The Use of Chitin for the Removal of Nitrates and Orthophosphates from Greenhouse Wastewater
Abstract
1. Introduction
2. Results and Discussion
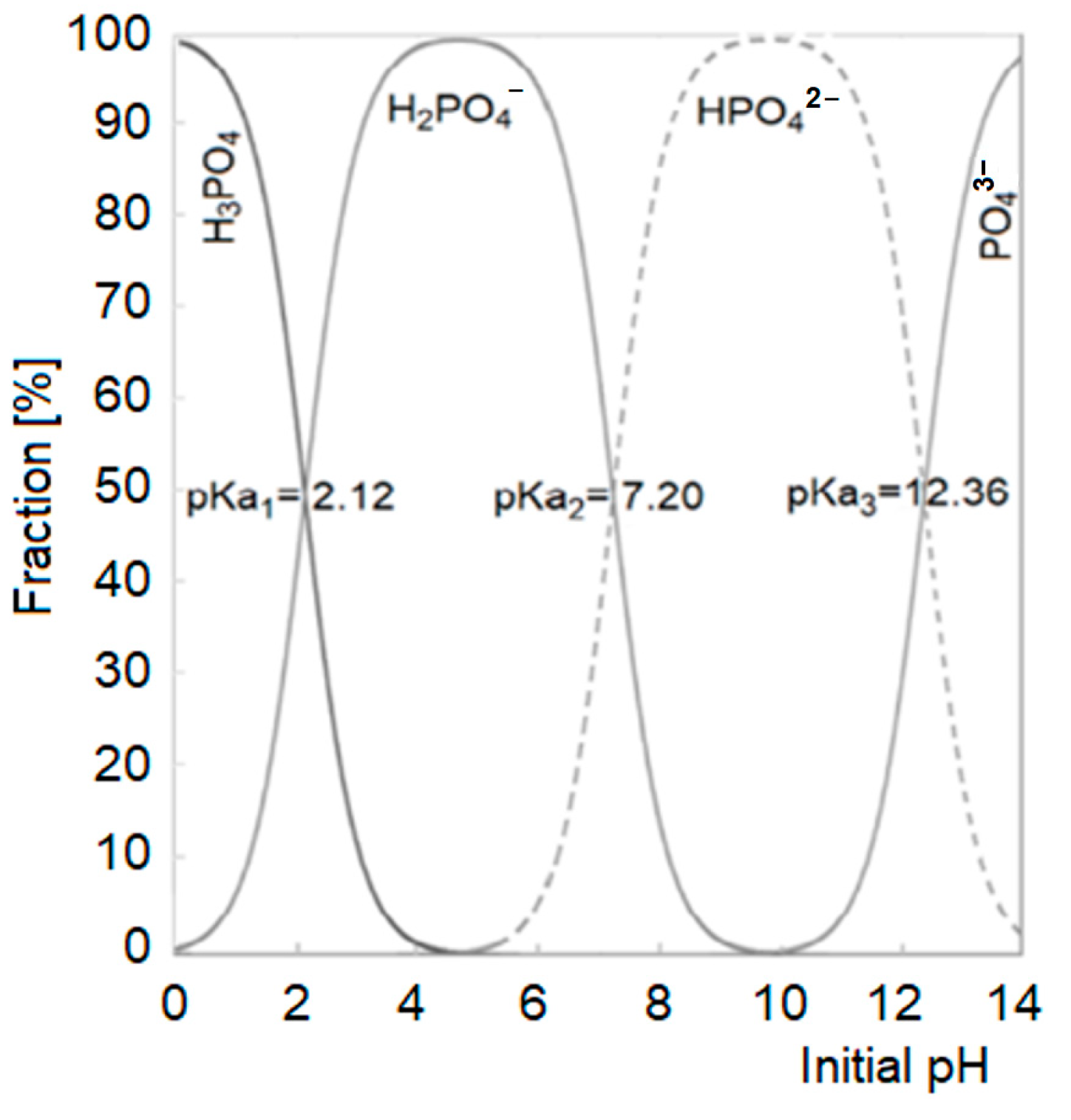
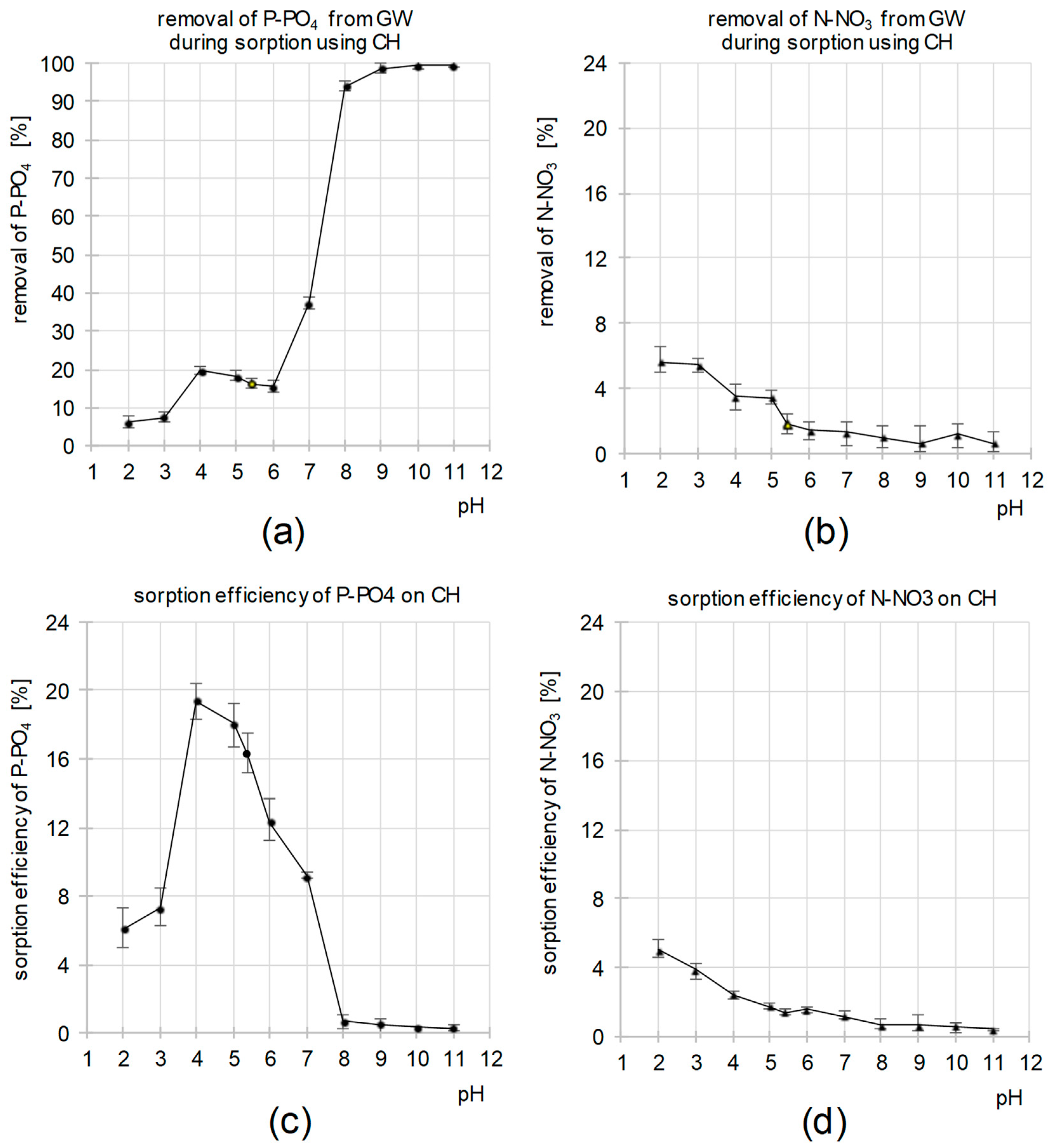
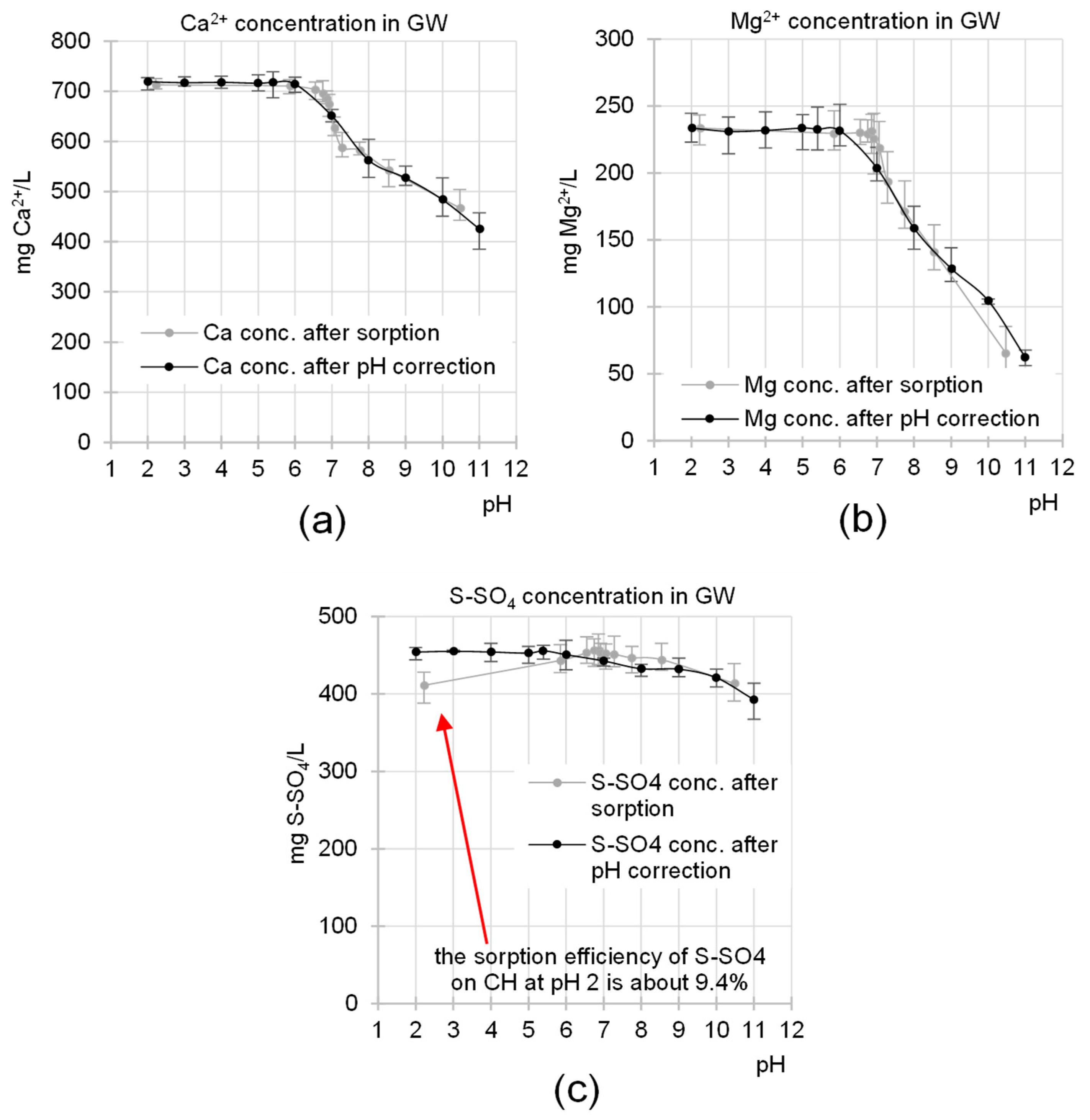
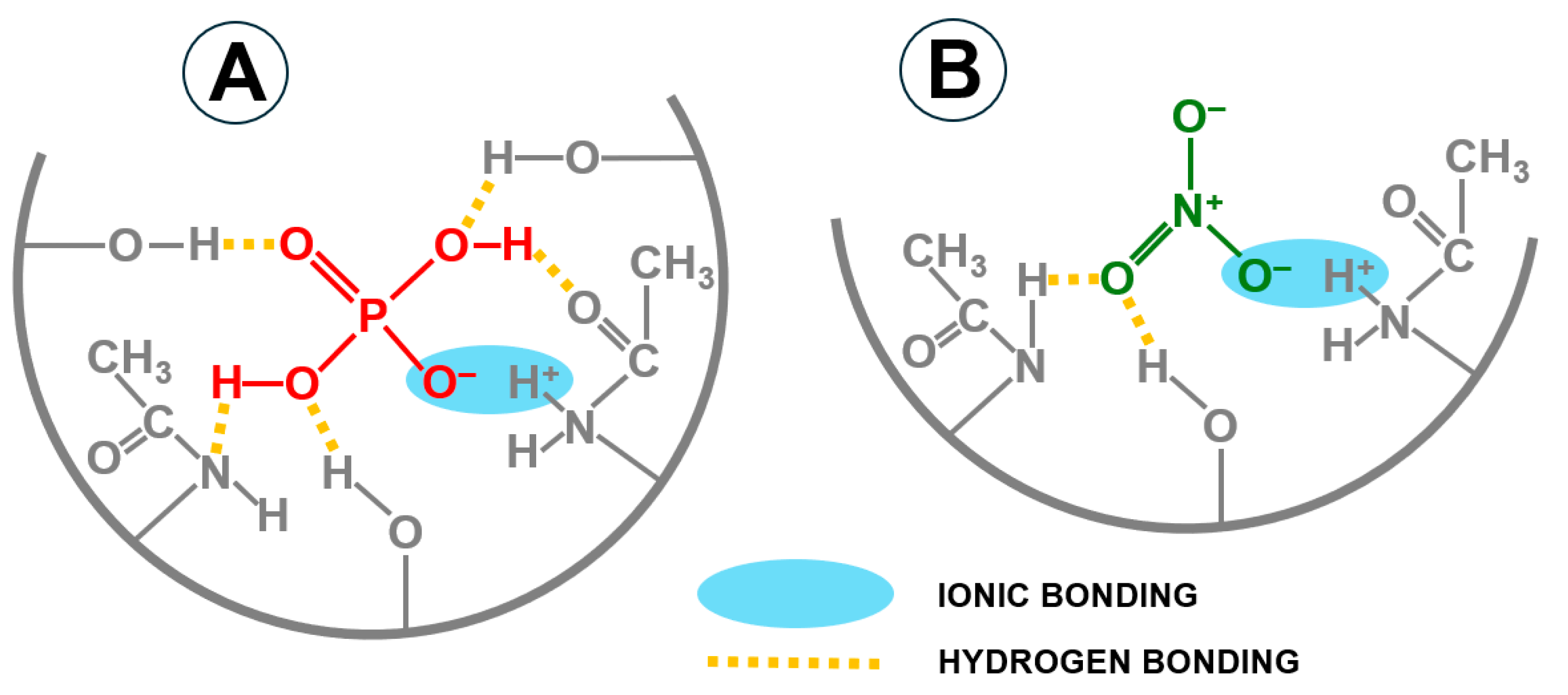
2.1. The Influence of pH on GW Composition and the Efficiency of Nutrient Sorption on CH
- -
- In the pH range of 5.4–7.2 (most orthophosphates are in the form of the H2PO4− anion)
- 2 H2PO4− + Ca2+ → Ca(H2PO4)2 (solubility in water ~20 g/L at 20 °C);
- 2 H2PO4− + Mg2+ → Mg(H2PO4)2 (solubility in water ~200 g/L at 20 °C).
