Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production
Abstract
1. Introduction
2. The PEC Process of Water Decomposition
2.1. Mechanisms
2.2. PEC Cell
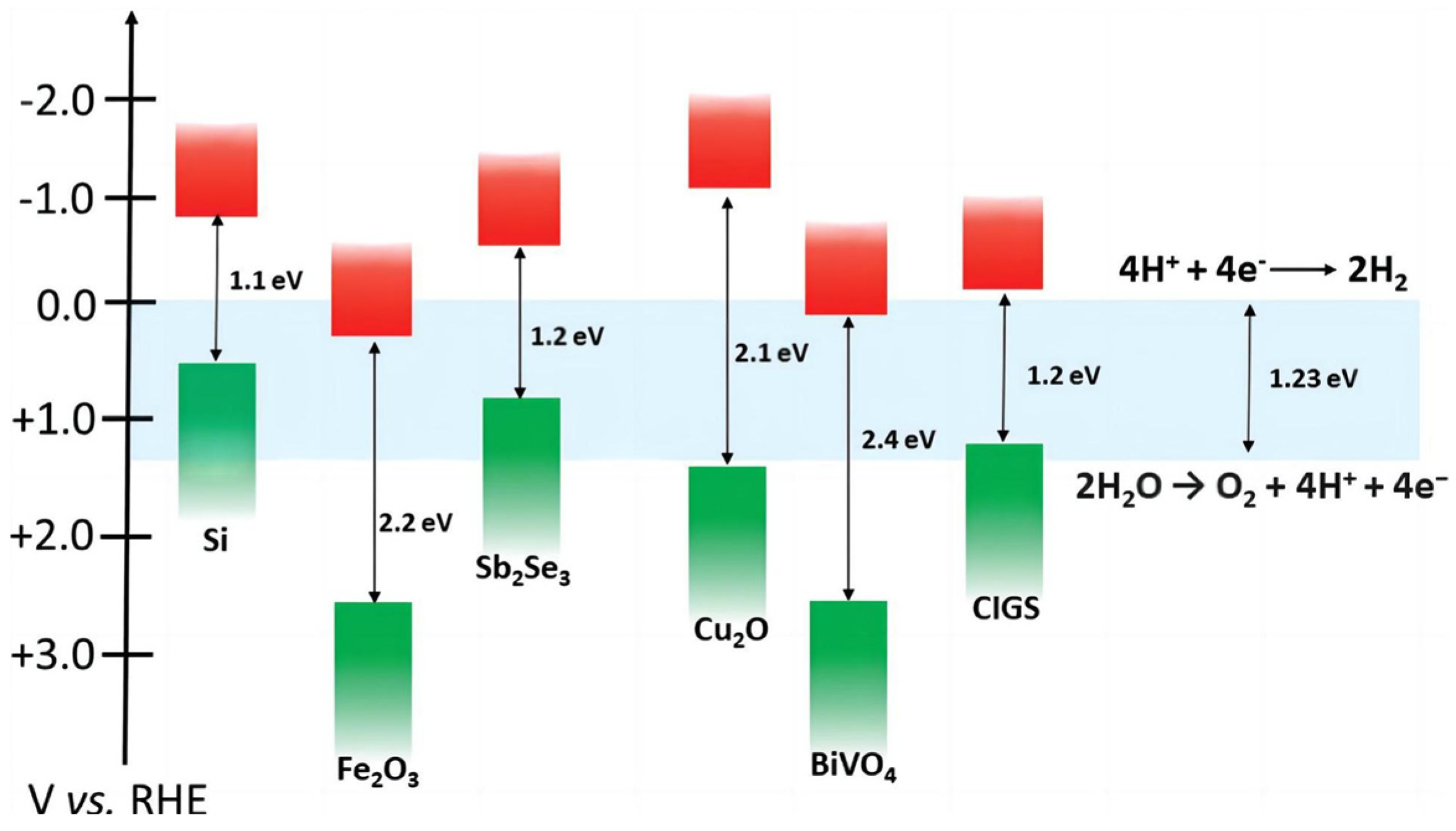
3. Semiconductor Materials for PECs
3.1. Metal Oxide Materials
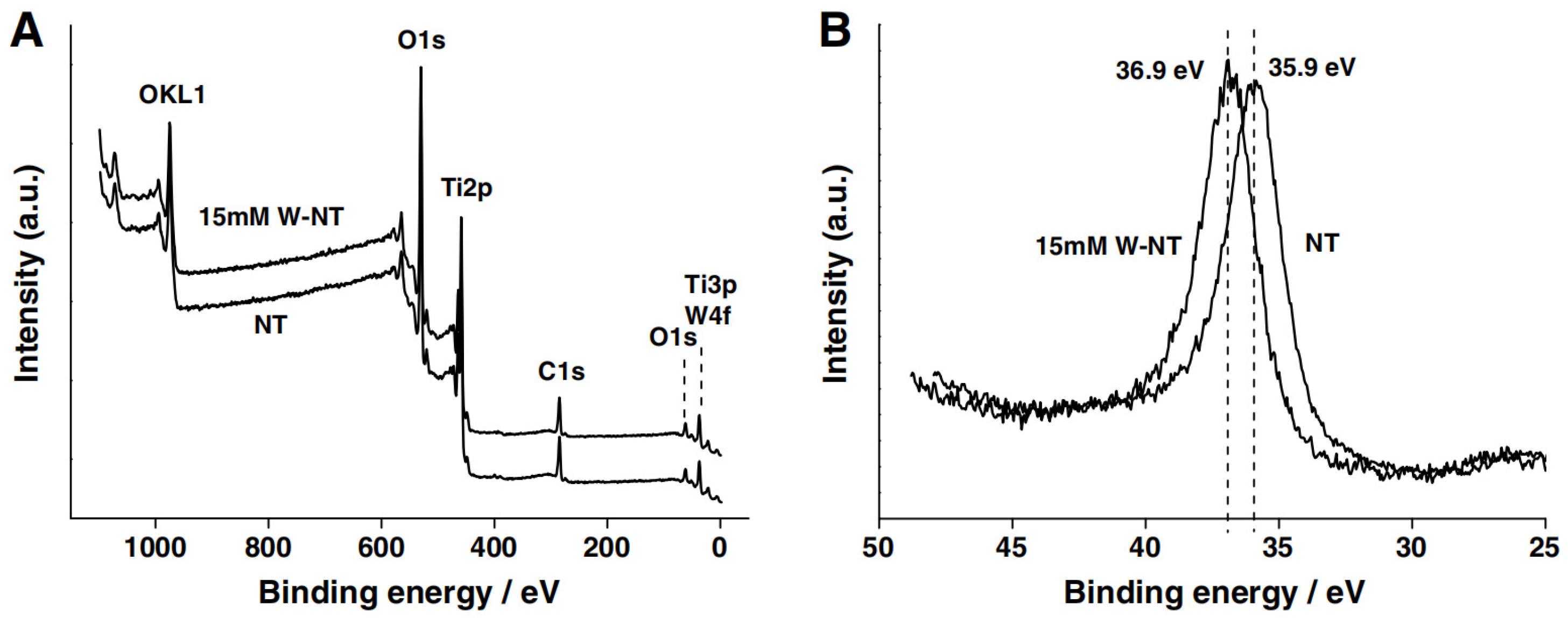
3.1.1. TiO2
- (1)
- Doping
- (2)
- TiO2-based composites
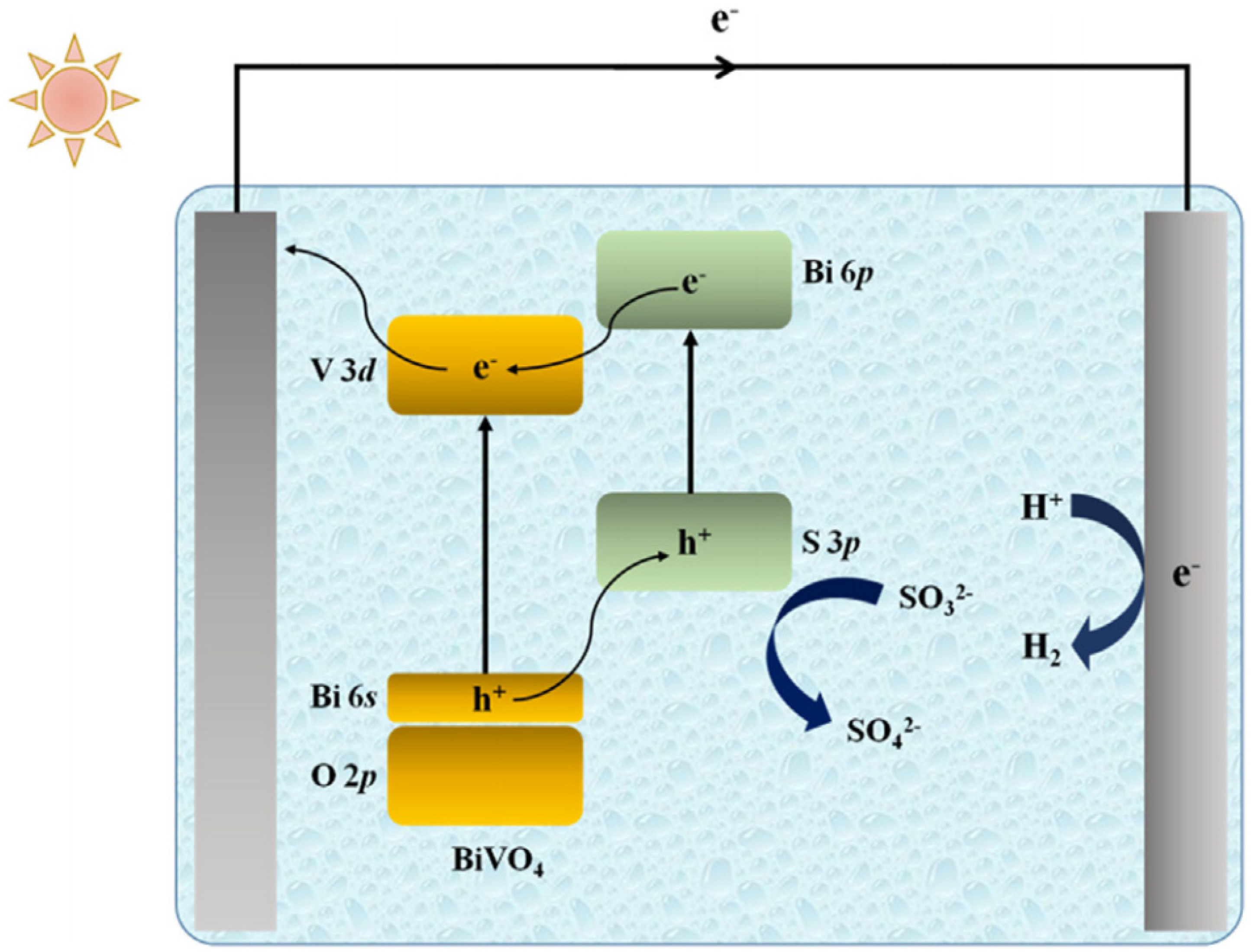
3.1.2. BiVO4
- (1)
- Doping
- (2)
- BiVO4-based composites
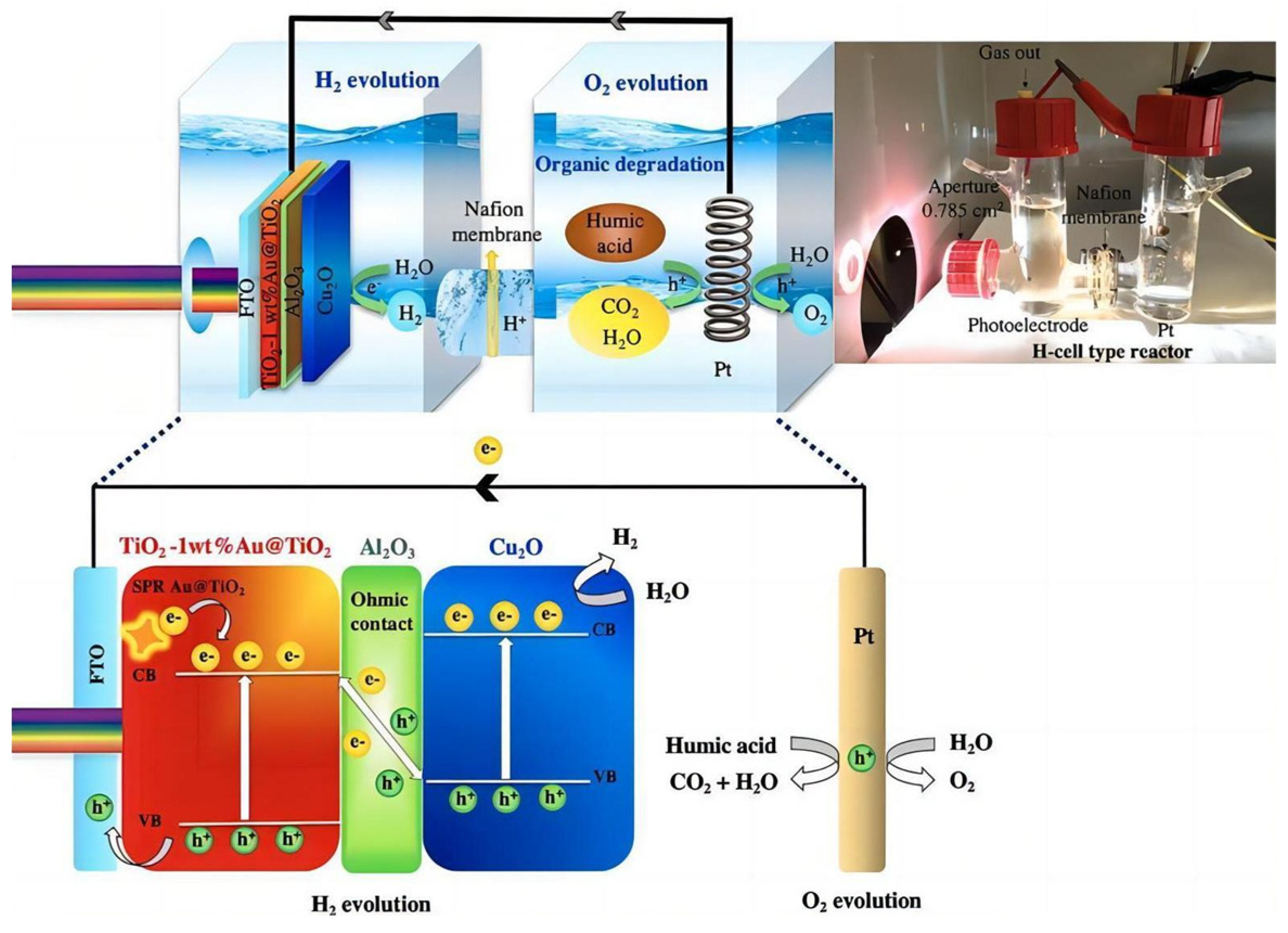
3.1.3. Cu2O
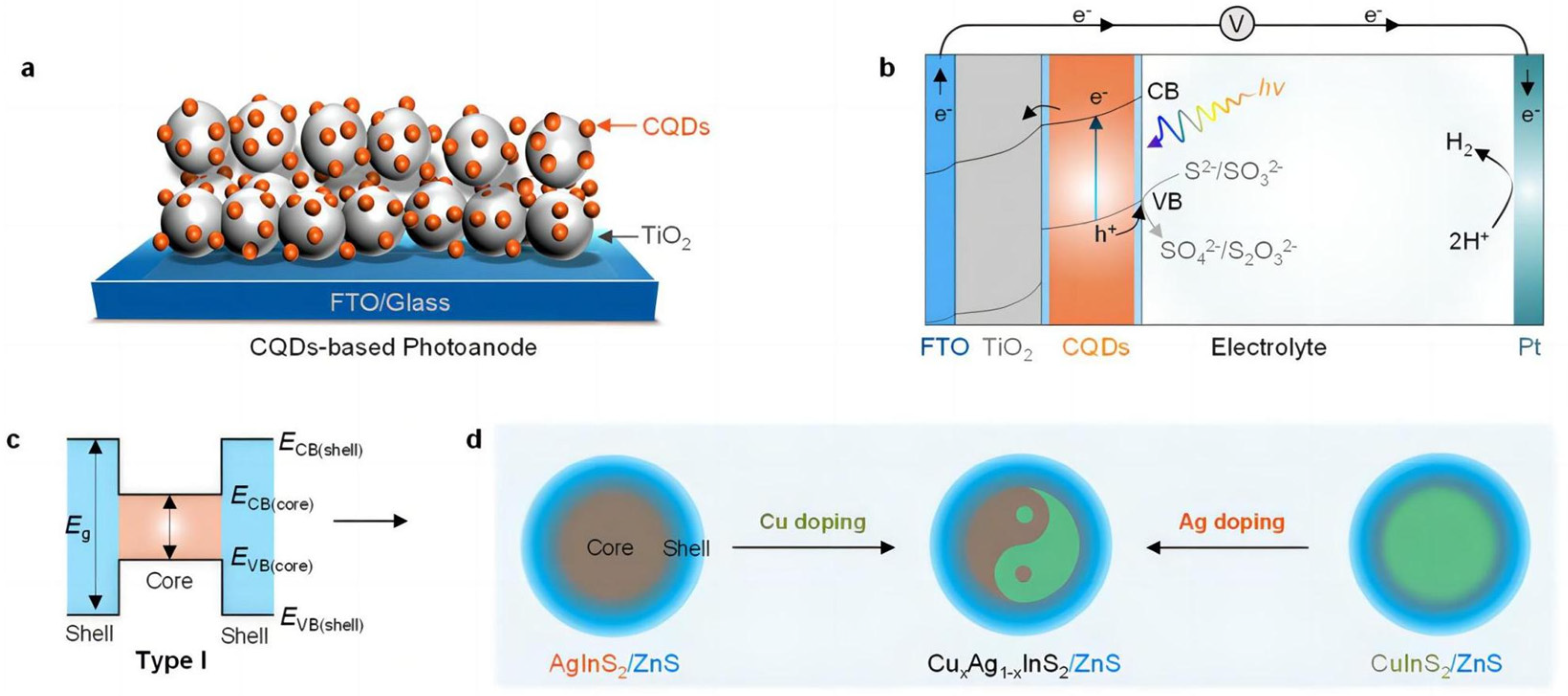
3.2. Sulfide Materials
3.2.1. Doping
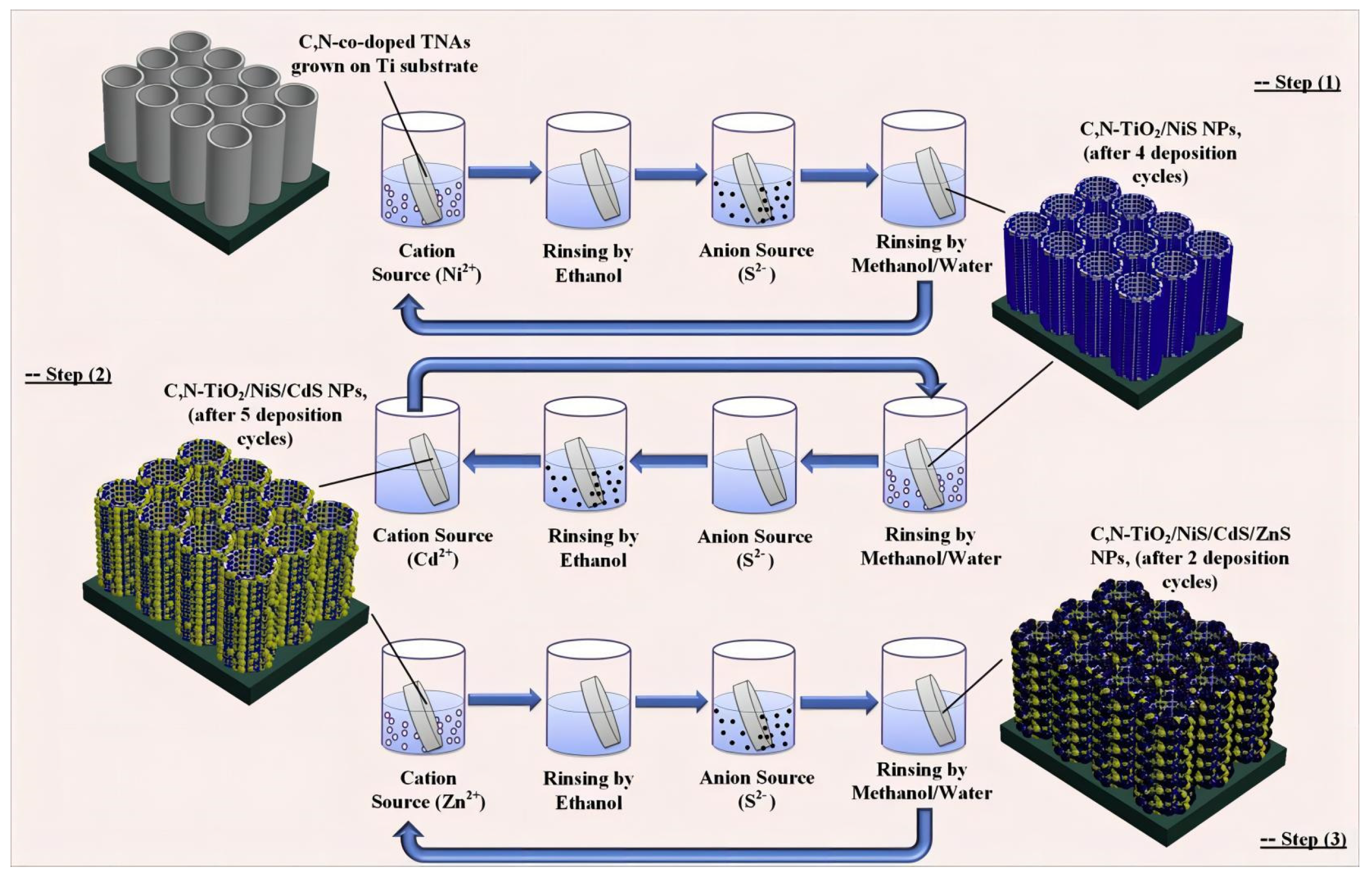
3.2.2. Sulfide-Based Composites
3.3. Graphite-Phase Carbon Nitride (g-C3N4)
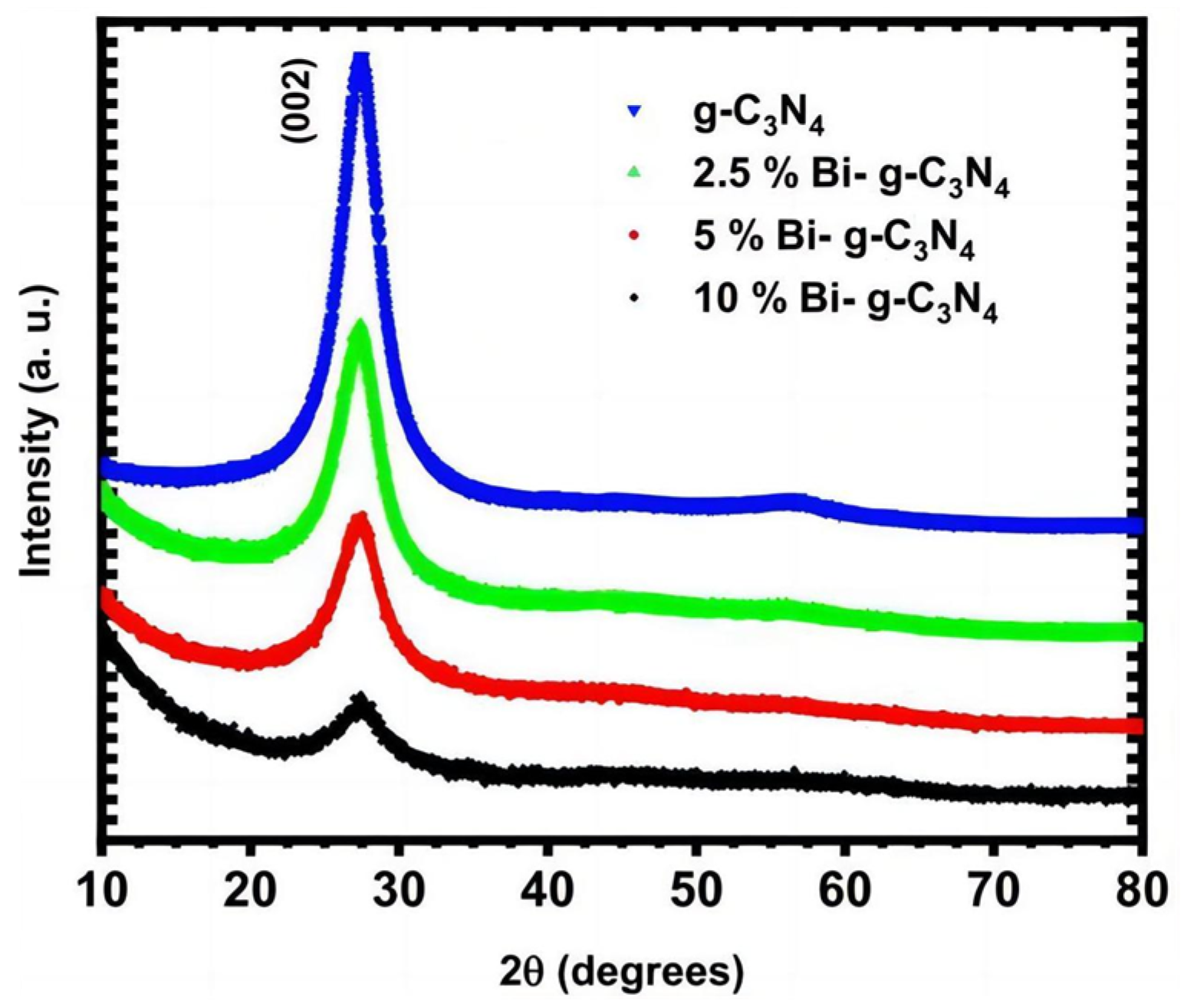
3.3.1. Doping
3.3.2. g-C3N4 Composites
4. Strategies to Improve the Efficiency of PEC Hydrogen Evolution
4.1. Morphological Control
4.2. Doping
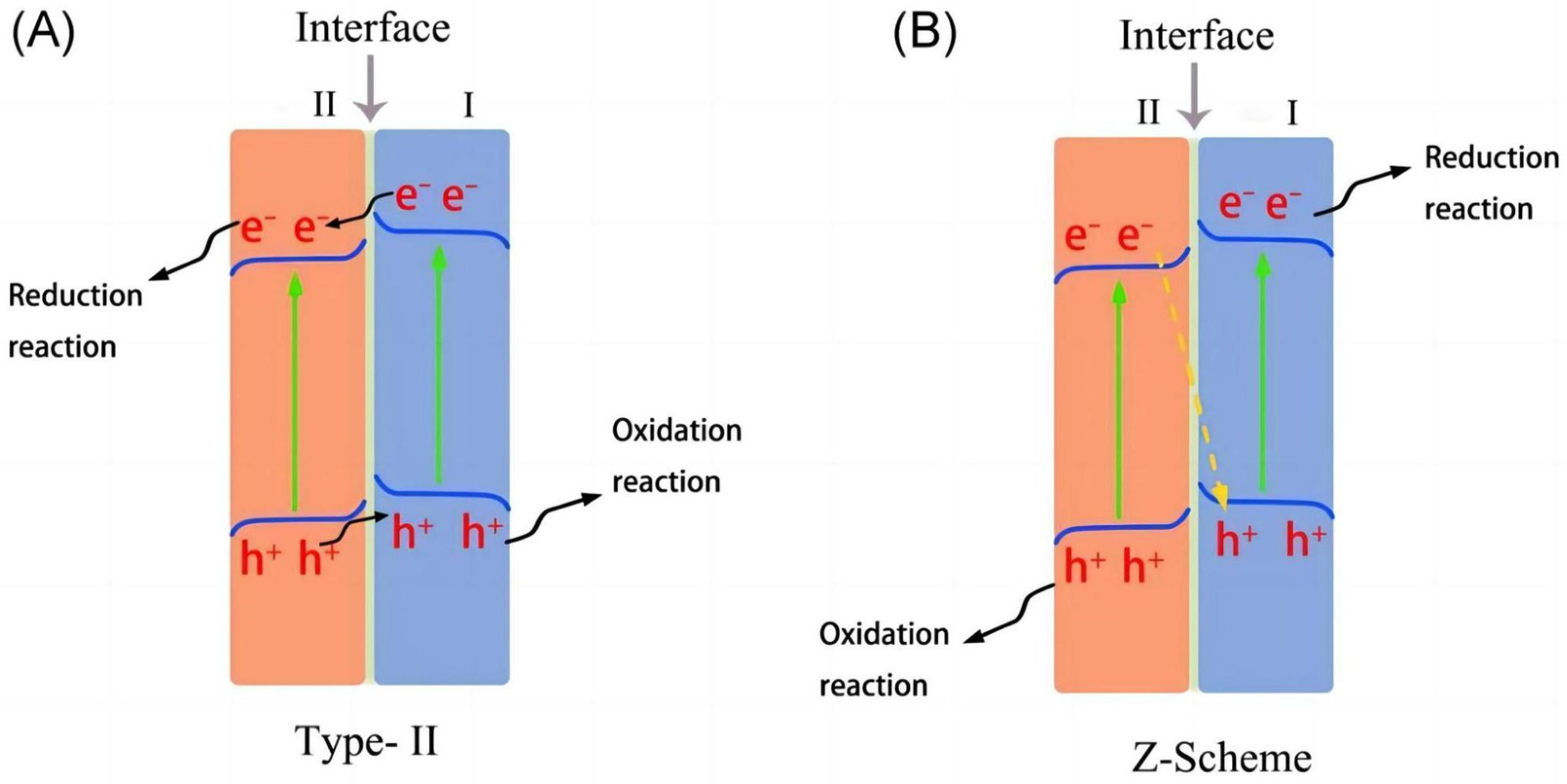
4.3. Heterojunctions
4.4. Surface Modification
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Mao, Z. Hydrogen Energy: Green Energy in 21st Century; Chemical Industry Press: Beijing, China, 2005; ISBN 9787502560393. [Google Scholar]
- Dragomir, G.; Șerban, A.; Năstase, G.; Brezeanu, A.I. Wind energy in Romania: A review from 2009 to 2016. Renew. Sustain. Energy Rev. 2016, 64, 129–143. [Google Scholar] [CrossRef]
- Menyah, K.; Wolde-Rufael, Y. CO2 emissions, nuclear energy, renewable energy and economic growth in the US. Energy Policy 2010, 38, 2911–2915. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Haussener, S.; Xiang, C.; Spurgeon, J.M.; Ardo, S.; Lewis, N.S.; Weber, A.Z. Modeling, simulation, and design criteria for photoelectrochemical water-splitting systems. Energy Environ. Sci. 2012, 5, 9922–9935. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V.; Ryabchuk, V.K.; Kuznetsov, V.N.; Artem’ev, Y.M.; Horikoshi, S. Why do hydrogen and oxygen yields from semiconductor-based photocatalyzed water splitting remain disappointingly low? Intrinsic and extrinsic factors impacting surface redox reactions. ACS Energy Lett. 2016, 1, 931–948. [Google Scholar] [CrossRef]
- Kenney, M.J.; Gong, M.; Li, Y.; Wu, J.Z.; Feng, J.; Lanza, M.; Dai, H. High-performance silicon photoanodes passivated with ultrathin nickel films for water oxidation. Science 2013, 342, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-B.; Kaiser, B.; Jaegermann, W. Novel photoelectrochemical behaviors of p-SiC films on Si for solar water splitting. J. Power Source 2014, 253, 41–47. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge carrier dynamics at TiO2 particles: Reactivity of free and trapped holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Pham, T.A.; Ping, Y.; Galli, G. Modelling heterogeneous interfaces for solar water splitting. Nat. Mater. 2017, 16, 401–408. [Google Scholar] [CrossRef]
