Practical Epoxidation of Olefins Using Air and Ubiquitous Iron-Based Fluorous Salen Complex
Abstract
1. Introduction
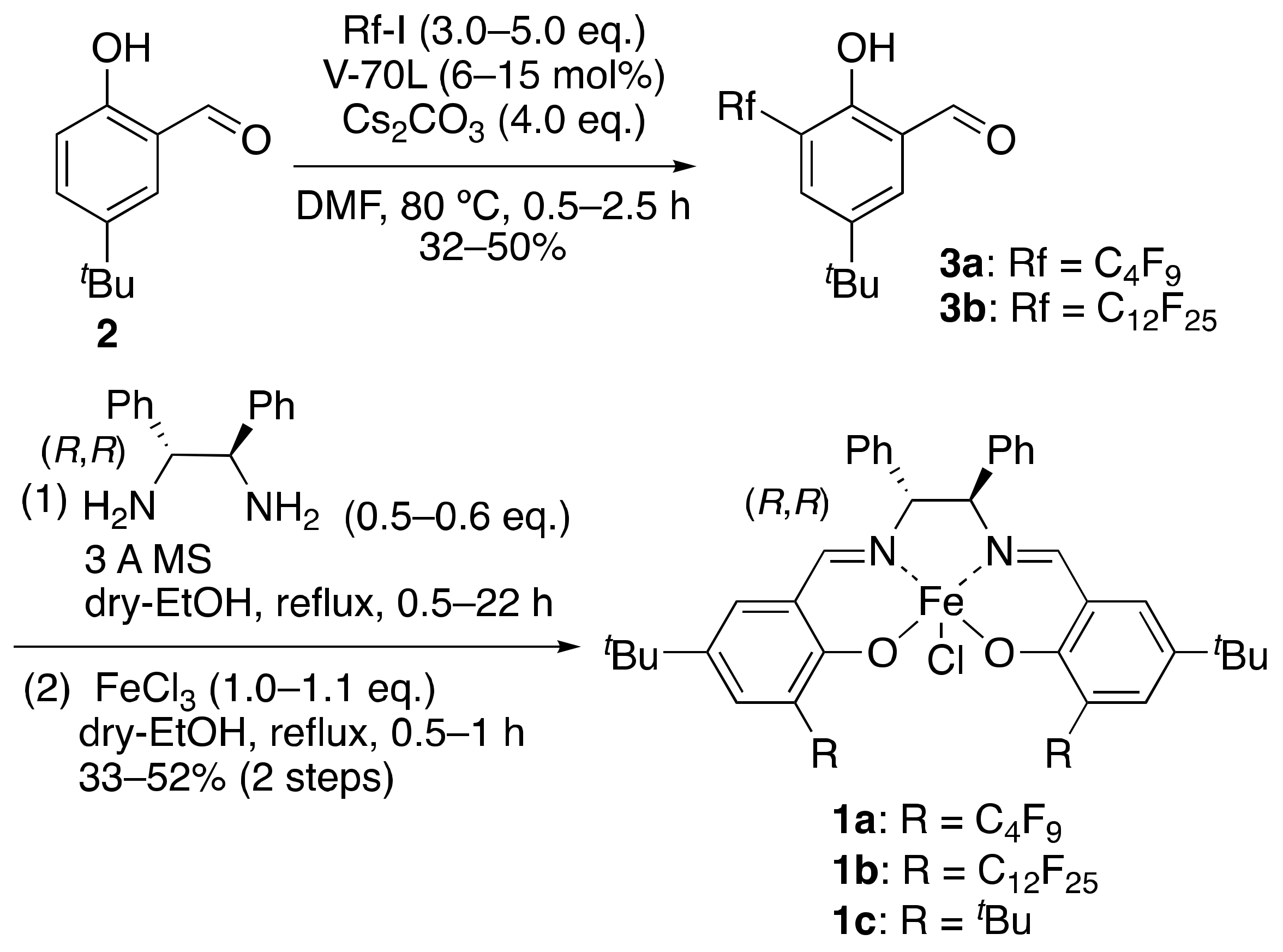
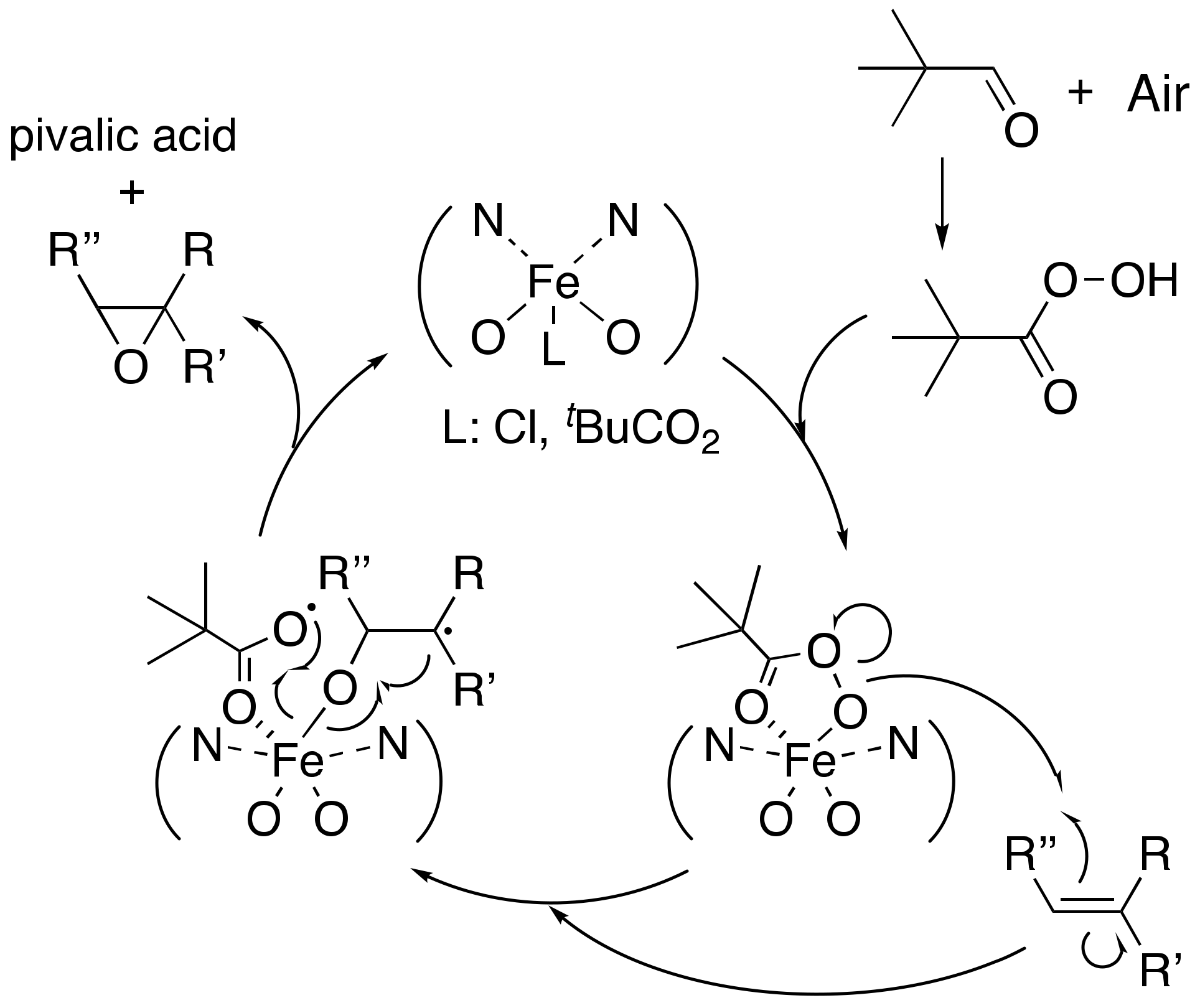
2. Results and Discussions
3. Conclusions
4. Experimental Section
4.1. Materials and Reagents
4.2. Analytical Instruments
4.3. General Procedure for the Epoxidation of Olefins Using Air
4.4. Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, K.; Haruna, S.; Imanaka, T.; Hamamoto, M.; Nishiyama, Y.; Ishii, Y. A convenient synthesis of epoxides from olefins using molecular oxygen in the absence of metal catalysts. Tetrahedron Lett. 1992, 33, 6827–6830. [Google Scholar] [CrossRef]
- Laszlo, P.; Levart, M. “Clayniac”-catalyzed epoxidation: The role of the aldehyde as co-reducer of molecular oxygen. Tetrahedron Lett. 1993, 34, 1127–1130. [Google Scholar] [CrossRef]
- Köckritz, A.; Blumenstein, M.; Martin, A. Epoxidation of methyl oleate with molecular oxygen in the presence of aldehydes. Eur. J. Lipid Sci. Technol. 2008, 110, 581–586. [Google Scholar] [CrossRef]
- Lassila, K.R.; Waller, F.J.; Werkheiser, S.E.; Wressell, A.L. Aldehyde/olefin cooxidations: Parallel epoxidation pathways and concerted decomposition of the peroxyacyl-olefin adduct. Tetrahedron Lett. 1994, 35, 8077–8080. [Google Scholar] [CrossRef]
- Yamada, T.; Imagawa, K.; Nagata, T.; Mukaiyama, T. Aerobic Enantioselective Epoxidation of Unfunctionalized Olefins Catalyzed by Optically Active Salen-Manganese(III) Complexes. Bull. Chem. Soc. Jpn. 1994, 67, 2248–2256. [Google Scholar] [CrossRef]
