Hetero-Diels–Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods
Abstract
1. Introduction
2. Results
2.1. Chemistry
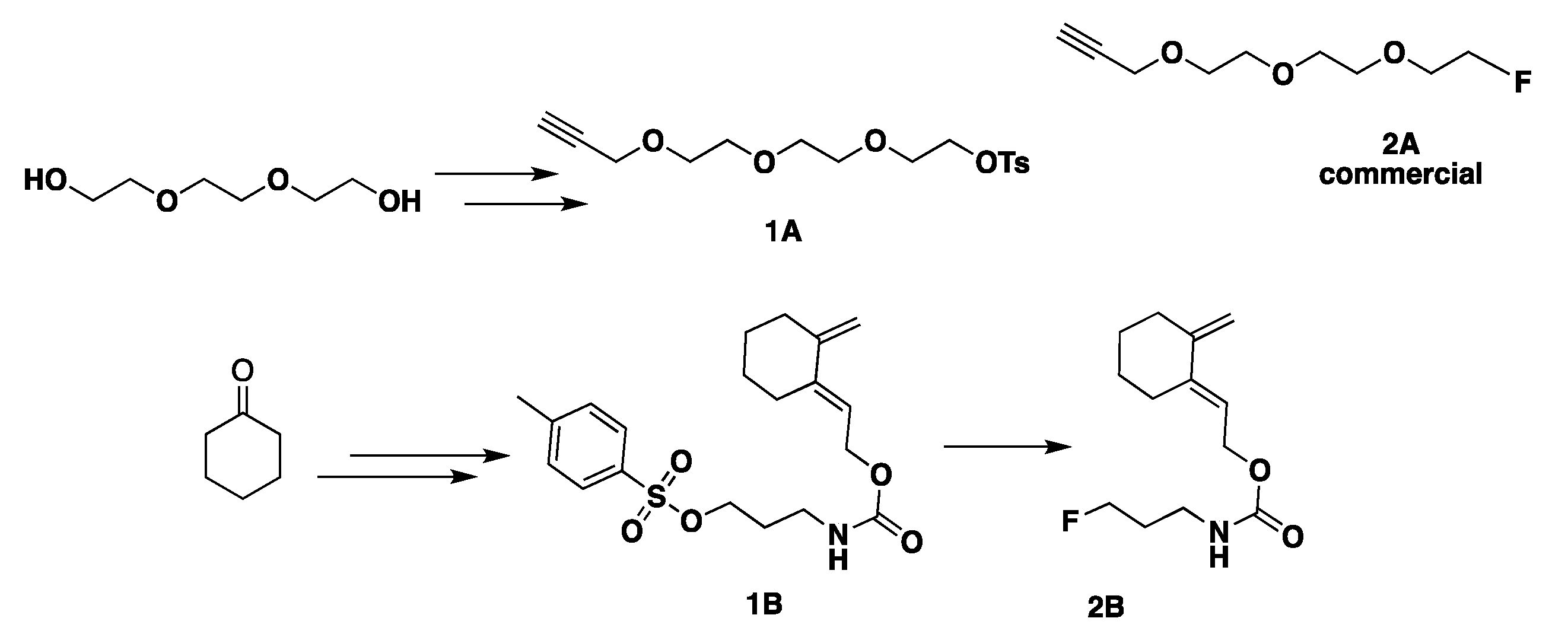
2.1.1. Non-Peptidic Cycloaddition Partners
- for CuAAC
- for HDA reaction
2.1.2. Synthesis of Peptides and Modified Peptides
- Synthesis of modified azido-peptides 3A–5A for CuAAC
- Synthesis of modified dithioester-peptides 3B–5B for HDA reactions
- Synthesis of reference peptides 6–8
2.2. Radiochemistry
2.2.1. Manual Synthesis
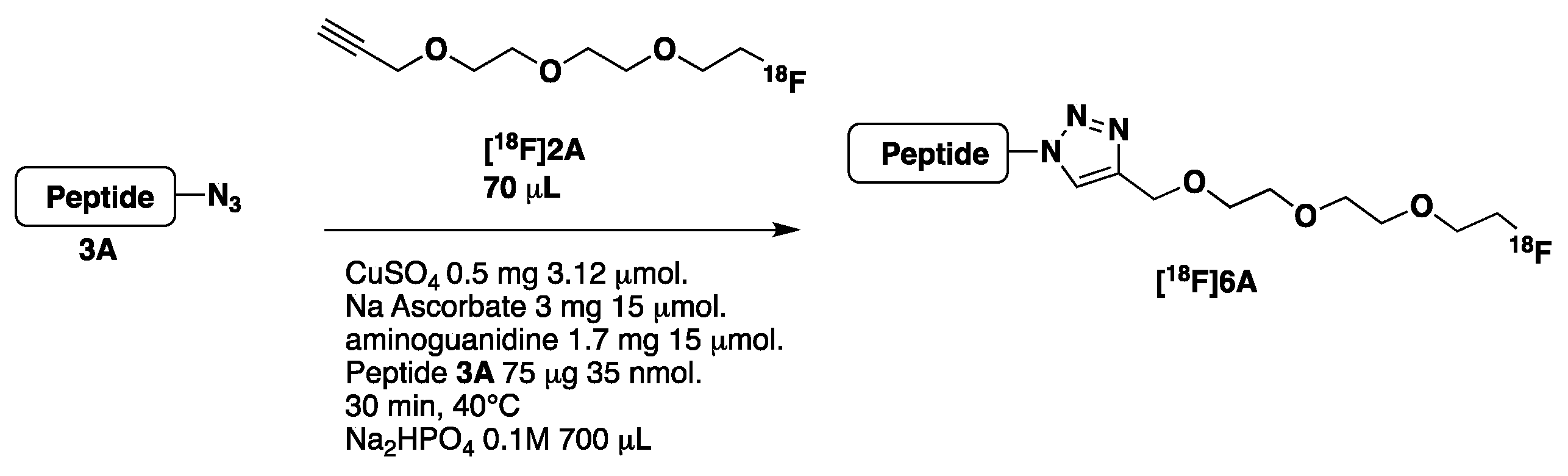
- Synthesis of [18F]2A and [18F]2B (Scheme 3, see also Supplementary Materials pages 12–13)

- Optimization of the CuAAC reaction using peptide 3A
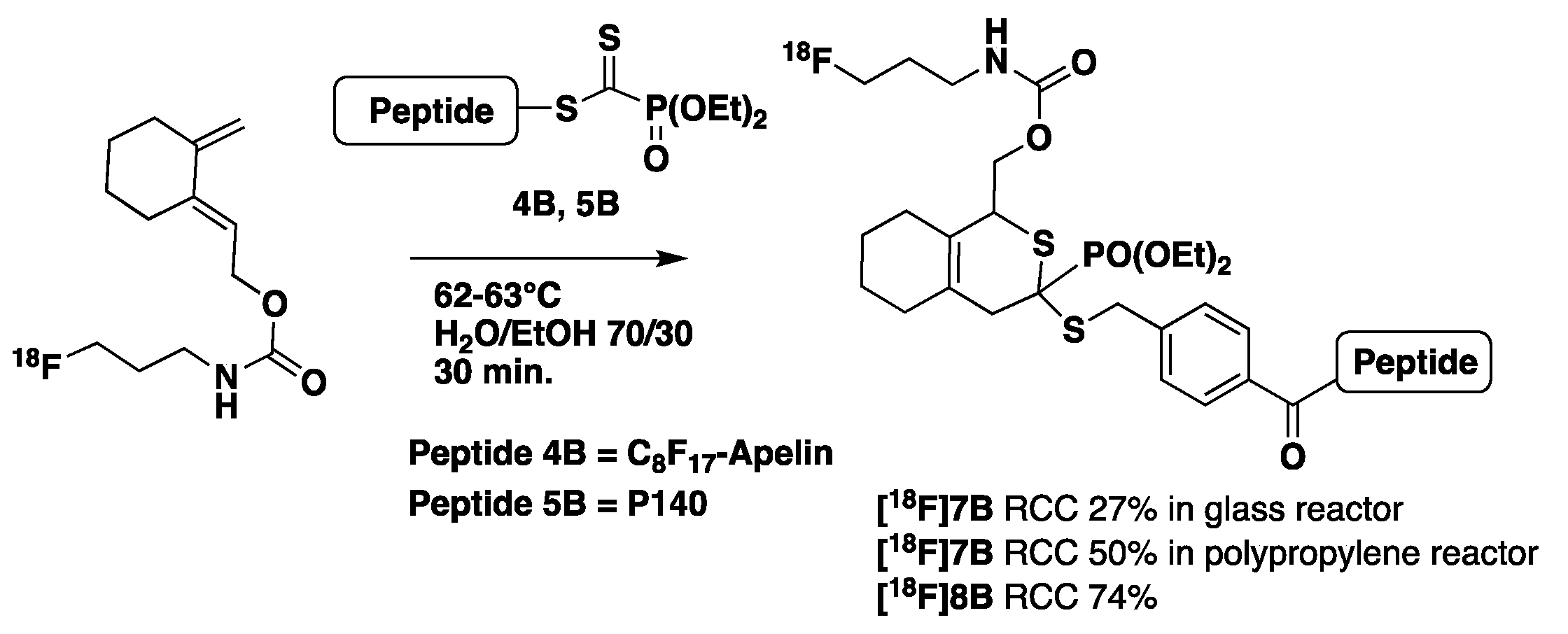
- Optimization of the HDA reaction (Scheme 6)
2.2.2. Automated Synthesis
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J. Radiolabeled Peptides in Imaging and Therapy: Basic and Clinical Perspectives. Curr. Med. Chem. 2020, 27, 6966–6967. [Google Scholar] [CrossRef]
- Kręcisz, P.; Czarnecka, K.; Kroĺicki, L.; Mikiciuk-Olasik, E.; Szymanśki, P. Radiolabeled Peptides and Antibodies in Medicine. Bioconjug. Chem. 2021, 32, 25–42. [Google Scholar] [CrossRef]
- Mohtavinejada, N.; Ardestani, M.S.; Khalaj, A.; Pormohammad, A.; Najafi, R.; Bitarafan-Rajabi, A.; Hajiramezanali, M.; Amanlou, M. Application of radiolabeled peptides in tumor imaging and therapy. Life Sci. 2020, 258, 118206. [Google Scholar] [CrossRef] [PubMed]
- Bispo, A.C.A.; Almeida, F.A.F.; Silva, J.B.; Mamede, M. Peptides Radiofluorination: Main Methods and Highlights. Int. J. Org. Chem. 2022, 12, 161–172. [Google Scholar] [CrossRef]
- Rangger, C.; Haubner, R. Radiolabelled Peptides for Positron Emission Tomography and Endoradiotherapy in Oncology. Pharmaceuticals 2020, 13, 22. [Google Scholar] [CrossRef]
- Failla, M.; Floresta, G.; Abbate, V. Peptide-based positron emission tomography probes: Current strategies for synthesis and radiolabelling. RSC Med. Chem. 2023, 14, 592–623. [Google Scholar] [CrossRef]
- Olberg, D.E.; Hjelstuen, O.K. Labeling strategies of peptides with ¹⁸F for positron emission tomography. Curr. Top. Med. Chem. 2010, 10, 1669–1679. [Google Scholar] [CrossRef]
- Morris, O.; Fairclough, M.; Grigg, J.; Prenant, C.; McMahon, A. A review of approaches to 18F radiolabelling affinity peptides and proteins. J. Label. Compd. Radiopharm. 2019, 62, 4–23. [Google Scholar] [CrossRef]
