Construction of Nanofibrillar Networked Wood Aerogels Derived from Typical Softwood and Hardwood: A Comparative Study on the In Situ Formation Mechanism of Nanofibrillar Networks
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of WAs
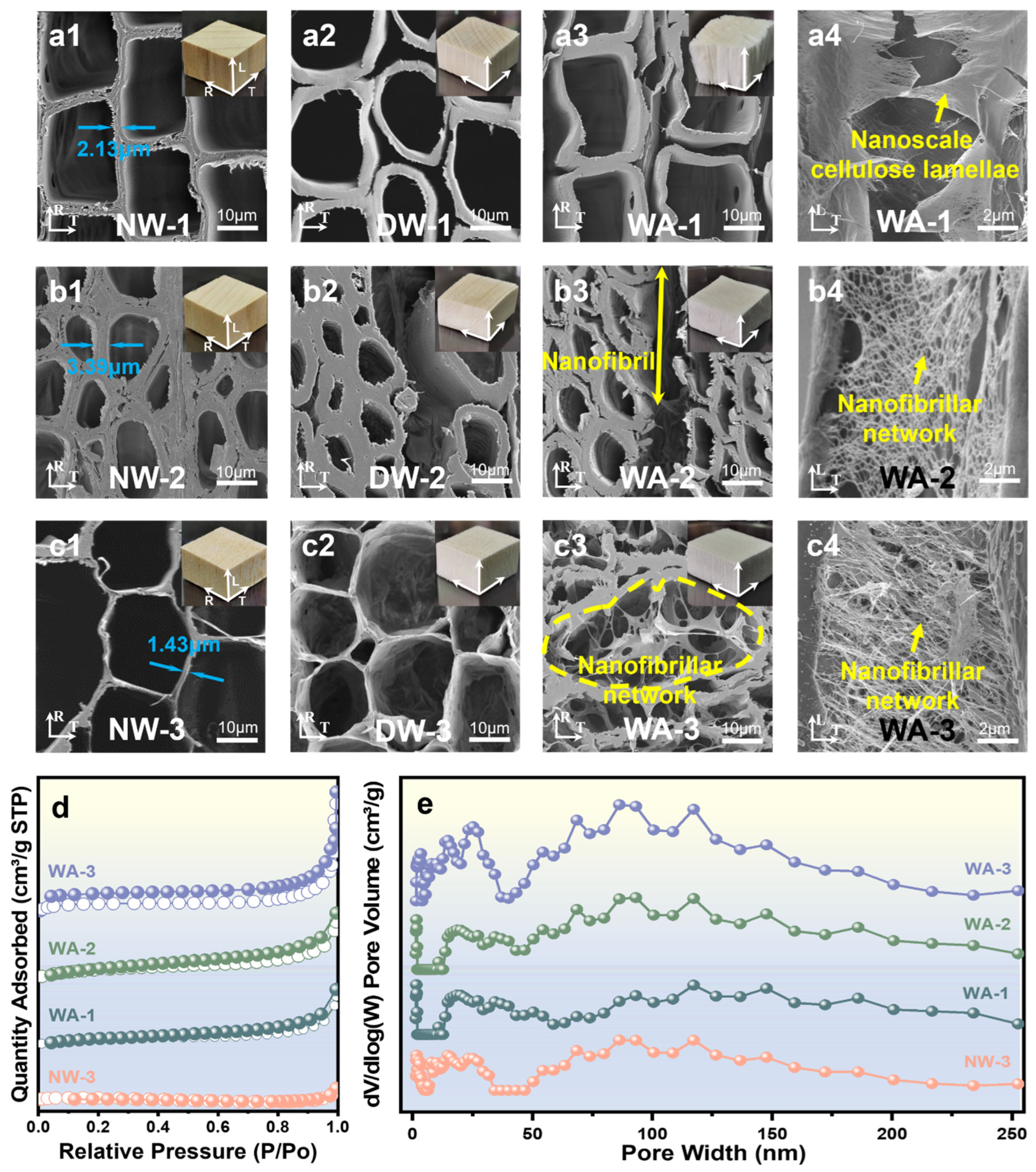
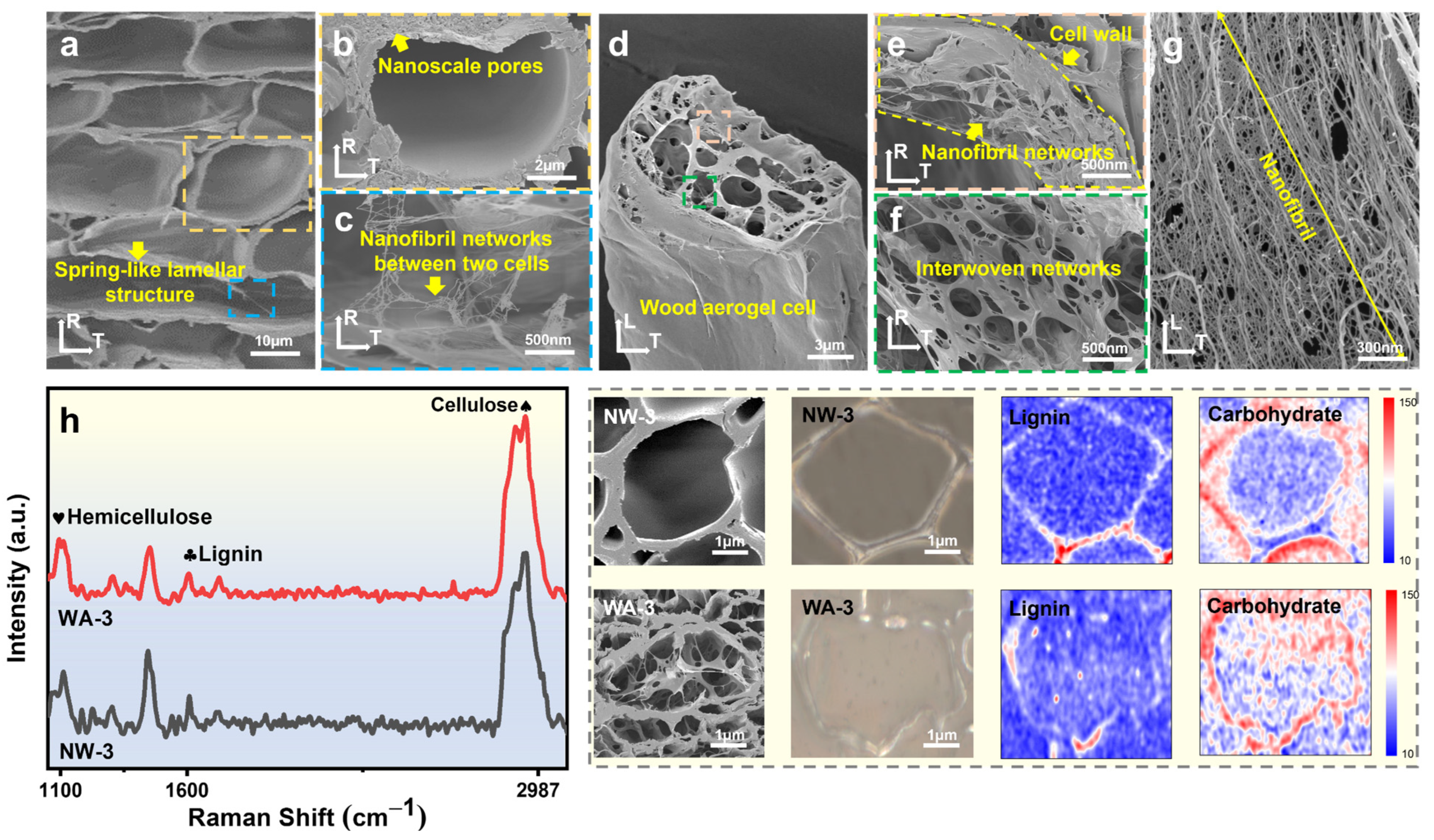
2.2. Morphology and Structure
2.3. Chemical and Structural Analysis
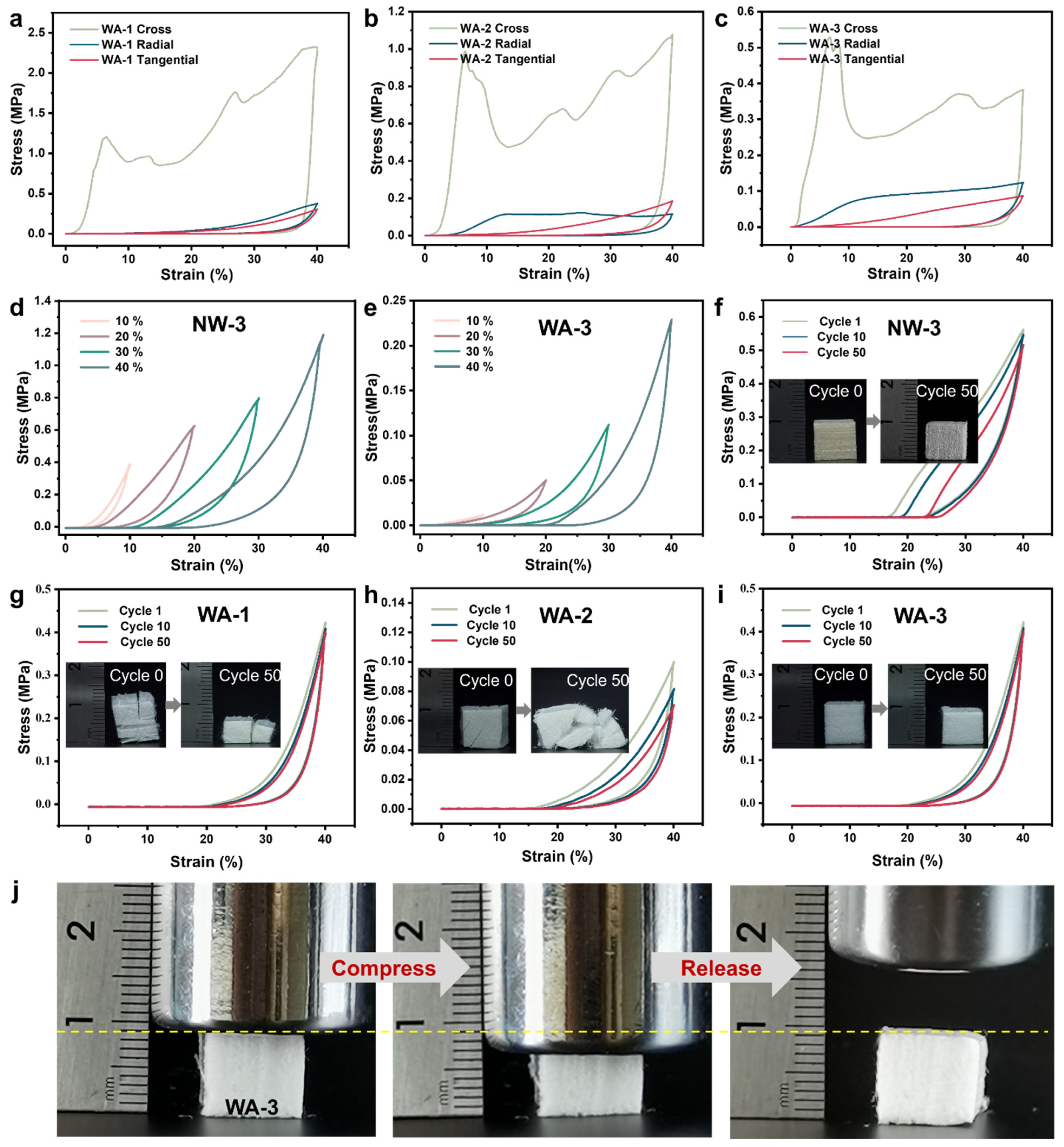
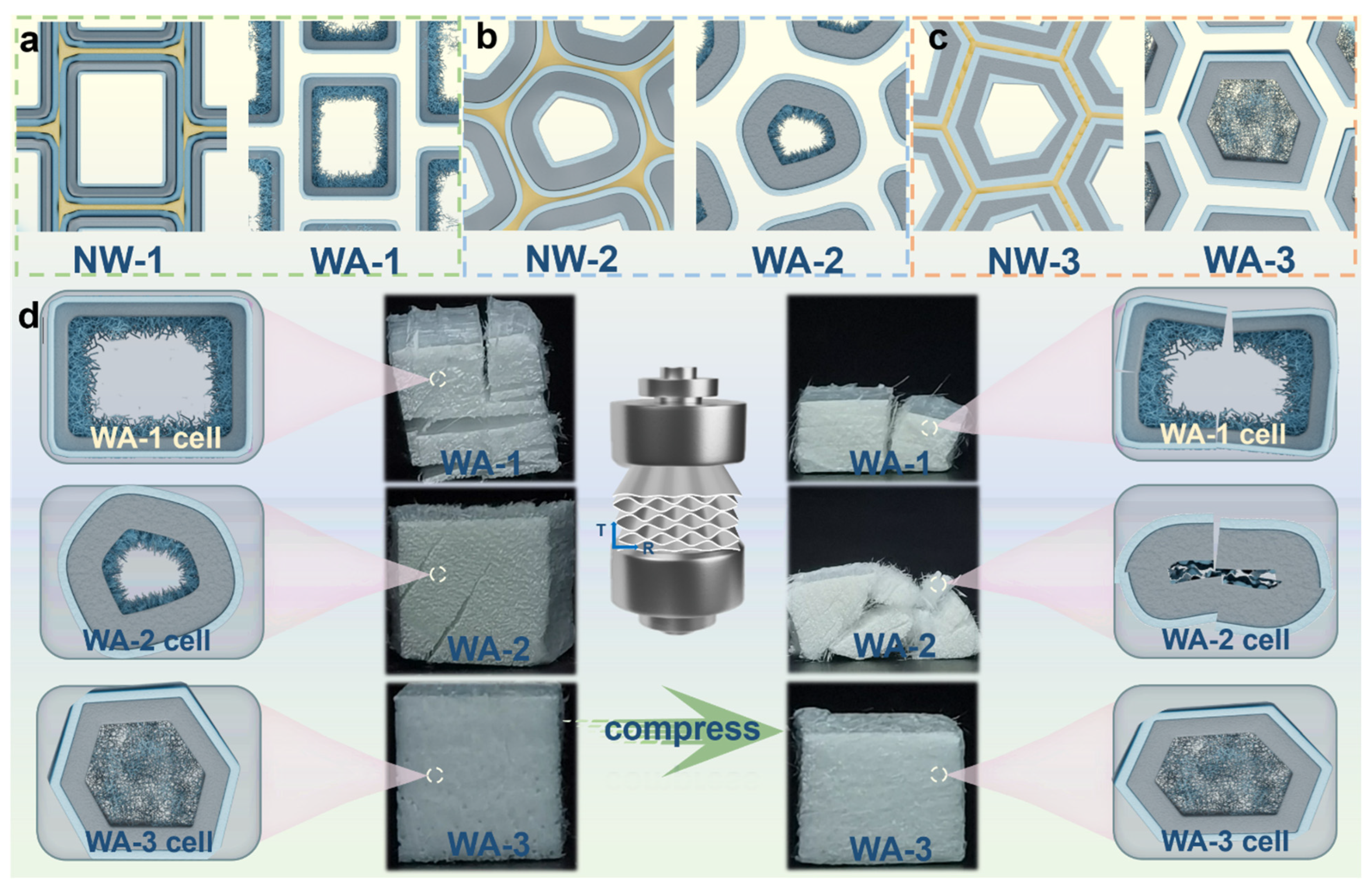
2.4. Mechanical Properties
2.5. Formation Mechanism of the Nanofibrillar Network
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of DW
3.3. Preparation of WAs
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NW | natural wood |
| DW | delignified wood |
| WA | wood aerogel |
| FD | freeze-drying |
| TNN | TEMPO-NaClO2-NaClO |
References
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Hu, P.; Wang, J.; Zhang, P.; Wu, F.; Cheng, Y.; Wang, J.; Sun, Z. Hyperelastic Kevlar Nanofiber Aerogels as Robust Thermal Switches for Smart Thermal Management. Adv. Mater. 2022, 35, e2207638. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Zhou, M.; Tan, S.; Peymanfar, R.; Aslibeiki, B.; Ji, G. Ultrabroad Microwave Absorption Ability and Infrared Stealth Property of Nano-Micro CuS@rGO Lightweight Aerogels. Nano-Micro Lett. 2022, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, Q.; Wang, F.; Hu, S.; Zhou, J.; Liu, Y.; Jiang, Z.; Wang, X.; Yu, Y.; Yang, H.; et al. Sustainable lignocellulose aerogel for air purifier with thermal insulation, flame retardancy, mechanical strength, and its life cycle assessment. Int. J. Biol. Macromol. 2024, 257, 128599. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, X.; Cai, Z.; Wei, S.; Fan, S.; Ge, Y.; Li, X.; Wu, Y. Strong, Lightweight, and Shape-Memory Bamboo-Derived All-Cellulose Aerogels for Versatile Scaffolds of Sustainable Multifunctional Materials. Small 2023, 20, e2305857. [Google Scholar] [CrossRef]