- Wentzel, B.B.; Alsters, P.L.; Feiters, M.C.; Nolte, R.J.M. Mechanistic Studies on the Mukaiyama Epoxidation. J. Org. Chem. 2004, 69, 3453–3464. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Yamada, T.; Nagata, T.; Imagawa, K. Asymmetric Aerobic Epoxidation of Unfunctionalized Olefins Catalyzed by Optically Active α-Alkoxycarbonyl-β-ketoiminato Manganese(III) Complexes. Chem. Lett. 1993, 22, 327–330. [Google Scholar] [CrossRef]
- Imagawa, K.; Nagata, T.; Yamada, T.; Mukaiyama, T. N-Alkyl lmidazoles as Effective Axial Ligands in the Aerobic Asymmetric Epoxidation of Unfunctionalized Olefins Catalyzed by Optically Active Manganese(III)-salen-type Complex. Chem. Lett. 1994, 23, 527–530. [Google Scholar] [CrossRef]
- Takai, T.; Hata, E.; Yamada, T.; Mukaiyama, T. Aerobic Epoxidation of Olefinic Compounds Catalyzed by Tris(1,3-diketonato)iron(III). Bull. Chem. Soc. Jpn. 1991, 64, 2513–2518. [Google Scholar] [CrossRef]
- Nagata, T.; Imagawa, K.; Yamada, T.; Mukaiyama, T. Optically Active N,N′-Bis(3-oxobutylidene)diaminatomanganese(III) Complexes as Novel and Efficient Catalysts for Aerobic Enantioselective Epoxidation of Simple Olefins. Bull. Chem. Soc. Jpn. 1995, 68, 1455–1465. [Google Scholar] [CrossRef]
- Hirao, T.; Moriuchi, T.; Ishikawa, T.; Nishimura, K.; Mikami, S.; Ohshiro, Y.; Ikeda, I. A novel catalytic system for oxygenation with molecular oxygen induced by transition metal complexes with a multidentate N-heterocyclic podand ligand. J. Mol. Catal. A Chem. 1996, 113, 117–130. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.-M.; Chen, Q.-Y. Fluorous Biphasic Catalytic Oxidation of Alkenes and Aldehydes with Air and 2-Methylpropanal in the Presence of (β-Perfluoroalkylated tetraphenylporphyrin)cobalt Complexes. Eur. J. Org. Chem. 2006, 2006, 2703–2706. [Google Scholar] [CrossRef]