- Shi, X.; Jeong, H.; Oh, S.J.; Ma, M.; Zhang, K.; Kwon, J.; Choi, I.T.; Choi, I.Y.; Kim, H.K.; Kim, J.K. Unassisted photoelectrochemical water splitting exceeding 7% solar-to-hydrogen conversion efficiency using photon recycling. Nat. Commun. 2016, 7, 11943. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Li, T.; He, J.; Peña, B.; Berlinguette, C.P. Curing BiVO4 photoanodes with ultraviolet light enhances photoelectrocatalysis. Angew. Chem. Int. Ed. 2016, 55, 1769–1772. [Google Scholar] [CrossRef]
- Monfort, O.; Pop, L.-C.; Sfaelou, S.; Plecenik, T.; Roch, T.; Dracopoulos, V.; Stathatos, E.; Plesch, G.; Lianos, P. Photoelectrocatalytic hydrogen production by water splitting using BiVO4 photoanodes. Chem. Eng. J. 2016, 286, 91–97. [Google Scholar] [CrossRef]
- Gu, Y.-E.; Zhang, Y.; Zhang, F.; Wei, J.; Wang, C.; Du, Y.; Ye, W. Investigation of photoelectrocatalytic activity of Cu2O nanoparticles for p-nitrophenol using rotating ring-disk electrode and application for electrocatalytic determination. Electrochim. Acta 2010, 56, 953–958. [Google Scholar] [CrossRef]
- e Silva, B.C.; Irikura, K.; Flor, J.B.S.; Dos Santos, R.M.M.; Lachgar, A.; Frem, R.C.G.; Zanoni, M.V.B. Electrochemical preparation of Cu/Cu2O-Cu (BDC) metal-organic framework electrodes for photoelectrocatalytic reduction of CO2. J. CO2 Util. 2020, 42, 101299. [Google Scholar] [CrossRef]
- Farooq, U.; Chaudhary, P.; Ingole, P.P.; Kalam, A.; Ahmad, T. Development of cuboidal KNbO3@ α-Fe2O3 hybrid nanostructures for improved photocatalytic and photoelectrocatalytic applications. ACS Omega 2020, 5, 20491–20505. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tian, Y.; Zhang, J. Construction of pn heterojunction film of Cu2O/α-Fe2O3 for efficiently photoelectrocatalytic degradation of oxytetracycline. J. Colloid Interface Sci. 2018, 526, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Muñoz-Portero, M.-J.; Garcia-Anton, J. Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem. Eng. J. 2018, 350, 1114–1124. [Google Scholar] [CrossRef]
- Mohite, S.; Ganbavle, V.; Rajpure, K. Photoelectrocatalytic activity of immobilized Yb doped WO3 photocatalyst for degradation of methyl orange dye. J. Energy Chem. 2017, 26, 440–447. [Google Scholar] [CrossRef]
- Rabell, G.O.; Cruz, M.A.; Juárez-Ramírez, I. Hydrogen production of ZnO and ZnO/Ag films by photocatalysis and photoelectrocatalysis. Mater. Sci. Semicond. Process. 2021, 134, 105985. [Google Scholar] [CrossRef]
- Sapkal, R.; Shinde, S.; Mahadik, M.; Mohite, V.; Waghmode, T.; Govindwar, S.; Rajpure, K.; Bhosale, C. Photoelectrocatalytic decolorization and degradation of textile effluent using ZnO thin films. J. Photochem. Photobiol. B 2012, 114, 102–107. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Wang, L. Crystal facet engineering of photoelectrodes for photoelectrochemical water splitting. Chem. Rev. 2019, 119, 5192–5247. [Google Scholar] [CrossRef]