- Xie, T.; Wang, Y.; Zhang, Q.; Shen, S.; Guo, W.; Chen, X.; Wang, Q.; Qu, L.; Li, C. Wood aerogels decorated amino-functionalized MIL-101(Cr) as efficient filter for multistage purification of wastewater. Chemosphere 2024, 350, 141052. [Google Scholar] [CrossRef]
- Wang, Z. Preparation of Wood Gel Materials and Their Anisotropic Applications. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2023. [Google Scholar]
- Dong, X.; Gan, W.; Shang, Y.; Tang, J.; Wang, Y.; Cao, Z.; Xie, Y.; Liu, J.; Bai, L.; Li, J.; et al. Low-value wood for sustainable high-performance structural materials. Nat. Sustain. 2022, 5, 628–635. [Google Scholar] [CrossRef]
- Garemark, J.; Perea-Buceta, J.E.; Felhofer, M.; Chen, B.; Cortes Ruiz, M.F.; Sapouna, I.; Gierlinger, N.; Kilpelainen, I.A.; Berglund, L.A.; Li, Y. Strong, Shape-Memory Lignocellulosic Aerogel via Wood Cell Wall Nanoscale Reassembly. ACS Nano 2023, 17, 4775–4789. [Google Scholar] [CrossRef]
- Garemark, J.; Perea-Buceta, J.E.; Rico Del Cerro, D.; Hall, S.; Berke, B.; Kilpelainen, I.; Berglund, L.A.; Li, Y. Nanostructurally Controllable Strong Wood Aerogel toward Efficient Thermal Insulation. ACS Appl. Mater. Interfaces 2022, 14, 24697–24707. [Google Scholar] [CrossRef]
- Garemark, J.; Yang, X.; Sheng, X.; Cheung, O.; Sun, L.; Berglund, L.A.; Li, Y. Top-Down Approach Making Anisotropic Cellulose Aerogels as Universal Substrates for Multifunctionalization. ACS Nano 2020, 14, 7111–7120. [Google Scholar] [CrossRef]