- -
- In the pH range of 7.2–11.0 (most orthophosphates are in the form of the HPO42− anion)
- HPO42− + Ca2+ → CaHPO4↓ (solubility in water ~0.1 g/L at 20 °C);
- HPO42− + Mg2+ → MgHPO4↓ (solubility in water ~0.25 g/L at 20 °C).
2.2. Kinetics of the Sorption of Nutrients onto CH
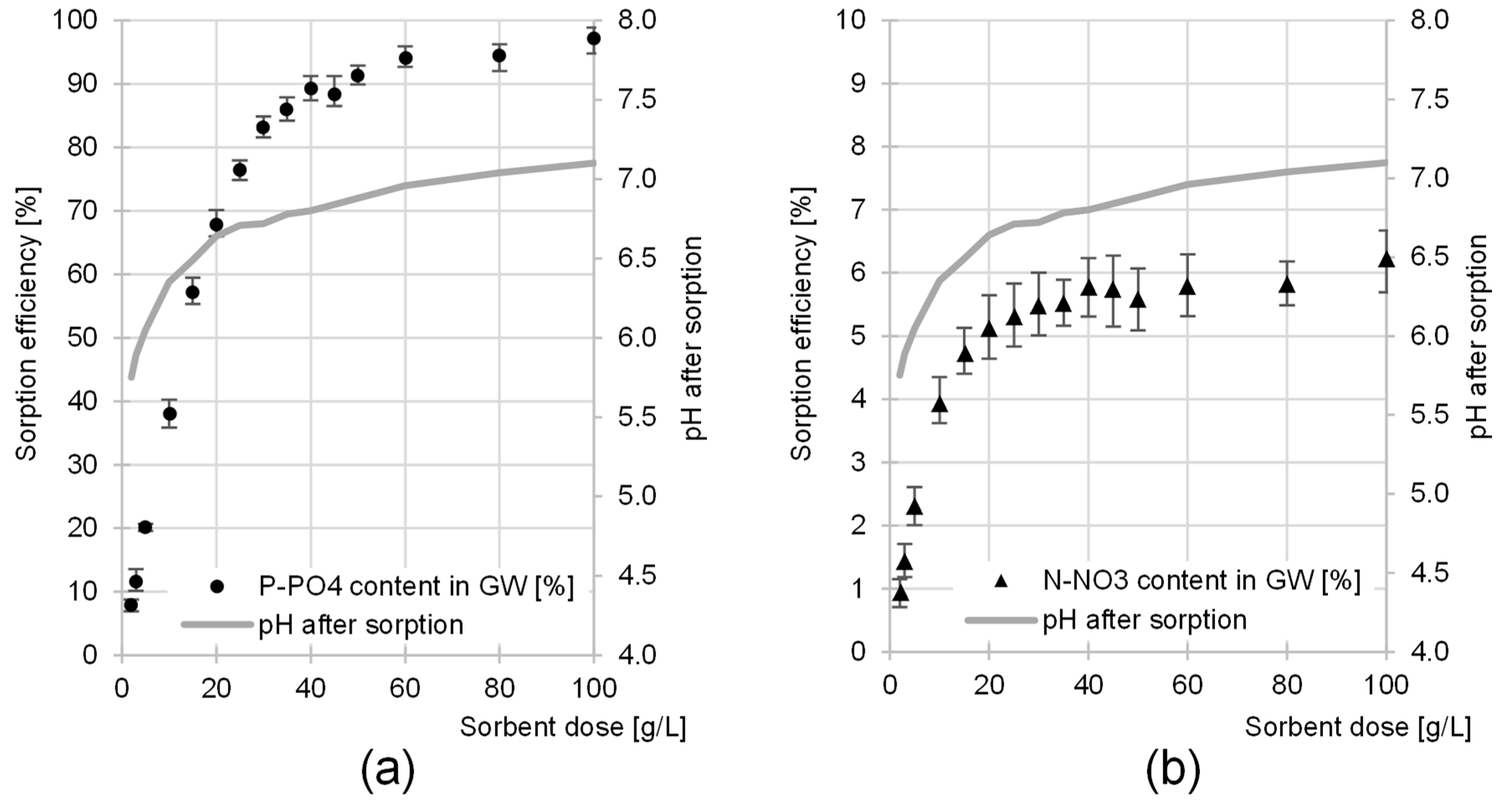
2.3. Influence of Sorbent Dose on the Efficiency of P-PO4 and N-NO3 Sorption from GW
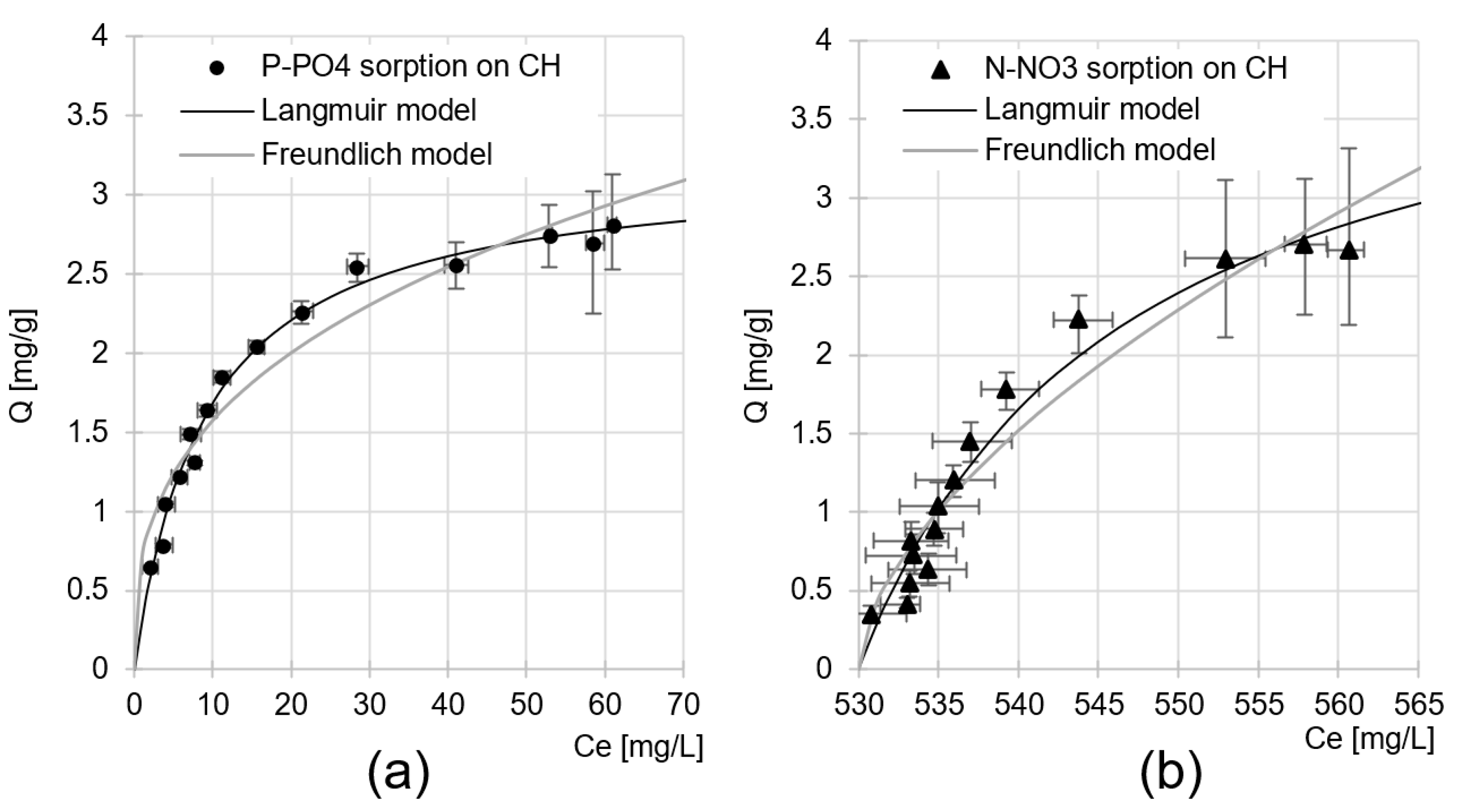
Maximum Nutrient Sorption Capacity of CH
3. Materials
3.1. Chitin Flakes (CH)
3.2. Greenhouse Wastewater (GW)
3.3. Chemical Reagents
- Sodium hydroxide (NaOH) ≥ 98.0% (powder)—used to correct the pH of wastewater;
- Hydrochloric acid (HCl) 37.0%—used to correct the pH of wastewater;
- Buffer solutions for calibrating the pH meter (pH 4 ± 0.05/pH 7 ± 0.05/pH 10 ± 0.05).
