Atractylenolide II Suppresses Glycolysis and Induces Apoptosis by Blocking the PADI3-ERK Signaling Pathway in Endometrial Cancer Cells
Abstract
1. Introduction
2. Results
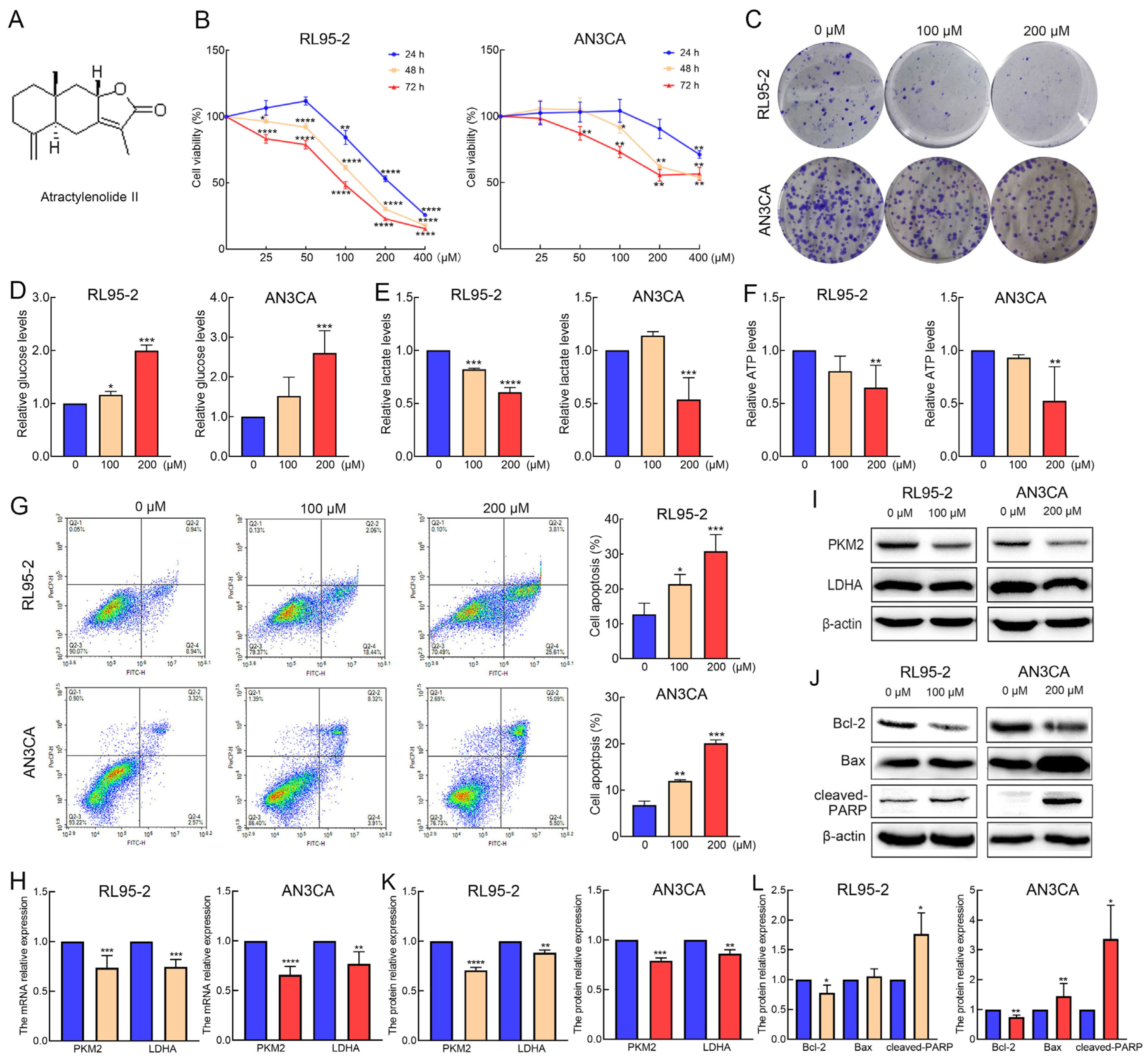
2.1. AT-II Suppresses Proliferation and Glycolysis in Endometrial Cancer Cells and Induces Their Apoptosis
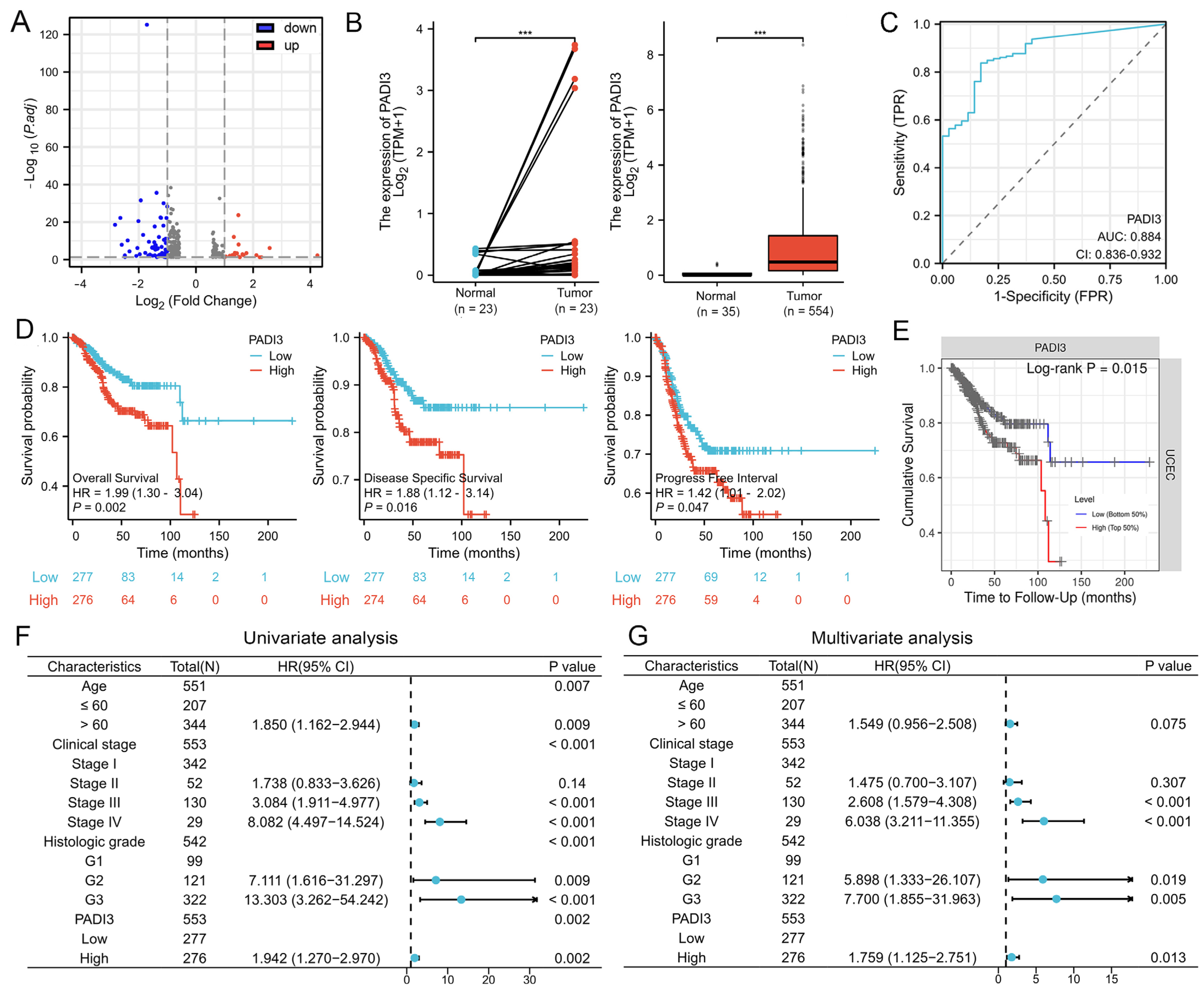
2.2. PADI3 Shows Good Prognostic Value and May Serve as a Novel Clinical Indicator for Endometrial Cancer
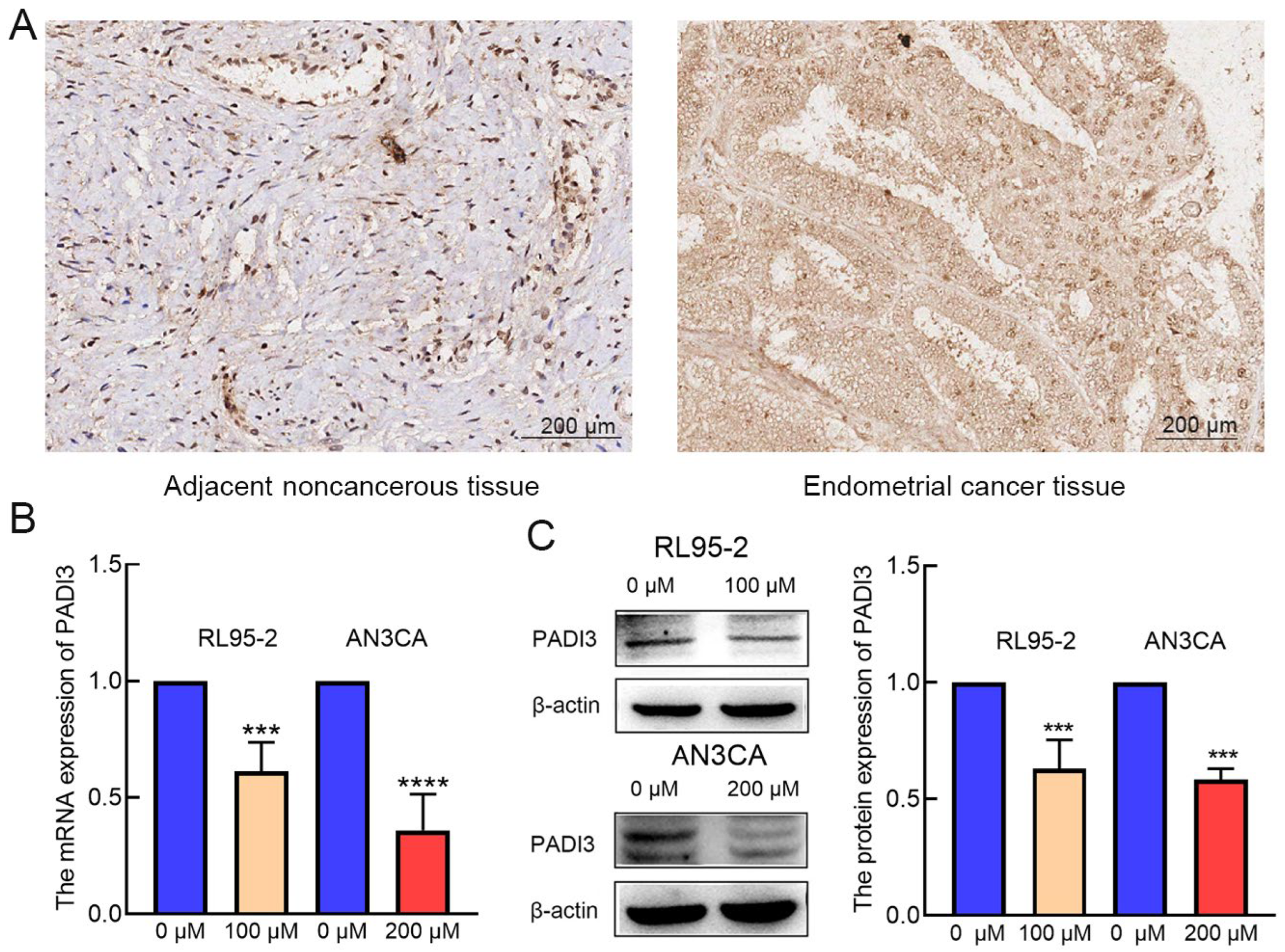
2.3. PADI3 Was Negatively Regulated by AT-II in RL95-2 and AN3CA Cells
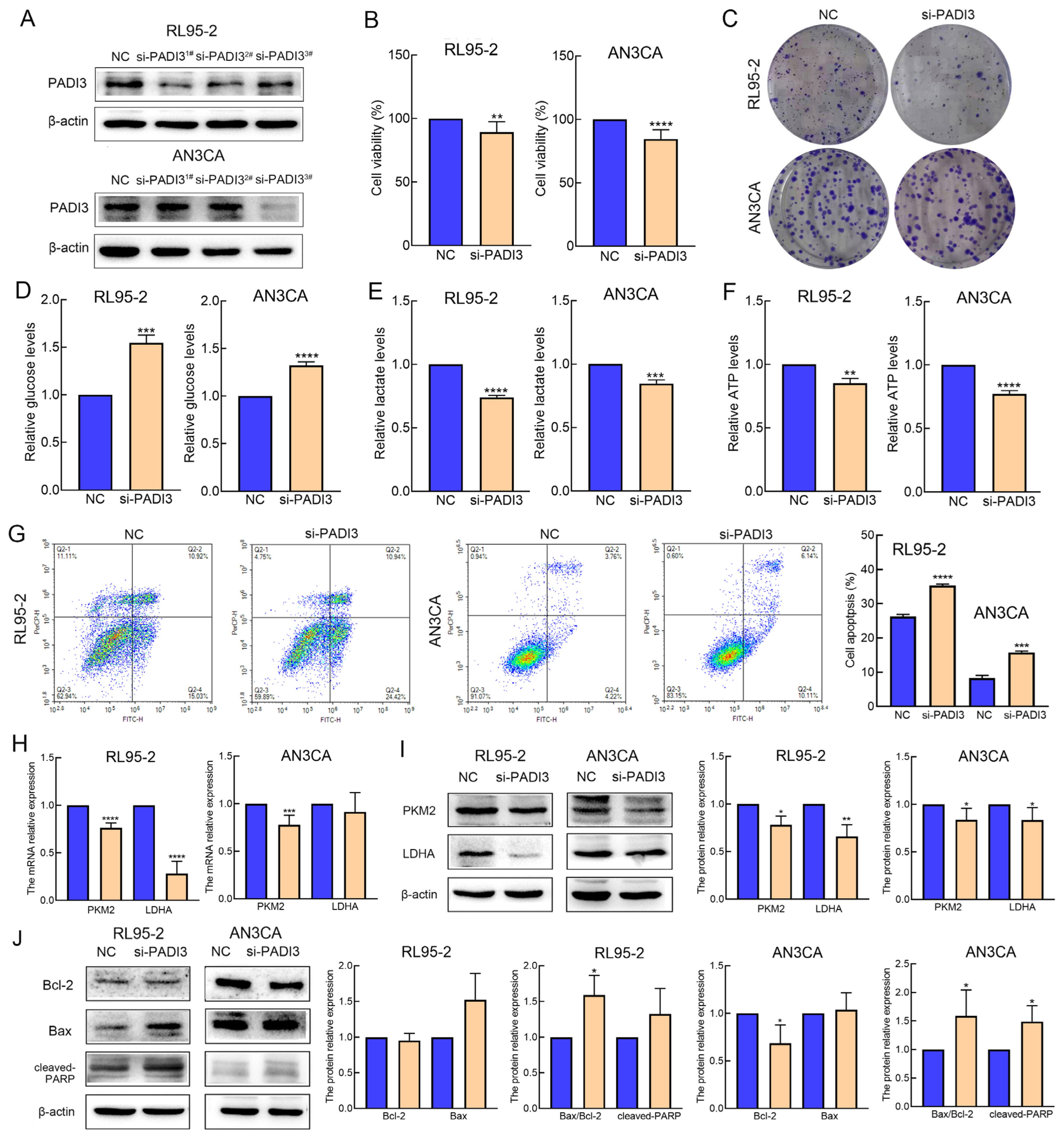
2.4. Knockdown of PADI3 Inhibits Proliferation and Glycolysis in RL95-2 and AN3CA Cells and Induces Their Apoptosis
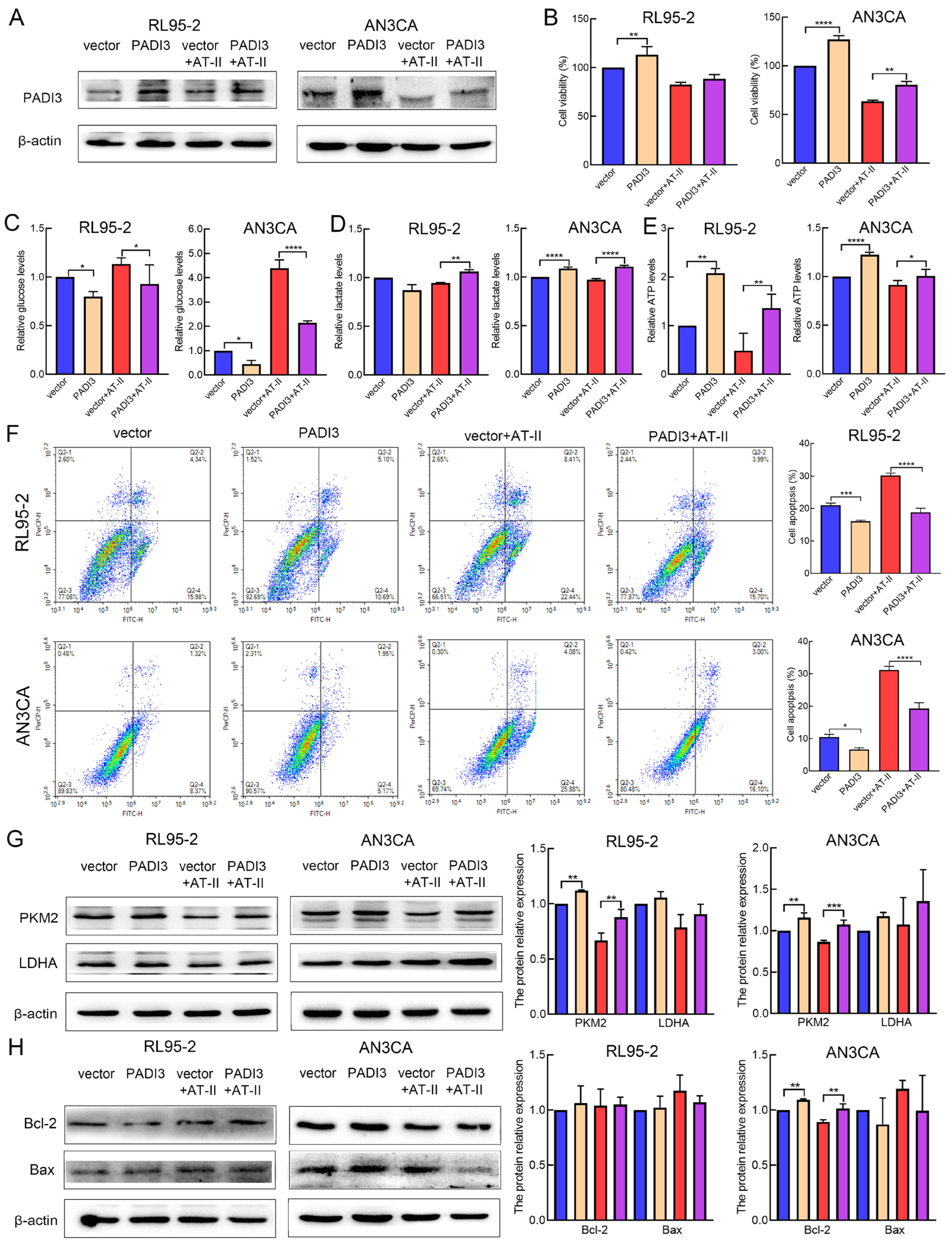
2.5. Overexpression of PADI3 Reverses the Roles of AT-II in RL95-2 and AN3CA Cells
2.6. AT-II Inhibits Glycolysis and Induces Apoptosis in Human Endometrial Cancer Cells by Inactivating the ERK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Glycolysis Analysis
4.6. Cell Apoptosis Assay
4.7. Western Blot
4.8. RNA Sequencing
4.9. Bioinformatics Analysis
4.10. Tissue Microarray and Immunohistochemistry Staining
4.11. Real-Time PCR
4.12. PADI3 siRNA and Overexpression Plasmid