- Lehtinen, C.; Brunow, G. Epoxidation of Terminal Alkenes with Oxygen and 2-Ethyl Hexanal, without Added Catalyst or Solvent. Org. Process Res. Dev. 1999, 3, 101–103. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Nobile, C.F. Aerobic epoxidation of olefins catalyzed by polymerizable β-ketoesterate complexes of iron(III), nickel(II) and cobalt(II). J. Mol. Catal. 1994, 94, 19–26. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Zhang, W.; Muci, A.R.; Ecker, J.R.; Deng, L. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar] [CrossRef]
- Nishida, T.; Miyafuji, A.; Ito, Y.N.; Katsuki, T. Enthalpy- and/or entropy-controlled asymmetric oxidation: Stereocontrolling factors in Mn–salen-catalyzed oxidation. Tetrahedron Lett. 2000, 41, 7053–7058. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Obayashi, R.; Watanabe, Y.; Miyazaki, H.; Miyata, I.; Suzuki, Y.; Yoshida, Y.; Shioiri, T.; Matsugi, M. Unprecedented Asymmetric Epoxidation of Isolated Carbon–Carbon Double Bonds by a Chiral Fluorous Fe(III) Salen Complex: Exploiting Fluorophilic Effect for Catalyst Design. Eur. J. Org. Chem. 2019, 2019, 2401–2408. [Google Scholar] [CrossRef]
- Matsugi, M.; Hasegawa, M.; Hasebe, S.; Takai, S.; Suyama, R.; Wakita, Y.; Kudo, K.; Imamura, H.; Hayashi, T.; Haga, S. Direct perfluoroalkylation of non-activated aromatic C–H bonds of phenols. Tetrahedron Lett. 2008, 49, 4189–4191. [Google Scholar] [CrossRef]
