ROS Scavenging Effect of Selected Isoflavones in Provoked Oxidative Stress Conditions in Human Skin Fibroblasts and Keratinocytes
Abstract
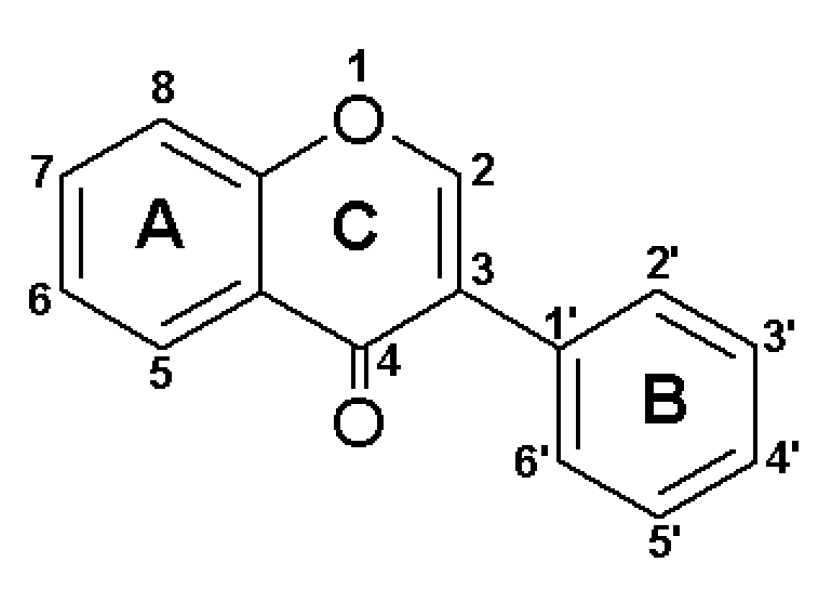
1. Introduction
2. Results and Discussion
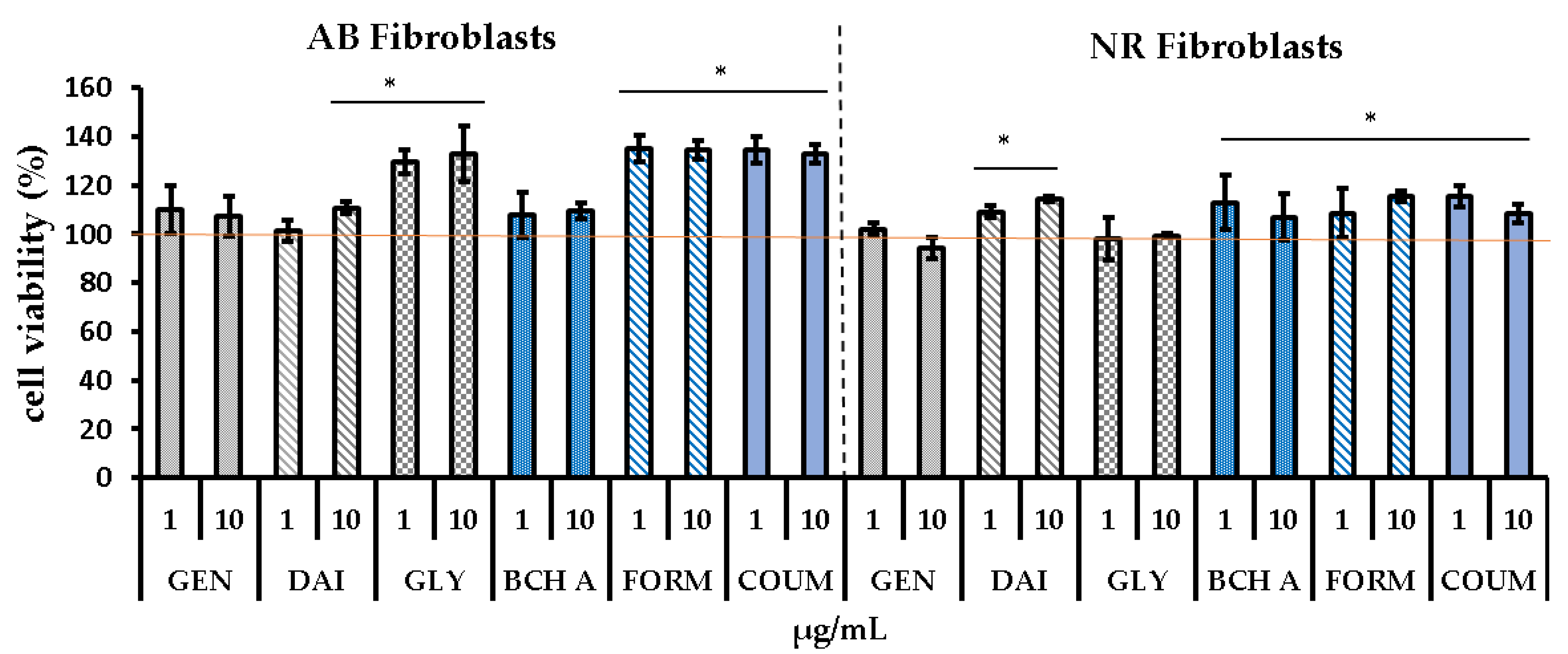
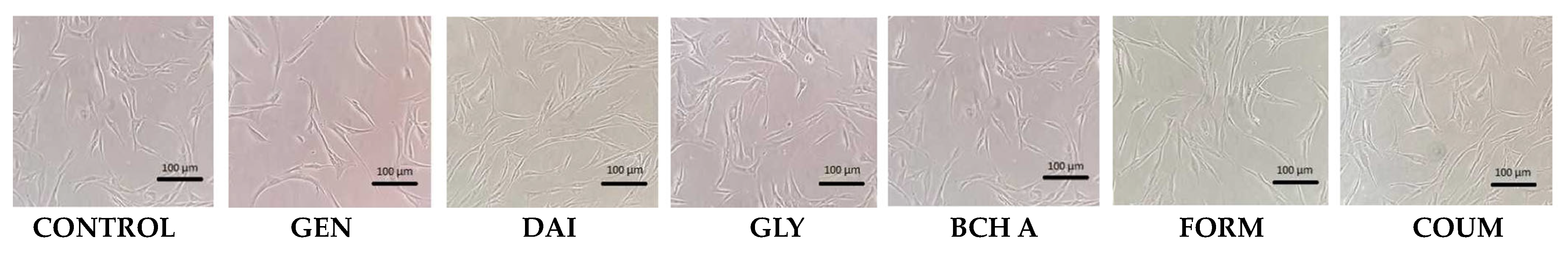
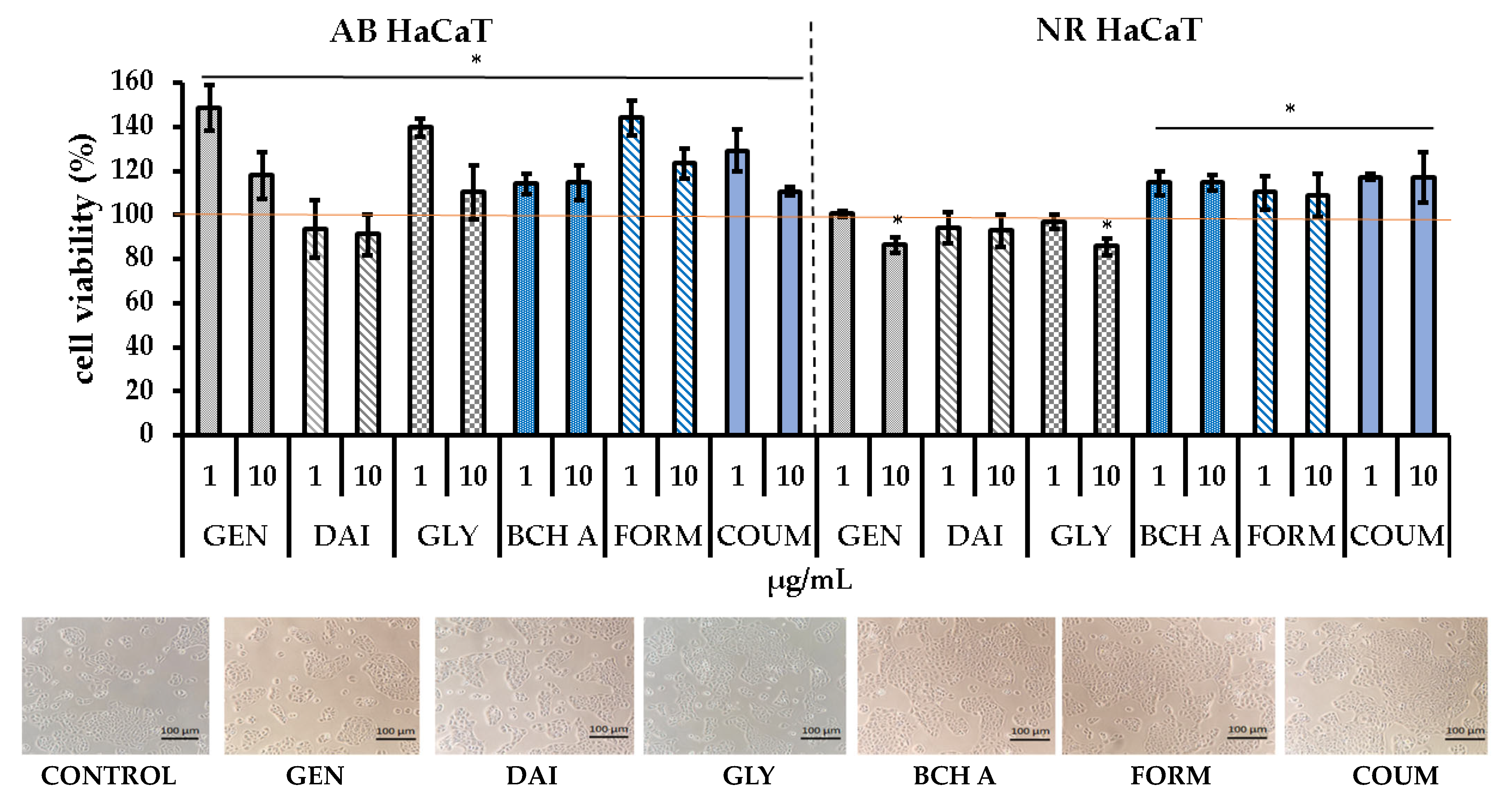
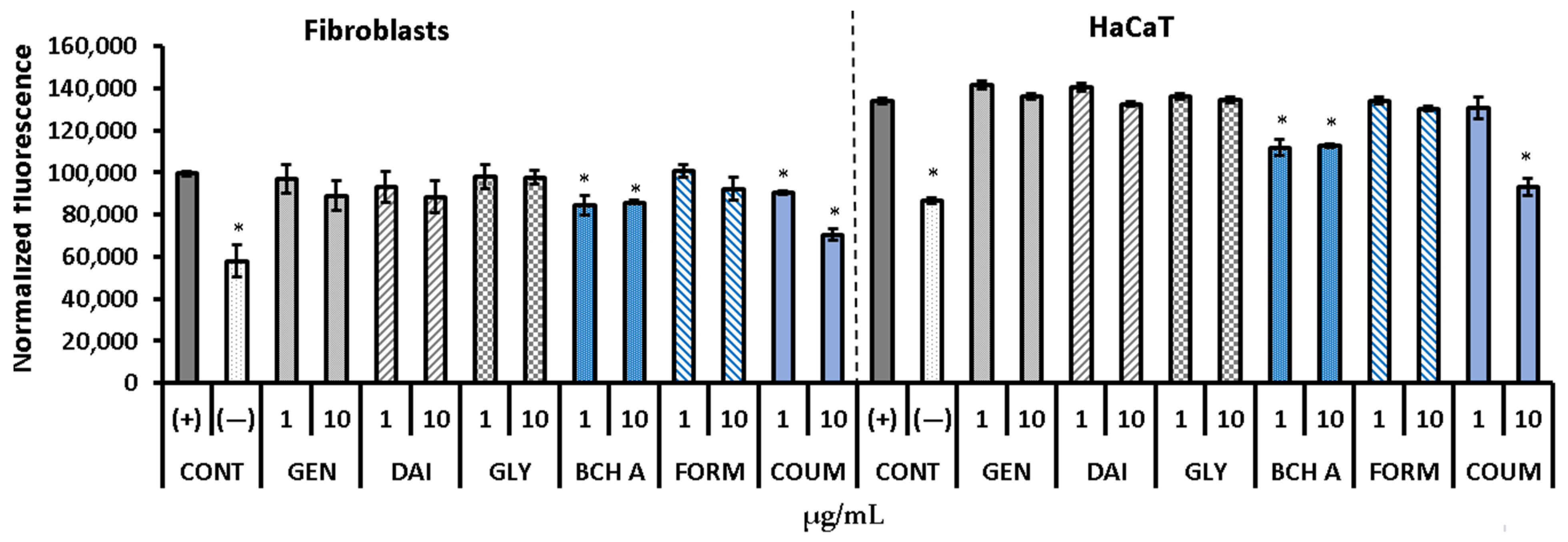
2.1. Cytotoxicity Assessment
2.2. Assessment of Antioxidant Activity
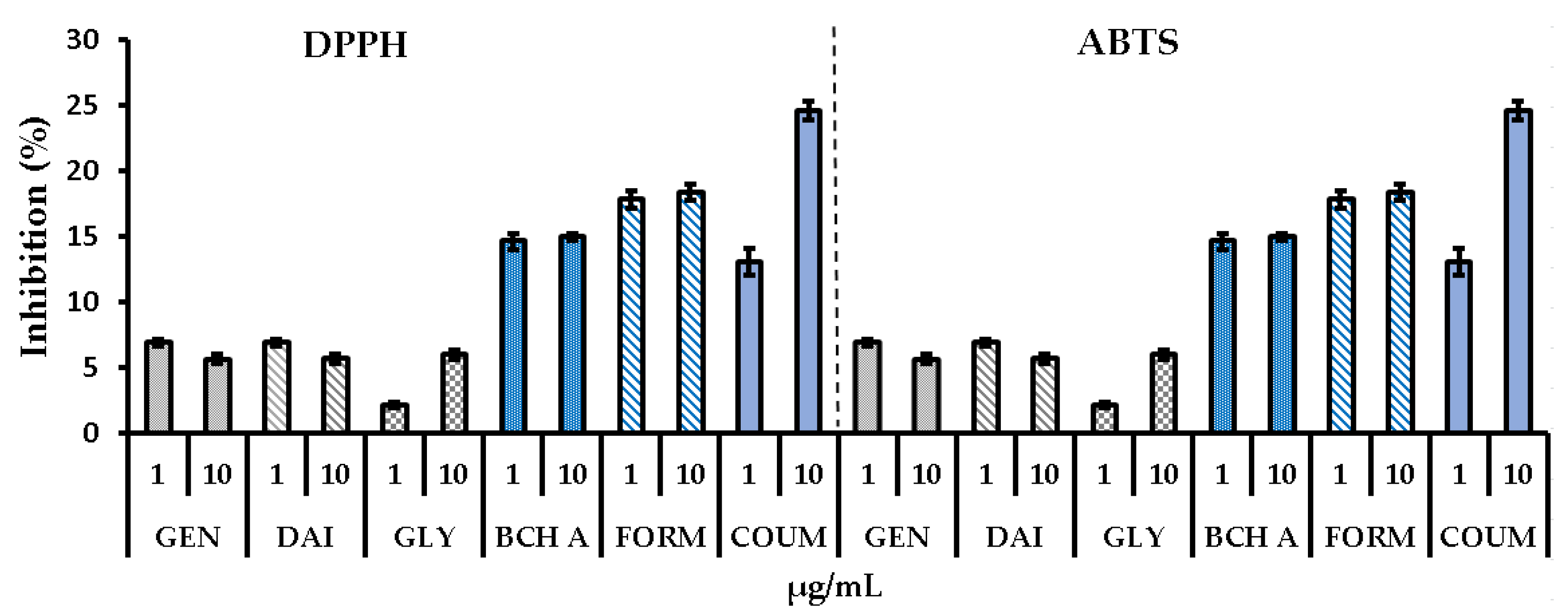
2.2.1. DPPH, ABTS, and FRAP Tests
2.2.2. Intracellular Reactive Oxygen Species (ROS) Levels
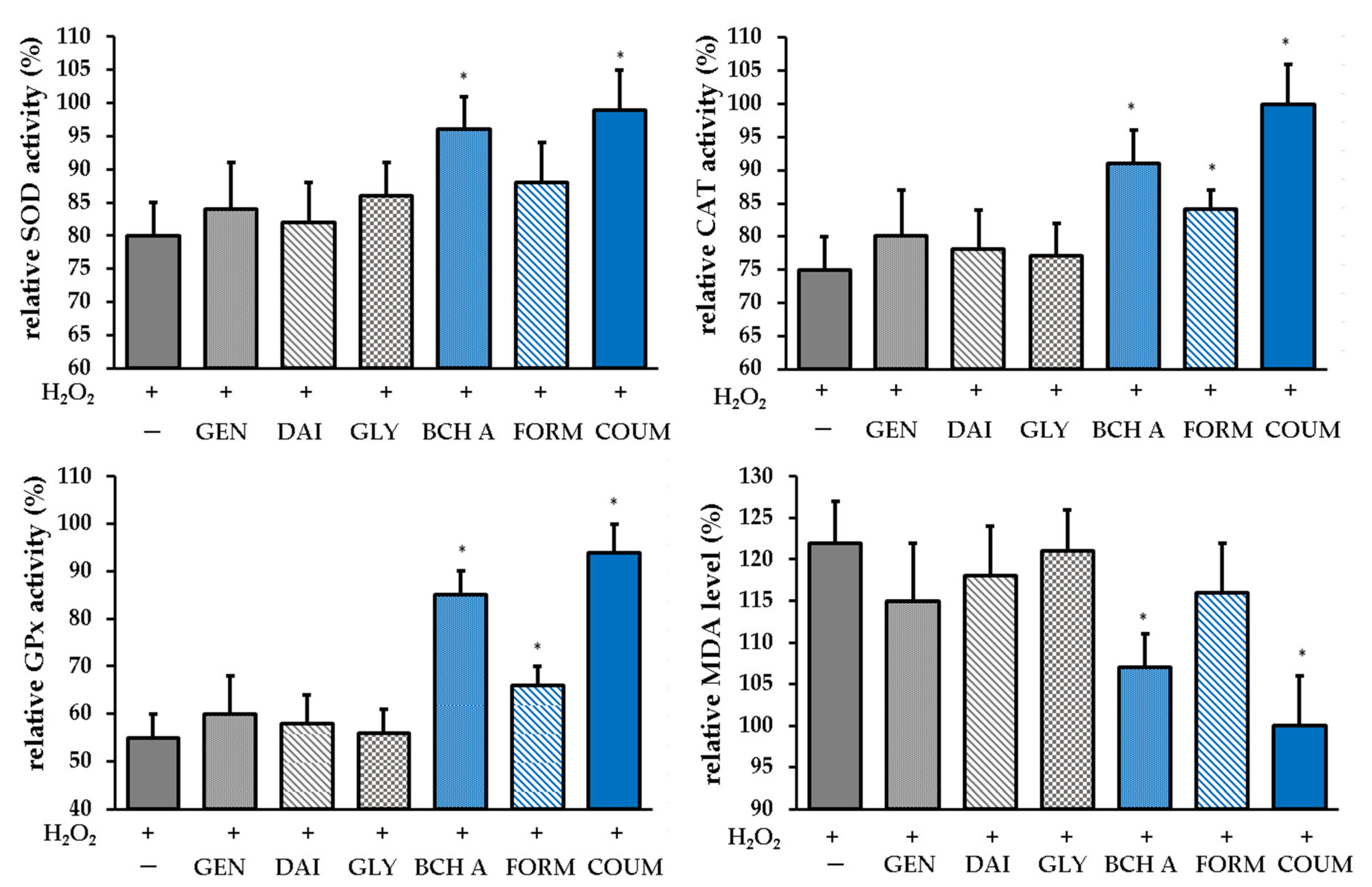
2.2.3. Antioxidant Enzyme Activity
3. Materials and Methods
3.1. Materials and Equipment
3.2. Cytotoxicity Analysis
3.2.1. Cell Culture
3.2.2. Alamar Blue Assay
3.2.3. Neutral Red Uptake Assay
3.3. Determination of Antioxidant Properties
3.3.1. DPPH and ABTS Scavenging Assays
3.3.2. Ferric Ion Reducing/Antioxidant Power (FRAP) Assay
3.3.3. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
3.3.4. Determination of Antioxidant Enzyme Activity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones Derived from Plant Raw Materials: Bioavailability, Anti-Cancer, Anti-Aging Potentials, and Microbiome Modulation. Crit. Rev. Food Sci. Nutr. 2023, 63, 261–287. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.; Zhang, H.; Liu, Z. Recent Advances in the Anti-aging Effects of Phytoestrogens on Collagen, Water Content, and Oxidative Stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Polito, F.; Marini, H.; Bitto, A.; Irrera, N.; Vaccaro, M.; Adamo, E.B.; Micali, A.; Squadrito, F.; Minutoli, L.; Altavilla, D. Genistein Aglycone, a Soy-derived Isoflavone, Improves Skin Changes Induced by Ovariectomy in Rats. Br. J. Pharmacol. 2012, 165, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-R.; Hung, C.-F.; Lin, Y.-K.; Fang, J.-Y. In Vitro and in Vivo Evaluation of Topical Delivery and Potential Dermal Use of Soy Isoflavones Genistein and Daidzein. Int. J. Pharm. 2008, 364, 36–44. [Google Scholar] [CrossRef]
- Moore, J.O.; Wang, Y.; Stebbins, W.G.; Gao, D.; Zhou, X.; Phelps, R.; Lebwohl, M.; Wei, H. Photoprotective Effect of Isoflavone Genistein on Ultraviolet B-Induced Pyrimidine Dimer Formation and PCNA Expression in Human Reconstituted Skin and Its Implications in Dermatology and Prevention of Cutaneous Carcinogenesis. Carcinogenesis 2006, 27, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Monfrecola, G.; Bevilacqua, M.A. Synergic Effect of Genistein and Daidzein on UVB-Induced DNA Damage: An Effective Photoprotective Combination. J. Biomed. Biotechnol. 