Exploring the Biological and Phytochemical Potential of Jordan’s Flora: A Review and Update of Eight Selected Genera from Mediterranean Region
Abstract
1. Introduction
2. Methods
3. Results
3.1. Biological Activities
3.1.1. Antioxidant Activities
- Crude extracts and essential oils:
- Pure compounds
3.1.2. Antimicrobial Activities
- Crude extracts and essential oils
- Pure compounds
3.1.3. Cytotoxic and Antiproliferative Activities
- Crude extracts and essential oils
- Pure compounds
3.1.4. Anti-Inflammatory Effect
- Crude extracts and essential oils
- Pure compounds
3.1.5. Antidiabetic Effects
- Crude extracts and essential oils
3.1.6. Antiulcer Agents
- Crude extracts and essential oils
3.1.7. Neuroprotective Effect
- Crude extracts and essential oils
3.1.8. Miscellaneous Bioactivities
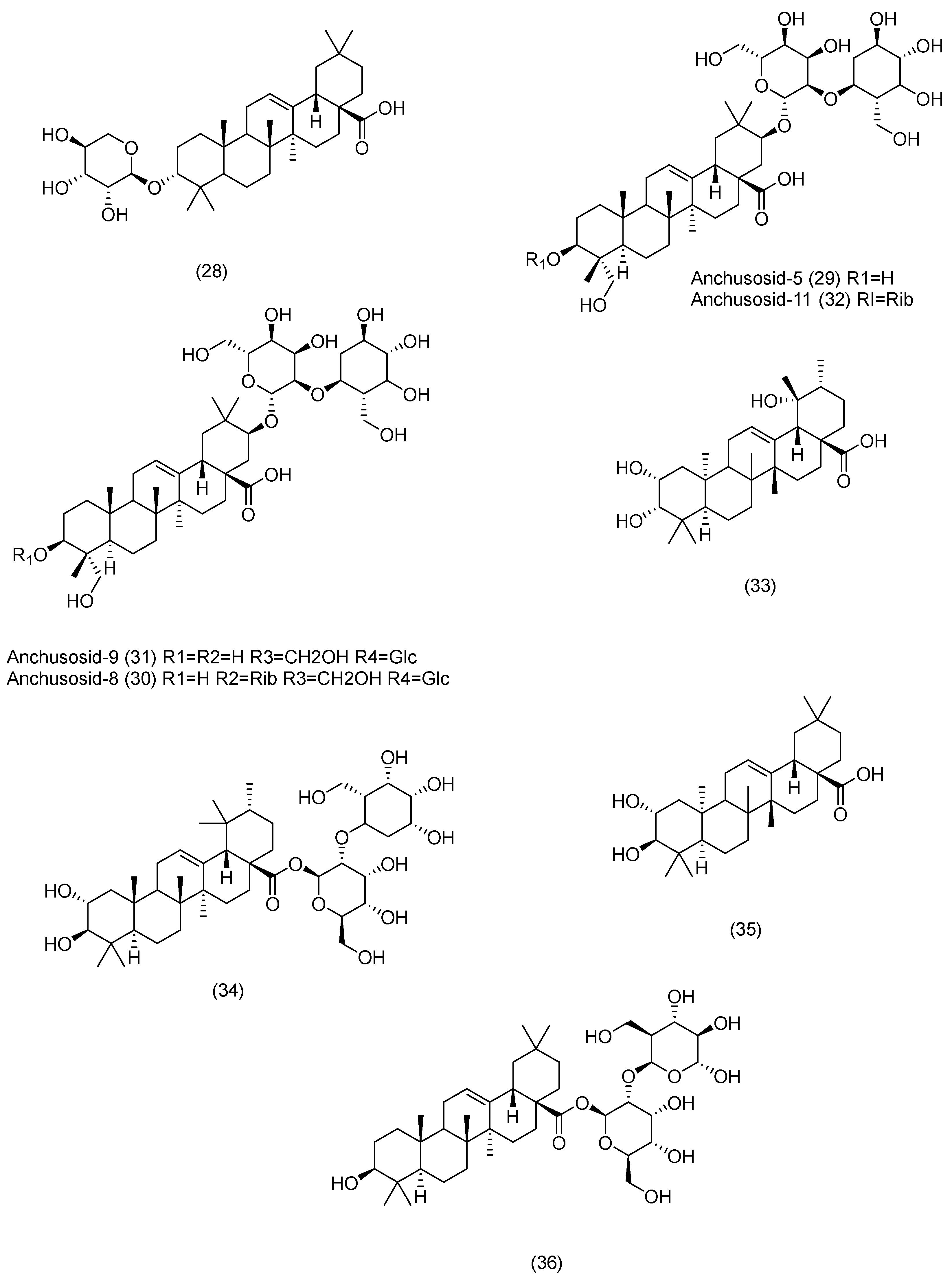
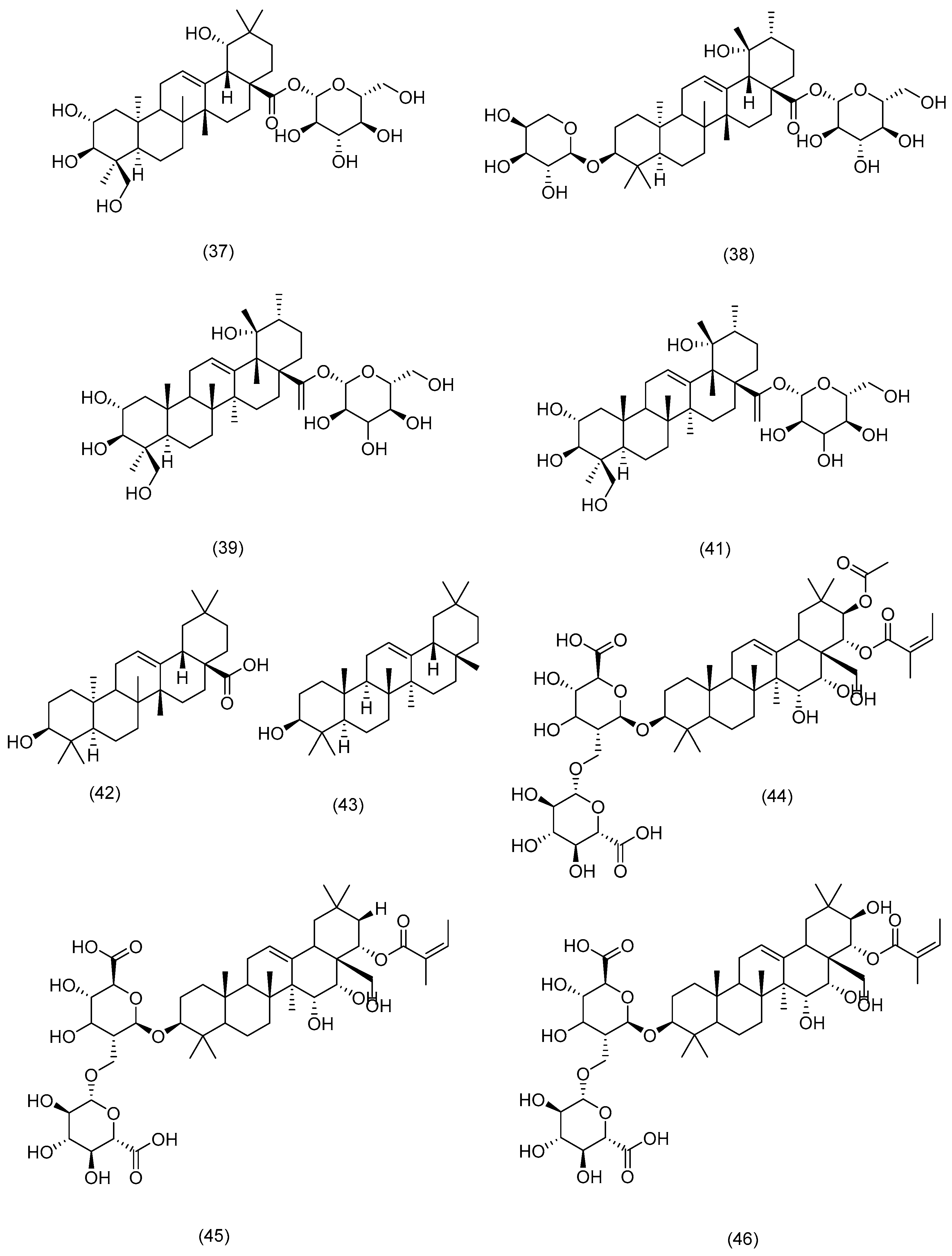
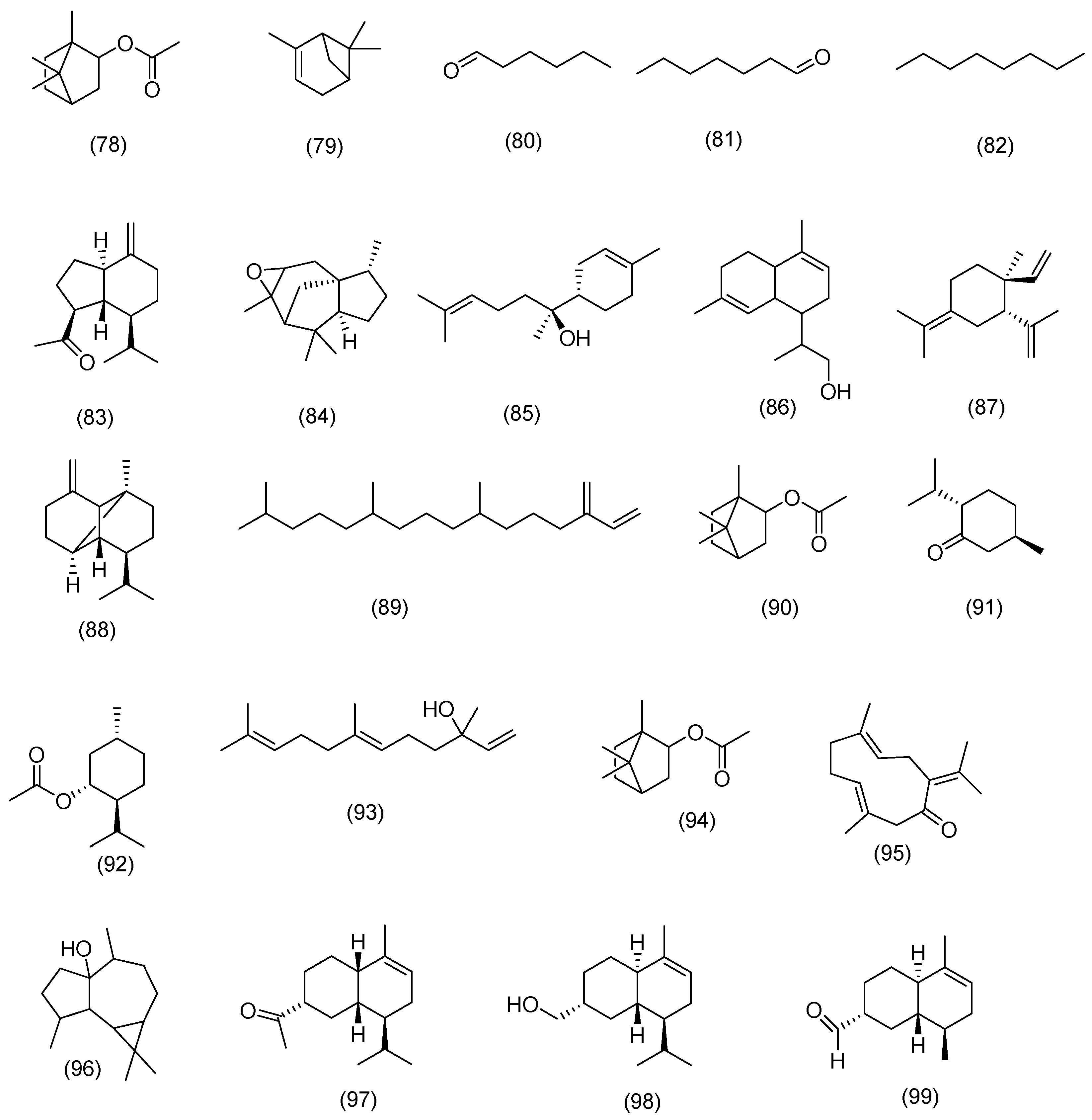
3.2. Phytochemical Constituents
3.2.1. Terpenoids
3.2.2. Phytosterols
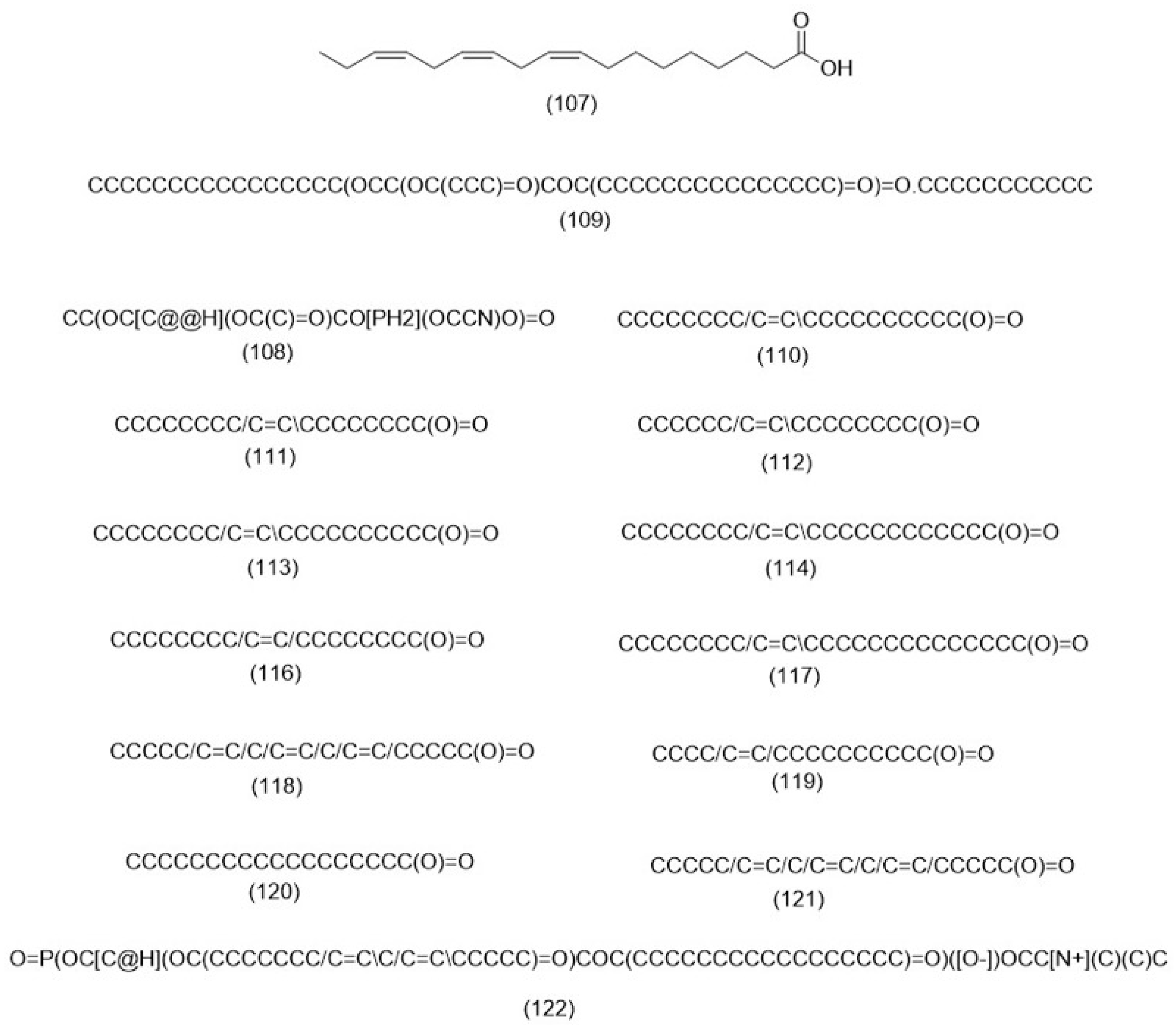
3.2.3. Fatty Acids
3.2.4. Phenolic Compounds
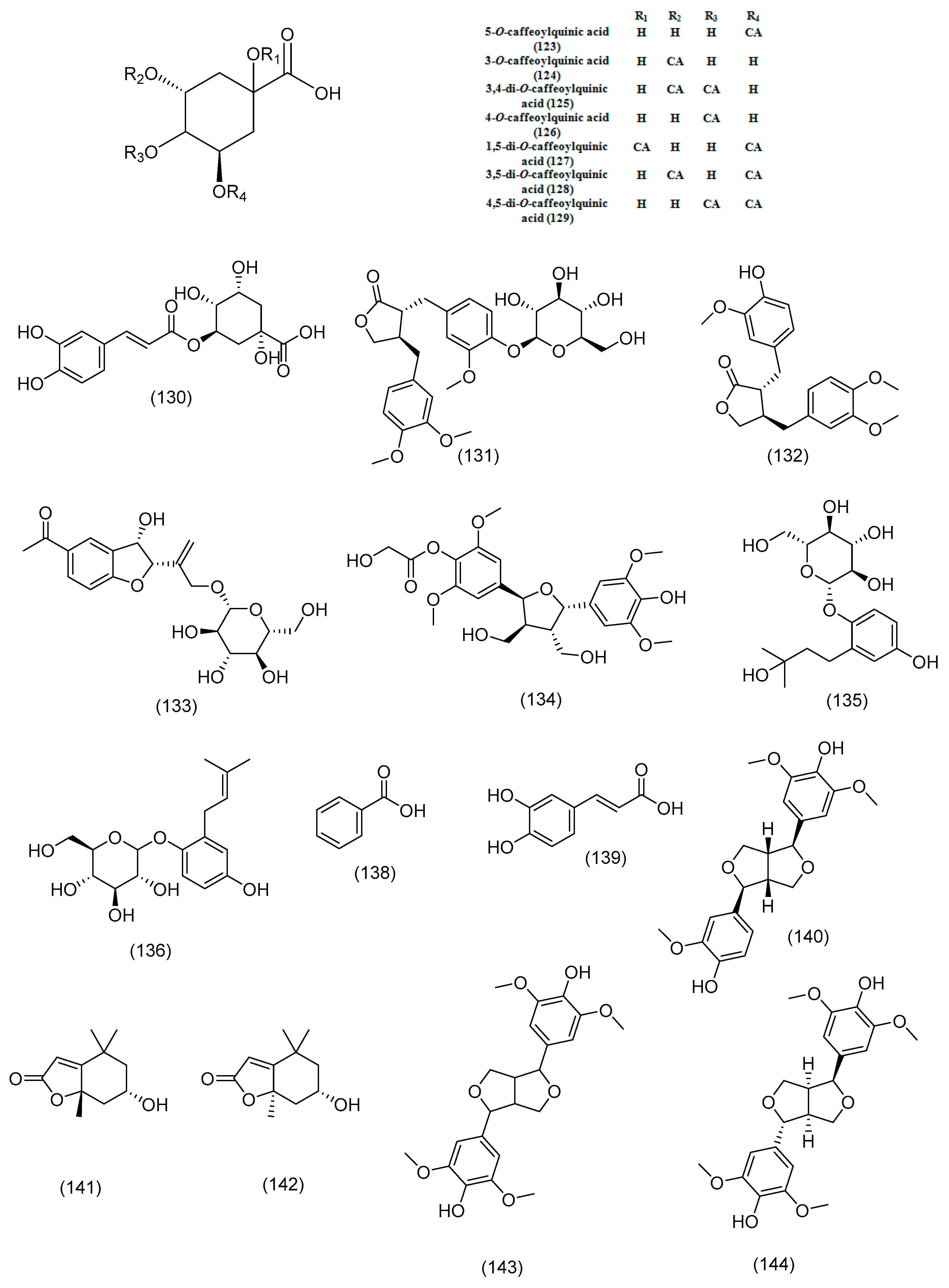
- Phenolic acids, lignans and coumarins
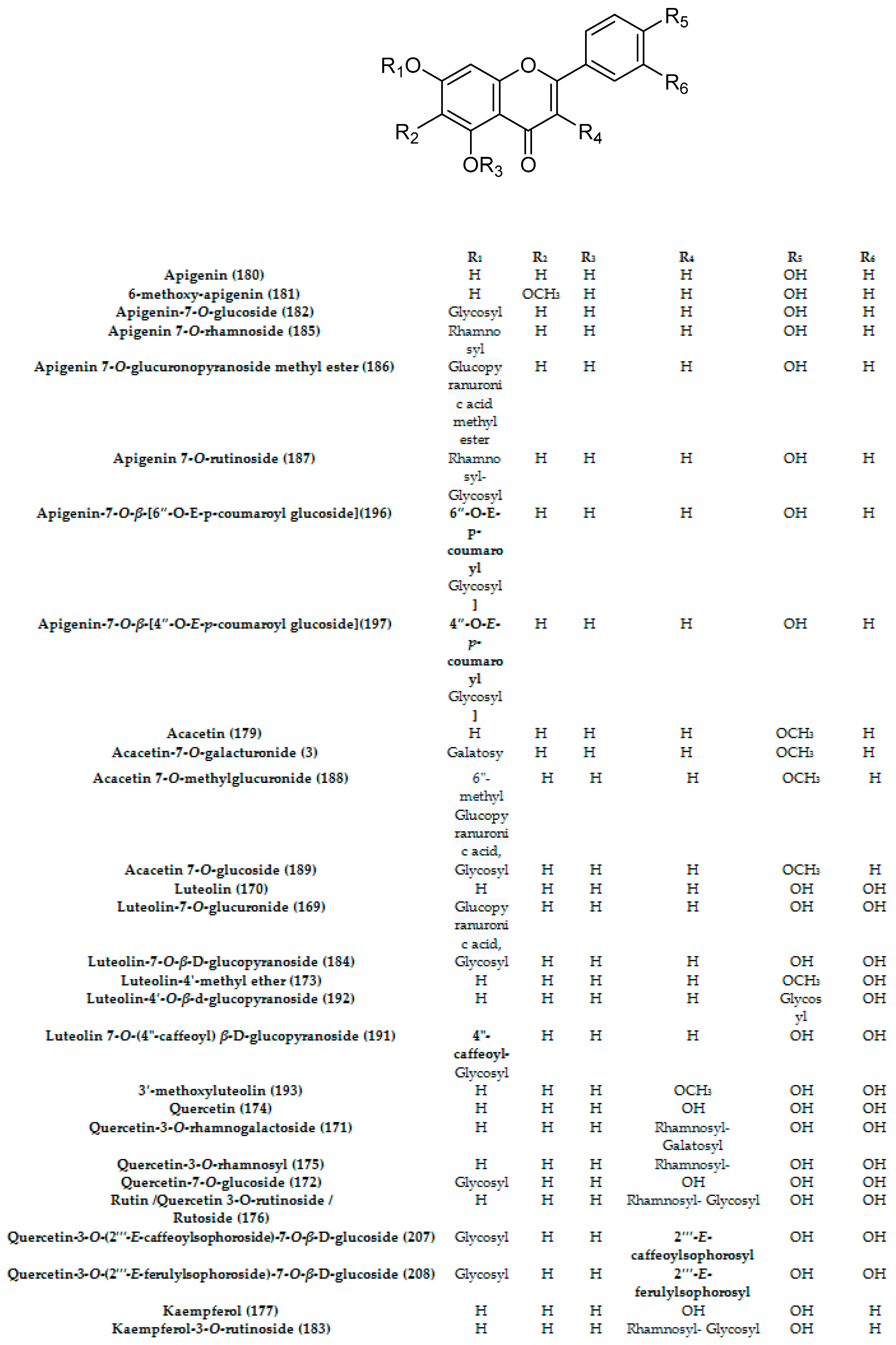
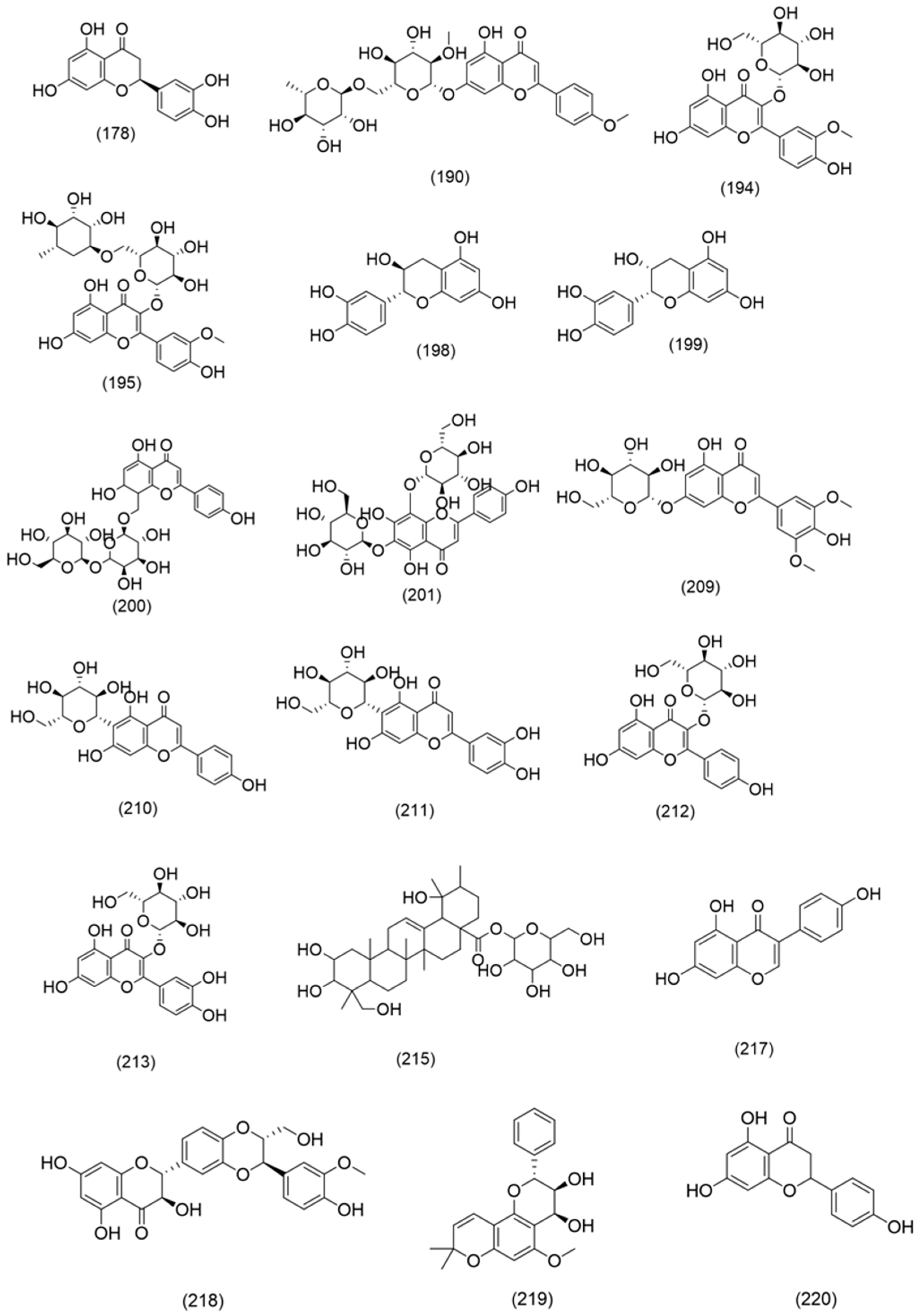
- Flavonoids
3.2.5. Alkaloids
3.2.6. Miscellaneous
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 12(S)HHTrE | 12-Hydroxyheptadecatrienoic acid |
| 5(S)-HETE | 5-Hydroxyeicosatetraenoic acid |
| A. baumannii | Acinetobacter baumannii |
| A. brassicae | Alternaria brassicae |
| A. brassicicola | Alternaria brassicicola |
| A. flavus | Aspergillus flavus |
| A. niger | Aspergillus niger |
| A375 | Human melanoma cell line |
| A375.S2 | Human Melanoma cell line |
| A549 | Adenocarcinomic human alveolar basal epithelial cells |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AChE | Acetylcholinesterase |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| B. cinerea | Botrytis cinerea |
| B. subtilis | Bacillus subtilis |
| B. cereus | Bacillus cereus |
| BChE | Butyrylcholinesterase |
| C. jejuni | Campylobacter jejuni |
| C. xerosis | Corynebacterium xerosis |
| C. albicans | Candida albicans |
| C32 | Amelanotic melanoma |
| CACO-2 | Colorectal adenocarcinoma |
| CAT | Catalase |
| CCl4 | Chemokine (C-C motif) ligands 4 |
| CH2Cl2 | Methylene chloride |
| COR-L23 | Large cell cancer |
| CUPRAC | Cupric ion-reducing antioxidant capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| E. faecalis | Enterococcus faecalis |
| ECC-1 cells | Endometrial cancer cells |
| ET-induced inflammation | Endotoxin-induced pro-inflammatory markers |
| ESI | Electrospray ionization |
| EtOAc | Ethyl acetate |
| F. oxysporum | Fusarium oxysporum |
| F. solani | Fusarium solani |
| FRAP | Ferric reducing ability of plasma |
| FTICR | Fourier transform ion cyclotron resonance mass spectrometry |
| FT-IR | Fourier transform infrared spectroscopy |
| GC-MS | Gas chromatography-mass spectrometry |
| GPX | Glutathione peroxidase |
| HCT-116 | Human colon cancer cell line |
| HCT-15 | Colorectal adenocarcinoma |
| HEK293 | Human embryonic kidney 293 cells |
| HeLa | Cervical cancer cells |
| Hep2 | Human laryngeal epidermoid carcinoma |
| HepG-2 | Hepatocellular carcinoma |
| HPLC | High-performance liquid chromatography |
| HR-MS | High-resolution mass spectrometry |
| HRT18 | Human rectum adenocarcinoma |
| HSL | The hormone-sensitive lipase |
| HT-29 | Human colon cancer cell line |
| IC50 | The half-maximal inhibitory concentration |
| iNOS | Inducible nitric oxide synthase |
| K. pneumoniae | Klebsiella pneumoniae |
| L. monocytogenes | Listeria monocytogenes |
| LC-MS | Liquid chromatography-mass spectrometry |
| LD | Lactate dehydrogenase |
| LPO | lipid peroxidation |
| LPS | Lipopolysaccharides |
| LTB4 | Leukotriene B4 |
| M. canis | Microsporum canis |
| M. fulvum | Microsporum fulvum |
| M. gypseum | Microsporum gypseum |
| MAE | Microwave-assisted extraction |
| MCF7 | Breast cancer epithelial cell line |
| MDA-MB-231 | Human breast carcinoma |
| MDBK | Madin–Darby bovine kidney cells |
| MeOH | Methanol |
| MI | Myocardial infarction |