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sobel, M.; Simpson, A.N.; Ferguson, S.E. Endometrial cancer. Can. Med. Assoc. J. 2021, 193, E1423. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Mathieu, L.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Zheng, R.S.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Gallo, A.; Catena, U.; Saccone, G.; Sardo, A.D.S. Conservative surgery in endometrial cancer. J. Clin. Med. 2021, 11, 183. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, Z.Y.; Han, J.; Cheng, W.M.; Su, B.; Mo, J.; Feng, X.J.; Feng, S.T.; Chen, G.J.; Huang, P.; et al. Antinociceptive effects of Shenling Baizhu through PI3K-Akt-mTOR signaling pathway in a mouse model of bone metastasis with small-cell lung cancer. Evid.-Based. Complement. Altern. 2020, 2020, 4121483. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, H.J.; Long, J.Y.; Song, J.W.; Xie, L.; Li, X.F. Atractylenolides (I, II, and III): A review of their pharmacology and pharmacokinetics. Arch. Pharm. Res. 2021, 44, 633–654. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Liu, Y.X.; Wang, J.G.; Jiang, Z.Y.; Zhang, L.; Cui, Y.; Zhao, D.Y.; Wang, Y.J. Atractylenolide II inhibits tumor-associated macrophages (TAMs)-induced lung cancer cell metastasis. Immunopharm. Immunot. 2022, 44, 227–237. [Google Scholar] [CrossRef]