- Knight, P.D.; O’Shaughnessy, P.N.; Munslow, I.J.; Kimberley, B.S.; Scott, P. Biaryl-bridged Schiff base complexes of zirconium alkyls: Synthesis structure and stability. J. Organomet. Chem. 2003, 683, 103–113. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirota, S.; Fujino, A.; Ishihara, K.; Shioiri, T.; Matsugi, M. Asymmetric Henry reaction using a double fluorous-tagged Co–salen complex. Tetrahedron Lett. 2022, 99, 153833. [Google Scholar] [CrossRef]
- Geng, X.-L.; Wang, Z.; Li, X.-Q.; Zhang, C. A Simple Method for Epoxidation of Olefins Using Sodium Chlorite as an Oxidant without a Catalyst. J. Org. Chem. 2005, 70, 9610–9613. [Google Scholar] [CrossRef]
- Ren, G.; Cui, X.; Yang, E.; Yang, F.; Wu, Y. Study on the Heck reaction promoted by carbene adduct of cyclopalladated ferrocenylimine and the related reaction mechanism. Tetrahedron 2010, 66, 4022–4028. [Google Scholar] [CrossRef]
- Yasuda, S.; Yorimitsu, H.; Oshima, K. Arylation of Styrenes with Aryliron Complexes [CpFe(CO)2Ar]. Organometallics 2010, 29, 2634–2636. [Google Scholar] [CrossRef]
- Saiyed, A.S.; Joshi, R.S.; Bedekar, A.V. Amino Oxazolines as Easily Accessible Water Stable Ligands for Palladium Catalysed Aqueous Heck Reaction. J. Chem. Res. 2011, 35, 408–411. [Google Scholar] [CrossRef]
- Robinson, M.W.C.; Pillinger, K.S.; Mabbett, I.; Timms, D.A.; Graham, A.E. Copper(II) tetrafluroborate-promoted Meinwald rearrangement reactions of epoxides. Tetrahedron 2010, 66, 8377–8382. [Google Scholar] [CrossRef]
- Li, X.-Q.; Li, C.; Song, F.-B.; Zhang, C. A First Homogeneous gold(III)-Catalysed Epoxidation of Aromatic Alkenes. J. Chem. Res. 2007, 2007, 722–724. [Google Scholar] [CrossRef]
- Saito, T.; Akiba, D.; Sakairi, M.; Ishikawa, K.; Otani, T. Chiral sulfide-mediated enantioselective epoxidation of aldehydes. Arkivoc 2004, 2004, 152–171. [Google Scholar] [CrossRef]