| MIC | Minimum inhibitory concentration |
| MRC-5 | Human fetal lung cell line |
| MTS | 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| MTT | Colorimetric assay for assessing cell metabolic activity |
| n-BuOH | 1 Butanol |
| NMR | Nuclear magnetic resonance |
| NO | Nitrogen oxide |
| ORAC | Oxygen radical absorbance capacity |
| P. aeruginosa | Pseudomonas aeruginosa |
| P. mirabilis | Proteus mirabilis |
| P. ultimum | Pythium ultimum |
| P. vulgaris | Proteus vulgaris |
| P. acnes | Propionibacterium acnes |
| PC-3 | Human prostate cancer cell line |
| PMNL | Polymorphonuclear leukocytes |
| RAW 264.7 | Murine macrophage cell line |
| RKO cancer cells | Colorectal cancer cell line |
| S. aureus | Staphylococcus aureus |
| S. bovis | Streptococcus bovis |
| S. cerevisiae | Saccharomyces cerevisiae |
| S. dysgalactiae | Streptococcus dysgalactiae |
| S. epidermidis | Staphylococcus epidermidis |
| S. pyogenes | Streptococcus pyogenes |
| S. typhi | Salmonella typhi |
| SFE | Supercritical fluid extraction |
| SNP | Silver nanoparticle assay |
| SOD | Superoxide dismutase |
| spp | Species |
| St. salivarius | Streptococcus salivarius |
| T. mentagrophytes | Trichophyton mentagrophytes |
| T. rubrum | Trichophyton rubrum |
| T. tonsurans | Trichophyton tonsurans |
| T47D | Human breast ductal carcinoma |
| TBA | Thiobarbituric acid assays |
| TEAC | Trolox equivalent antioxidant capacity assay |
| UAE | Ultrasound-assisted extraction |
| UV | Ultraviolet |
| WEHI | Fibrosarcoma cell line |
| WM1361A | Primary melanoma cell line |
References
- Aburjai, T.; Hudaib, M.; Tayyem, R.; Yousef, M.; Qishawi, M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007, 110, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Sawsan, A.O. A list of flowering wild plants in Tafila Province, Jordan. Int. J. Biodivers Conserv. 2014, 6, 28–40. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Singh, S.P.; Bhakuni, R.S. Secondary metabolites of Chrysanthemum genus and their biological activities. Curr. Sci. 2005, 89, 1489–1501. [Google Scholar]
- Liu, F.; Ong, E.S.; Li, S.F.Y. A green and effective approach for characterisation and quality control of Chrysanthemum by pressurized hot water extraction in combination with HPLC with UV absorbance detection. Food Chem. 2013, 141, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Mao, Y.; Yao, N.; Zhang, X. Development of microwave-assisted extraction followed by headspace solid-phase microextraction and gas chromatography–mass spectrometry for quantification of camphor and borneol in Flos Chrysanthemi indici. Anal. Chim. Acta 2006, 575, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Oran, S.A.; Al-Eisawi, D.M. Ethnobotanical survey of the medicinal plants in the central mountains (North-South) in Jordan. J. Biodivers. Environ. Sci. 2015, 6, 2220–6663. [Google Scholar]
- Bruno, M.; Maggio, A.; Rosselli, S.; Safder, M.; Bancheva, S. The Metabolites of the Genus Onopordum (Asteraceae): Chemistry and Biological Properties. Curr. Org. Chem. 2011, 15, 888–927. [Google Scholar] [CrossRef]
- von Löwenstern, A.B.; Al-Eisawi, D.M.; Garbari, F. Studies on the flora of Jordan 14. The species of the Hisma Basin (Wadi Rum desert). Webbia 2000, 55, 195–277. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Yaghmour, R.M.-R.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Al-Eisawi, D.M.H. Vegetation community analysis in Mujib Biosphere Reserve, Jordan. J. Nat. Hist. 2014, 1, 35–58. [Google Scholar]
- Ouchbani, T.; Ouchbani, S.; Bouhfid, R.; Merghoub, N.; Guessous, A.R.; Mzibri, M.E.; Essassi, E.M. Chemical composition and antiproliferative activity of Senecio leucanthemifolius poiret essential oil. J. Essent. Oil Bear. Plants 2011, 14, 815–819. [Google Scholar] [CrossRef]
- Du, Z.-Z.; Yang, X.-W.; Han, H.; Cai, X.-H.; Luo, X.-D. A new flavone C-glycoside from Clematis rehderiana. Molecules 2010, 15, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Kumar, S.; Sharma, A. The genus Clematis (Ranunculaceae): Chemical and pharmacological perspectives. J. Ethnopharmacol. 2012, 143, 116–150. [Google Scholar] [CrossRef]