- Dou, S.H.; Yang, C.; Zou, D.F.; Da, W.; Masood, M.; Adlat, S.; Baima, Y.J.; Nasser, M.I.; Li, B.; Jiang, N. Atractylenolide II induces cell cycle arrest and apoptosis in breast cancer cells through ER pathway. Pak. J. Pharm. Sci. 2021, 34, 1449–1458. [Google Scholar] [PubMed]
- Zhang, R.J.; Wang, Z.J.; Yu, Q.Y.; Shen, J.; He, W.J.; Zhou, D.Q.; Yu, Q.Q.; Fan, J.W.; Gao, S.R.; Duan, L.H. Atractylenolide II reverses the influence of lncRNA XIST/miR-30a-5p/ROR1 axis on chemo-resistance of colorectal cancer cells. J. Cell Mol. Med. 2019, 23, 3151–3165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nasser, M.I.; Adlat, S.; Jiang, M.M.; Jiang, N.; Gao, L. Atractylenolide II induces apoptosis of prostate cancer cells through regulation of AR and JAK2/STAT3 signaling pathways. Molecules 2018, 23, 3298. [Google Scholar] [CrossRef]
- Tian, S.; Yu, H.D. Atractylenolide II inhibits proliferation, motility and induces apoptosis in human gastric carcinoma cell lines HGC-27 and AGS. Molecules 2017, 22, 1886. [Google Scholar] [CrossRef]