- Yang, W.; Moon, J. Recent advances in earth-abundant photocathodes for photoelectrochemical water splitting. ChemSusChem 2019, 12, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; An, X.; Liu, R.; Xiong, L.; Tang, J.; Hu, C.; Liu, H.; Qu, J. Interface-modulated nanojunction and microfluidic platform for photoelectrocatalytic chemicals upgrading. Appl. Catal. B 2021, 282, 119541. [Google Scholar] [CrossRef]
- Ray, C.; Pal, T. Retracted Article: Recent advances of metal–metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Gong, J.; Pu, W.; Yang, C.; Zhang, J. Novel one-step preparation of tungsten loaded TiO2 nanotube arrays with enhanced photoelectrocatalytic activity for pollutant degradation and hydrogen production. Catal. Commun. 2013, 36, 89–93. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, G.; Yan, K. TiO2 nanotubes for hydrogen generation by photocatalytic water splitting in a two-compartment photoelectrochemical cell. Int. J. Hydrogen Energy 2011, 36, 15502–15508. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Wang, C.; Ao, Y. Polymeric carbon nitride coated Nb-TiO2 nanorod arrays with enhanced photoelectrocatalytic activity under visible light irradiation. Inorg. Chem. Commun. 2019, 101, 113–116. [Google Scholar] [CrossRef]
- See, A.; Bartynski, R. Inverse photoemission study of the defective TiO2 (110) surface. J. Vac. Sci. Technol. A Vac. Surf. Film. 1992, 10, 2591–2596. [Google Scholar] [CrossRef]
- Thomas, A.; Flavell, W.; Mallick, A.; Kumarasinghe, A.; Tsoutsou, D.; Khan, N.; Chatwin, C.; Rayner, S.; Smith, G.; Stockbauer, R. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and X-ray absorption spectroscopy. Phys. Rev. B 2007, 75, 035105. [Google Scholar] [CrossRef]
- Khan, M.A.; Woo, S.I.; Yang, O.-B. Hydrothermally stabilized Fe (III) doped titania active under visible light for water splitting reaction. Int. J. Hydrogen Energy 2008, 33, 5345–5351. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Wang, Q.; Jia, Y.; Tan, X.; Du, X.; Gao, S. Solvothermal fabrication and construction of highly photoelectrocatalytic TiO2 NTs/Bi2MoO6 heterojunction based on titanium mesh. J. Colloid Interface Sci. 2019, 556, 92–101. [Google Scholar] [CrossRef] [PubMed]
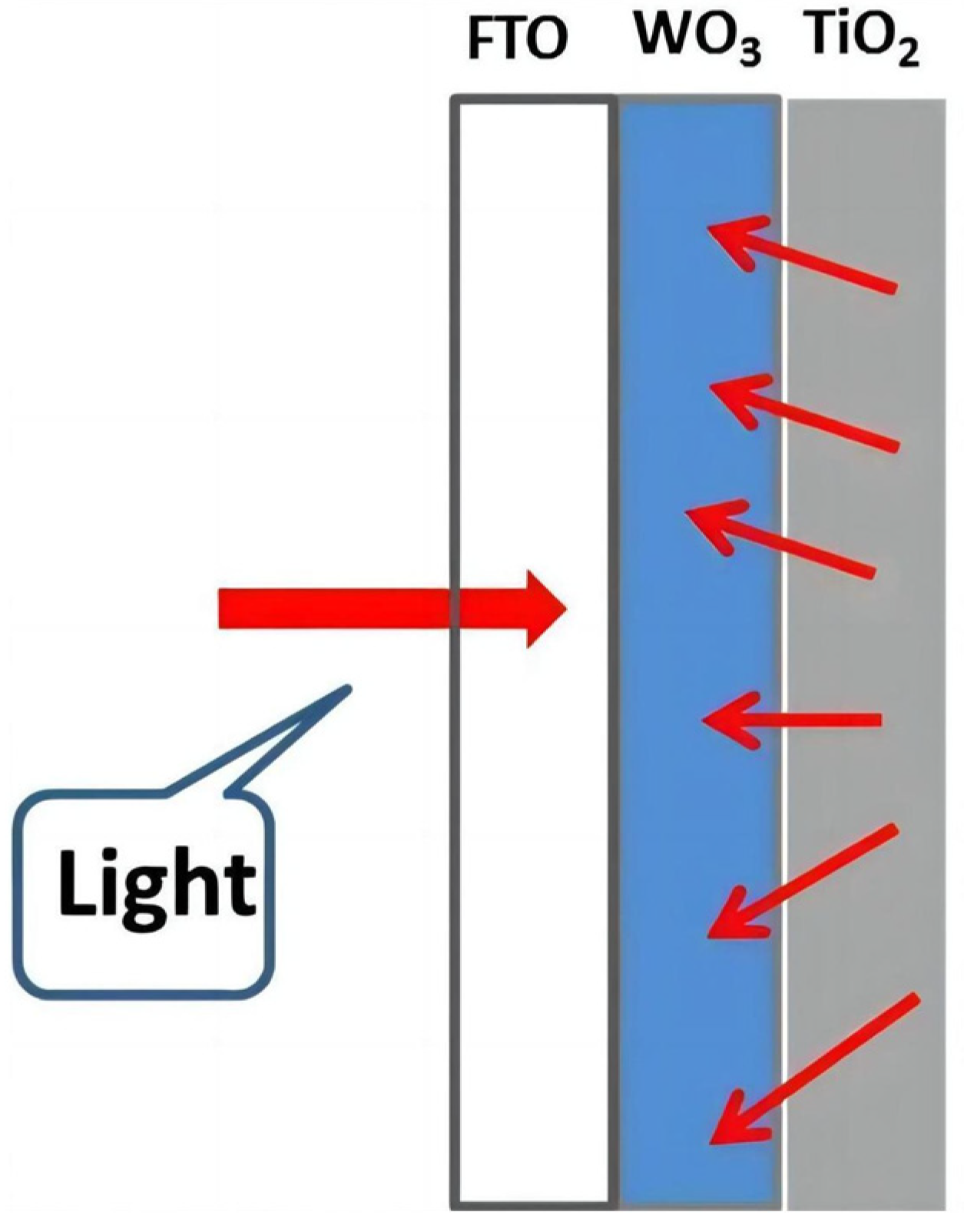
- Adamopoulos, P.M.; Papagiannis, I.; Raptis, D.; Lianos, P. Photoelectrocatalytic hydrogen production using a TiO2/WO3 bilayer photocatalyst in the presence of ethanol as a fuel. Catalysts 2019, 9, 976. [Google Scholar] [CrossRef]
- Tatsuma, T.; Saitoh, S.; Ngaotrakanwiwat, P.; Ohko, Y.; Fujishima, A. Energy storage of TiO2-WO3 photocatalysis systems in the gas phase. Langmuir 2002, 18, 7777–7779. [Google Scholar] [CrossRef]
- Ngaotrakanwiwat, P.; Tatsuma, T. Optimization of energy storage TiO2-WO3 photocatalysts and further modification with phosphotungstic acid. J. Electroanal. Chem. 2004, 573, 263–269. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ngaotrakanwiwat, P.; Tatsuma, T. Energy storage TiO2-MoO3 photocatalysts. Electrochim. Acta 2004, 49, 2025–2029. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tatsuma, T. Oxidative energy storage ability of a TiO2-Ni(OH)2 bilayer photocatalyst. Langmuir 2005, 21, 12357–12361. [Google Scholar] [CrossRef] [PubMed]