- Guan, H.; Cheng, Z.; Wang, X. Highly Compressible Wood Sponges with a Spring-like Lamellar Structure as Effective and Reusable Oil Absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fu, S. Photochromic holo-cellulose wood-based aerogel grafted azobenzene derivative by SI-ATRP. Carbohydr. Polym. 2021, 259, 117736. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Biswas, S.K.; Okubayashi, S. Polyethylenimine-Impregnated Mesoporous Delignified Wood with High Mechanical Strength for CO2/N2 Selective Adsorption. ACS Appl. Nano Mater. 2020, 3, 5499–5508. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, D.; Sun, Q.; Liu, Y.; Zhao, H. Lignocellulose aerogel from wood-ionic liquid solution (1-allyl-3-methylimidazolium chloride) under freezing and thawing conditions. Biomacromolecules 2011, 12, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cao, J.; Guo, F.; Guo, Z.; Zhou, P.; Wang, R.; Liang, S.; Pang, Q.; Wei, B.; Jiao, Y.; et al. Nanostructured carboxylated-wood aerogel membrane for high-efficiency removal of Cu (II) ions from wastewater. Chem. Eng. J. 2023, 468. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Isogai, A. Oxidation of bleached wood pulp by TEMPO/NaClO/NaClO2 system: Effect of the oxidation conditions on carboxylate content and degree of polymerization. J. Wood Sci. 2010, 56, 227–232. [Google Scholar] [CrossRef]
- Li, K.; Wang, S.; Chen, H.; Yang, X.; Berglund, L.A.; Zhou, Q. Self-Densification of Highly Mesoporous Wood Structure into a Strong and Transparent Film. Adv. Mater. 2020, 32, e2003653. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Li, K.; Koskela, S.; Berglund, L.A.; Zhou, Q. Strong, transparent, and thermochromic composite hydrogel from wood derived highly mesoporous cellulose network and PNIPAM. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106757. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, B.; Wang, C.; Chen, Y.; Chen, K.; Chen, X.; Li, Y.; Sun, Q. Anisotropic Nature of Lightweight Wooden Metamaterials with Mechanical/Thermomechanical Multistability. Adv. Funct. Mater. 2023, 33, 2307242. [Google Scholar] [CrossRef]
- Chen, H. Preparation of Biomass-Based Aerogel Electrode Materials and Study on the Performance of Electrocatalytic Hydrogen and Oxygen Evolution. Master’s Thesis, Qingdao University, Qingdao, China, 2023. [Google Scholar]
- Gao, R. Preparation of Biomimetic Wood Aerogel and Its Application in the Field of Water Environment Purification. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- He, W.; Qiang, H.; Liang, S.; Guo, F.; Wang, R.; Cao, J.; Guo, Z.; Pang, Q.; Wei, B.; Sun, J. Hierarchically porous wood aerogel/polypyrrole(PPy) composite thick electrode for supercapacitor. Chem. Eng. J. 2022, 446, 137331. [Google Scholar] [CrossRef]
- Jin, H.; Nishiyama, Y.; Wada, M.; Kuga, S. Nanofibrillar cellulose aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 63–67. [Google Scholar] [CrossRef]
- Sun, C.; Tan, H.; Zhang, Y. Simulating the pyrolysis interactions among hemicellulose, cellulose and lignin in wood waste under real conditions to find the proper way to prepare bio-oil. Renew. Energy 2023, 205, 851–863. [Google Scholar] [CrossRef]
- Stubbs, C.; Baban, N.; Robertson, D.; Alzube, L.; Cook, D.; Geitmann, A. Plant Biomechanics:From Structure to Function at Multiple Scales. In Bending Stress in Plant Stems: Models and Assumptions; Springer: Cham, Switzerland, 2018; pp. 49–77. [Google Scholar] [CrossRef]