- El-Oqlah, A.A.; Lahham, J.N. A checklist of vascular plants of Ajlun mountain (Jordan). Candollea 1985, 40, 377–387. [Google Scholar]
- Aslam, M.S.; Choudhary, B.A.; Uzair, M.; Ijaz, A.S. The genus Ranunculus: A phytochemical and ethnopharmacological review. Int. J. Pharm. Pharm. Sci. 2012, 4, 15–22. [Google Scholar]
- Al-Quran, S. Taxonomical and pharmacological survey of therapeutic plants in Jordan. J. Nat. Prod. 2008, 1, 10–26. [Google Scholar]
- Ali-Shtayeh, M.S.; Yaniv, Z.; Mahajna, J. Ethnobotanical survey in the Palestinian area: A classification of the healing potential of medicinal plants. J. Ethnopharmacol. 2000, 73, 221–232. [Google Scholar] [CrossRef]
- Dafni, A.; Yaniv, Z.; Palevitch, D. Ethnobotanical survey of medicinal plants in northern Israel. J. Ethnopharmacol. 1984, 10, 295–310. [Google Scholar] [CrossRef]
- Lardos, A. The botanical materia medica of the Iatrosophikon—A collection of prescriptions from a monastery in Cyprus. J. Ethnopharmacol. 2006, 104, 387–406. [Google Scholar] [CrossRef]
- Wang, P.; Su, Z.; Yuan, W.; Deng, G.; Li, S. Phytochemical constituents and pharmacological activities of Eryngium L. (Apiaceae). Pharm Crop. 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Erdem, S.A.; Nabavi, S.F.; Orhan, I.E.; Daglia, M.; Izadi, M.; Nabavi, S.M. Blessings in disguise: A review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J. Pharm. Sci. 2015, 23, 1–22. [Google Scholar] [CrossRef]
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Kennouche, S.; Bicha, S.; Bentamene, A.; Crèche, J.; Benayache, F.; Benayache, S. In vitro antioxidant activity, phenolic and flavonoid contents of different polarity extracts from Chrysanthemum segetum L. growing in Algeria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1522–1525. [Google Scholar]
- Abd-Allah, W.E.; Radwan, H.M.; Shams, K.A.; Ismail, S.I.; Ali, S.M. The lipid and volatile oil of the seed and aerial parts of Onopordum alexandrinum Boiss. growing in Egypt and their antioxidant activity. Egypt Pharm. J. 2012, 11, 49. [Google Scholar]
- Salamaa, M.M.; Ezzat, S.M.; Sleem, A.A.; Salama, M.M.; Ezzat, S.M.; Sleem, A.A. A new hepatoprotective flavone glycoside from the flowers of Onopordum alexandrinum growing in Egypt. Zeitschrift für Naturforsch C. 2011, 66, 251–259. [Google Scholar] [CrossRef]
- Ferchichi, L.; Chohra, D.; Mellouk, K.; Alsheikh, S.M.; Cakmak, Y.S.; Zengin, G. Chemical composition and antioxidant activity of essential oil from the aerial parts of Clematis cirrhosa L.(Ranunculaceae) growing in Algeria. Ann. Rom. Soc. Cell Biol. 2021, 25, 1314–1324. [Google Scholar]
- Saidi, R.; Chawech, R.; Baccouch, N.; Jarraya, R.M. Study toward antioxidant activity of Clematis flammula extracts: Purification and identification of two flavonoids-glucoside and trisaccharide. S. Afr. J. Bot. 2019, 123, 208–213. [Google Scholar] [CrossRef]
- Chohra, D.; Ferchichi, L.; Cakmak, Y.S.; Zengin, G.; Alsheikh, S.M. Phenolic profiles, antioxidant activities and enzyme inhibitory effects of an Algerian medicinal plant (Clematis cirrhosa L.). S. Afr. J. Bot. 2020, 132, 164–170. [Google Scholar] [CrossRef]
- Shahid, S.; Riaz, T.; Asghar, M.N. Screening of Ranunculus sceleratus for enzyme inhibition, antibacterial and antioxidant activities. Bangladesh J. Pharmacol. 2015, 10, 436–442. [Google Scholar] [CrossRef][Green Version]
- Neag, T.; Toma, C.-C.; Olah, N.; Ardelean, A. Polyphenols profile and antioxidant activity of some Romanian Ranunculus species. Stud. Univ. Babes-Bolyai Chem. 2017, 62, 75–88. [Google Scholar] [CrossRef]
- Solanki, S.; Prasad, D.; Singh, A.K. Antioxidant determination and thin layer chromatography of extract Withania somnifera, Terminalia arjuna, Bacopa monnieri, Ranunculus sceleratus and Acalypha indica. Eur. J. Mol. Clin. Med. 2020, 7, 4394–4408. [Google Scholar]
- Serag, M.S.; Khedr, A.; El-Amier, Y.A.; El-Afify, S.M. Bioactive constituents and allelopathic activities of the invasive weed Ranunculus sceleratus L. Nile Delta, Egypt. J. Exp. Sci. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Aktumsek, A.; Ceylan, O.; Uysal, S. Screening of possible in vitro neuroprotective, skin care, antihyperglycemic, and antioxidative effects of Anchusa undulata L. subsp. hybrida (Ten.) Coutinho from Turkey and its fatty acid profile. Int. J. Food Prop. 2015, 18, 1491–1504. [Google Scholar] [CrossRef]
