1. Introduction
Red mud (RM) is a bauxite residue produced in the alumina industry. On average, 1–1.5 tons of RM are produced for every ton of alumina produced [
1,
2]. The abandoned RM not only occupies a large area of land but also contains thorium, potassium, and other radioactive elements [
3,
4], which are hazardous solid wastes. In addition, the RM is an alkaline material and the alkali dissolves in the rainwater and contaminates the surface water and groundwater, causing severe environmental pollution [
5]. Therefore, multichannel and massive recycling of RM resources is necessary. RM has a small particle diameter and a pore frame structure. It has a substantially larger pore ratio than typical soil with a large specific surface area. Hematite, goethite, and other minerals can also be found in the RM [
6,
7,
8]. Pretreated RM can adsorb radioactive substances such as Co
2+ and Sr
2+ [
9], heavy metal ions such as Cu
2+ and Pb
2+ [
10], non-metallic hazardous compounds such as PO
43− and As
3+, and some organic contaminants [
11,
12]. It can also be used for wastewater decolorization and clarity [
13,
14] and for calcination in the range of 500 °C–800 °C to produce macroporous iron and carbon-combined calcined and carbon-calcined red mud. The testing results show that RM calcined with iron and carbon additives achieved the highest U adsorption capacity (59.45 mg·g
−1) under proper calcination temperature (i.e., 600 °C), pH of 2.5, and optimum reaction conditions. Lyu, F, et al. [
15] used a hydrothermal approach to modify RM by adsorbing Pb(II) ions in aqueous solutions using colloidal silica and sodium hydroxide under mild conditions. According to the experimental results, the saturated adsorption capacity of the modified RM for Pb(II) ion is ~564.97 mg·g
−1, and the Langmuir constant KL is 0.23, which suggests that the adsorption process is favorable.
Using RM as the primary adsorbent, a spherical RM particle adsorbent with a double-layer structure was prepared in this study. The RM particle adsorbents have a high adsorption capacity and strong mechanical properties for phosphorus in phosphate ore flotation wastewater. The experimental results show that the composition of the raw material has a great influence on the adsorption efficiency of the adsorbent. Many factors affect the performance of the adsorbent, since most parameters of the adsorbent change nonlinearly, traditional methods are inadequate to determine the optimal formulation for adsorbents. The current material formulation optimization methods mainly include the orthogonal experimental methods [
16] and the response surface methodology (RSM) [
17]. For complex systems with multivariable, nonlinear, and highly coupled variables, the traditional data analysis methods cannot satisfy the requirements. In 1986, Romelhart and McClelland proposed the error back-propagation (BP) algorithm. Since the training of the multilayer feedforward network often uses this algorithm, the multilayer feedforward network is often called the BP network. Many scientists [
18,
19,
20] developed removal rate and adsorption capacity models using artificial neural network methods. The results show that these models can predict the removal rate and adsorption capacity of ferricyanide using active RM by changing input variables. Jie [
21] used RSM and artificial neural network (ANN) to study the effects of HCl concentration, temperature, and time on the adsorption of phosphorus by activated bauxite; the results show that the prediction accuracy of the artificial neural network is better than that of RSM. Therefore, the BP neural network can be well applied to multifactor formulation optimization, realizing the transformation from a linear model to a nonlinear model, and can predict the experimental results well.
Research showed that many factors affect the adsorption of RM adsorbent, including the preparation methods, raw material formulation, drug system, operating conditions, and adsorption state. By establishing a multi-input and multi-output comprehensive evaluation model based on a BP neural network model, the sensitive factors of the adsorption process are obtained; this allows a comprehensive evaluation of the adsorption performance. With regard to the parameter optimization for adsorption, it is a dynamic process with many influencing parameters, using a neural network to optimize the parameters can greatly reduce the number of tests, modify the test’s precision, and improve the efficiency.
In the adsorption theory of adsorbents, generally good adsorbents, along with the research of adsorption, have to be considered for desorption, regeneration, and utilization [
22]. Judging the combined effect of the influencing factors on the RM adsorbent is mainly based on the following: the adsorbent must have a large specific surface area, high adsorption capacity and removal amount, and a certain degree of water resistance strength, be capable of regeneration and reuse, cheap, and not pollute the environment. Therefore, in this paper, fly ash (FA) is used to adjust the adsorption capacity, hydrogen peroxide foaming agent is used to adjust the specific surface area, and surfactant is used to adjust the wettability. In contrast, cement and sodium sulfate are used to enhance the mechanical properties of red mud absorbent, so as to make the pulverization rate of red mud in water lower, thus achieving the purpose of reuse. This paper presents an integrated computational intelligence approach based on a hybrid strategy of neural networks and a multiobjective evolutionary algorithm. The BP neural network was used to establish a multi-input and multi-output neural network model to optimize the formulation. The experimental data were trained to obtain a neural network model that reflected the nonlinear mapping relationship between the parameter vector space and target vector space during parameter optimization. The trained neural network model was embedded into the multiobjective evolutionary algorithm, which is used as the individual fitness evaluation function in the evolutionary process. Hence, the multiobjective evolutionary algorithm could be directly applied in product parameter optimization design.
3. Materials and Methods
3.1. Test Materials
RM, fly ash (FA), aluminate cement (A2C), and manganese dioxide (MnO2) are the main raw materials of the particle adsorbents in this study. The moisture content of RM was 30%. After drying at 50 °C for 12 h, the RM was ground by a ball mill and screened using a 0.075 mm sieve. For FA, the burning loss was ≤5%, SiO2 content was ≥30%, and SO3 content was ≤2%; for the aluminate cement, Al2O3 accounted for 50%; CaO, 30%; SiO2, 10%; and Fe2O3, 3%. MnO2 was of industrial grade, with a purity of 97%.