- Fu, Q.; Ansari, F.; Zhou, Q.; Berglund, L.A. Wood Nanotechnology for Strong, Mesoporous, and Hydrophobic Biocomposites for Selective Separation of Oil/Water Mixtures. ACS Nano 2018, 12, 2222–2230. [Google Scholar] [CrossRef]
- Shi, J.; Xing, D.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of Torrefaction on the Pyrolysis Behavior and Bio-Oil Properties of Rice Husk by Using TG-FTIR and Py-GC/MS. Energy Fuels 2014, 28, 5857–5863. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Morgano, M.T.; Mätzing, H.; Goos, E.; Leibold, H.; Merz, D.; Riedel, U.; Stapf, D. Comparison of wood pyrolysis kinetic data derived from thermogravimetric experiments by model-fitting and model-free methods. Energy Convers. Manag. 2020, 212, 112818. [Google Scholar] [CrossRef]
- Arseneau, D.F. The differential thermal analysis of wood. Can. J. Chem. 1961, 39, 1915–1919. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved cellulose X-ray diffraction analysis using Fourier series modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Abe, K.; Yamamoto, H. Mechanical interaction between cellulose microfibril and matrix substance in wood cell wall determined by X-ray diffraction. J. Wood Sci. 2005, 51, 334–338. [Google Scholar] [CrossRef]
- Li, H.; Zong, Y.; He, J.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Wood-inspired high strength and lightweight aerogel based on carbon nanotube and nanocellulose fiber for heat collection. Carbohydr. Polym. 2022, 280, 119036. [Google Scholar] [CrossRef] [PubMed]