3.4. Laboratory Equipment
- EX2202 precision balance (OHAUS, Nänikon, Switzerland)—for preparing solutions and weighing the sorbent;
- HI 221 pH-meter (Hanna Instruments, Woonsocket, RI, USA)—for the measurement and correction of the solutions’ pH;
- Laboratory shaker SK-71 (JEIO TECH, Daejeon, Republic of Korea)—(for the process of sorption);
- Multi-Channel Stirrer MS-53M (JEIO TECH, Daejeon, Republic of Korea)—for the process of sorption.
4. Methodology
4.1. Research on pH Correction Influence on GW Composition
4.2. Research on pH Influence on the Efficiency of Nutrient Sorption from GW
4.3. Determination of the pHPZC of CH
4.4. Research on the Kinetics of Nutrient Sorption from GW
4.5. Research on CH Dose Influence on the Sorption Efficiency of P-PO4 and N-NO3 from GW
Notes to Section 4.1, Section 4.2, Section 4.3, Section 4.4 and Section 4.5
- The preparation of the solutions and the weighing of the sorbents in laboratory flasks or beakers were carried out using a precision balance with an accuracy of 0.001 g.
- Beakers with GW were weighed before and after pH correction in order to take into account the change in volume of the GW sample caused by the addition of acidifying/alkalizing agents later in the calculations.
- The mixing parameters, set on a shaker or a multi-station mixer, ensured the distribution of CH throughout the GW volume.
- Nutrient concentrations in the GW were determined according to Polish standards: for P-PO4—PN-EN 6878:2006 and for N-NO3—PN-73/C-04576/06.
- The concentrations of other substances (sulfates, calcium and magnesium ions) were determined using HACH cell tests (HACH LANGE Sp. z o. o., Wrocław, Poland).
- When selecting the optimum sorption pH value, the effectiveness of phosphorus binding was taken into account due to the primary economic importance of this element.
- All analytical series were carried out in triplicate.
- During analyses, the temperature in the laboratory was kept constant at 20 °C.
4.6. Computation Methods
- QS—mass of sorbed nutrient [mg/g];
- C0—initial concentration of nutrient in GW [mg/L];
- CS—concentration of nutrient after sorption [mg/L];
- V—volume of GW [L];
- m—mass of CH [g].
- Q—instantaneous value of sorbed nutrient [mg/g];
- qe—the amount of nutrient sorbed at the equilibrium state [mg/g];
- t—time of sorption [min];
- k1—pseudo-first-order adsorption rate constant [1/min];
- k2—pseudo-second-order adsorption rate constant [g/(mg·min)];
- kid—intraparticle diffusion model adsorption rate constant [mg/(g·min0.5)].
- QS—mass of sorbed nutrient [mg/g];
- Qmax—maximum sorption capacity in Langmuir equation [mg/g];
- KC—constant in Langmuir equation [L/mg];
- K—the equilibrium sorption constant in the Freundlich model;
- n—Freundlich equilibrium constant;
- C—concentration of the dye remaining in the solution [mg/L].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koukounaras, A. Advanced Greenhouse Horticulture: New Technologies and Cultivation Practices. Horticulturae 2020, 7, 1. [Google Scholar] [CrossRef]
- Geng, Y.; Bashir, M.A.; Zhao, Y.; Luo, J.; Liu, X.; Li, F.; Wang, H.; Raza, Q.U.A.; Rehim, A.; Zhang, X.; et al. Long-Term Fertilizer Reduction in Greenhouse Tomato-Cucumber Rotation System to Assess N Utilization, Leaching, and Cost Efficiency. Sustainability 2022, 14, 4647. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, Y.; Wang, J.; Yang, W.; Wang, Y. Reducing Chemical Fertilizer Application in Greenhouse Vegetable Cultivation under Different Residual Levels of Nutrient. Agriculture 2023, 13, 1174. [Google Scholar] [CrossRef]
- Jamal-Uddin, A.T.; Matsuura, T.; Al-Daoud, F.; Zytner, R.G. Treatment and Recycle of Greenhouse Nutrient Feed Water Applying Hydrochar and Activated Carbon Followed by Reverse Osmosis. Water 2022, 14, 3573. [Google Scholar] [CrossRef]