- Yasomanee, J.; Bandara, J. Multi-electron storage of photoenergy using Cu2O–TiO2 thin film photocatalyst. Sol. Energy Mater. Sol. Cells 2008, 92, 348–352. [Google Scholar] [CrossRef]
- Xiong, L.; Ouyang, M.; Yan, L.; Li, J.; Qiu, M.; Yu, Y. Visible-light energy storage by Ti3+ in TiO2/Cu2O bilayer film. Chem. Lett. 2009, 38, 1154–1155. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, F.; Yan, L.; Yan, N.; Yang, X.; Qiu, M.; Yu, Y. Bifunctional photocatalysis of TiO2/Cu2O composite under visible light: Ti3+ in organic pollutant degradation and water splitting. J. Phys. Chem. Solids 2011, 72, 1104–1109. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Zou, Z.; Abe, R.; Abe, Y.; Arakawa, H. Photoelectrochemical decomposition of water on nanocrystalline BiVO4 film electrodes under visible light. Chem. Commun. 2003, 2908–2909. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Zhao, X.; Zhao, Z.; Li, Z.; Zou, Z. Formation energy and photoelectrochemical properties of BiVO4 after doping at Bi3+ or V5+ sites with higher valence metal ions. PCCP 2013, 15, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.F.; Han, L.; Smets, A.H.; Zeman, M.; Dam, B.; Van De Krol, R. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 2013, 4, 2195. [Google Scholar] [CrossRef]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.-S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef]
- Liang, Y.; Tsubota, T.; Mooij, L.P.; van de Krol, R. Highly improved quantum efficiencies for thin film BiVO4 photoanodes. J. Phys. Chem. C 2011, 115, 17594–17598. [Google Scholar] [CrossRef]
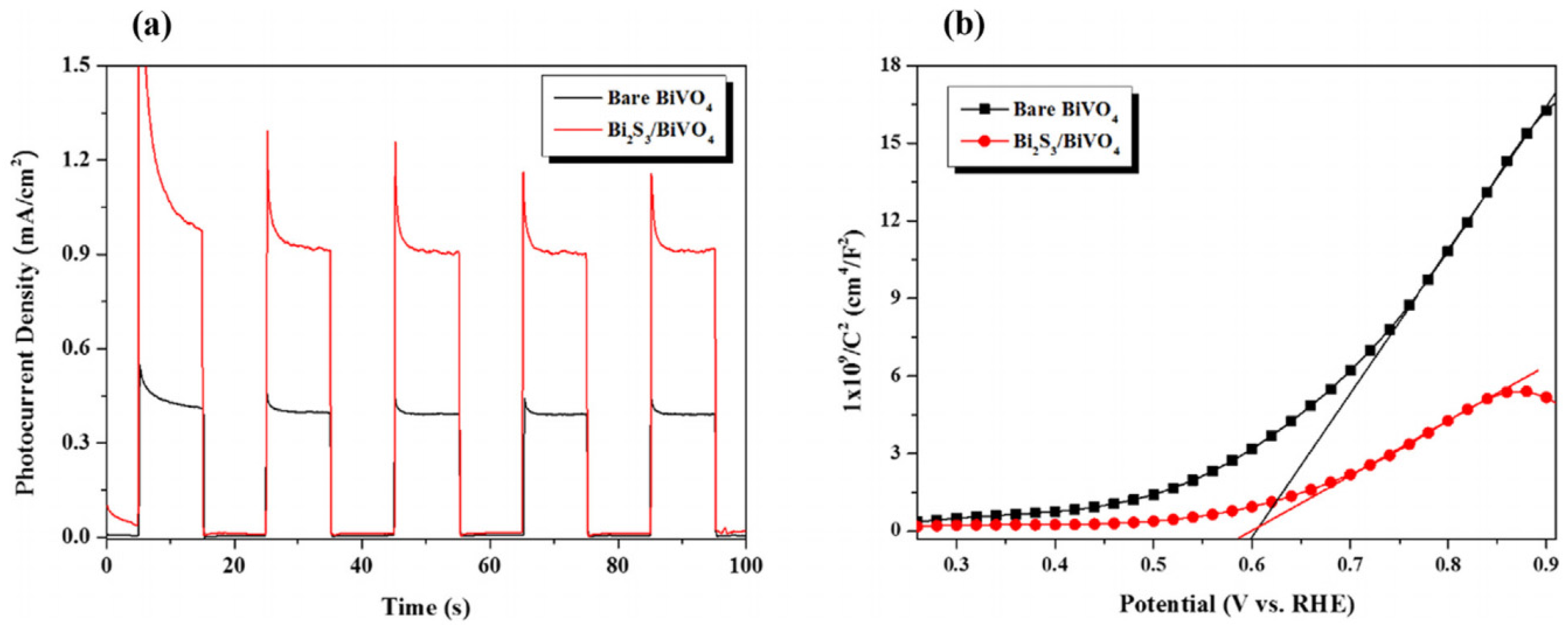
- Li, F.; Leung, D.Y. Highly enhanced performance of heterojunction Bi2S3/BiVO4 photoanode for photoelectrocatalytic hydrogen production under solar light irradiation. Chem. Eng. Sci. 2020, 211, 115266. [Google Scholar] [CrossRef]
- Kondo, J.N. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 357–358. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, H.; Ba, X.; Zhang, W.; Yu, Y. Cu2O-Based Nanocomposites for Environmental Protection: Relationship between Structure and Photocatalytic Activity, Application, and Mechanism. In Nanomaterials for Environmental Protection; Kharisov, B.I., Kharissova, O.V., Dias, H.V.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 41–70. ISBN 9781118496978. [Google Scholar]
