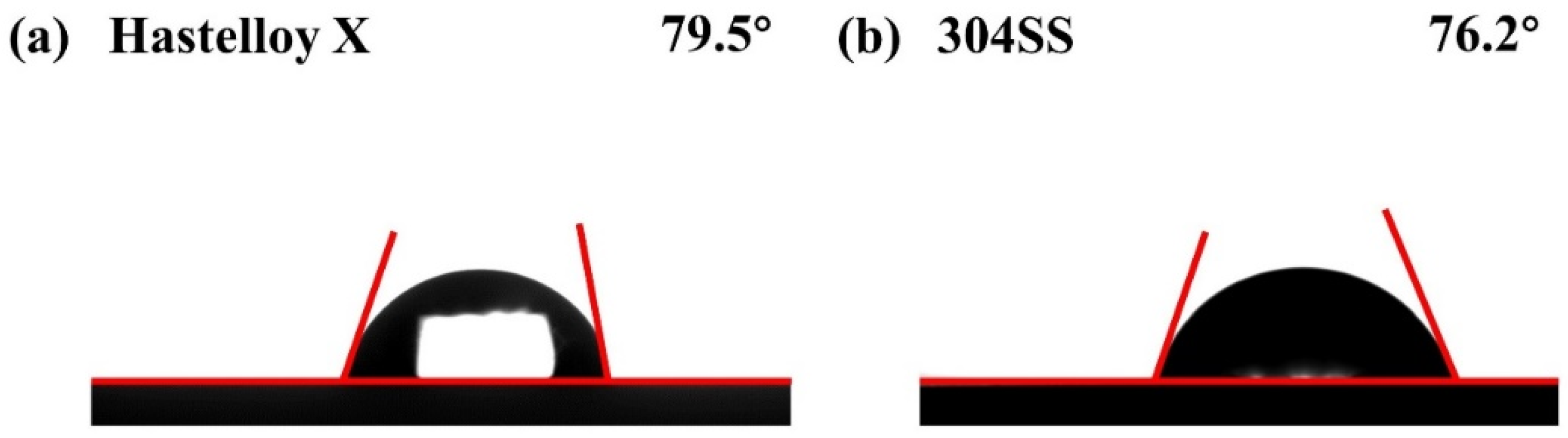
2.4. Hydrophobicity
2.5. Interfacial Contact Resistance (ICR)
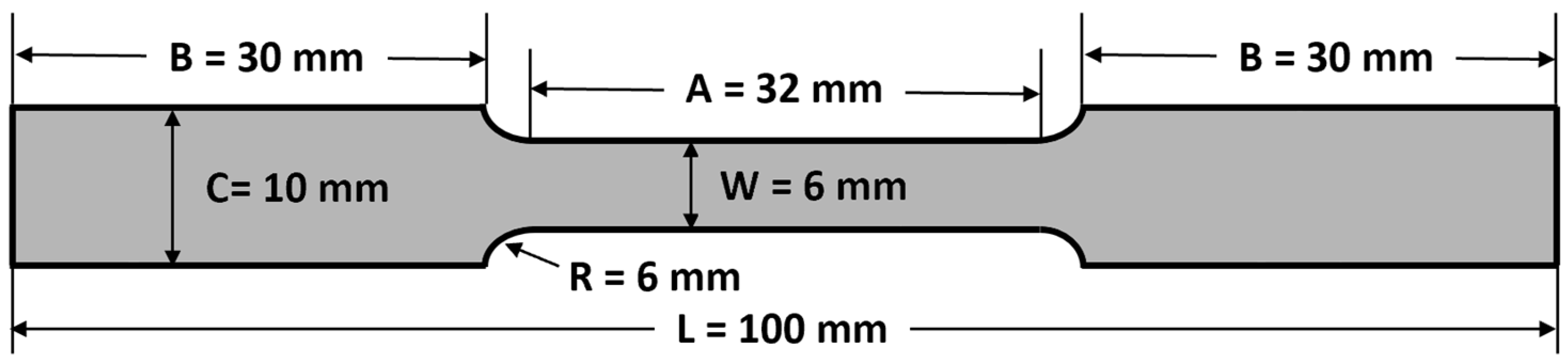
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Liu, F.; Liu, F.; Huang, C.; Zheng, H.; Zhang, Q.; Zheng, Y.; Gao, J. Microstructural evolution and cracking behavior of Hastelloy X superalloy fabricated by laser directed energy deposition. J. Alloys Compd. 2022, 905, 164179. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Yao, J.; Man, C.; Wang, L.; Xiao, K.; Li, X. Anisotropic response in mechanical and corrosion properties of hastelloy X fabricated by selective laser melting. Constr. Build. Mater. 2019, 221, 720–729. [Google Scholar] [CrossRef]
- Karapuzha, A.S.; Fraser, D.; Schliephake, D.; Dietrich, S.; Zhu, Y.; Wu, X.; Huang, A. Room and elevated temperature tensile and fatigue behaviour of additively manufactured Hastelloy X. Mater. Sci. Eng. A 2023, 882, 145479. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Y.; Yang, Z.; Cui, D.; He, F.; Li, J.; Wang, Z.; Lin, X.; Wang, J. Cracking mechanism of Hastelloy X superalloy during directed energy deposition additive manufacturing. Addit. Manuf. 2022, 55, 102792. [Google Scholar] [CrossRef]
- Yin, Y.; Li, H.; Pan, S.; Zhang, J.; Han, Q.; Yang, S. Electrochemical behaviour of passivation film formed on SLM-fabricated Hastelloy X superalloy surface in 10 wt% NaNO3 solution. Corros. Sci. 2022, 206, 110494. [Google Scholar] [CrossRef]
- Yoon, D.; Heo, I.; Kim, J.; Chang, S.; Chang, S. Hold Time-low cycle fatigue behavior of nickel based Hastelloy X at elevated temperatures. Int. J. Precis. Eng. Manuf. 2019, 20, 147–157. [Google Scholar] [CrossRef]
- Kim, I.; Choi, B.; Jung, J.; Do, J.; Jo, C. Effect of microstructural characteristics on the low cycle fatigue behaviors of cast Ni-base superalloys. Mater. Charact. 2015, 106, 375–381. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Huang, P.; Chen, Z.; Zhang, J.; Wu, M.; Liu, Y.; Zhang, F.; Chen, Y.; Chen, X. Stainless steel bipolar plate fuel cell with different flow field structures prepared by laser additive manufacturing. Int. J. Heat Mass Transf. 2022, 183, 122186. [Google Scholar] [CrossRef]