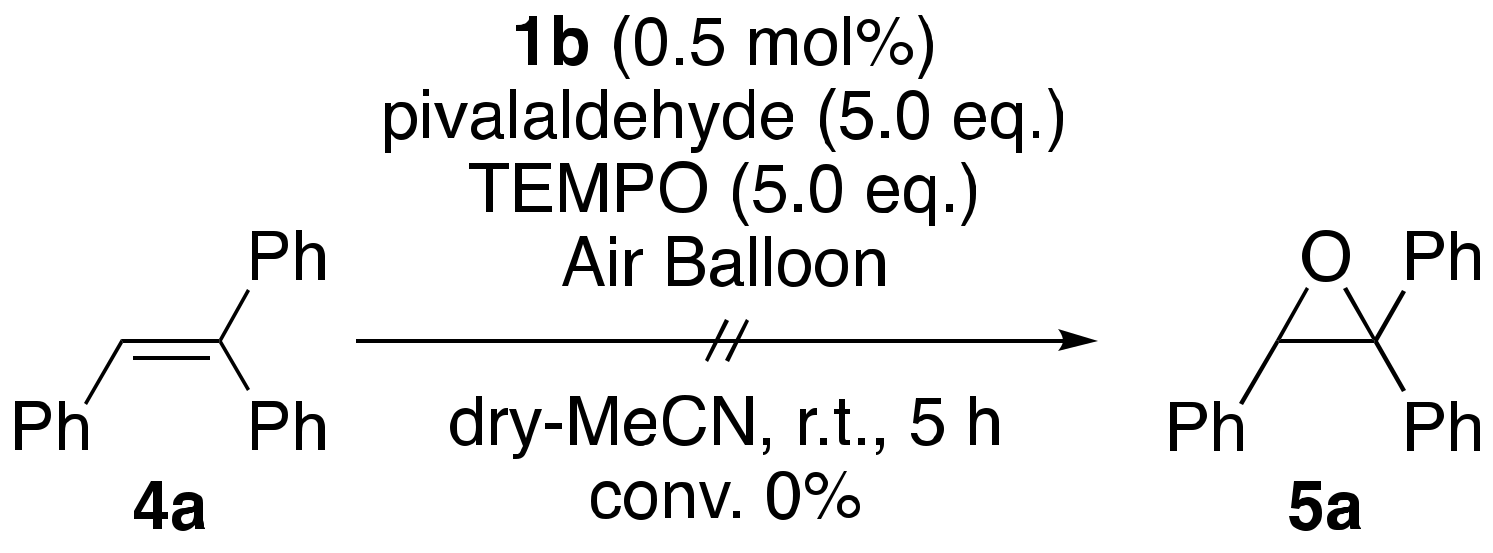
 | ||
|---|---|---|
| Entry | Catalyst (mol%) | Conv. (%) a |
| 1 | 1a (1) | 73 |
| 2 | 1b (1) | 100 (98) b |
| 3 | 1b (0.5) | 100 (95) b |
| 4 | 1b (0.1) | 6 |
| 5 | 1c (0.5) | 53 |
| 6 | - | 5 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Aldehyde | Time (h) | Conv. (%) a | Entry | Aldehyde | Time (h) | Conv. (%) a |
| 1 | 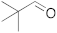 | 5 | 100 | 7 | 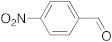 | 24 | n.r. b |
| 2 | 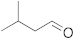 | 24 | 82 | 8 | 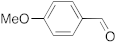 | 24 | trace |
| 3 |  | 24 | 10 | 9 | 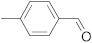 | 24 | n.r. b |
| 4 | 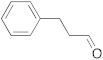 | 24 | trace | 10 | 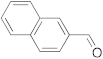 | 24 | n.r. b |
| 5 | 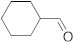 | 24 | trace | 11 | 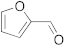 | 24 | trace |
| 6 |  | 24 | n.r. b | 12 | 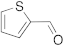 | 24 | trace |
 | |||
|---|---|---|---|
| Entry | Solvent | Time (h) | Conv. (%) a |
| 1 | dry-MeCN | 5 | 100 |
| 2 | tBuOH | 8 | 100 |
| 3 | dry-MeOH | 24 | trace |
| 4 | dry-DMF | 24 | trace |
| 5 | dry-THF | 24 | trace |
| 6 | dry-DCM | 24 | 96 |
| 7 | 2,3,4,5,6-pentafluorotoluene | 24 | 98 |
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Olefin | Epoxide | Time (h) | Conv. (%) a | Entry | Olefin | Epoxide | Time (h) | Conv. (%) a |
| 1 | 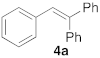 | 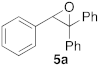 | 5 | 100 | 6 | 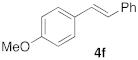 | 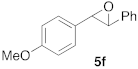 | 5 | 100 |