The main reagents used in the test were hydrogen peroxide (H
2O
2), HCl (concentration 36–38%), ICP test standard solution, HNO
3 (concentration 68%), sodium dodecyl benzene sulfonate (SDSB), and sodium silicate (Na
2SiO
4), all of which were analytically pure. Furthermore, commercially pure hydroxypropyl methylcellulose (referred to as HPMC, 2 million mPa·s) and KH-602 silane-coupling agent were used. The adsorption activity site of the adsorbent was improved by adding FA [
29,
30], which was rich in Fe and Al compounds and larger than the surface. Foaming catalysts like H
2O
2 [
31] and MnO
2 [
32] were added to increase the specific surface area of the adsorbent. The pH value, Zeta potential, and pore size distribution of the adsorbent were changed due to HCl [
33] activation. The anionic surfactant and wetting agent sodium dodecylbenzene sulfonate (SDBS) [
34] were used to alter the adsorbent’s wettability and surface activity, while also increasing its adsorption capacity. Combining the reinforcing agent (A2C + HPMC + Na
2SiO
4 + KH-602) enhanced the adsorbent’s strength and immersion loss rate [
35,
36].
For the test, phosphorus-containing wastewater was collected from a phosphate ore dressing plant in Guizhou, China. The recycling water containing flotation tailings was collected; this water contained 1278 mg·L−1 of phosphorous. To prepare RM particle adsorbents, the recycling water containing flotation tailings diluted 10 times was selected as the test water. This choice is based on the measurement range of the ICP instrument (Thermo Fisher Scientific, Waltham, MA, USA, model ICP-7400).
The test equipment included a PQ10 granulating disk, electronic balance (model: BL-2000F), ball mill (XMGB Φ 305 × 3.5), SHBY-40B constant temperature and humidity standard curing box, SHBY-40B water bath