2011, 2011, 692846. [Google Scholar] [CrossRef]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Giannini, V.; Monfrecola, G.; Bevilacqua, M.A. A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms. Int. J. Mol. Sci. 2012, 13, 16444–16456. [Google Scholar] [CrossRef]
- Chiu, T.-M.; Huang, C.-C.; Lin, T.-J.; Fang, J.-Y.; Wu, N.-L.; Hung, C.-F. In Vitro and in Vivo Anti-Photoaging Effects of an Isoflavone Extract from Soybean Cake. J. Ethnopharmacol. 2009, 126, 108–113. [Google Scholar] [CrossRef]
- Brand, R.M.; Jendrzejewski, J.L. Topical Treatment with (−)-Epigallocatechin-3-Gallate and Genistein after a Single UV Exposure Can Reduce Skin Damage. J. Dermatol. Sci. 2008, 50, 69–72. [Google Scholar] [CrossRef]
- Shyong, E.Q.; Lu, Y.; Lazinsky, A.; Saladi, R.N.; Phelps, R.G.; Austin, L.M.; Lebwohl, M.; Wei, H. Effects of the Isoflavone 4′,5,7-Trihydroxyisoflavone (Genistein) on Psoralen plus Ultraviolet A Radiation (PUVA)-Induced Photodamage. Carcinogenesis 2002, 23, 317–321. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hanamizu, T.; Sone, T.; Chiba, K.; Kinoshita, T.; Yoshikawa, S. Topical Application of Bifidobacterium-Fermented Soy Milk Extract Containing Genistein and Daidzein Improves Rheological and Physiological Properties of Skin. J. Cosmet. Sci. 2004, 55, 473–479. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hanamizu, T.; Iizuka, R.; Chiba, K. Bifidobacterium-Fermented Soy Milk Extract Stimulates Hyaluronic Acid Production in Human Skin Cells and Hairless Mouse Skin. Skin Pharmacol. Physiol. 2003, 16, 108–116. [Google Scholar] [CrossRef]
- Giorgini, S.; Greco, A.; Cristina Melli, M.; Lotti, T.M. Phytoestrogens in Dermatocosmetology. Clinical and Instrumental Trial Conducted on a Cream Made from Glicine and Other Bioflavonoids in Spontaneous Menopausal Women. Ital J Dermatol Venerol. 2006, 141, 409–414. [Google Scholar]
- Patriarca, M.T.; Barbosa De Moraes, A.R.; Nader, H.B.; Petri, V.; Martins, J.R.M.; Gomes, R.C.T.; Soares, J.M. Hyaluronic Acid Concentration in Postmenopausal Facial Skin after Topical Estradiol and Genistein Treatment: A Double-Blind, Randomized Clinical Trial of Efficacy. Menopause 2013, 20, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Ferraz Carbonel, A.A.; De Moraes, A.R.B.; Simões, R.S.; Sasso, G.R.D.S.; Goes, L.; Nunes, W.; Simões, M.J.; Patriarca, M.T. Collagen Concentration on the Facial Skin of Postmenopausal Women after Topical Treatment with Estradiol and Genistein: A Randomized Double-Blind Controlled Trial. Gynecol. Endocrinol. 2017, 33, 845–848. [Google Scholar] [CrossRef]