- Scroggie, K.R.; Perkins, M.V.; Chalker, J.M. Reaction of [18F]Fluoride at Heteroatoms and Metals for Imaging of Peptides and Proteins by Positron Emission Tomography. Front. Chem. 2021, 9, 687678. [Google Scholar] [CrossRef]
- Okarvi, S. Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur. J. Nucl. Med. 2001, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Haider, A.; Jeppesen, T.E.; Josephson, L.; Linag, S.H. Radiochemistry for positron emission tomography. Nat. Commun. 2023, 14, 3257. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Wuest, F. 18F-Labeled Peptides: The Future Is Bright. Molecules 2014, 19, 20536–20556. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Maecke, H.R. Radiopharmaceutical development of radiolabelled peptides. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Becaud, J.; Mu, L.; Karramkam, M.; Schubiger, P.A.; Ametamey, S.M.; Graham, K.; Stellfeld, T.; Lehmann, L.; Borkowski, S.; Berndorff, D.; et al. Direct one-step 18F-labeling of peptides via nucleophilic aromatic substitution. Bioconjug. Chem. 2009, 20, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pourghiasian, M.; Radtke, M.A.; Lau, J.; Pan, J.; Dias, G.M.; Yapp, D.; Lin, K.-S.; Bénard, F.; Perrin, D.M. An Organotrifluoroborate for Broadly Applicable One-Step 18F-Labeling. Angew. Chem. Int. Ed. Engl. 2014, 53, 11876–11880. [Google Scholar] [CrossRef]
- Yuan, Z.; Nodwell, M.B.; Yang, H.; Malik, N.; Merkens, H.; Bénard, F.; Martin, R.E.; Schaffer, P.; Britton, R. Site-Selective, Late-Stage C-H 18F-Fluorination on Unprotected Peptides for Positron Emission Tomography Imaging. Angew. Chem. Int. Ed. 2018, 57, 12733–12736. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.; Ma, G.; Rickmeier, J.; McDaniel, J.W.; Petzold, R.; Neumann, C.C.; Murphy, J.M.; Ritter, T. Deoxyfluorination of phenols for chemoselective 18F-labeling of peptides. Nat. Protoc. 2023, 8, 3614–3651. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.W.; Stauber, J.M.; Doud, E.A.; Spokoyny, A.M.; Murphy, J.M. An Organometallic Gold(III) Reagent for 18F Labeling of Unprotected Peptides and Sugars in Aqueous Media. Org. Lett. 2022, 24, 5132–5136. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Mou, Z.; Feng, W.; Li, Z.; Yang, H.; Chen, X.; Lv, S.; Li, Z. Direct 18F-Labeling of Biomolecules via Spontaneous Site-Specific Nucleophilic Substitution by F− on Phosphonate Prostheses. Org. Lett. 2021, 23, 4261–4266. [Google Scholar] [CrossRef]