- Li, C.; He, J.; Xiao, Y.; Li, Y.; Delaunay, J.-J. Earth-abundant Cu-based metal oxide photocathodes for photoelectrochemical water splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Wu, Z.; Fei, H.; Wang, D. MoS2/Cu2O nanohybrid as a highly efficient catalyst for the photoelectrocatalytic hydrogen generation. Mater. Lett. 2019, 256, 126622. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Yun, J.-H.; Butburee, T.; Chen, H.; Thaweesak, S.; Lyu, M.; Wang, S.; Wang, L. Bifunctional photoelectrochemical process for humic acid degradation and hydrogen production using multi-layered p-type Cu2O photoelectrodes with plasmonic Au@TiO2. J. Hazard. Mater. 2021, 402, 123533. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Shen, L.; Takata, T.; Domen, K.; Gupta, A.; Yanagisawa, K.; Grimes, C.A. Facile Cd-thiourea complex thermolysis synthesis of phase-controlled CdS nanocrystals for photocatalytic hydrogen production under visible light. J. Phys. Chem. C 2007, 111, 17527–17534. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kudo, A. Visible-Light-Induced H2 Evolution from an Aqueous Solution Containing Sulfide and Sulfite over a ZnS–CuInS2–AgInS2 Solid-Solution Photocatalyst. Angew. Chem. Int. Ed. 2005, 117, 3631–3634. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Y.; Liu, L.; Hu, J.; Liang, Y. Microwave-assisted synthesis of CdS intercalated K4Nb6O17 and its photocatalytic activity for hydrogen production. Appl. Catal. A 2012, 417, 111–118. [Google Scholar] [CrossRef]
- Tian, H.; Liang, J.; Ma, X.; Cao, L.; Hu, X.; Gao, M.; Yang, H.; Liang, Z. Enhanced Photoelectrocatalytic H2 Evolution over Two-Dimensional MoS2 Nanosheets Loaded on Cu-Doped CdS Nanorods. ChemElectroChem 2019, 6, 714–723. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.; Ji, Y.; Chi, N.; Cong, Y. Preparation of a novel Fe2O3-MoS2-CdS ternary composite film and its photoelectrocatalytic performance. Electrochim. Acta 2018, 285, 230–240. [Google Scholar] [CrossRef]
- Barpuzary, D.; Khan, Z.; Vinothkumar, N.; De, M.; Qureshi, M. Hierarchically grown urchinlike CdS@ZnO and CdS@Al2O3 heteroarrays for efficient visible-light-driven photocatalytic hydrogen generation. J. Phys. Chem. C 2012, 116, 150–156. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Xiao, L.; Kurbanjan, D.; Nie, H.; Du, H. Cation exchange synthesis of porous Cd1−xZnxS twinned nanosheets for visible light highly active H2 evolution. J. Am. Ceram. Soc. 2022, 105, 1405–1416. [Google Scholar] [CrossRef]
- Guo, H.; Yang, P.; Hu, J.; Jiang, A.; Chen, H.; Niu, X.; Zhou, Y. Band Structure Engineering and Defect Passivation of CuxAg1-xInS2/ZnS Quantum Dots to Enhance Photoelectrochemical Hydrogen Evolution. ACS Omega 2022, 7, 9642–9651. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, C.; Meng, H.; Yu, J. Visible-Light Photocatalytic Hydrogen Production Activity of ZnIn2S4 Microspheres Using Carbon Quantum Dots and Platinum as Dual Co-catalysts. Chem. Asian J. 2014, 9, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chen, W.; Liu, J.; An, J.; Wang, L.; Sillanpãã, M. The photoelectrocatalytic performance of ZnIn2S4 nanosheets and microspheres grown on flexible graphite felt. J. Electroanal. Chem. 2019, 845, 144–153. [Google Scholar] [CrossRef]