thermostatic oscillator, JJ-1 enhanced electric agitator, HY-4 speed-regulating multipurpose oscillator, and self-made dynamic adsorption and desorption integrated equipment.
3.2. Preparation Method of the RM Adsorbent
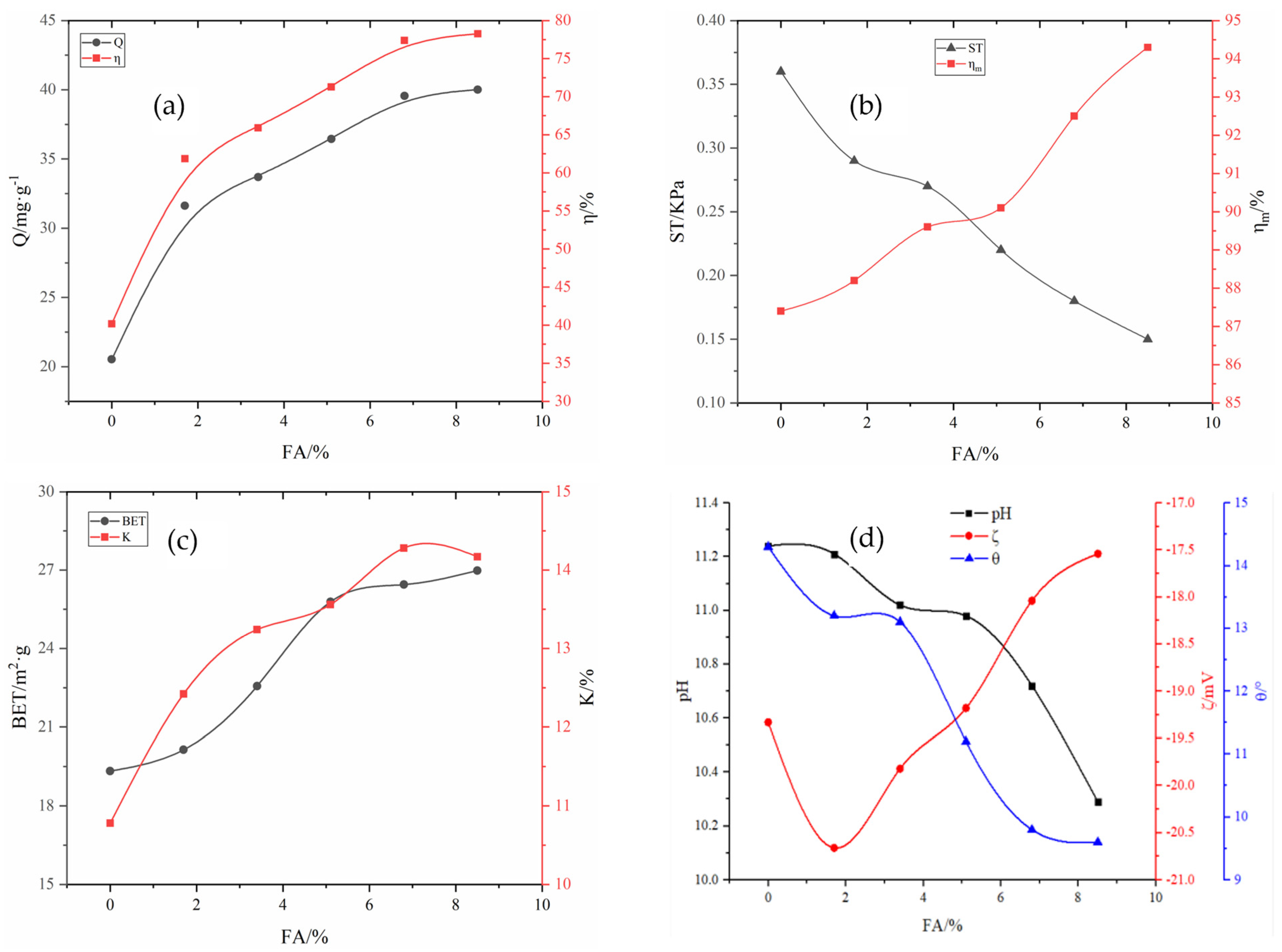
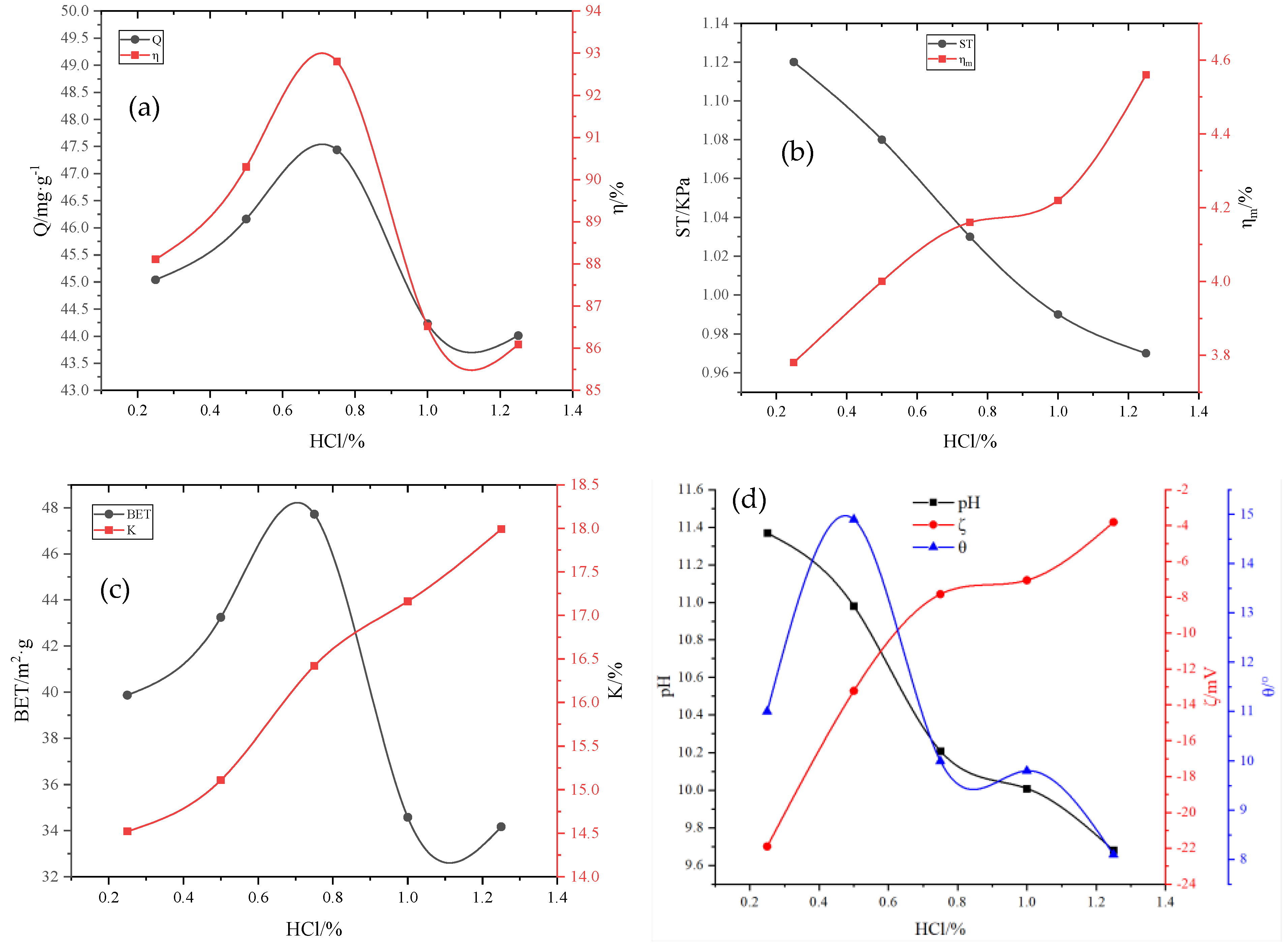
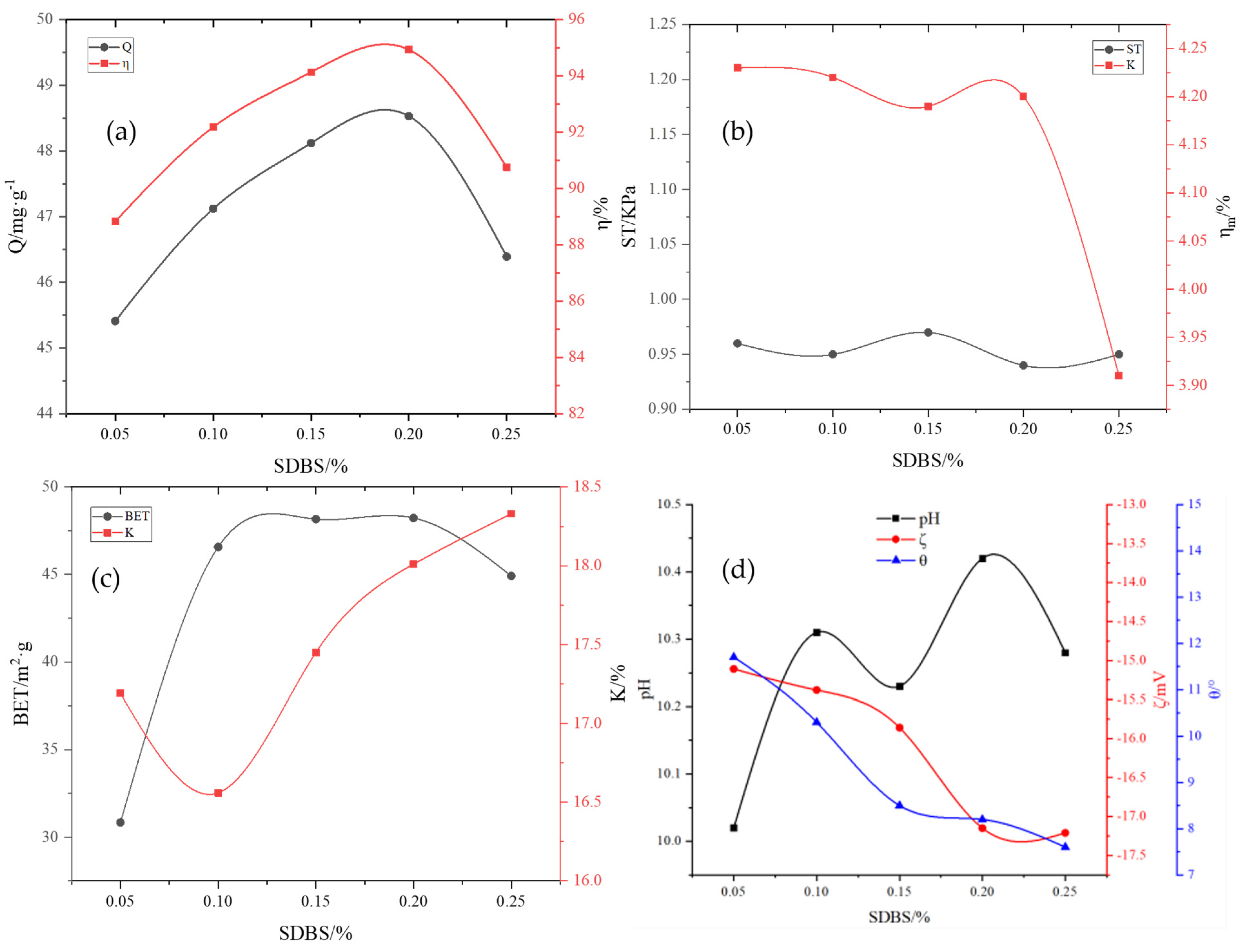
Preparation of the main material for the adsorbent powder: RM and aluminate cement were added to the agitator with a mass ratio of 6:4. After mixing the materials evenly, the mixture was placed in a granulating disk, and deionized water was sprayed into the agitator while rotating until the mixture was formed. Subsequently, a 1 mm sieve was used to remove circular particles. After sieving, the mixture was dried in a 40 °C electric blast-drying oven till the water content was less than 5%, resulting in the formation of nucleating granule support. In the high-speed