- Fu, X.Q.; Chou, G.X.; Kwan, H.Y.; Tse, A.K.W.; Zhao, L.H.; Yuen, T.K.; Gao, H.H.; Yu, H.; Chao, X.J.; Su, T.; et al. Inhibition of STAT3 signalling contributes to the antimelanoma action of atractylenolide II. Exp. Dermatol. 2014, 23, 855–857. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lai, Y.J.; Chou, Y.C.; Lin, Y.J.; Yu, M.H.; Ou, Y.C.; Chu, P.W.; Wu, C.C.; Wang, Y.C.; Chao, T.K. Pyruvate Kinase M2 expression: A potential metabolic biomarker to differentiate endometrial precancer and cancer that is associated with poor outcomes in endometrial carcinoma. Int. J. Environ. Res. Public Health 2019, 16, 4589. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liu, H.; Liu, Z.X.; Ding, Y.; Ledoux, S.P.; Wilson, G.L.; Voellmy, R.; Lin, Y.F.; Lin, W.S.; Nahta, R.; et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011, 71, 4585–4597. [Google Scholar] [CrossRef]
- Coassolo, S.; Davidson, I. Regulation of glycolysis and cancer cell proliferation by PKM2 citrullination. Mol. Cell Oncol. 2021, 8, 1927446. [Google Scholar] [CrossRef]
- Coassolo, S.; Davidson, G.; Negroni, L.; Gambi, G.; Daujat, S.; Romier, C.; Davidson, I. Citrullination of pyruvate kinase M2 by PADI1 and PADI3 regulates glycolysis and cancer cell proliferation. Nat. Commun. 2021, 12, 1718. [Google Scholar] [CrossRef]