- Li, H.; Guo, P.; Zhang, D.; Liu, L.; Wang, Z.; Ma, G.; Xin, Y.; Ke, P.; Saito, H.; Wang, A. Interface-induced degradation of amorphous carbon films/stainless steel bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2020, 469, 228269. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, D.; Guo, P.; Li, H.; Xin, Y.; Wang, Z.; Wang, A. Phase orientation improved the corrosion resistance and conductivity of Cr2AlC coatings for metal bipolar plates. J. Mater. Sci. Technol. 2022, 105, 36–44. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Wang, H.; Yuan, X.-Z.; Wang, G.; Pan, M. A review on performance degradation of proton exchange membrane fuel cells during startup and shutdown processes: Causes, consequences, and mitigation strategies. J. Power Sources 2012, 205, 10–23. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Wu, X.; Ouyang, C.; Lei, T.; Yang, P.; Liu, C.; Wang, J.; Ouyang, T.; Huang, Q. Development of Ti bipolar plates with carbon/PTFE/TiN composites coating for PEMFCs. Int. J. Hydrogen Energy 2018, 43, 20947–20958. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Jiang, Y.; Wang, Y.; Ye, C.; Du, C.-F. Probing the tribocorrosion behaviors of three nickel-based superalloys in sodium chloride solution. Tribol. Int. 2022, 172, 107581. [Google Scholar] [CrossRef]
- Shahwaz; Nath, P.; Sen, I. A critical review on the microstructure and mechanical properties correlation of additively manufactured nickel-based superalloys. J. Alloys Compd. 2022, 907, 164530. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Chen, Y.; Feng, K.; Yan, H.; Song, H.; Luo, C.; Hu, Z. Real-time monitoring of the corrosion behaviour of the 304SS in HCl solution using BPNN with joint image recognition and electrochemical noise. Corros. Sci. 2024, 228, 111779. [Google Scholar] [CrossRef]
- Jinoop, A.; Paul, C.; Bindra, K. Laser assisted direct energy deposition of Hastelloy-X. Opt. Laser Technol. 2019, 109, 14–19. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z.J. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Kaoumi, D.; Liu, J. Deformation induced martensitic transformation in 304 austenitic stainless steel: In-situ vs. ex-situ transmission electron microscopy characterization. Mater. Sci. Eng. A 2018, 715, 73–82. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Yang, H.; Huang, H.; Ji, S.; Ruan, J.; Liu, Z. Corrosion behavior of CoCrNi medium-entropy alloy compared with 304 stainless steel in H2SO4 and NaOH solutions. Corros. Sci. 2020, 177, 108973. [Google Scholar] [CrossRef]
- Ghiaasiaan, R.; Muhammad, M.; Gradl, P.R.; Shao, S.; Shamsaei, N. Superior tensile properties of Hastelloy X enabled by additive manufacturing. Mater. Res. Lett. 2021, 9, 308–314. [Google Scholar] [CrossRef]