- Mollavali, M.; Falamaki, C.; Rohani, S. Efficient light harvesting by NiS/CdS/ZnS NPs incorporated in C, N-co-doped-TiO2 nanotube arrays as visible-light sensitive multilayer photoanode for solar applications. Int. J. Hydrogen Energy 2018, 43, 9259–9278. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Di, Y.; Antonietti, M.; Li, H.; Chen, X.; Wang, X. Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids. Chem. Mater. 2010, 22, 5119–5121. [Google Scholar] [CrossRef]
- Wu, F.; Ma, Y.; Hu, Y.H. Near infrared light-driven photoelectrocatalytic water splitting over P-doped g-C3N4. ACS Appl. Energy Mater. 2020, 3, 11223–11230. [Google Scholar] [CrossRef]
- El Rouby, W.M.; Aboubakr, A.E.A.; Khan, M.D.; Farghali, A.A.; Millet, P.; Revaprasadu, N. Synthesis and characterization of Bi-doped g-C3N4 for photoelectrochemical water oxidation. Sol. Energy 2020, 211, 478–487. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Kuang, P.-Y.; Su, Y.-Z.; Chen, G.-F.; Luo, Z.; Xing, S.-Y.; Li, N.; Liu, Z.-Q. g-C3N4 decorated ZnO nanorod arrays for enhanced photoelectrocatalytic performance. Appl. Surf. Sci. 2015, 358, 296–303. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4@ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol. J. Hazard. Mater. 2018, 344, 369–380. [Google Scholar] [CrossRef]
- Wei, Z.; Liang, F.; Liu, Y.; Luo, W.; Wang, J.; Yao, W.; Zhu, Y. Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl. Catal. B 2017, 201, 600–606. [Google Scholar] [CrossRef]
- Karimi-Nazarabad, M.; Goharshadi, E.K. Highly efficient photocatalytic and photoelectrocatalytic activity of solar light driven WO3/g-C3N4 nanocomposite. Sol. Energy Mater. Sol. Cells 2017, 160, 484–493. [Google Scholar] [CrossRef]
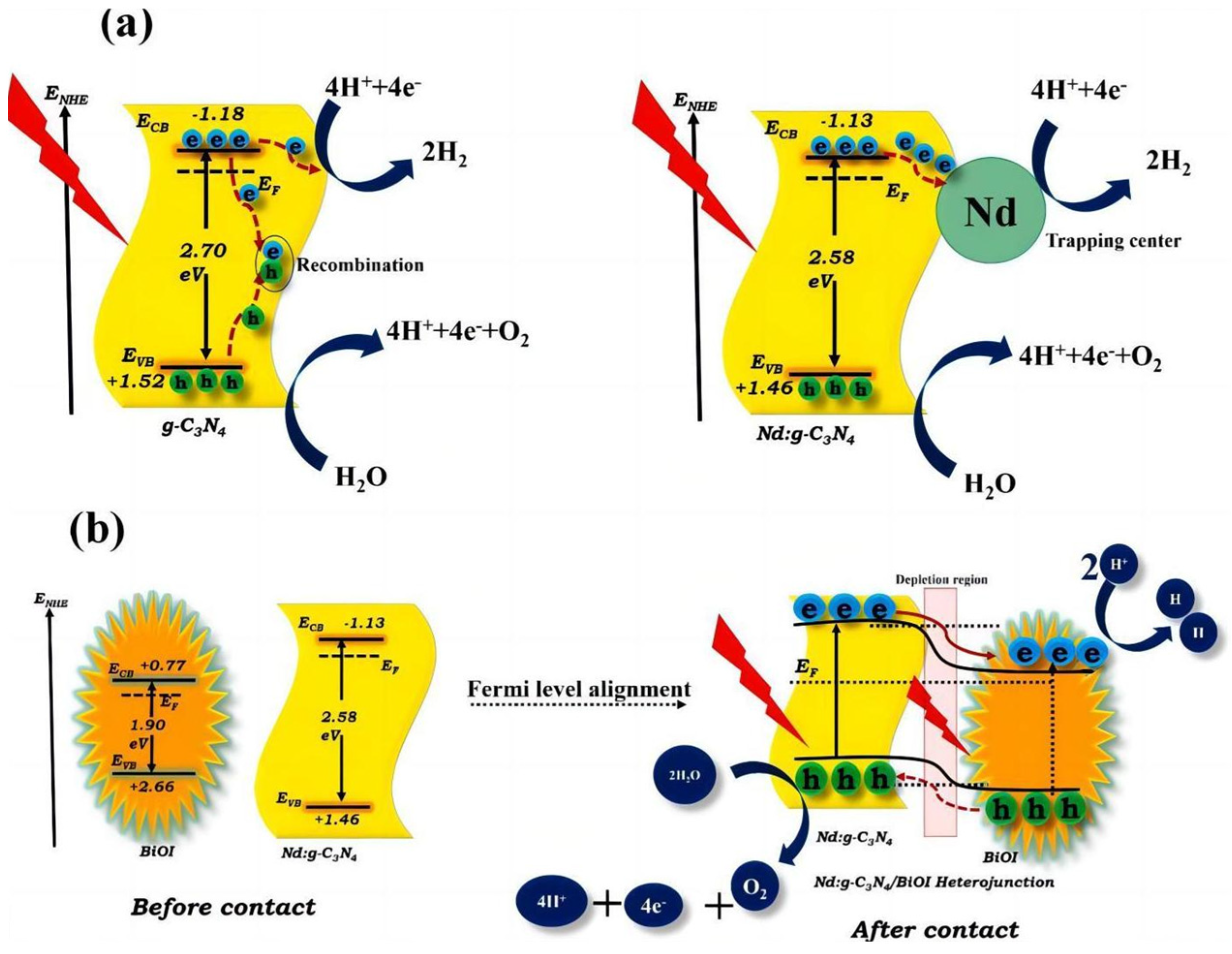
- Velusamy, P.; Sathiya, M.; Liu, Y.; Liu, S.; Babu, R.R.; Aly, M.A.S.; Elangovan, E.; Chang, H.; Mao, L.; Xing, R. Investigating the effect of Nd3+ dopant and the formation of g-C3N4/BiOI heterostructure on the microstructural, optical and photoelectrocatalytic properties of g-C3N4. Appl. Surf. Sci. 2021, 561, 150082. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Chen, S.; Fan, X.; Quan, X.; Yu, H. Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. J. Membr. Sci. 2017, 541, 153–161. [Google Scholar] [CrossRef]
- Hu, J.; Yu, C.; Zhai, C.; Hu, S.; Wang, Y.; Fu, N.; Zeng, L.; Zhu, M. 2D/1D heterostructure of g-C3N4 nanosheets/CdS nanowires as effective photo-activated support for photoelectrocatalytic oxidation of methanol. Catal. Today 2018, 315, 36–45. [Google Scholar] [CrossRef]
- Zhai, C.; Sun, M.; Zeng, L.; Xue, M.; Pan, J.; Du, Y.; Zhu, M. Construction of Pt/graphitic C3N4/MoS2 heterostructures on photo-enhanced electrocatalytic oxidation of small organic molecules. Appl. Catal. B 2019, 243, 283–293. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, D.; Chen, X.; Li, R.; Jiang, T.; Wang, W.; Li, G.; Leung, D.Y. g-C3N4 photoanode for photoelectrocatalytic synergistic pollutant degradation and hydrogen evolution. Appl. Surf. Sci. 2019, 467, 658–665. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Ma, H.; Dong, G. Enhancing the yield of hydrogen peroxide and phenol degradation via a synergistic effect of photoelectrocatalysis using a g-C3N4/ACF electrode. Int. J. Hydrog. Energy 2018, 43, 19500–19509. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI-g-C3N4 composite photocatalysts. J. Mater. Chem. 2012, 22, 11843–11850. [Google Scholar] [CrossRef]