- Johnson, G.L.; Stuhlmiller, T.J.; Angus, S.P.; Zawistowski, J.S.; Graves, L.M. Molecular pathways: Adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer. Clin. Cancer Res. 2014, 20, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Giannoni, E.; Fiore, S.; Ferrari, K.J.; Fernández-Pérez, D.; Isella, C.; Granchi, C.; Minutolo, F.; Sottile, A.; Comoglio, P.M.; et al. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab. 2018, 28, 848–865.e6. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ai, Z.Y.; Chen, J.; Yi, J.; Liu, Z.; Zhao, H.S.; Wei, H.L. Glycometabolic adaptation mediates the insensitivity of drug-resistant K562/ADM leukaemia cells to adriamycin via the AKT-mTOR/c-Myc signalling pathway. Mol. Med. Rep. 2017, 15, 1869–1876. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, Y.P.; Liu, Z.X.; Guo, X.Q.; Miao, Z.W.; Ma, S.P. Atractylenolide I induces apoptosis and suppresses glycolysis by blocking the JAK2/STAT3 signaling pathway in colorectal cancer cells. Front. Pharmacol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Ma, Y.D.; Lai, X.H.; Wen, Z.L.; Zhou, Z.L.; Yang, M.K.; Chen, Q.Q.; Wang, X.; Mei, F.; Yang, L.; Yin, T.M.; et al. Design, synthesis and biological evaluation of novel modified dual-target shikonin derivatives for colorectal cancer treatment. Bioorg. Chem. 2023, 139, 106703. [Google Scholar] [CrossRef]