- Taban, K.; Eruygur, N.; Üstün, O. Biological activity studies on the aqueous methanol extract of Anchusa undulata L. subsp. hybrida (Ten.) Coutinho. J. Res. Pharm. 2018, 21, 357–364. [Google Scholar] [CrossRef]
- Al-Khateeb, E.H.; Al-Assi, G.A.; Shakya, A.K.; Al-Rawi, N.; Shalan, N. Antioxidant potential of Pistacia Vera L. fruit hull, Anchusa Strigosa flowers and Ilex paraguariensis A. St.-Hil. leaves extract. Orient. J. Chem. 2019, 35, 982. [Google Scholar] [CrossRef]
- Khomsi, M.E.; Imtara, H.; Kara, M.; Hmamou, A.; Assouguem, A.; Bourkhiss, B.; Tarayrah, M.; AlZain, M.N.; Alzamel, N.M.; Noman, O.; et al. Antimicrobial and Antioxidant Properties of Total Polyphenols of Anchusa italica Retz. Molecules 2022, 27, 416. [Google Scholar] [CrossRef] [PubMed]
- Farhan, H.; Malli, F.; Rammal, H.; Hijazi, A.; Bassal, A.; Ajouz, N.; Badran, B. Phytochemical screening and antioxidant activity of Lebanese Eryngium creticum L. Asian Pac. J. Trop. Biomed. 2012, 2, S1217–S1220. [Google Scholar] [CrossRef]
- Dammous, M.; Farhan, H.; Rammal, H.; Hijazi, A.; Bassal, A.; Fayyad-Kazan, H.; Makhour, Y.; Badran, B. Chemical composition of Lebanese Eryngium creticum L. Int. J. Sci. 2014, 3, 40–53. [Google Scholar]
- Hijazi, A.; Al Masri, D.S.; Farhan, H.; Nasser, M.; Rammal, H.; Annan, H. Effect of different ethanol concentrations, using different extraction techniques, on the antioxidant capacity of Lebanese Eryngium creticum. J. Pharm. Chem. Biol. Sci. 2015, 3, 262–271. [Google Scholar]
- Darriet, F.; Andreani, S.; De Cian, M.; Costa, J.; Muselli, A. Chemical variability and antioxidant activity of Eryngium maritimum L. essential oils from Corsica and Sardinia. Flavour Fragr. J. 2014, 29, 3–13. [Google Scholar] [CrossRef]
- Ben Lajnef, H.; Ferioli, F.; Pasini, F.; Politowicz, J.; Khaldi, A.; Filippo D’Antuono, L.; Caboni, M.F.; Nasri, N. Chemical composition and antioxidant activity of the volatile fraction extracted from air-dried fruits of Tunisian Eryngium maritimum L. ecotypes. J. Sci. Food Agric. 2018, 98, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Matejić, J.S.; Stojanović-Radić, Z.Z.; Krivošej, Z.Đ.; Zlatković, B.K.; Marin, P.D.; Džamić, A.M. Biological activity of extracts and essential oils of two Eryngium (Apiaceae) species from the Balkan peninsula. Acta Medica Median. 2019, 58, 24–31. [Google Scholar] [CrossRef]
- Traversier, M.; Gaslonde, T.; Lecso, M.; Michel, S.; Delannay, E. Comparison of extraction methods for chemical composition, antibacterial, depigmenting and antioxidant activities of Eryngium maritimum. Int. J. Cosmet. Sci. 2020, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hoa, N.T.; Vo, Q.V. The radical scavenging activity of muriolide in physiological environments: Mechanistic and kinetic insights into double processes. RSC Adv. 2021, 11, 33245–33252. [Google Scholar] [CrossRef]
- Raziq, N.; Saeed, M.; Ali, M.S.; Shahid, M.; Lateef, M.; Zafar, S. Muricazine, a new hydrazine derivative from Ranunculus muricatus L. with antioxidant, lipoxygenase and urease inhibitory activities. Nat. Prod. Res. 2022, 36, 961–966. [Google Scholar] [CrossRef]
- Moloney, M.G. Natural products as a source for novel antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Alvarez-Castellanos, P.P.; Bishop, C.D.; Pascual-Villalobos, M.J. Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry 2001, 57, 99–102. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Hudaib, M.M.; Tawaha, K.A.; Bashatwah, R.M. Studies on the in vitro antiproliferative, antimicrobial, antioxidant, and acetylcholinesterase inhibition activities associated with Chrysanthemum coronarium essential oil. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Zaher, A.M.; Sultan, R.; Ramadan, T.; Amro, A. New antimicrobial and cytotoxic benzofuran glucoside from Senecio glaucus L. Nat. Prod. Res. 2021, 36, 136–141. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Statti, G.A.; Tundis, R.; Conforti, F.; Bonesi, M.; Autelitano, G.; Houghton, P.J.; Miljkovic-Brake, A.; Menichini, F. Antibacterial and antifungal activity of Senecio inaequidens DC. and Senecio vulgaris L. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 777–779. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Statti, G.A.; Houghton, P.J.; Miljkovic-Brake, A.; Menichini, F. In vitro hypoglycemic and antimicrobial activities of Senecio leucanthemifolius Poiret. Nat. Prod. Res. 2007, 21, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Zazharskyi, V.V.; Davydenko, P.; Kulishenko, O.; Borovik, I.V.; Zazharska, N.M.; Brygadyrenko, V.V. Antibacterial and fungicidal activities of ethanol extracts of 38 species of plants. Biosyst. Divers. 2020, 28, 281–289. [Google Scholar] [CrossRef]
- Ourabah, A.; Atmani-Kilani, D.; Debbache-Benaida, N.; Kolesova, O.; Azib, L.; Yous, F.; Benloukil, M.; Botta, B.; Atmani, D.; Simonetti, G. Anti-Candida albicans biofilm activity of extracts from two selected indigenous Algerian plants: Clematis flammula and Fraxinus angustifolia. J. Herb. Med. 2020, 20, 100319. [Google Scholar] [CrossRef]
- Terzioglu, S.; Yasar, A.; Yayli, N.; Yilmaz, N.; Karaoglu, S.; Yayli, N. Antimicrobial activity and essential oil compositions of two Ranunculus species from Turkey: R. constantinopolitanus and R. arvensis. Asian J. Chem. 2008, 20, 3277. [Google Scholar]
- Sharma, K.K.; Kotoky, J.; Kalita, J.C.; Barthakur, R. Evaluation of antidermatophytic activity of Ranunculus sceleratus and Pongamia pinnata available in North Eastern Region of India. Asian Pac. J. Trop. Biomed. 2012, 2, S808–S811. [Google Scholar] [CrossRef]
- Jaish, B.M. In vitro Antifungal Activity of Some Higher Plant Extracts against Alternaria brassicae (Berk.) Sacc. and A. brassicicola (Schw.) Wiltsh. Bull. Pure Appl. Sci.-Bot. 2018, 37, 108–111. [Google Scholar]
- Al-Snafi, A.E. Pharmacological and toxicological effects of the Ranunculus species (Ranunculus arvensis and Ranunculus sceleratus) grown in Iraq. Int. J. Biol. Pharm. Sci. Arch. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Hachelaf, A.; Zellagui, A.; Touil, A.; Rhouati, S. Chemical composition and analysis antifungal properties of Ranunculus arvensis L. Pharmacophore 2013, 4, 89–91. [Google Scholar]
- Hachelaf, A.; Touil, A.; Zellagui, A.; Rhouati, S. Antioxidant and antibacterial activities of essential oil extracted from Ranunculus arvensis L. Der Pharma Chem. 2015, 7, 170–173. [Google Scholar]
- Khan, M.Z.; Jan, S.; Khan, F.U.; Noor, W.; Khan, Y.M.; Shah, A.; Chaudhary, M.I.; Ali, F.; Khan, K.; Ullah, W.; et al. Phytochemical screening and biological activities of Ranunculus arvensis. Int. J. Biosci. 2017, 11, 15–21. [Google Scholar]
- Nazir, S.; Tahir, K.; Naz, R.; Khan, Z.; Khan, A.; Islam, R.; Rehman, A.U. In vitro screening of Ranunculus muricatus for potential cytotoxic and antimicrobial activities. J. Pharmacol. 2014, 8, 427–431. [Google Scholar]
- Al-Salihi, F.G.; Al-Ameri, A.K.; Al-Juobory, T.S. Antimicrobial activity of total lipids extracted from Anchusa strigosa Lab. J. Surra Man Raa 2007, 3, 11–20. [Google Scholar]
- Al-Salihi, F.; Yasseen, A.I.; Al-Salihi, S.F. Antimicrobial activity of volatile oil and fixed oil extracted from Anchusa strigosa Lab. Tikrit J. Pure Sci. 2009, 14, 21–24. [Google Scholar]
- Al-Aymi, H.A. Evaluation of antimicrobial activity of watery and alcoholic extracts for Anchusa strigosa on growth of gram positive pathogenic bacteria isolated from pharyngitis and tonsillits cases. Iraqi J. Vet. Med. 2007, 31, 87–103. [Google Scholar] [CrossRef]
- Boussoualim, A. Anti-bacterial and β-lactamase inhibitory effects of Anchusa azurea and Globularia alypum extracts. Res. J. Pharm. Biol. Chem. Sci. 2014, 1, 742. [Google Scholar]
- Makki, R.; Dirani, Z.E.; Rammal, H.; Sweidan, A.; Al Bazzal, A.; Chokr, A. Antibacterial activity of two Lebanese plants: Eryngium creticum and Centranthus longiflorus. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Abou-Jawdah, Y.; Sobh, H.; Salameh, A. Antimycotic activities of selected plant flora, growing wild in Lebanon, against phytopathogenic fungi. J. Agric. Food Chem. 2002, 50, 3208–3213. [Google Scholar] [CrossRef]