powder-dispersing machine, the adsorbent powder was prepared by adding RM, 1.7–8.5% FA, 2–10% A2C, 10–20% MnO2, and other powders according to the ratio.
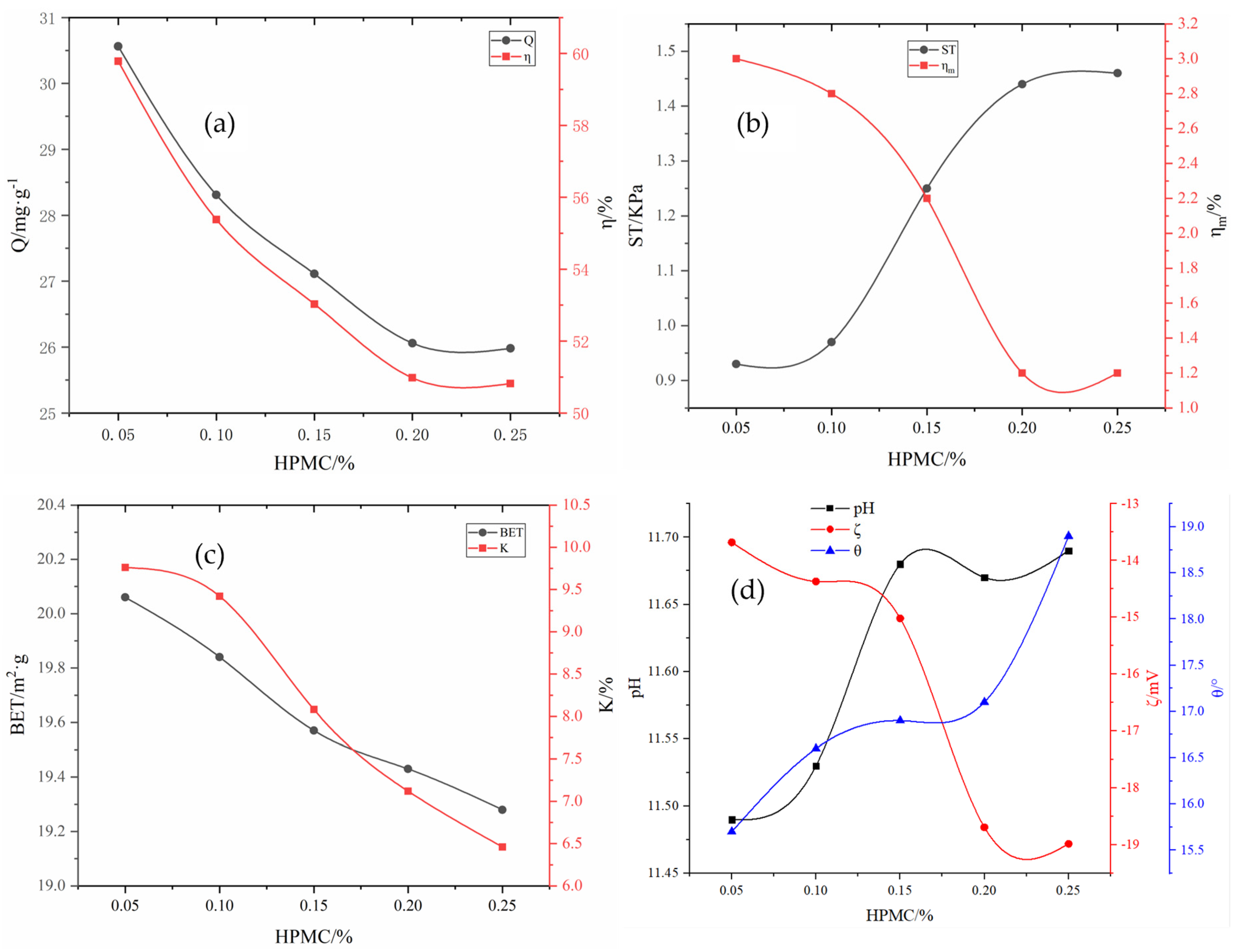
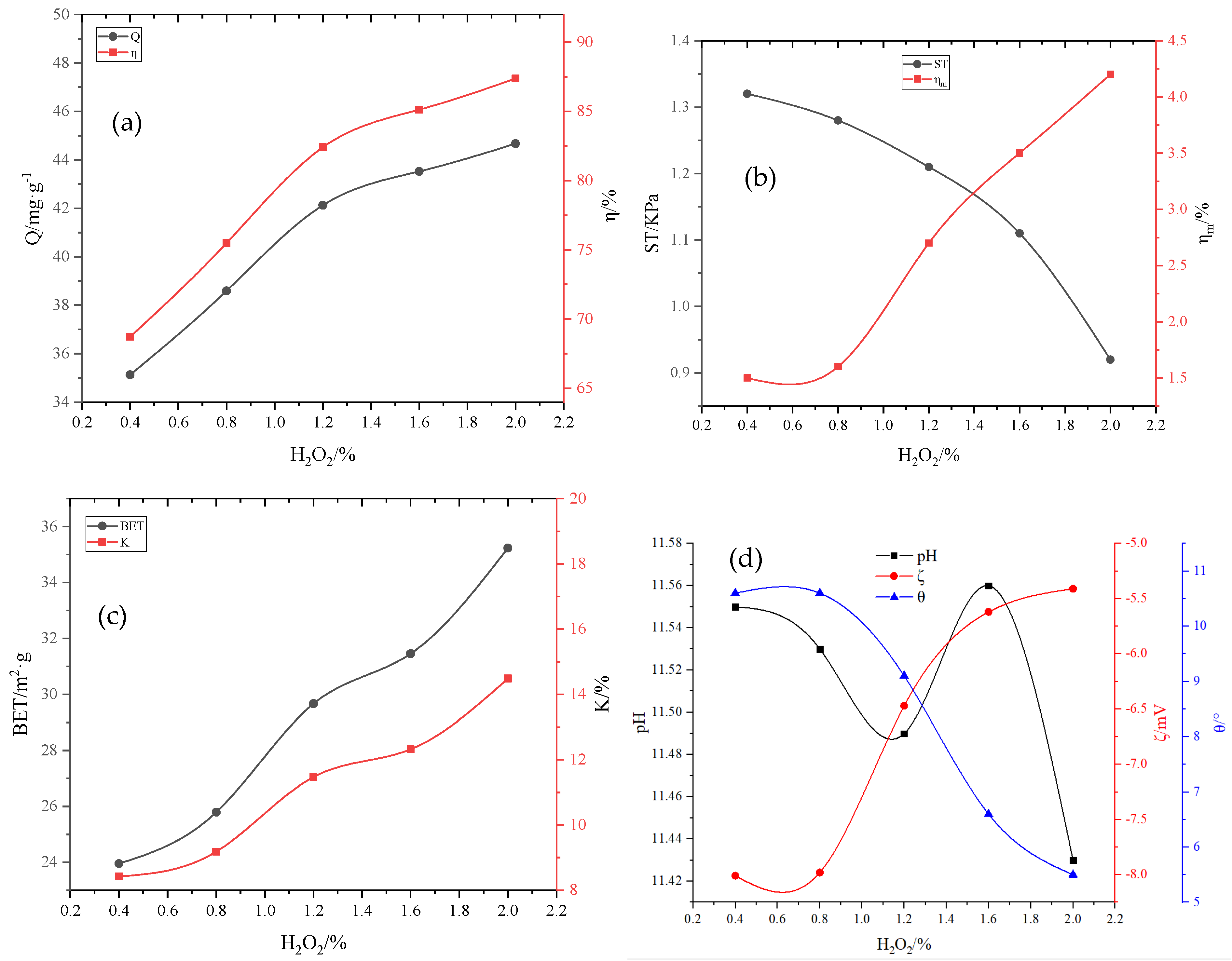
Preparation of the active binder: KH-602, hydroxypropyl methylcellulose (HPMC), and Na2SiO4 powders were added into the deionized water with a water–binder ratio of 3:1, with mass ratios of 0.1–0.4%, 0.5–2.5%, and 2–4%, respectively. Thereafter, the mixture was magnetically stirred for 5 min, and then it was kept standing for ~24 h for later use; subsequently, H2O2 was added according to the mass ratio of 0.4–2.0%, followed by magnetic stirring for 5 min and standing for ~24 h; HCl and SDBS were added with mass ratios of 0.25–1.2% and 0.05–0.25%, respectively, followed by magnetic stirring for 5 min and standing for ~24 h, resulting in the formation of the active binder.