- White, S.A.; Owen, J.S.; Majsztrik, J.C.; Oki, L.R.; Fisher, P.R.; Hall, C.R.; Lea-Cox, J.D.; Thomas Fernandez, R. Greenhouse and Nursery Water Management Characterization and Research Priorities in the USA. Water 2019, 11, 2338. [Google Scholar] [CrossRef]
- Castañeda-Escobar, L.A.; Hernández-Orduña, M.G.; Lara-Capistrán, L.; Pulido-Herrera, V. Analysis of Disinfection in Greenhouse Soils with Medium-Temperature Steam Produced by Solar Energy. Appl. Sci. 2023, 13, 11055. [Google Scholar] [CrossRef]
- Mielcarek, A.; Kłobukowska, K.; Rodziewicz, J.; Janczukowicz, W.; Bryszewski, K.Ł. Water Nutrient Management in Soilless Plant Cultivation versus Sustainability. Sustainability 2023, 16, 152. [Google Scholar] [CrossRef]
- Álvarez-Gil, M.; Blanco-Vieites, M.; Suárez-Montes, D.; Casado-Bañares, V.; Delgado-Ramallo, J.F.; Rodríguez, E. Revolutionizing Agriculture: Leveraging Hydroponic Greenhouse Wastewater for Sustainable Microalgae-Based Biostimulant Production. Sustainability 2023, 15, 14398. [Google Scholar] [CrossRef]
- Mekonnen, D.T.; Alemayehu, E.; Lennartz, B. Removal of Phosphate Ions from Aqueous Solutions by Adsorption onto Leftover Coal. Water 2020, 12, 1381. [Google Scholar] [CrossRef]
- Christensen, M.L.; Cvitanich, C.; Quist-Jensen, C.A.; Thau, M.; Malmgren-Hansen, B. Precipitation and Recovery of Phosphorus from the Wastewater Hydrolysis Tank. Sci. Total Environ. 2022, 813, 151875. [Google Scholar] [CrossRef]
- Mohammadi, R.; Ramasamy, D.L.; Sillanpää, M. Enhancement of Nitrate Removal and Recovery from Municipal Wastewater through Single- and Multi-Batch Electrodialysis: Process Optimisation and Energy Consumption. Desalination 2021, 498, 114726. [Google Scholar] [CrossRef]
- Scholes, R.C.; Vega, M.A.; Sharp, J.O.; Sedlak, D.L. Nitrate Removal from Reverse Osmosis Concentrate in Pilot-Scale Open-Water Unit Process Wetlands. Environ. Sci. 2021, 7, 650–661. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Mckay, G.; Kochkodan, V.; Ali Atieh, M.; Al-Ansari, T. A State of the Art Review on Phosphate Removal from Water by Biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Zhang, P.; Shen, J.; Wang, S.; Li, Y. Development of Iron-Based Biochar for Enhancing Nitrate Adsorption: Effects of Specific Surface Area, Electrostatic Force, and Functional Groups. Sci. Total Environ. 2023, 856, 159037. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Hameed, B.H.; Khan, M.A. Recent Advances on Activated Carbon-Based Materials for Nitrate Adsorption: A Review. J. Anal. Appl. Pyrolysis 2023, 169, 105856. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Mckay, G.; Atieh, M.A.; Al-Ansari, T. Review of Phosphate Removal from Water by Carbonaceous Sorbents. J. Environ. Manag. 2021, 287, 112245. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Li, Y.; Arai, Y. Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions. Soil. Syst. 2020, 4, 36. [Google Scholar] [CrossRef]
- Duan, S.; Tong, T.; Zheng, S.; Zhang, X.; Li, S. Achieving Low-Cost, Highly Selective Nitrate Removal with Standard Anion Exchange Resin by Tuning Recycled Brine Composition. Water Res. 2020, 173, 115571. [Google Scholar] [CrossRef]