- Landoulsi, A.; Roumy, V.; Duhal, N.; Skhiri, F.H.; Rivière, C.; Sahpaz, S.; Neut, C.; Benhamida, J.; Hennebelle, T. Chemical Composition and Antimicrobial Activity of the Essential Oil from Aerial Parts and Roots of Eryngium barrelieri Boiss. and Eryngium glomeratum Lamk. from Tunisia. Chem. Biodivers. 2016, 13, 1720–1729. [Google Scholar] [CrossRef]
- Kikowska, M.; Kalemba, D.; Dlugaszewska, J.; Thiem, B. Chemical composition of essential oils from rare and endangered species—Eryngium maritimum L. and E. alpinum L. Plants 2020, 9, 417. [Google Scholar] [CrossRef]
- Kholkhal, W.; Ilias, F.; Bekhechi, C.; Bekkara, F.A. Eryngium maritimum: A rich medicinal plant of polyphenols and flavonoids compounds with antioxidant, antibacterial and antifungal activities. Curr. Res. J. Biol. Sci. 2012, 4, 437–443. [Google Scholar]
- Thiem, B.; Goslinska, O.; Kikowska, M.; Budzianowski, J. Antimicrobial activity of three Eryngium L. species (Apiaceae). Herba Pol. 2010, 56, 52–59. [Google Scholar]
- Celik, B.Ö.; Kara, E.M.; Guzel, C.B.; Genç, G.E.; Genç, I.; Anil, S.K.; Melikoğlu, G. Evaluation of antimicrobial and cytotoxic effects of four turkish species of Eryngium L. Bangladesh J. Bot. 2019, 48, 271–278. [Google Scholar] [CrossRef]
- Seca, A.M.; Pinto, D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Abu-rish, E.Y.; Kasabri, V.; Hudaib, M.M.; Mashalla, S.H.; AlAlawi, L.H.; Tawaha, K.; Mohammad, M.K.; Mohamed, Y.S.; Bustanji, Y. Evaluation of antiproliferative activity of some traditional anticancer herbal remedies from Jordan. Trop. J. Pharm. Res. 2016, 15, 469–474. [Google Scholar] [CrossRef]
- El-Najjar, N.; Saliba, N.; Talhouk, S.; Gali-Muhtasib, H. Onopordum cynarocephalum induces apoptosis and protects against 1, 2 dimethylhydrazine-induced colon cancer. Oncol. Rep. 2007, 17, 1517–1523. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Russo, A.; Cardile, V.; Caggia, S.; Arnold, N.A.; Mari, A.; Piacente, S.; Rosselli, S.; Senatore, F.; et al. Phytochemical profile and apoptotic activity of Onopordum cynarocephalum. Planta Med. 2012, 78, 1651–1660. [Google Scholar] [CrossRef]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Houghton, P.J.; Menichini, F. Biological properties of different extracts of two Senecio species. Int. J. Food Sci. Nutr. 2006, 57, 1–8. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Statti, G.A.; Menichini, F.; Houghton, P.J. In-vitro antiproliferative effects on human tumor cell lines of extracts and jacaranone from Senecio leucanthemifolius Poiret. J. Pharm. Pharmacol. 2005, 57, 897–901. [Google Scholar] [CrossRef]
- Dina, A.; Jose, I.R.-S.; Ro, J.L.; Fadil, B.; Djebbar, A. Antioxidant potential, cytotoxic activity and phenolic content of Clematis flammula leaf extracts. J. Med. Plants Res. 2011, 5, 589–598. [Google Scholar]
- Rammal, H.; Farhan, H.; Mohsen, H.; Hijazi, A.; Kobeissy, A.; Daher, A.; Badran, B. Antioxidant, cytotoxic properties and phytochemical screening of two Lebanese medicinal plants. Int. Res. J. Pharm. 2013, 4, 132–136. [Google Scholar]
- Dirani, Z.; Makki, R.; Rammal, H.; Naserddine, S.; Hijazi, A.; Kazan, H.F.; Nasser, M.; Daher, A.; Badran, B. The antioxidant and anti-tumor activities of the Lebanese Eryngium creticum L. IJBPAS 2014, 3, 2199–2222. [Google Scholar]
- Landoulsi, A.; Hennebelle, T.; Bero, J.; Rivière, C.; Sahpaz, S.; Quetin-Leclercq, J.; Neut, C.; Benhamida, J.; Roumy, V. Antimicrobial and Light-Enhanced Antimicrobial Activities, Cytotoxicity and Chemical Variability of All Tunisian Eryngium Species. Chem. Biodivers. 2020, 17, e1900543. [Google Scholar] [CrossRef]
- Yurdakök, B.; Baydan, E. Cytotoxic effects of Eryngium kotschyi and Eryngium maritimum on Hep2, HepG2, Vero and U138 MG cell lines. Pharm. Biol. 2013, 51, 1579–1585. [Google Scholar]
- Choi, J.M.; Lee, E.O.; Lee, H.J.; Kim, K.H.; Ahn, K.S.; Shim, B.S.; Kim, N.I.; Song, M.C.; Baek, N.I.; Kim, S.H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phyther. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Wehling, M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: Management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014, 70, 1159–1172. [Google Scholar] [CrossRef]