| 2 |  | 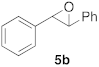 | 7 | 100 | 7 | 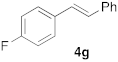 | 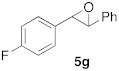 | 5 | 100 |
| 3 | 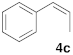 | 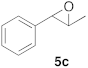 | 6 | 100 | 8 | 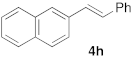 | 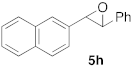 | 24 | 52 |
| 4 | 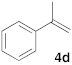 |  | 7 | 100 | 9 b | 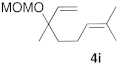 | 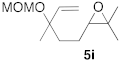 | 5 | 100 (5i:5i’ = 100:0) |
| 5 | 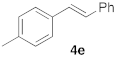 | 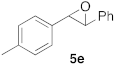 | 6 | 100 | 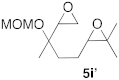 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y.; Kanoh, M.; Kobayashi, H.; Shioiri, T.; Matsugi, M. Practical Epoxidation of Olefins Using Air and Ubiquitous Iron-Based Fluorous Salen Complex. Molecules 2024, 29, 966. https://doi.org/10.3390/molecules29050966
Kato Y, Kanoh M, Kobayashi H, Shioiri T, Matsugi M. Practical Epoxidation of Olefins Using Air and Ubiquitous Iron-Based Fluorous Salen Complex. Molecules. 2024; 29(5):966. https://doi.org/10.3390/molecules29050966
Chicago/Turabian StyleKato, Yamato, Miho Kanoh, Hina Kobayashi, Takayuki Shioiri, and Masato Matsugi. 2024. "Practical Epoxidation of Olefins Using Air and Ubiquitous Iron-Based Fluorous Salen Complex" Molecules 29, no. 5: 966. https://doi.org/10.3390/molecules29050966
APA StyleKato, Y., Kanoh, M., Kobayashi, H., Shioiri, T., & Matsugi, M. (2024). Practical Epoxidation of Olefins Using Air and Ubiquitous Iron-Based Fluorous Salen Complex. Molecules, 29(5), 966. https://doi.org/10.3390/molecules29050966