- Moraes, A.B.; Haidar, M.A.; Soares, J.M.; Simões, M.J.; Baracat, E.C.; Patriarca, M.T. The Effects of Topical Isoflavones on Postmenopausal Skin: Double-Blind and Randomized Clinical Trial of Efficacy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 188–192. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant Activity of Phytoestrogenic Isoflavones. Free Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant Activities of Isoflavones and Their Biological Metabolites in a Liposomal System. Arch. Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.G. Antioxidant Efficacy of Phytoestrogens in Chemical and Biological Model Systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Seo, D.B.; Shin, H.-J.; Lee, S.-J. Effect of Aspergillus Oryzae-Challenged Germination on Soybean Isoflavone Content and Antioxidant Activity. J. Agric. Food Chem. 2012, 60, 2807–2814. [Google Scholar] [CrossRef]
- Borawska, M.H.; Czechowska, S.K.; Markiewicz, R.; Hayirli, A.; Olszewska, E.; Sahin, K. Cell Viability of Normal Human Skin Fibroblast and Fibroblasts Derived from Granulation Tissue: Effects of Nutraceuticals. J. Med. Food 2009, 12, 429–434. [Google Scholar] [CrossRef]
- Sehdev, V.; Lai, J.C.K.; Bhushan, A. Biochanin A Modulates Cell Viability, Invasion, and Growth Promoting Signaling Pathways in HER-2-Positive Breast Cancer Cells. J. Oncol. 2009, 2009, 121458. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone Genistein: Photoprotection and Clinical Implications in Dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [CrossRef] [PubMed]
- Pawlicka, M.A.; Zmorzyński, S.; Popek-Marciniec, S.; Filip, A.A. The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 12360. [Google Scholar] [CrossRef] [PubMed]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and Immunosenescence as Part of Skin Aging—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic Pathway of Natural Antioxidants, Antioxidant Enzymes and ROS Providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Rüfer, C.E.; Kulling, S.E. Antioxidant Activity of Isoflavones and Their Major Metabolites Using Different in Vitro Assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Relative Antioxidant Activity of Soybean Isoflavones and Their Glycosides. Food Chem. 2005, 90, 735–741. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Duchnik, E.; Kruk, J.; Baranowska-Bosiacka, I.; Pilutin, A.; Maleszka, R.; Marchlewicz, M. Effects of the Soy Isoflavones, Genistein and Daidzein, on Male Rats’ Skin. Adv. Dermatol. Allergol. 2019, 36, 760–766. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wu, W.; Chen, H.C.; Fang, H. Genistein Protects against UVB-Induced Senescence-like Characteristics in Human Dermal Fibroblast by p66Shc down-Regulation. J. Dermatol. Sci. 2010, 58, 19–27. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements In-Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity Determined in Vitro by Morphological Alterations and Neutral Red Absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Sowa, I.; Szczepanek, D.; Wójciak, M. Comparative Study of Cytotoxicity and Antioxidant, Anti-Aging and Antibacterial Properties of Unfermented and Fermented Extract of Cornus Mas L. Int. J. Mol. Sci. 2023, 24, 13232. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Paduch, R.; Mołdoch, J.; Szczepanek, D.; Szkutnik, J.; Sowa, P.; Tyszczuk-Rotko, K.; Blicharski, T.; Wójciak, M. Antioxidant and Cytotoxic Potential of Carlina Vulgaris Extract and Bioactivity-Guided Isolation of Cytotoxic Components. Antioxidants 2023, 12, 1704. [Google Scholar] [CrossRef] [PubMed]
- Evangelista-Vargas, S.; Santiani, A. Detection of Intracellular Reactive Oxygen Species (Superoxide Anion and Hydrogen Peroxide) and Lipid Peroxidation during Cryopreservation of Alpaca Spermatozoa. Reprod. Domest. Anim. 2017, 52, 819–824. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, M.; Drozdowski, P.; Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z.; Kubrak, T.; Sowa, I. ROS Scavenging Effect of Selected Isoflavones in Provoked Oxidative Stress Conditions in Human Skin Fibroblasts and Keratinocytes. Molecules 2024, 29, 955. https://doi.org/10.3390/molecules29050955
Wójciak M, Drozdowski P, Ziemlewska A, Zagórska-Dziok M, Nizioł-Łukaszewska Z, Kubrak T, Sowa I. ROS Scavenging Effect of Selected Isoflavones in Provoked Oxidative Stress Conditions in Human Skin Fibroblasts and Keratinocytes. Molecules. 2024; 29(5):955. https://doi.org/10.3390/molecules29050955
Chicago/Turabian StyleWójciak, Magdalena, Piotr Drozdowski, Aleksandra Ziemlewska, Martyna Zagórska-Dziok, Zofia Nizioł-Łukaszewska, Tomasz Kubrak, and Ireneusz Sowa. 2024. "ROS Scavenging Effect of Selected Isoflavones in Provoked Oxidative Stress Conditions in Human Skin Fibroblasts and Keratinocytes" Molecules 29, no. 5: 955. https://doi.org/10.3390/molecules29050955
APA StyleWójciak, M., Drozdowski, P., Ziemlewska, A., Zagórska-Dziok, M., Nizioł-Łukaszewska, Z., Kubrak, T., & Sowa, I. (2024). ROS Scavenging Effect of Selected Isoflavones in Provoked Oxidative Stress Conditions in Human Skin Fibroblasts and Keratinocytes. Molecules, 29(5), 955. https://doi.org/10.3390/molecules29050955