- Ogihara, H.; Wang, H.; Saji, T. Electrodeposition of Ni–B/SiC composite films with high hardness and wear resistance. Appl. Surf. Sci. 2014, 296, 108–113. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhong, J.C.; Zhang, M.; Hou, B.R.; Zhang, W.M.; Zhao, C.W. Mechanical properties of Hastelloy X and C-276 and their application in hydrogen fuel cell bipolar plates. Mech. Eng. 2023, 45, 1274–1280. [Google Scholar] [CrossRef]
- Zhong, J.; Hou, B.; Zhang, W.; Guo, Z.; Zhao, C. Investigation on the physical and electrochemical properties of typical Ni-based alloys used for the bipolar plates of proton exchange membrane fuel cells. Heliyon 2023, 9, e16276. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Xin, Y.; Xu, L.; Guo, M.; Huang, L.; Zhang, Y.; Zhao, Y.; Liu, Y.; Li, L.; Xue, L.; et al. Effects of fluoride ions on corrosion performance and surface properties of SS304 in simulated PEMFC cathodic environments. Renew. Energy 2023, 212, 769–778. [Google Scholar] [CrossRef]
- Fuel Cell Technical Team Roadmap (2017). Available online: https://www.energy.gov/eere/vehicles/us-drive-partnership-plan-roadmaps-and-accomplishments (accessed on 12 December 2023).
- Yin, Q.; Zhang, K.; Fu, X.-Z.; Wang, X.-Z.; Luo, J.-L. Rapid coating preparation strategy for chromium nitride coated titanium bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2022, 47, 31435–31445. [Google Scholar] [CrossRef]
- Liu, T.; Diao, P. Nickel foam supported Cr-doped NiCo2O4/FeOOH nanoneedle arrays as a high-performance bifunctional electrocatalyst for overall water splitting. Nano Res. 2020, 13, 3299–3309. [Google Scholar] [CrossRef]
- Kannan, A.R.; Shanmugam, N.S.; Palguna, Y.; Girinath, B.; Lee, W.; Yoon, J. Effect of double-side welding on the microstructural characteristics and mechanical performance of dissimilar AA6061-AA5052 aluminium alloys. Mater. Lett. 2023, 331, 133444. [Google Scholar] [CrossRef]
- Li, D.W.; Liu, J.X.; Sun, Y.T.; Huang, W.Q.; Li, N.; Yang, L.H. Microstructure and mechanical degradation of K403 Ni-based superalloy from ultra-long-term serviced turbine blade. J. Alloys Compd. 2023, 957, 170378. [Google Scholar] [CrossRef]
- Jin, J.; Liu, H.; Zheng, D.; Zhu, Z. Effects of Mo content on the interfacial contact resistance and corrosion properties of CrN coatings on SS316L as bipolar plates in simulated PEMFCs environment. Int. J. Hydrogen Energy 2018, 43, 10048–10060. [Google Scholar] [CrossRef]


| Alloys | θ111 (°) | θ200 (°) | θ220 (°) | a (nm) |
|---|---|---|---|---|
| Hastelloy X | 21.77 | 25.35 | 37.27 | 0.3603 |
| 304SS | 21.74 | 25.34 | 37.34 | 0.3464 |
| Alloys | Eocp (V) | Ecorr (V) | Icorr (A·cm−2) |