- Virshette, S.J.; Patil, M.K.; Somkuwar, A.P. A review on medicinal plants used as anti-inflammatory agents. J. Pharmacogn. Phytochem. 2019, 8, 1641–1646. [Google Scholar]
- Servi, H. Chemical composition and biological activities of essential oils of two new chemotypes of Glebionis Cass. Turk. J. Chem. 2021, 45, 1559–1566. [Google Scholar] [CrossRef]
- Talhouk, R.S.; Esseili, M.A.; Kogan, J.; Atallah, M.R.; Talhouk, S.N.; Homaidan, F.R. Inhibition of endotoxin-induced pro-inflammatory markers by water extracts of Onopordum cynarocephalum and Achillea damascena. J. Med. Plants Res. 2009, 3, 686–698. [Google Scholar]
- Yous, F.; Atmani-Kilani, D.; Debbache-Benaida, N.; Cheraft, N.; Sebaihi, S.; Saidene, N.; Benloukil, M.; Atmani, D. Anti-ulcerogenic and proton pump (H+, K+ ATPase) inhibitory activity of Clematis flammula L. extract. S. Afr. J. Bot. 2018, 119, 390–399. [Google Scholar] [CrossRef]
- Marrelli, M.; De Marco, C.T.; Statti, G.; Neag, T.A.; Toma, C.-C.; Conforti, F. Ranunculus species suppress nitric oxide production in LPS-stimulated RAW 264.7 macrophages. Nat. Prod. Res. 2022, 36, 2859–2863. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, P.; Uttra, A.M.; Asif, H.; Younis, W.; Hasan, U.H.; Irfan, H.M.; Sharif, A. Evaluation of anti-inflammatory and analgesic activities of aqueous methanolic extract of Ranunculus muricatus in albino mice. Pak. J. Pharm. Sci. 2020, 33, 1121–1126. [Google Scholar]
- Alallan, L.; Agha, M.I.H.; Omerein, A.N.; Al Balkhi, M.H. Anti-arthritic effects of Anchusa strigosa extracts on complete Freund’s adjuvant-induced arthritis in rats. J. Pharmacogn. Phytochem. 2018, 7, 679–685. [Google Scholar]
- Kuruuzum-Uz, A.; Suleyman, H.; Cadirci, E.; Guvenalp, Z.; Demirezer, L.O.; Omur Demirezer, L. Investigation on anti-inflammatory and antiulcer activities of Anchusa azurea extracts and their major constituent rosmarinic acid. Z. Für Naturforsch C. 2012, 67, 360–366. [Google Scholar]
- Conea, S.; Parvu, A.E.; Bolboaca, S. Anti-inflammatory effects of Eryngium planum L. and Eryngium maritimum L.(Apiaceae) Extracts in turpentine-oil induced acute inflammation in Rats. Inflammation 2016, 5, 10. [Google Scholar]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19. [Google Scholar] [CrossRef]
- Miller, N.; Joubert, E. Critical assessment of in vitro screening of α-glucosidase inhibitors from plants with acarbose as a reference standard. Planta Med. 2022, 88, 1078–1091. [Google Scholar] [CrossRef]
- Muhammed, A.; Arı, N. Antidiabetic activity of the aqueous extract of Anchusa strigosa Lab in streptozotocin diabetic rats. Int. J. Pharm. 2012, 2, 445–449. [Google Scholar]
- Kasabri, V.; Abu-Dahab, R.; Afifi, F.U.; Naffa, R.; Majdalawi, L. Modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption with Achillea santolina, Eryngium creticum and Pistacia atlantica extracts: In vitro evaluation. J. Exp. Integr. Med. 2012, 2, 245–254. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef]
- Adinortey, M.B.; Ansah, C.; Galyuon, I.; Nyarko, A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013, 2013, 796405. [Google Scholar] [CrossRef]
- Mishra, A.P.; Ankit, B.; Suresh, C. A comprehensive review on the screening models for the pharmacological assessment of antiulcer drugs. Curr. Clin. Pharmacol. 2019, 14, 175–196. [Google Scholar] [CrossRef]
- Abbas, M.; Disi, A.; Al-Khalil, S. Isolation and Identification of anti-ulcer components from Anchusa strigosa root. Jordan J. Pharm. Sci. 2009, 2, 131–139. [Google Scholar]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Higuchi, M.; Saido, T.C. Metabolism of amyloid-β peptide and Alzheimer’s disease. Pharmacol. Ther. 2005, 108, 129–148. [Google Scholar] [CrossRef]
- Khan, A.Q.; Ahmad, T.; Mushtaq, M.N.; Malik, M.N.H.; Naz, H.; Ahsan, H.; Asif, H.; Noor, N.; Rahman, M.S.U.; Dar, U.; et al. Phytochemical analysis and cardiotonic ativity of methanolic extract of Ranunculus muricactus Linn. in isolated rabbit heart. Acta Pol. Pharm. 2016, 73, 949–954. [Google Scholar]