- Archibald, S.J.; Allott, L. The aluminium-[18F]fluoride revolution: Simple radiochemistry with a big impact for radiolabelled biomolecules. EJNMMI Radiopharm. Chem. 2021, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Helbert, H.; Simeth, N.A.; Crespi, S.; Spoelstra, G.B.; van Dijl, J.M.; van Oosten, M.; Nazario, L.R.; Van der Born, D.; Luurtsema, G.; et al. Ultrafast Photoclick Reaction for Selective 18F-Positron Emission Tomography Tracer Synthesis in Flow. J. Am. Chem. Soc. 2021, 143, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Verhoog, S.; Kee, C.W.; Wang, Y.; Khotavivattana, T.; Wilson, T.C.; Kersemans, V.; Smart, S.; Tredwell, M.; Davis, B.G.; Gouverneur, V. 18F-Trifluoromethylation of Unmodified Peptides with 5-18F-(Trifluoromethyl)dibenzothiophenium Trifluoromethanesulfonate. J. Am. Chem. Soc. 2018, 140, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, L.; Liu, H.; Zhang, Y.; Mou, Z.; Chen, X.; Li, J.; He, F.; Li, Z. Simplified one-pot 18F-labeling of biomolecules with in situ generated fluorothiophosphate synthons in high molar activity. Theranostics 2023, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Martin Aubert, S.; Denis, C.; Dubois, S.; Nozach, H.; Truillet, C.; Kuhnast, B. Fluorine-18 and Radiometal Labeling of Biomolecules via Disulfide Rebridging. Bioconjug. Chem. 2023, 34, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Haskali, M.B.; Roselt, P.D.; Hicks, R.J.; Hutton, C.A. Automated preparation of 2-[18F]fluoropropionate labeled peptides using a flexible, multi-stage synthesis platform (iPHASE Flexlab). J. Label. Compd. Radiopharm. 2018, 61, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Maschaued, S.; Prante, O. Sweetening Pharmaceutical Radiochemistry by 18F-Fluoroglycosylation: Recent Progress and Future Prospects. Pharmaceuticals 2021, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.S.; Ma, L.; Vasdev, N.; Liang, S.H. 18F-Labeling of Sensitive Biomolecules for Positron Emission Tomography. Chem. Eur. J. 2017, 23, 15553–15577. [Google Scholar] [CrossRef]
- Schirrmacher, R.; Wängler, B.; Bailey, J.; Bernard-Gauthier, V.; Schirrmacher, E.; Wängler, C. Small Prosthetic Groups in 18F-Radiochemistry: Useful Auxiliaries for the Design of 18F-PET Tracers. Semin. Nucl. Med. 2017, 47, 474–492. [Google Scholar] [CrossRef]
- Chiotellis, A.; Ahmed, H.; Betzel, T.; Tanriver, M.; White, C.J.; Song, H.; Da Ros, S.; Schibli, R.; Bode, J.W.; Ametamey, S.M. Chemoselective 18F-incorporation into pyridyl acyltrifluoroborates for rapid radiolabelling of peptides and proteins at room temperature. Chem. Commun. 2020, 56, 723–726. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meyer, J.P.; Adumeau, P.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: The First 10 Years. Bioconjug Chem. 2016, 27, 2791–2807. [Google Scholar] [CrossRef] [PubMed]