- Dey, S.; Haripavan, N.; Basha, S.R.; Babu, G.V. Removal of Ammonia and Nitrates from Contaminated Water by Using Solid Waste Bio-Adsorbents. Curr. Res. Chem. Biol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Auteri, N.; Scalenghe, R.; Saiano, F. Phosphorus Recovery from Agricultural Waste via Cactus Pear Biomass. Heliyon 2023, 9, e19996. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Omer, A.M.; El-Aqapa, H.G.; Gaber, N.M.; Attia, N.F.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Abd El-Monaem, E.M. Chitosan Based Adsorbents for the Removal of Phosphate and Nitrate: A Critical Review. Carbohydr. Polym. 2021, 274, 118671. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bui, H.M.; Ward, H.; Dau, T.; City, M.; Duong Province, B. Adsorption of Nitrate and Phosphate in an Aqueous Solution on Composites of PVA and Chitosan Prepared from a Somanniathelphusa Sinensis Shell. Water Supply 2021, 21, 765–779. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Kuczajowska-Zadrożna, M.; Mielcarek, A. The Use of Cross-Linked Chitosan Beads for Nutrients (Nitrate and Orthophosphate) Removal from a Mixture of P-PO4, N-NO2 and N-NO3. Int. J. Biol. Macromol. 2017, 104, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Foley, S.R.; Wilson, L.D. An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media. Molecules 2022, 27, 978. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Mielcarek, A.; Janczukowicz, W.; Rodziewicz, J.; Majkowska-Gadomska, J.; Chojnowska, M. Hydrogel Chitosan Sorbent Application for Nutrient Removal from Soilless Plant Cultivation Wastewater. Environ. Sci. Pollut. Res. 2018, 25, 18484–18497. [Google Scholar] [CrossRef]
- Klimiuk, E.; Gusiatin, Z.; Kabardo, K. The Effectiveness of Surfactants Adsorption onto Chitin and Dye-Modified Chitin. Pol. J. Environ. Stud. 2006, 15, 95–104. [Google Scholar]
- Chen, R.F.; Liu, T.; Rong, H.W.; Zhong, H.T.; Wei, C.H. Effect of Organic Substances on Nutrients Recovery by Struvite Electrochemical Precipitation from Synthetic Anaerobically Treated Swine Wastewater. Membranes 2021, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, P.; Filipkowska, U.; Jóźwiak, T.; Kuczajowska-Zadrożna, M. Phosphate Removal from Aqueous Solutions by Chitin and Chitosan in Flakes. Prog. Chem. Appl. Chitin Deriv. 2016, 21, 192–202. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Mielcarek, A. Sorption of Nutrients (Orthophosphate, Nitrate III and V) in an Equimolar Mixture of P–PO4, N–NO2 and N–NO3 Using Chitosan. Arab. J. Chem. 2019, 12, 4104–4117. [Google Scholar] [CrossRef]
- Filipkowska, U.; Jóźwiak, T.; Szymczyk, P. Application of Cross-Linked Chitosan for Phosphate Removal from Aqueous Solutions. Prog. Chem. Appl. Chitin Deriv. 2014, 19, 5–14. [Google Scholar] [CrossRef]
- El Ouardi, M.; Qourzal, S.; Alahiane, S.; Assabbane, A.; Douch, J. Effective Removal of Nitrates Ions from Aqueous Solution Using New Clay as Potential Low-Cost Adsorbent. J. Encapsulation Adsorpt. Sci. 2015, 5, 178–190. [Google Scholar] [CrossRef]
- Koné, H.; Assémian, A.S.; Tiho, T.; Adouby, K.; Yao, K.B.; Drogui, P. Borassus Aethiopum Activated Carbon Prepared for Nitrate Ions Removal. J. Appl. Water Eng. Res. 2022, 10, 64–77. [Google Scholar] [CrossRef]
- Lekene, R.B.N.; Kouotou, D.; Ankoro, N.O.; Kouoh, A.P.M.S.; Ndi, J.N.; Ketcha, J.M. Development and Tailoring of Amino-Functionalized Activated Carbon Based Cucumerupsi Manni Naudin Seed Shells for the Removal of Nitrate Ions from Aqueous Solution. J. Saudi Chem. Soc. 2021, 25, 101316. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, Á.; Gonzalez-Delgado, Á.D. Removal of Nitrate Ions Using Thermally and Chemically Modified Bioadsorbents. Appl. Sci. 2021, 11, 8455. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, L.; Chang, N.; Liu, J.; Duan, C.; Zhou, Q.; Li, X.; Wang, X. Removal of Phosphate from Water by Activated Carbon Fiber Loaded with Lanthanum Oxide. J. Hazard. Mater. 2011, 190, 848–855. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Ji, M.; Choi, Y.H.; Jung, W.; Lee, S.H.; Kim, S.J.; Lee, G.; Suk, H.; Kim, H.S.; Min, B.; et al. Removal of Nitrate from Water by Adsorption onto Zinc Chloride Treated Activated Carbon. Sep. Sci. Technol. 2008, 43, 886–907. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Mielcarek, A. Application of Cross-Linked Chitosan for Nitrate Nitrogen (V) Removal from Aqueous Solutions. Prog. Chem. Appl. Chitin Deriv. 2014, 19, 41–52. [Google Scholar] [CrossRef][Green Version]