- Paul, S.K.; Chowdhury, K.D.; Dey, S.R.; Paul, A.; Haldar, R. Exploring the possibility of drug repurposing for cancer therapy targeting human lactate dehydrogenase A: A computational approach. J. Biomol. Struct. Dyn. 2023, 41, 9967–9976. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.M.; Huang, Z.B.; Tan, Y.F.; Zhang, C.Y.; Zhang, S.W.; Zhang, Y.; Zhang, X.P. A new labdane diterpenoid from Scoparia dulcis improving pancreatic function against islets cell apoptotic by Bax/Bcl-2/Caspase-3 pathway. J. Ethnopharmacol. 2023, 322, 117571. [Google Scholar] [CrossRef]
- Tilvawala, R.; Thompson, P.R. Peptidyl arginine deiminases: Detection and functional analysis of protein citrullination. Curr. Opin. Struct. Biol. 2019, 59, 205–215. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Zendman, A.J.W.; Venrooij, W.J.V.; Pruijin, G.J.M. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Yuzhalin, A.E. Citrullination in Cancer. Cancer Res. 2019, 79, 1274–1284. [Google Scholar] [CrossRef]
- Uysal-Onganer, P.; D′Alessio, S.; Mortoglou, M.; Kraev, L.; Lange, S. Peptidylarginine deiminase inhibitor application, using Cl-Amidine, PAD2, PAD3 and PAD4 isozyme-specific inhibitors in pancreatic cancer cells, reveals roles for PAD2 and PAD3 in cancer invasion and modulation of extracellular vesicle signatures. Int. J. Mol. Sci. 2021, 22, 1396. [Google Scholar] [CrossRef]
- Chang, X.T.; Chai, Z.B.; Zou, J.R.; Wang, H.X.; Wang, Y.; Zheng, Y.B.; Wu, H.; Liu, C.Y. PADI3 induces cell cycle arrest via the Sirt2/AKT/p21 pathway and acts as a tumor suppressor gene in colon cancer. Cancer Biol. Med. 2019, 16, 729–742. [Google Scholar] [CrossRef]
- Chai, Z.B.; Wang, L.; Zheng, Y.B.; Liang, N.; Wang, X.W.; Zheng, Y.Y.; Zhang, Z.W.; Zhao, C.X.; Zhu, T.T.; Liu, C.Y. PADI3 plays an antitumor role via the Hsp90/CKS1 pathway in colon cancer. Cancer Cell Int. 2019, 19, 277. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol. Res. 2019, 142, 151–168. [Google Scholar] [CrossRef]
- Hu, H.R.; Dong, Z.; Wang, X.X.; Bai, L.C.; Lei, Q.; Yang, J.; Li, L.; Li, Q.; Liu, L.C.; Zhang, Y.L.; et al. Dehydrocorydaline inhibits cell proliferation, migration and invasion via suppressing MEK1/2-ERK1/2 cascade in melanoma. OncoTargets Ther. 2019, 12, 5163–5175. [Google Scholar] [CrossRef]