- Kettenbach, K.; Schieferstein, H.; Ross, T.L. 18F-Labeling Using Click Cycloadditions. BioMed. Res. Int. 2014, 2014, 361329. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Cornejo, M.A.; Hoang, T.T.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: An Update. Bioconjug. Chem. 2023, 34, 1925–1950. [Google Scholar] [CrossRef] [PubMed]
- Presolski, S.I.; Hong, V.P.; Finn, M.G. Copper-Catalyzed Azide–Alkyne Click Chemistry for Bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Fuaad, A.A.; Azmi, F.; Skwarczynski, M.; Toth, I. Peptide Conjugation via CuAAC ‘Click’ Chemistry. Molecules 2013, 18, 13148–13174. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Marik, J. Preparation of 18F-labeled peptides using the copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition. Nat. Protoc. 2011, 6, 1718–1725. [Google Scholar] [CrossRef]
- Sirion, U.; Kim, H.J.; Lee, J.H.; Seo, J.W.; Lee, B.S.; Lee, S.J.; Ohb, S.J.; Chi, D.Y. An efficient F-18 labeling method for PET study: Huisgen 1,3-dipolar cycloaddition of bioactive substances and F-18-labeled compounds. Tetrahedron Lett. 2007, 48, 3953–3957. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. [Google Scholar] [CrossRef]
- Campbell-Verduyn, L.S.; Mirfeizi, L.; Schoonen, A.K.; Dierckx, R.A.; Elsinga, P.H.; Feringa, B.L. Strain-Promoted Copper-Free “Click” Chemistry for 18F Radiolabeling of Bombesin. Angew. Chem. Int. Ed. 2011, 50, 11117–11120. [Google Scholar] [CrossRef]
- Evans, H.L.; Slade, R.L.; Carroll, L.; Smith, G.; Nguyen, Q.D.; Iddon, L.; Kamaly, N.; Stockmann, H.; Leeper, F.J.; Aboagye, E.O.; et al. Copper-free click-a promising tool for pre-targeted PET imaging. Chem. Commun. 2012, 48, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Sachin, K.; Jadhav, V.H.; Kim, E.M.; Kim, H.L.; Lee, S.B.; Jeong, H.J.; Lim, S.T.; Sohn, M.H.; Kim, D.W. F-18 labeling protocol of peptides based on chemically orthogonal strain-promoted cycloaddition under physiologically friendly reaction conditions. Bioconjug. Chem. 2012, 23, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.D.; Hausner, S.H.; Sutcliffe, J.L. Copper-Free Click for PET: Rapid 1,3-Dipolar Cycloadditions with a Fluorine-18 Cyclooctyne. ACS Med. Chem. Lett. 2011, 2, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Alanizi, A.A.; Sorlin, A.M.; Parker, M.F.L.; López-Álvarez, M.; Qin, H.; Lee, S.H.; Blecha, J.; Rosenberg, O.S.; Engel, J.; Ohliger, M.A.; et al. Bioorthogonal Radiolabeling of Azide-Modified Bacteria Using [18F]FB-sulfo-DBCO. Bioconjug Chem. 2024, 35, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Specklin, S.; Richard, M.; Hinnen, F.; Génermont, K.; Kuhnast, B. [18F]FPyZIDE: A versatile prosthetic reagent for the fluorine-18 radiolabeling of biologics via copper-catalyzed or strain-promoted alkyne-azide cycloadditions. J. Label. Comp. Radiopharm. 2019, 62, 95–108. [Google Scholar] [CrossRef]
- Liu, S.; Hassink, M.; Selvaraj, R.; Yap, L.P.; Park, R.; Wang, H.; Chen, X.; Fox, J.M.; Li, Z.; Conti, P.S. Efficient 18F labeling of cysteine-containing peptides and proteins using tetrazine-trans-cyclooctene ligation. Mol. Imaging. 2013, 12, 121–128. [Google Scholar] [CrossRef]
- Allott, L.; Amgheib, A.; Barnes, C.; Braga, M.; Brickute, D.; Wang, N.; Fu, R.; Ghaem-Maghami, S.; Aboagye, E.O. Radiolabelling an 18F biologic via facile IEDDA "click" chemistry on the GE FASTLab™ platform. React. Chem. Eng. 2021, 6, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Allott, L.; Chen, C.; Braga, M.; Leung, S.F.J.; Wang, N.; Barnes, C.; Brickute, D.; Carroll, L.; Aboagye, E.O. Detecting hypoxia in vitro using 18F-pretargeted IEDDA "click" chemistry in live cells. RSC Adv. 2021, 11, 20335–20341. [Google Scholar] [CrossRef]
- Beaufrez, J.; Guillouet, S.; Seimbille, Y.; Perrio, C. Synthesis, Fluorine-18 Radiolabeling, and In Vivo PET Imaging of a Hydrophilic Fluorosulfotetrazine. Pharmaceuticals 2023, 16, 636. [Google Scholar] [CrossRef]
- Xu, M.; Ma, X.; Pigga, J.E.; Zhang, H.; Wang, S.; Zhao, W.; Deng, H.; Wu, A.M.; Liu, R.; Wu, Z.; et al. Development of 18F-Labeled hydrophilic trans-cyclooctene as a bioorthogonal tool for PET probe construction. Chem. Commun. 2023, 59, 14387–14390. [Google Scholar] [CrossRef]
- Zientek, S.H.; Thompson, S.; Sephton, S.M.; Aigbirhio, F.I. The inverse electron demand Diels–Alder cycloaddition with carbon-11 and fluorine-18: A gateway to pretargeted imaging across the blood–brain barrier. J. Label. Compd. Radiopharm. 2023, 66, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Otaru, S.; Imlimthan, S.; Sarparanta, M.; Helariutta, K.; Wähälä, K.; Airaksinen, A.J. Evaluation of Organo [18F]Fluorosilicon Tetrazine as a Prosthetic Group for the Synthesis of PET Radiotracers. Molecules 2020, 25, 1208. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Truillet, C.; Tran, V.L.; Liu, H.; Porte, K.; Audisio, D.; Roche, M.; Jego, B.; Cholet, S.; Fenaille, F.; et al. New fluorine-18 pretargeting PET imaging by bioorthogonal chlorosydnone–cycloalkyne click reaction. Chem. Commun. 2019, 55, 10400–10403. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, M.K.; Lai, B.T.; Loredo, J.M.; Wilson, J.A.; Eliasen, A.M.; LaBerge, N.A.; Nason, M.; Cantu, A.L.; Luton, B.K.; Xu, S.; et al. Positron Emission Tomography Tracer Design of Targeted Synthetic Peptides via 18F-Sydnone Alkyne Cycloaddition. Bioconjug. Chem. 2021, 32, 2073–2082. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, X.; Zhang, X.; Xiong, Q.; Jiang, S.; Yu, Z. Synthesis and evaluation of photo-activatable β-diarylsydnone-l-alanines for fluorogenic photo-click cyclization of peptides. Org. Biomol. Chem. 2019, 17, 6777–6781. [Google Scholar] [CrossRef]
- Li, S.; Cai, H.; He, J.; Chen, H.; Lam, S.; Cai, T.; Zhu, Z.; Bark, S.J.; Cai, C. Extent of the Oxidative Side Reactions to Peptides and Proteins During the CuAAC Reaction. Bioconjug. Chem. 2016, 27, 2315–2322. [Google Scholar] [CrossRef]
- Pischetsrieder, M. Reaction of L-Ascorbic Acid with L-Arginine Derivatives. J. Agric. Food Chem. 1996, 44, 2081–2085. [Google Scholar] [CrossRef]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. Engl. 2009, 48, 9879–9883. [Google Scholar] [CrossRef] [PubMed]
- Maujean, T.; Marchand, P.; Wagner, P.; Riché, S.; Boisson, F.; Girard, N.; Bonnet, D.; Gulea, M. Hetero-Diels-Alder Click Reaction of Dithioesters for a Catalyst-Free Indirect 18F-Radiolabelling of Peptides. Chem. Commun. 2022, 58, 11151–11154. [Google Scholar] [CrossRef]
- Qian, Y.; Schürmann, M.; Janning, P.; Hedberg, C.; Waldmann, H. Activity-Based Proteome Profiling Probes Based on Woodward’s Reagent K with Distinct Target Selectivity. Angew. Chem. Int. Ed. 2016, 55, 7766–7771. [Google Scholar] [CrossRef]
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front. Physiol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Gerbier, R.; Alvear-Perez, R.; Margathe, J.-F.; Flahault, A.; Couvineau, P.; Gao, J.; De Mota, N.; Dabire, H.; Li, B.; Ceraudo, E.; et al. Development of original metabolically stable apelin-17 analogs with diuretic and cardiovascular effects. FASEB J. 2017, 31, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Galvão, I.; Mastrippolito, D.; Talamini, L.; Aganetti, M.; Rocha, V.; Verdot, C.; Mendes, V.; Soares De Oliveira, V.L.; Dias Braga, A.; Dantas, V.; et al. The therapeutic effect of phosphopeptide P140 attenuates inflammation induced by uric acid crystals in gout arthritis mouse model. Cells 2022, 11, 3709. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Wang, F.; Tasset, I.; Schall, N.; Page, N.; Briand, J.P.; Cuervo, A.M.; Muller, S. Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide. Autophagy 2015, 11, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Maujean, T.; Wagner, P.; Valencia, C.; Riché, S.; Iturrioz, X.; Villa, P.; Girard, N.; Karpenko, J.; Gulea, M.; Bonnet, D. Rapid and Highly Selective Fluorescent Labeling of Peptides via a Thia-Diels-Alder Cycloaddition: Application to Apelin. Bioconjug. Chem. 2023, 34, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Louis, B.; Nail, V.; Nachar, O.; Bouhlel, A.; Moyon, A.; Balasse, L.; Simoncini, S.; Chabert, A.; Fernandez, S.; Brige, P.; et al. Design and preclinical evaluation of a novel apelin-based PET radiotracer targeting APJ receptor for molecular imaging of angiogenesis. Angiogenesis 2023, 3, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Drake, C.; Ippisch, R.C.; Moore, M.; Sutcliffe, J.L. Fully automated peptide radiolabeling from [18F] fluoride. RSC Adv. 2019, 9, 8638–8649. [Google Scholar] [CrossRef] [PubMed]
- Axispharma. Available online: https://axispharm.com/product-category/peg-linkers/propargyl-peg/ (accessed on 14 May 2024).
- Broadpharma. Available online: https://broadpharm.com/product-categories/click-chemistry-reagents (accessed on 14 May 2024).
- Cao, J.; Liu, Y.; Zhang, L.; Du, F.; Ci, Y.; Zhang, Y.; Xiao, H.; Yao, X.; Shi, S.; Zhu, L.; et al. Synthesis of novel PEG-modified nitroimidazole derivatives via “hot-click” reaction and their biological evaluation as potential PET imaging agent for tumors. J. Radioanal. Nucl. Chem. 2017, 312, 263–276. [Google Scholar] [CrossRef]
- Schieferstein, H.; Betzel, T.; Fischer, C.R.; Ross, T.L. 18F-click labeling and preclinical evaluation of a new 18F-folate for PET imaging. EJNMMI Res. 2013, 3, 68. [Google Scholar] [CrossRef]
- Li, Z.-B.; Wu, Z.; Chen, K.; Chin, F.T.; Chen, X. Click Chemistry for 18F-Labeling of RGD Peptides and microPET Imaging of Tumor Integrin αvβ3 Expression. Bioconjug. Chem. 2007, 18, 1987–1994. [Google Scholar] [CrossRef]
- Jourdain de Muizon, C.; Ramanoudjame, S.M.; Esteoulle, L.; Ling, C.; Brou, G.; Anton, N.; Vandamme, T.; Delsuc, M.A.; Bonnet, D.; Kieffer, B. Self-organization Properties of a GPCR-Binding Peptide with a Fluorinated Tail Studied by Fluorine NMR Spectroscopy. Chembiochem 2021, 22, 657–661. [Google Scholar] [CrossRef] [PubMed]