Preparation of RM particle adsorbents: First, 10–20% nucleating particle support was added to the granulating disk, and the adsorbent powder was added while rotating. The active binder was slowly sprayed in continuously till the adsorptive active coating attained a certain thickness. After rotating for another 10 min, the adsorbent was screened and placed in the cement concrete standard curing box under constant temperature and humidity for 3 days and finally dried at 40 °C in the electric blast-drying oven. Thus, the RM particle adsorbents were obtained.
The purpose of this study is to build a neural network model using the limited experimental data available from the experiment and then predict more data points by the model, so the experiment mainly obtains limited data points. The model can achieve non-linear interpolation through the relationship of these data points; the input training data of the network are single-factor test data and orthogonal test data of the RM particle adsorbents, and the output data are performance test data of non-thermal activated RM particle adsorbents. In all, 131 sets of training data were used. Among them, the first 31 groups of training data were single-factor test data. In the single-factor test, 300 g of RM with a water–cement ratio of 1:3, 2% Na
2SiO
4, and 0.1% KH-602 were used as blank tests. They were added as indicated in
Table 8. After the #02 to #06 tests, the amount of RM added was set to 285 g, and the FA concentration was set to 5.1%. Then, the concentrations of A2C, HPMC, H
2O
2, MnO
2, and HCl were determined as 8%, 0.25%, 1.6%, 0.32%, and 0.75%, respectively. The last 100 groups of training data were 11-factor 5-level orthogonal test data, where the concentrations of aluminate cement and Na
2SiO
4 were determined as 8% and 2%, respectively. Then, the orthogonal test was performed by changing the amount of RM, FA, H
2O
2, MnO
2, HPMC, HCl, and SDBS, with a water–cement ratio of 1:3.
In order to obtain the ideal BP neural network prediction model, there must be a sufficient number of input and output data pairs. In this study, a total of 131 adsorbents of red mud particles were prepared by arranging one-way and orthogonal tests based on the input and output data pairs of the BP neural network and formulation requirements.
3.3. Performance Tests of the RM Adsorbent
After the preparation of the adsorbent, it is necessary to test nine properties—specific surface area (BET), wetting angle (
), Zeta potential (
), adsorption capacity (Q) [
27], compressive strength (ST) [
37], removal rate (
) [
38], immersion pulverization rate(K), water absorption rate, and pH—of the adsorbent, of which the adsorption capacity, compressive strength, removal rate, and pulverization rate are the important basic indexes to measure the particle adsorbents. Zeta potential [
39] is a useful metric for determining the adsorption system’s stability and electrochemical properties. Colloids with high Zeta potential are stable, while those with low Zeta potential tend to flocculate. The pH value [
40] is the main influencing factor of Zeta potential. There is a good correlation between wettability and adsorption capacity, and the better the wettability [
41] of the adsorbent surface, the better the adsorption effect. The larger specific surface area [
42] is beneficial to the adsorption effect of the adsorbent, and the specific surface area is related to the water absorption [
43] and adsorption capacity.
The removal rate
η of phosphorus in the solution, the adsorption capacity Q of the adsorbent, the pulverization rate of the adsorbent
, and the water absorption rate K of the adsorbent were calculated according to Equations (1) and (4) as follows:
where
η (%) represents the removal rate of phosphorus in the solution;
(mg·L
−1) represents the concentration of phosphorus in the solution before adsorption;
(mg·L
−1) represents the concentration of phosphorus in the solution after adsorption;
(mg·g
−1) represents the adsorption capacity of adsorbent per unit mass;
(L) represents the volume of the solution;
(g) represents the mass of the adsorbent after drying;
(%) represents the pulverization rate of the adsorbent;
(g) represents the drying mass of the adsorbent after adsorption;
(g) represents adsorbing capacity;
(%) represents the water absorption rate;
(g) represents the mass of deionized water absorbed by the adsorbent.
Micro metrics measured the specific surface area of particle adsorbents ASAP2020 N2 adsorption/desorption physical adsorption instrument (BET), and the specific surface area (SBET) was calculated using the Brunauer–Emmett–Teller method.
The Zeta potential () on the surface of the adsorbent before and after adsorption was determined using the nanoparticle size/Zeta potential analyzer (Beckman Coulter, Pasadena, CA, USA, model: DELSA Nano C). The test sample was put into a beaker, and a certain volume of deionized water was added. The suspension was prepared with a water–cement mass ratio of 100:1. After magnetic stirring for 10 min, the suspension was fully dispersed and then left standing for 6 h. Subsequently, the supernatant was collected into the sample cell and placed in a potentiometer to determine the Zeta potential.
The wetting angle of the particle adsorbents was measured by the LAUDA wetting angle measuring instrument/contact angle measuring instrument (LAUDA Scientific, GmbH, Germany, model: LSA60). Since a block sample reflects the real wetting angle of the mineral better than a powder-pressed sample, 1 × 1 × 1 cm cubic mineral blocks that were ground with sandpaper, washed with distilled water, and dried naturally before being used were adopted for all the wetting angle tests. An ore cube was immersed in the solution to be tested for 30 min and then taken out to measure the wetting angle by the floating bubble method. The measurement was repeated five times to obtain the average value.
Particle strength ST (KPa) was measured by the APT-3 particle strength tester.
The pH value of the solution is affected by the content of the adsorbent formulation. The pH value of the phosphorus-containing wastewater after adsorption is measured using a pH meter.
3.4. Establishment and Application of the BP Neural Network Model
The main objective of adsorbent formulation optimization was to improve the mechanical adsorbent properties while satisfying the adsorption capacity. The preparation conditions clearly indicate that there exists certain coupling and nonlinearity among the various adsorbent properties, and the orthogonal test method cannot satisfy the formulation optimization. Therefore, the neural network was used for nonlinear formulation optimization to obtain the relationship between the appropriate ratio and the optimal performance.