- Zhou, H.; Tan, Y.; Gao, W.; Zhang, Y.; Yang, Y. Selective Nitrate Removal from Aqueous Solutions by a Hydrotalcite-like Absorbent FeMgMn-LDH. Sci. Rep. 2020, 10, 16126. [Google Scholar] [CrossRef] [PubMed]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef]
- Banu, H.T.; Meenakshi, S. One Pot Synthesis of Chitosan Grafted Quaternized Resin for the Removal of Nitrate and Phosphate from Aqueous Solution. Int. J. Biol. Macromol. 2017, 104, 1517–1527. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.Y.; Yue, Q.Y.; Zhong, Q.Q. Preparation of Agricultural By-Product Based Anion Exchanger and Its Utilization for Nitrate and Phosphate Removal. Bioresour. Technol. 2010, 101, 8558–8564. [Google Scholar] [CrossRef]
- Saad, R.; Belkacemi, K.; Hamoudi, S. Adsorption of Phosphate and Nitrate Anions on Ammonium-Functionalized MCM-48: Effects of Experimental Conditions. J. Colloid. Interface Sci. 2007, 311, 375–381. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Mereta, S.T.; Feyisa, F.F. Simultaneous Removal of Nitrate and Phosphate from Wastewater Using Solid Waste from Factory. Appl. Water Sci. 2019, 9, 28. [Google Scholar] [CrossRef]
- Ragheb, S.M. Phosphate Removal from Aqueous Solution Using Slag and Fly Ash. HBRC J. 2013, 9, 270–275. [Google Scholar] [CrossRef]
- Orlando, U.S.; Baes, A.U.; Nishijima, W.; Okada, M. Preparation of Agricultural Residue Anion Exchangers and Its Nitrate Maximum Adsorption Capacity. Chemosphere 2002, 48, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, T.; Filipkowska, U.; Bakuła, T.; Bralewska-Piotrowicz, B.; Karczmarczyk, K.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Szyryńska, N.; Lewczuk, B. The Use of Chitin from the Molts of Mealworm (Tenebrio Molitor) for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Materials 2023, 16, 545. [Google Scholar] [CrossRef] [PubMed]


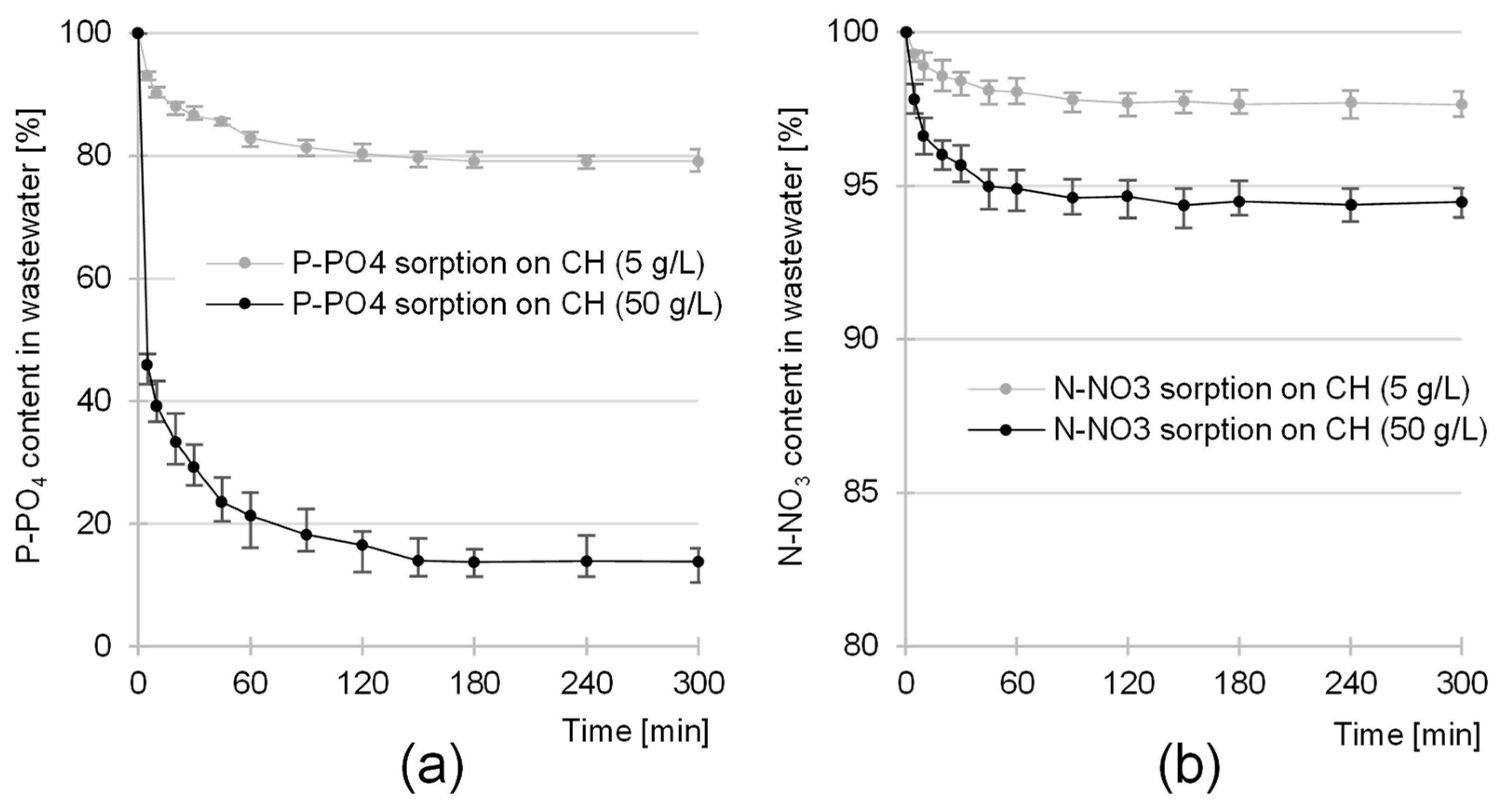
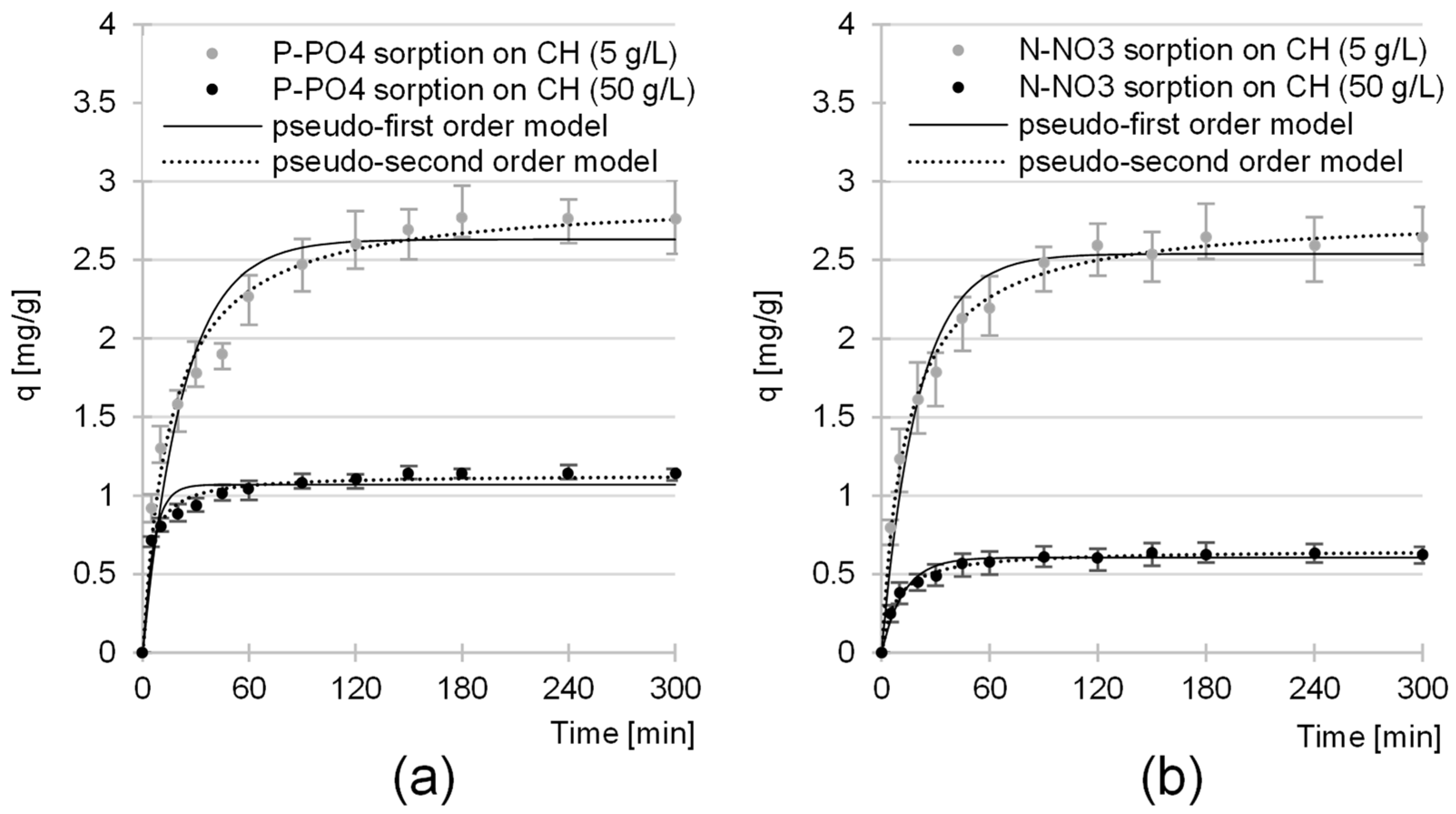
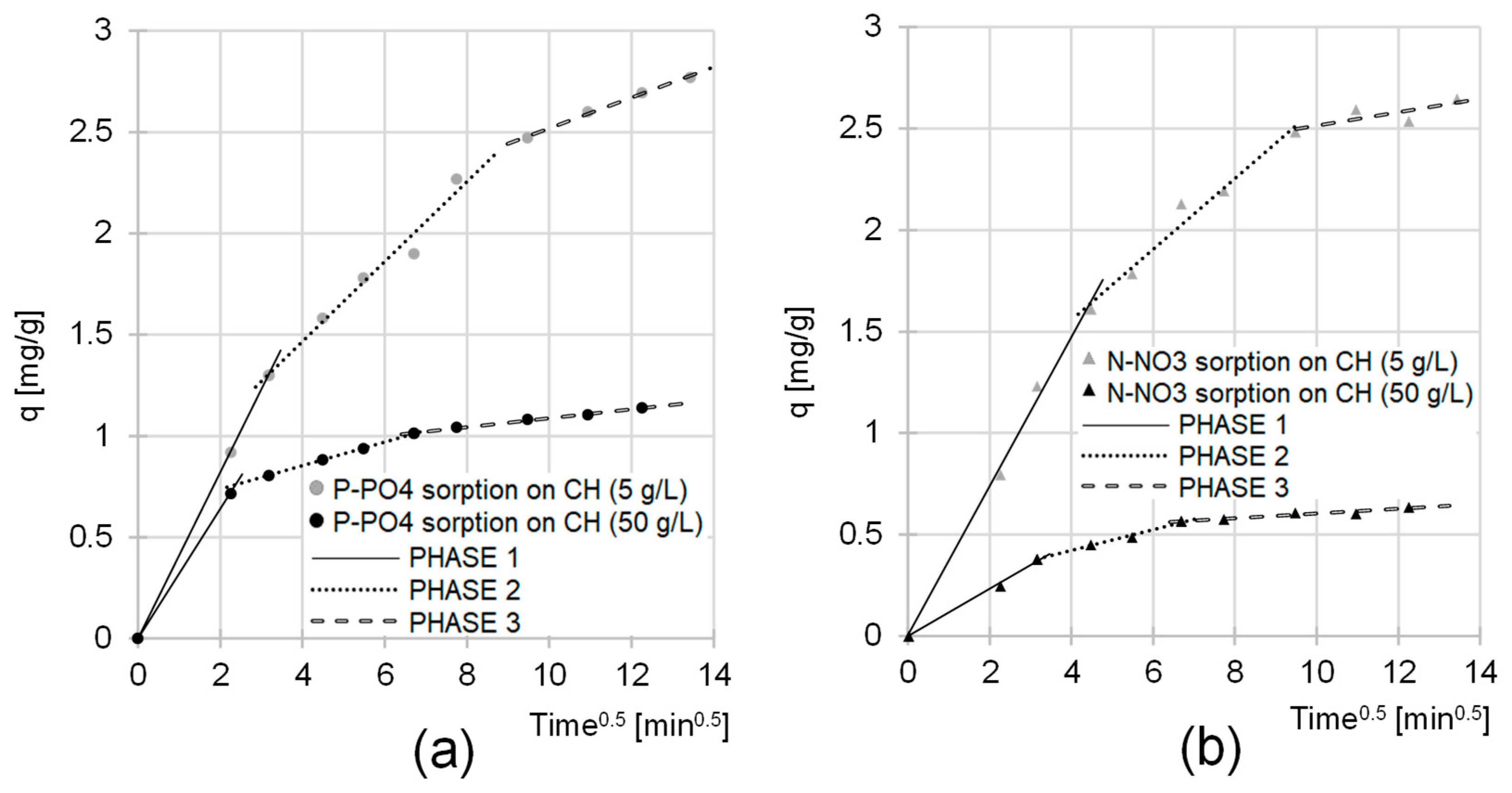
| Sorbate | CH Dose | Pseudo-First Order Model | Pseudo-Second Order Model | Exp. Data | Equil. Time | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | qe, cal. | R2 | k2 | qe, cal. | R2 | qe, exp. | |||
| [g/L] | [1/min] | [mg/g] | - | [g/mg·min] | [mg/g] | - | [mg/g] | [min] | |
| P-PO4 | 5 | 0.0437 | 2.63 | 0.9370 | 0.0222 | 2.90 | 0.9799 | 2.77 | 180 |
| 50 | 0.1651 | 1.07 | 0.9326 | 0.2297 | 1.13 | 0.9832 | 1.14 | 150 | |
| N-NO3 | 5 | 0.0494 | 2.54 | 0.9707 | 0.0256 | 2.79 | 0.9943 | 2.64 | 180 |
| 50 | 0.0809 | 0.61 | 0.9698 | 0.1892 | 0.65 | 0.9952 | 0.63 | 150 | |
| Sorbate | CH Dose | Phase 1 | Phase 2 | Phase 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kd1 | Dur. Time | R2 | kd2 | Dur. Time | R2 | kd3 | Dur. Time | R2 | ||
| [g/L] | * | [min] | - | * | [min] | - | * | [min] | - | |
| P-PO4 | 5 | 0.4112 | 10 | 0.(9) | 0.1973 | 50 | 0.9713 | 0.0758 | 120 | 0.9955 |
| 50 | 0.3200 | 5 | 0.(9) | 0.0583 | 40 | 0.9995 | 0.0221 | 105 | 0.9925 | |
| N-NO3 | 5 | 0.3664 | 20 | 0.9954 | 0.1743 | 70 | 0.9732 | 0.0329 | 90 | 0.8238 |
| 50 | 0.1105 | 10 | 0.(9) | 0.0516 | 35 | 0.9915 | 0.0115 | 105 | 0.9077 | |
| Sorbate | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qmax | KC | R2 | k | n | R2 | |
| [mg/g] | [L/mg] | - | - | - | - | |
| P-PO4 | 3.20 | 0.112 | 0.9870 | 0.707 | 0.347 | 0.9359 |
| N-NO3 | 3.04 | 0.058 | 0.9627 | 0.875 | 0.352 | 0.9523 |
| Type of the Sorbent | Type of Solution | Sorbate | Qmax (mg/g) | pH | Time [h] | Temp. | Source |
|---|---|---|---|---|---|---|---|