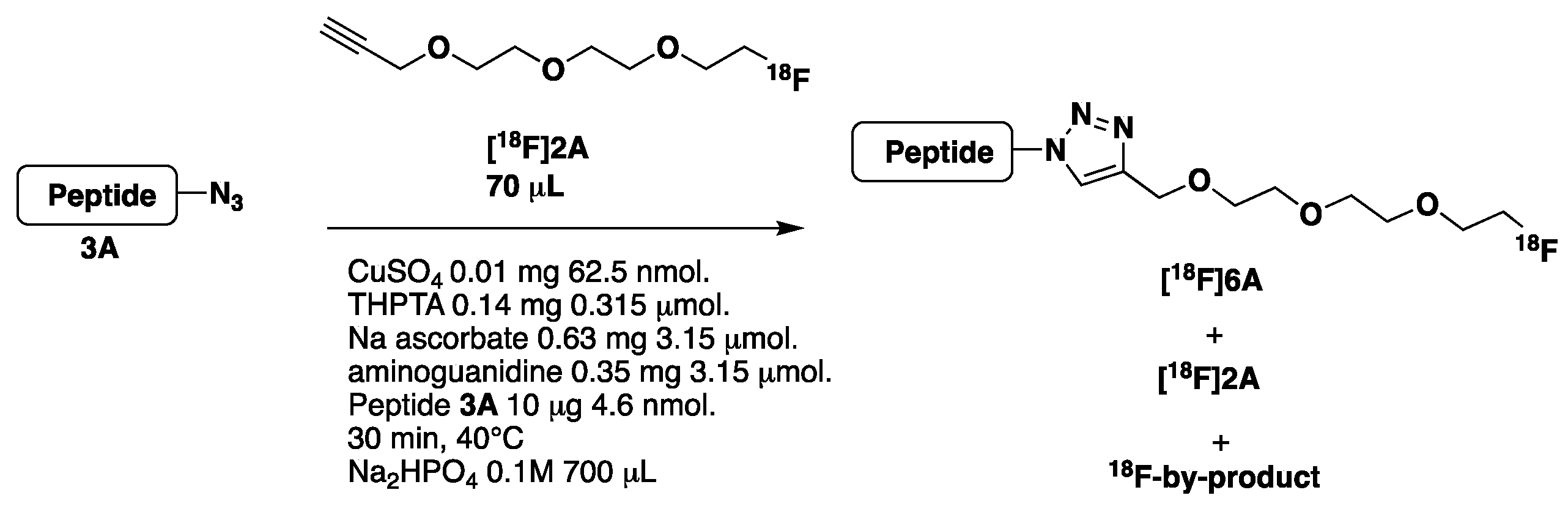
| Entry | Reagents | [18F]6A 3 Rt = 13.3 min | [18F]2A 3 Rt = 15.7 min | 18F-by-Product 3 Rt =11.9 min |
|---|---|---|---|---|
| 1 | CuSO4, THPTA, Na Ascorbate, Aminoguanidine, peptide 3A | 9.7% | 0.15% | 90.2% |
| 2 1 | CuSO4, THPTA, Na Ascorbate, Aminoguanidine 1 | 0% | 1.2% | 98.8% |
| 3 2 | CuSO4, Na Ascorbate, Aminoguanidine 2 | 0% | 91.8% | 0% |
| Method | Step 1 | Step 2 | Mean Isolated Yield DC 1 (NDC) 2 | Time (min) |
|---|---|---|---|---|
| CuAAC | 1A 3 mg CH3CN/DMSO, 10 min 115 °C HPLC + C18 Sep-Pak and evaporation | Peptide 3A, 4A, 5A, 0.3–0.5 mg/[18F]-2A CuSO4 (0.8 mg), Na Ascorbate (6.3 mg) aminoguanidine (3.6 mg), sodium gentisate (2 mg) H2O/DMF (90/10 V =1.2 mL) or PB 3 0.05 M (1.2 mL) 55 °C, 30 min C18 Sep-Pak purification | [18F]6A 47% (22%) n = 7 [18F]7A 37% (17.5%) n = 3 [18F]8A 30% (13.5%) n = 5 mean DC 39.3% n = 15 | 120 |
| HDA | 1B 5 mg CH3CN, 10 min 110 °C HPLC + C18 Sep-Pak | Peptide 3B, 4B 4, 5B, 2.5–3.5 mg/[18F]-2B H2O/EtOH (70/30, V = 5.2 mL) HPLC + C18 Sep-Pak | [18F]6B 30% (12.7%) n = 3 [18F]7B 38.7% (15%) n = 4 [18F]8B 40% (14.8%) n = 6 mean DC 37.6% n = 13 | 145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maujean, T.; Ramanoudjame, S.M.; Riché, S.; Le Guen, C.; Boisson, F.; Muller, S.; Bonnet, D.; Gulea, M.; Marchand, P. Hetero-Diels–Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods. Molecules 2024, 29, 3198. https://doi.org/10.3390/molecules29133198
Maujean T, Ramanoudjame SM, Riché S, Le Guen C, Boisson F, Muller S, Bonnet D, Gulea M, Marchand P. Hetero-Diels–Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods. Molecules. 2024; 29(13):3198. https://doi.org/10.3390/molecules29133198
Chicago/Turabian StyleMaujean, Timothé, Sridévi M. Ramanoudjame, Stéphanie Riché, Clothilde Le Guen, Frédéric Boisson, Sylviane Muller, Dominique Bonnet, Mihaela Gulea, and Patrice Marchand. 2024. "Hetero-Diels–Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods" Molecules 29, no. 13: 3198. https://doi.org/10.3390/molecules29133198
APA StyleMaujean, T., Ramanoudjame, S. M., Riché, S., Le Guen, C., Boisson, F., Muller, S., Bonnet, D., Gulea, M., & Marchand, P. (2024). Hetero-Diels–Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods. Molecules, 29(13), 3198. https://doi.org/10.3390/molecules29133198