|---|---|---|---|
| Hastelloy X | −0.23 | −0.23 | (1.01 ± 0.01) × 10−4 |
| 304SS | −0.45 | −0.34 | (7.36 ± 0.03) × 10−3 |
| Ni | Fe | Cr | Mo | Co | Al | O | |
|---|---|---|---|---|---|---|---|
| Before electrochemical testing | 49.28 | 20.10 | 23.36 | 5.09 | 1.96 | 0.2 | / |
| After electrochemical testing | 52.04 | 16.84 | 15.79 | 3.92 | 1.97 | 0.03 | 9.40 |
| Ni | Fe | Cr | Si | O | |
|---|---|---|---|---|---|
| Before electrochemical testing | 7.23 | 72.62 | 18.96 | 0.64 | / |
| After electrochemical testing | 5.95 | 61.41 | 16.47 | 0.62 | 15.55 |
| Alloys | CPE1/Ω−1·cm−2·sn | n1 | Rf/Ω·cm2 | CPE2/Ω−1·cm−2·sn | n2 | Rct/Ω·cm2 |
|---|---|---|---|---|---|---|
| Hastelloy X | (1.9 ± 0.1) × 10−3 | 0.651 ± 0.002 | 100.7 ± 5.2 | (1.4 ± 0.1) × 10−3 | 0.920 ± 0.012 | 47.58 ± 0.43 |
| 304SS | / | / | / | (1.1 ± 0.2) × 10−2 | 0.915 ± 0.023 | 9.57 ± 0.50 |
| Alloys | Yield Strength 0.2% Offset (MPa) | Ultimate Tensile Strength (MPa) | Uniform Elongation (%) | Elastic Modulus (GPa) | Hardness (HV) |
|---|---|---|---|---|---|
| Hastelloy X | 445.5 ± 2.3 | 823.9 ± 5.1 | 56.5 ± 1.3 | 203.8 ± 5.7 | 262.7 ± 1.2 |
| 304SS | 127.3 ± 4.6 | 616.3 ± 4.3 | 61.4 ± 2.5 | 273.7 ± 3.8 | 215.3 ± 6.5 |
| Alloys | ICR (mΩ·cm2) |
|---|---|
| Hastelloy X | 7.4 ± 0.3 |
| 304SS | 144.8 ± 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Liu, Z.; Zhang, M.; Liu, F.; Li, W.; Hou, B.; Zhang, W.; Zhao, C.; Gong, M. Investigation of the Performance of Hastelloy X as Potential Bipolar Plate Materials in Proton Exchange Membrane Fuel Cells. Molecules 2024, 29, 1299. https://doi.org/10.3390/molecules29061299
Zhong J, Liu Z, Zhang M, Liu F, Li W, Hou B, Zhang W, Zhao C, Gong M. Investigation of the Performance of Hastelloy X as Potential Bipolar Plate Materials in Proton Exchange Membrane Fuel Cells. Molecules. 2024; 29(6):1299. https://doi.org/10.3390/molecules29061299
Chicago/Turabian StyleZhong, Jiacheng, Zimeng Liu, Meng Zhang, Feng Liu, Wenjin Li, Beirui Hou, Wenmin Zhang, Chunwang Zhao, and Mingxing Gong. 2024. "Investigation of the Performance of Hastelloy X as Potential Bipolar Plate Materials in Proton Exchange Membrane Fuel Cells" Molecules 29, no. 6: 1299. https://doi.org/10.3390/molecules29061299
APA StyleZhong, J., Liu, Z., Zhang, M., Liu, F., Li, W., Hou, B., Zhang, W., Zhao, C., & Gong, M. (2024). Investigation of the Performance of Hastelloy X as Potential Bipolar Plate Materials in Proton Exchange Membrane Fuel Cells. Molecules, 29(6), 1299. https://doi.org/10.3390/molecules29061299