| Chitosan hydrogel beads modified with epichlorohydrin | deionized water + sodium phosphate | P-PO4 | 139.4 | 3 | 2 | 22 | [30] |
| deionized water + sodium nitrate | N-NO3 | 38.47 | 3 | 2 | 22 | [37] | |
| equimolar mixture of nutrients based on deionized water | mixture P-PO4; N-NO2; N-NO3 | 62.01 (total N + P) 38.22; 13.09; 10.70 | 3 | 2 | 22 | [23] | |
| greenhouse wastewater (60.8 mg P-PO4/L; 621 mg N-NO3/L) | mixture P-PO4; N-NO3 | 60.9 (total N + P) 55.9; 5.0 | 2 | 3 | 22 | [25] | |
| Biochar-MgAl LDH Nanocomposites | synthetic wastewater (50 mg P-PO4/L; 50 mg N-NO3/L) | mixture P-PO4; N-NO3 | 102.95 (total N + P) 73.69; 28.26 | 6 | 24 | 25 | [39] |
| Chitosan resin | synthetic wastewater (100 mg P-PO4/L; 100 mg N-NO3/L) | mixture P-PO4; N-NO3 | 85.93 (total N + P) 37.08; 48.85 | 3 | 1 | 30 | [40] |
| Chitosan hydrogel beads (unmodified) | deionized water + sodium phosphate | P-PO4 | 44.40 | 4 | 2 | 22 | [30] |
| deionized water + sodium nitrate | N-NO3 | 12.71 | 2 | 4 | 22 | [37] | |
| equimolar mixture of nutrients based on deionized water | mixture P-PO4; N-NO2; N-NO3 | 25.84 (total N + P) 15.72; 5.22; 4.90; | 4 | 1 | 22 | [29] | |
| greenhouse wastewater (60.8 mg P-PO4/L; 621 mg N-NO3/L) | mixture P-PO4; N-NO3; | 24.99 (total N + P) 5.3419.65 | 4 | 3 | 22 | [25] | |
| Aminated wheat straw | synthetic wastewater (50 mg P-PO4/L; 60 mg N-NO3/L) | mixture P-PO4; N-NO3 | 26.83 (total N + P) 14.9 111.92 | 3 | 4 | 20 | [41] |
| Aminated silica MCM-48 | synthetic wastewater (700 mg P-PO4/L; 700 mg N-NO3/L) | mixture P-PO4; N-NO3 | 21.38 (total N + P) 13.52 7.86 | 4 | 25 | [42] | |
| Waste residue from alum manufacturing (quartz, kaolin, aluminum Hydroxide) | synthetic wastewater (19.3 mg P-PO4/L; 5.1 mg N-NO3/L) | mixture P-PO4; N-NO3 | 13.17 (total N + P) 13.10; 0.07 | 4–8 | 1.5 | 25 | [43] |
| Chitosan flakes | deionized water + potassium phosphate | P-PO4 | 6.64 | 4 | 0.66 | 22 | [28] |
| Chitin flakes (CH) | greenhouse wastewater (66.2 mg P-PO4/L; 566 mg N-NO3/L) | mixture P-PO4; N-NO3 | 6.24 (total N + P) 3.20; 3.04 | 4 | 3.0 | 20 | This work |
| Slag | industrial wastewater (40 mg P-PO4/L; 32 mg N-NO3/L) | mixture P-PO4; N-NO3; | 3.28 (total N + P) 2.51; 0.77; | 5 | 0.5 | 27 | [44] |
| Fly ash | industrial wastewater (40 mg P-PO4/L; 32 mg N-NO3/L) | mixture P-PO4; N-NO3 | 2.76 (total N + P) 2.53; 0.23; | 7 | 0.5 | 27 | [44] |
| Bagasse (agricultural residues) | mixture of nutrients based on deionized water (38 mg P-PO4/L; 37 mg N-NO2/L; 37 mg N-NO3/L) | mixture P-PO4; N-NO2; N-NO3 | 0.72 (total N + P) 0.16; 0.31; 0.25; | 6.5 | 24 | 30 | [45] |
| Component of GW | P-PO4 [mg/L] | N-NO3 [mg/L] | S-SO4 [mg/L] | Cl− [mg/L] | Ca2+ [mg/L] | Mg2+ [mg/L] | K+ [mg/L] | Hardness [°dH] | pH |
|---|---|---|---|---|---|---|---|---|---|
| Content | 66.2 | 566.0 | 456.0 | 13.7 | 721.0 | 230.0 | 980.6 | 11.3 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, T.; Mielcarek, A.; Filipkowska, U. The Use of Chitin for the Removal of Nitrates and Orthophosphates from Greenhouse Wastewater. Molecules 2024, 29, 1289. https://doi.org/10.3390/molecules29061289
Jóźwiak T, Mielcarek A, Filipkowska U. The Use of Chitin for the Removal of Nitrates and Orthophosphates from Greenhouse Wastewater. Molecules. 2024; 29(6):1289. https://doi.org/10.3390/molecules29061289
Chicago/Turabian StyleJóźwiak, Tomasz, Artur Mielcarek, and Urszula Filipkowska. 2024. "The Use of Chitin for the Removal of Nitrates and Orthophosphates from Greenhouse Wastewater" Molecules 29, no. 6: 1289. https://doi.org/10.3390/molecules29061289
APA StyleJóźwiak, T., Mielcarek, A., & Filipkowska, U. (2024). The Use of Chitin for the Removal of Nitrates and Orthophosphates from Greenhouse Wastewater. Molecules, 29(6), 1289. https://doi.org/10.3390/molecules29061289