3.4.1. Determination of Input and Output Training Datasets
The design of 131 red mud adsorbent formulations according to
Section 3.2 was used as input data for the BP neural network, and the performance tested in
Section 3.3 was used as output data. When the input value is too large, the neurons will be in a saturated state, thus losing the ability to learn. Therefore, the input value should be normalized, and the input value should be processed from 0 to 1. The normalized function is shown in Equation (5) [
44].
The input and output data sets were normalized. Similarly, the predicted results were normalized and then output. The training parameters were set after normalizing the training data. Among the 131 experimental data, 84 data were randomly selected as training data for neural network training and 47 data were used as testing data for network testing.
3.4.2. Establishment of a BP Network Optimization Model
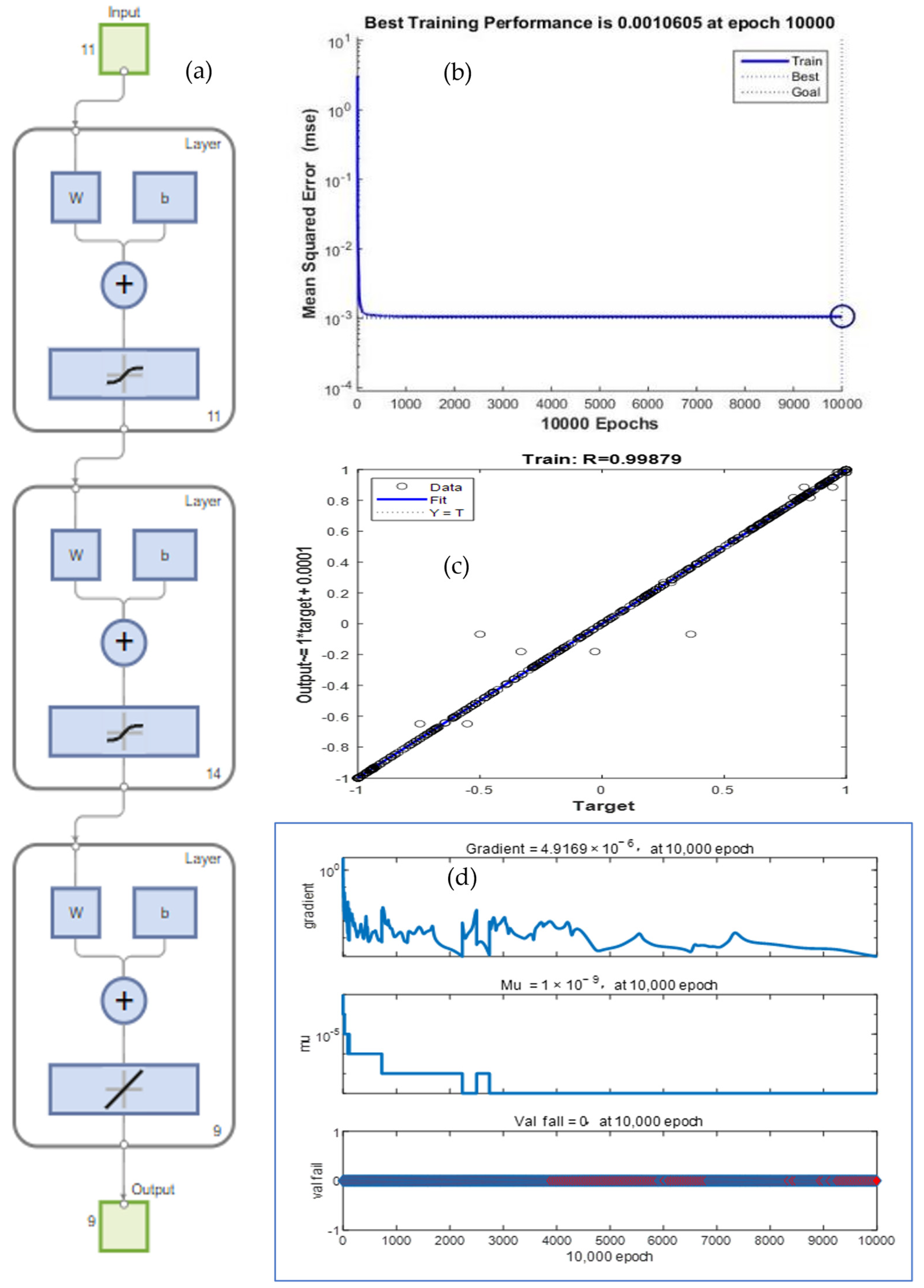
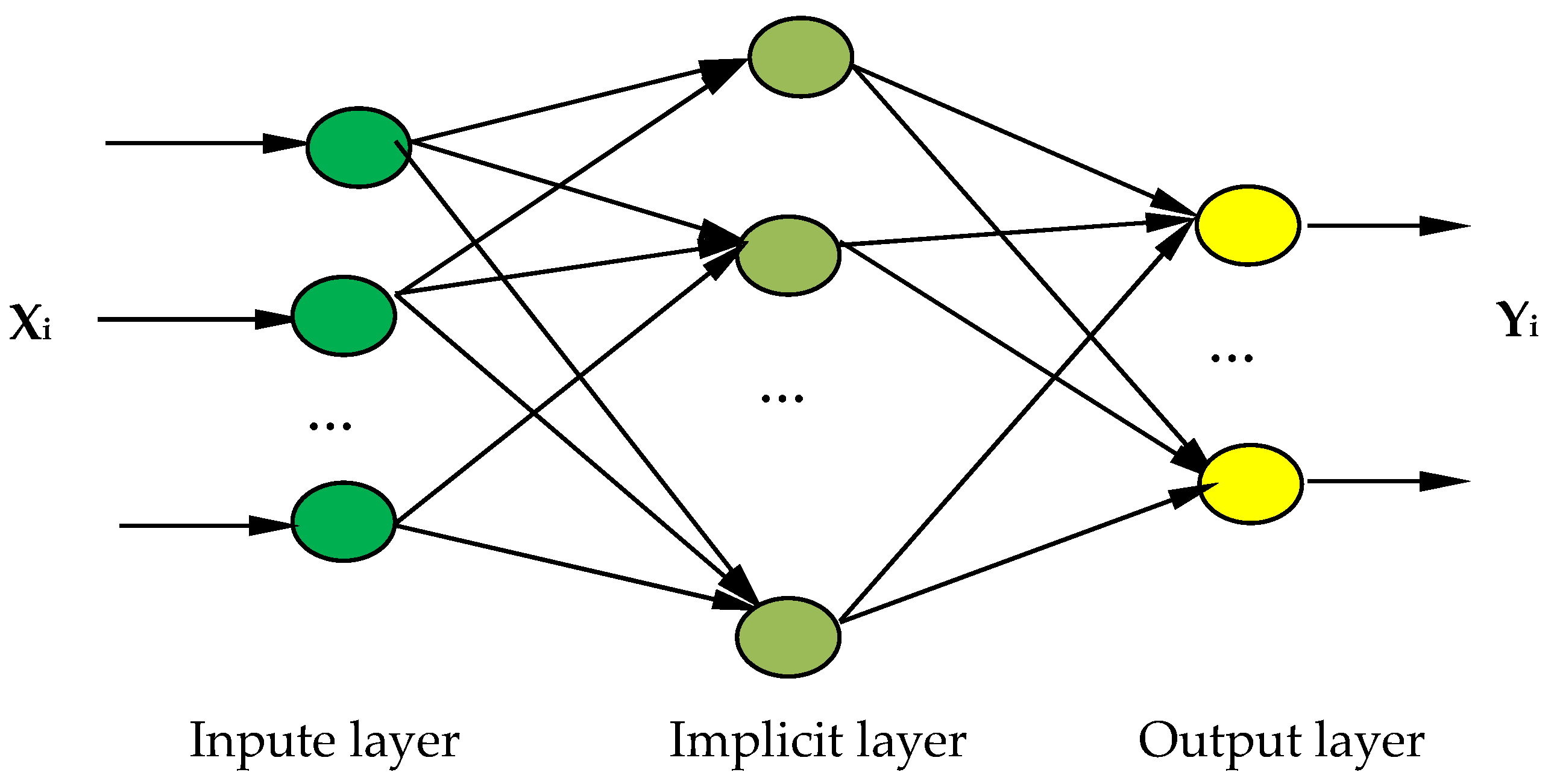
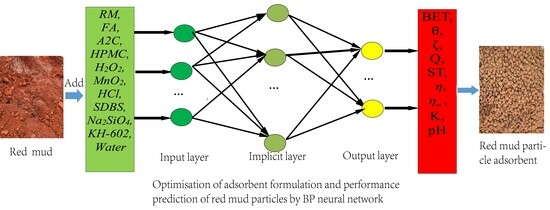
The BP neural network optimization model of the nonthermally active RM particle adsorbents is shown in
Figure 9. The model has an 11-input-9-output structure. The designed neural network has a three-layered structure, including the input layer, output layer, and hidden layer, where X is the input layer, representing the parameter change of each formulation. Y represents the amount of change in each performance index caused by a change in X and is used to investigate the influence of the change amount of each factor on the performance index of the adsorbent. The following formulation was used to select the number of neurons in the hidden layer in Equation (6) [
44].
where m represents the number of nodes in the input layer; l represents the number of nodes in the hidden layer; n represents the number of nodes in the output layer; and
is a constant between 1 and 10; in this paper, m = 11, n = 9,
= 10, l = 14.
The input–output relationship is represented as follows:
X = {RM, FA, Water–Cement Ratio, A2C, HPMC, Na2SiO4, KH-602, H2O2, MnO2, HCl, SDBS},
Y = {specific surface area, wetting angle, Zeta potential, adsorption capacity, removal rate, compressive strength, immersion pulverization rate, water absorption rate, pH}.
The output layer transfer function chooses the linear function. In this paper, the Sigmoid function is selected as an intermediate layer transfer function, and the training strategy is a gradient reduction method [
45].
3.4.3. Training and Testing
While the BP neural network model is built, the network is programmed and trained, and MATLAB R2022b software was used to set parameters as follows: numberOfSample is 84, numberOfTestSample is 47, numberOfForcastSample is 9, numberOfHiddenNeure is 14, inputDimension is 11, outputDimension is 9, net.trainParam.show is 10, net.trainParam.epochs is 10,000, net.trainParam.lr is 0.035, net.trainParam.goal is 10
−3, and net.divideFcn is ‘’. After the network is trained, the network is tested with 47 sets of test samples, and the testing data are given in
Table 3. The network is evaluated using mean square error (MSE, Equation (7)), root mean square error (RMSE, Equation (8)), and coefficient of determination (R
2, Equation (9)) [
46].
where
n is the number of input/output data pairs;
is the predicted value;
is the experimental value; and
is the average of predicted values.
3.4.4. Formulation Optimization
While the network has been trained and tested, the model can be used to predict and analyze the results of the experiment [
44]. During formulation optimization, 11 input parameters and 9 output parameters were optimized. By fixing the ratio of 11 agents and changing the dosage of the agent to be optimized, the value was calculated according to the range of the dosage from small to large with a certain step size, and the changes in the output parameters at each change point were compared. Then, according to the target output value of the RM particle adsorbents, the optimal ratio was derived. The range of the optimized parameters is shown in
Table 6.