- Li, G.; Lian, Z.; Wang, W.; Zhang, D.; Li, H. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 2016, 19, 446–454. [Google Scholar] [CrossRef]
- Brillet, J.; Gratzel, M.; Sivula, K. Decoupling feature size and functionality in solution-processed, porous hematite electrodes for solar water splitting. Nano Lett. 2010, 10, 4155–4160. [Google Scholar] [CrossRef]
- Wen, X.; Luo, W.; Zou, Z. Photocurrent improvement in nanocrystalline Cu2ZnSnS4 photocathodes by introducing porous structures. J. Mater. Chem. A 2013, 1, 15479–15485. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Wang, H.; Chan, K.M.; Yu, J.C. Biocompatible anatase single-crystal photocatalysts with tunable percentage of reactive facets. Cryst. Growth Des. 2010, 10, 1130–1137. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Wang, H.; Xihong, L.; Li, Y. Chemically modified nanostructures for photoelectrochemical water splitting. J. Photochem. Photobiol. C 2014, 19, 35–51. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Schülli, T.U.; Bai, Y.; Monny, S.A.; Du, A.; Wang, L. Identifying copper vacancies and their role in the CuO based photocathode for water splitting. Angew. Chem. 2019, 131, 17768–17773. [Google Scholar] [CrossRef]
- Chang, S.M.; Liu, W.S. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B 2014, 156, 466–475. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, S.; Jung, H.S.; Kim, J.Y.; Shin, H.; Hong, K.S. Effects of heterojunction on photoelectrocatalytic properties of ZnO-TiO2 films. Int. J. Hydrogen Energy 2007, 32, 3137–3140. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Zhang, W.; Jin, B.; Feng, Q.; Huang, J.; Jiao, Z. Advances in Z-scheme semiconductor photocatalysts for the photoelectrochemical applications: A review. Carbon Energy 2022, 4, 294–331. [Google Scholar] [CrossRef]
- Pop, L.C.; Dracopoulos, V.; Lianos, P. Photoelectrocatalytic hydrogen production using nanoparticulate titania and a novel Pt/Carbon electrocatalyst: The concept of the “Photoelectrocatalytic Leaf”. Appl. Surf. Sci. 2015, 333, 147–151. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, J.; Zhu, B.; Yu, J. Shape-dependent photocatalytic hydrogen evolution activity over a Pt nanoparticle coupled g-C3N4 photocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 19457–19463. [Google Scholar] [CrossRef]
- Agegnehu, A.K.; Pan, C.J.; Rick, J.; Lee, J.F.; Su, W.N.; Hwang, B.J. Enhanced hydrogen generation by cocatalytic Ni and NiO nanoparticles loaded on graphene oxide sheets. J. Mater. Chem. 2012, 22, 13849–13854. [Google Scholar] [CrossRef]