- Burgert, I.; Keplinger, T. Plant micro- and nanomechanics: Experimental techniques for plant cell-wall analysis. J. Exp. Bot. 2013, 64, 4635–4649. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.; Jin, C.; Gao, R.; Liu, B.; Huang, Y.; Yu, Y.; Ren, J.; Deng, Y.; Tao, X.; et al. Natural Wood Structure Inspires Practical Lithium–Metal Batteries. ACS Energy Lett. 2021, 6, 2103–2110. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xu, F. Chemical Characteristics of Wood Cell Wall with an Emphasis on Ultrastructure: A Mini-Review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Tsuguyuki, S.; Satoshi, K.; Yoshiharu, N.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Tahiri, C.; Vignon, M.R. TEMPO-oxidation of cellulose: Synthesis and characterisation of polyglucuronans. Cellulose 2000, 7, 177–188. [Google Scholar] [CrossRef]
- Xu, F.; Chen, S.; Zhang, X. Combining Raman Imaging and Multivariate Analysis to Visualize Lignin, Cellulose, and Hemicellulose in the Plant Cell Wall. J. Vis. Exp. 2017, 10, 55910. [Google Scholar] [CrossRef]
- Gibson, L.J. The Structure of Cellular Solids; Cambridge University Press: Cambridge, UK, 1999; pp. 15–51. [Google Scholar] [CrossRef]
- Coste, R.; Soliman, M.; Bercu, N.B.; Potiron, S.; Lasri, K.; Aguié-Béghin, V.; Tetard, L.; Chabbert, B.; Molinari, M. Unveiling the impact of embedding resins on the physicochemical traits of wood cell walls with subcellular functional probing. Compos. Sci. Technol. 2021, 201, 108485. [Google Scholar] [CrossRef]


| Sample | DTGmax (°C) | Cf (wt%) |
|---|---|---|
| NW-1 | 383.4 | −81.29 |
| WA-1 | 328.9 | −78.49 |
| NW-2 | 382.9 | −86.03 |
| WA-2 | 328.9 | −78.19 |
| NW-3 | 342.5 | −80.80 |
| WA-3 | 309.9 | −73.85 |
| Sample | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| NW-1 | 48.2385 | 8.6179 | 35.2846 |
| DW-1 | 81.6735 | 6.5331 | 0.0562 |
| NW-2 | 49.7124 | 20.0036 | 14.9353 |
| DW-2 | 65.2821 | 9.6136 | 5.1515 |
| NW-3 | 56.8344 | 25.4290 | 7.2296 |