- Xue, T.; Liu, X.Q.; Zhang, M.; E, Q.K.; Liu, S.T.; Zou, M.S.; Li, Y.; Ma, Z.N.; Han, Y.; Thompson, P.; et al. PADI2-catalyzed MEK1 citrullination activates ERK1/2 and promotes IGF2BP1-mediated SOX2 mRNA stability in endometrial cancer. Adv. Sci. 2021, 8, 2002831. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Ren, L.; Liu, C.; Wang, Z. Atractylenolide II Suppresses Glycolysis and Induces Apoptosis by Blocking the PADI3-ERK Signaling Pathway in Endometrial Cancer Cells. Molecules 2024, 29, 939. https://doi.org/10.3390/molecules29050939
Tian S, Ren L, Liu C, Wang Z. Atractylenolide II Suppresses Glycolysis and Induces Apoptosis by Blocking the PADI3-ERK Signaling Pathway in Endometrial Cancer Cells. Molecules. 2024; 29(5):939. https://doi.org/10.3390/molecules29050939
Chicago/Turabian StyleTian, Shuang, Lili Ren, Chao Liu, and Zhe Wang. 2024. "Atractylenolide II Suppresses Glycolysis and Induces Apoptosis by Blocking the PADI3-ERK Signaling Pathway in Endometrial Cancer Cells" Molecules 29, no. 5: 939. https://doi.org/10.3390/molecules29050939
APA StyleTian, S., Ren, L., Liu, C., & Wang, Z. (2024). Atractylenolide II Suppresses Glycolysis and Induces Apoptosis by Blocking the PADI3-ERK Signaling Pathway in Endometrial Cancer Cells. Molecules, 29(5), 939. https://doi.org/10.3390/molecules29050939