- Xu, S.; Du, A.J.; Liu, J.; Ng, J.; Sun, D.D. Highly efficient CuO incorporated TiO2 nanotube photocatalyst for hydrogen production from water. Int. J. Hydrogen Energy 2011, 36, 6560–6568. [Google Scholar] [CrossRef]
- Kim, S.; Han, D.S.; Park, H. Reduced titania nanorods and Ni–Mo–S catalysts for photoelectrocatalytic water treatment and hydrogen production coupled with desalination. Appl. Catal. B 2021, 284, 119745. [Google Scholar] [CrossRef]
















| Photoelectrocatalyst | Modification Strategy | Rate (µmol h−1 g−1) | Incident Light (nm) | Ref. |
|---|---|---|---|---|
| BiVO4/FTO | morphological control | 150 | UV-Vis | [22] |
| ZnO/Ag | surface modification | 10 | UV-Vis | [29] |
| W-TiO2 NTs | doping | 24.97 | UV-Vis | [36] |
| TiO2 nanotube arrays | morphological control | 97 | UV-Vis | [37] |
| Nb-TiO2/g-C3N4 | doping/heterojunction | 43.26 | >400 | [38] |
| Fe3+-TiO2 | doping | 12.5 | >400 | [41] |
| TiO2/WO3/FTO | heterojunction | 210 | UV-Vis | [43] |
| ITO/Cu2O/TiO2 | heterojunction | 12.15 | UV-Vis | [48] |
| TiO2/Cu2O | protective layer/Ti3+ | 0.068 | >420 | [50] |
| N-BiVO4 | doping | 3.7 | UV-Vis | [57] |
| Bi2S3/BiVO4 | heterojunction | 33.4 | UV-Vis | [59] |
| MoS2/Cu2O | heterojunction | 12.3 | UV-Vis | [64] |
| TiO2-1 wt% Au@TiO2/Al2O3/Cu2O | heterojunction/surface modification | 147 | UV-Vis | [65] |
| MoS2/Cu-CdS | doping/heterojunction | 1115 | UV-Vis | [69] |
| CdS/ZnO | heterojunction | 1008 | >400 | [71] |
| Cd0.5Zn0.5S | morphological control | 14,440 | >420 | [72] |
| Pt/C-ZnIn2S4 | morphological control/surface modification | 1032.2 | >400 | [74] |
| FTO/P-g-C3N4 | doping | 1.27 | >800 | [80] |
| Nd-doped g-C3N4/BiOI | doping/heterojunction | 288 | >420 | [88] |
| g-C3N4/reduction graphene oxide/nickel foam | heterojunction/morphological control | 6000 | >420 | [92] |
| Pt-TiO2/C | morphological control/surface modification | 300 | UV-enhanced light | [109] |
| Ni-Mo-S/reduced titania nanorods | surface modification | 40 | UV-Vis | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Zhang, Y.; Zhang, X.; Yang, H.; Xie, R.; Zhang, S.; Lv, Y.; Xiong, L. Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production. Molecules 2024, 29, 289. https://doi.org/10.3390/molecules29020289
Fu W, Zhang Y, Zhang X, Yang H, Xie R, Zhang S, Lv Y, Xiong L. Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production. Molecules. 2024; 29(2):289. https://doi.org/10.3390/molecules29020289
Chicago/Turabian StyleFu, Weisong, Yan Zhang, Xi Zhang, Hui Yang, Ruihao Xie, Shaoan Zhang, Yang Lv, and Liangbin Xiong. 2024. "Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production" Molecules 29, no. 2: 289. https://doi.org/10.3390/molecules29020289
APA StyleFu, W., Zhang, Y., Zhang, X., Yang, H., Xie, R., Zhang, S., Lv, Y., & Xiong, L. (2024). Progress in Promising Semiconductor Materials for Efficient Photoelectrocatalytic Hydrogen Production. Molecules, 29(2), 289. https://doi.org/10.3390/molecules29020289