| DW-3 | 63.2754 | 11.6244 | 2.8822 |
| Sample | Relative Content of Sodium Carboxylate (%) | Crystallinity (%) |
|---|---|---|
| NW-1 | 0.7 | 80.21 |
| DW-1 | 1.1 | 46.76 |
| WA-1 | 4.7 | 74.58 |
| NW-2 | 0.8 | 86.20 |
| DW-2 | 1.2 | 58.82 |
| WA-2 | 4.5 | 98.58 |
| NW-3 | 1.2 | 83.33 |
| DW-3 | 1.2 | 61.19 |
| WA-3 | 5.3 | 89.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Qing, Y.; Li, Z.; Li, L.; Luo, S.; Wu, Y.; Chen, D.; Wu, Y.; Tian, C. Construction of Nanofibrillar Networked Wood Aerogels Derived from Typical Softwood and Hardwood: A Comparative Study on the In Situ Formation Mechanism of Nanofibrillar Networks. Molecules 2024, 29, 938. https://doi.org/10.3390/molecules29050938
Yan W, Qing Y, Li Z, Li L, Luo S, Wu Y, Chen D, Wu Y, Tian C. Construction of Nanofibrillar Networked Wood Aerogels Derived from Typical Softwood and Hardwood: A Comparative Study on the In Situ Formation Mechanism of Nanofibrillar Networks. Molecules. 2024; 29(5):938. https://doi.org/10.3390/molecules29050938
Chicago/Turabian StyleYan, Wenjing, Yan Qing, Zhihan Li, Lei Li, Sha Luo, Ying Wu, Deng Chen, Yiqiang Wu, and Cuihua Tian. 2024. "Construction of Nanofibrillar Networked Wood Aerogels Derived from Typical Softwood and Hardwood: A Comparative Study on the In Situ Formation Mechanism of Nanofibrillar Networks" Molecules 29, no. 5: 938. https://doi.org/10.3390/molecules29050938
APA StyleYan, W., Qing, Y., Li, Z., Li, L., Luo, S., Wu, Y., Chen, D., Wu, Y., & Tian, C. (2024). Construction of Nanofibrillar Networked Wood Aerogels Derived from Typical Softwood and Hardwood: A Comparative Study on the In Situ Formation Mechanism of Nanofibrillar Networks. Molecules, 29(5), 938. https://doi.org/10.3390/molecules29050938