- Wang, S.; Zhao, Y.; Song, J.; Wang, R.; Gao, L.; Zhang, L.; Fang, L.; Lu, Y.; Du, G. Total flavonoids from Anchusa italica Retz. improve cardiac function and attenuate cardiac remodeling post myocardial infarction in mice. J. Ethnopharmacol. 2020, 257, 112887. [Google Scholar] [CrossRef]
- Torki, A.; Khalaji-Pirbalouty, V.; Lorigooini, Z.; Rafieian-Kopaei, M.; Sadeghimanesh, A.; Rabiei, Z. Anchusa italica extract: Phytochemical and neuroprotective evaluation on global cerebral ischemia and reperfusion. Braz. J. Pharm. Sci. 2018, 54, 1–9. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Kiss, T.; Tóth, B.; Csupor, D. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020, 19, 1449–1470. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Lee, K.D.; Yang, M.S.; Ha, T.J.; Park, K.M.; Park, K.H. Isolation and identification of dihydrochrysanolide and its 1-epimer from Chrysanthemum coronarium L. Biosci. Biotechnol. Biochem. 2002, 66, 862–865. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Wu, B.-L.; Zou, H.-L.; Qin, F.-M.; Li, H.-Y.; Zhou, G.-X. New Ent-Kaurane-Type Diterpene Glycosides and Benzophenone from Ranunculus muricatus Linn. Molecules 2015, 20, 22445–22453. [Google Scholar] [CrossRef]
- Sugimoto, S.; Yamano, Y.; Desoukey, S.Y.; Katakawa, K.; Wanas, A.S.; Otsuka, H.; Matsunami, K. Isolation of sesquiterpene–amino acid conjugates, onopornoids A–D, and a flavonoid glucoside from Onopordum alexandrinum. J. Nat. Prod. 2019, 82, 1471–1477. [Google Scholar] [CrossRef]
- Koz, O.; Pizza, C.; Kirmizigül, S.; Kırmızıgül, S. Triterpene and flavone glycosides from Anchusa undulata subsp. hybrida. Nat. Prod. Res. 2009, 23, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kuruüzüm-Uz, A.; Güvenalp, Z.; Kazaz, C.; Salih, B.; Demirezer, L.Ö. Four new triterpenes from Anchusa azurea var. azurea. Helv. Chim. Acta 2010, 93, 457–465. [Google Scholar] [CrossRef]
- Chen, K.-K.; Xie, Z.-J.; Dai, W.; Wang, Q. A new oleanolic-type triterpene glycoside from Anchusa italica. Nat. Prod. Res. 2017, 31, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.C.; Liu, Y.; Zheng, M.Z.; Zhang, R.Y.; Li, M.X.; Bao, F.Y.; Li, H.; Chen, L.X. Triterpenoids from Anchusa italica and their protective effects on hypoxia/reoxygenation induced cardiomyocytes injury. Bioorg. Chem. 2020, 97, 103714. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Bader, A.; Siciliano, T.; Morelli, I.; De Tommasi, N. New pyrrolizidine alkaloids and glycosides from Anchusa strigosa. Planta Med. 2003, 69, 835–841. [Google Scholar] [PubMed]
- Kowalczyk, M.; Masullo, M.; Thiem, B.; Piacente, S.; Stochmal, A.; Oleszek, W. Three new triterpene saponins from roots of Eryngium planum. Nat. Prod. Res. 2014, 28, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Cioni, P.L.; Morelli, I. Differences in the fragrance of pollen, leaves, and floral part of Garland (Chysanthemum coronarium) and composition of the essential oils from flowerheads and leaves. J. Agric. Food Chem. 2003, 51, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Senatore, F.; Rigano, D.; De Fusco, R.; Bruno, M. Composition of the essential oil from flowerheads of Chrysanthemum coronarium L. (Asteraceae) growing wild in Southern Italy. Flavour Fragr. J. 2004, 19, 149–152. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Laconi, S.; Deidda, D.; Maxia, A. Chemical and biological comparisons on supercritical extracts of Tanacetum cinerariifolium (Trevir) Sch. Bip. with three related species of chrysanthemums of Sardinia (Italy). Nat. Prod. Res. 2009, 23, 190–199. [Google Scholar] [CrossRef]
- Andreani, S.; Paolini, J.; Costa, J.; Muselli, A. Essential-Oil Composition and Chemical Variability of Senecio vulgaris L. from Corsica. Chem. Biodivers. 2015, 12, 752–766. [Google Scholar] [CrossRef]
- Saidi, R.; Ghrab, F.; Kallel, R.; El Feki, A.; Boudawara, T.; Chesné, C.; Ammar, E.; Jarraya, R.M. Tunisian Clematis flammula essential oil enhances wound healing: GC-MS analysis, biochemical and histological assessment. J. Oleo Sci. 2018, 67, 1483–1499. [Google Scholar] [CrossRef]
- Boroomand, N.; Sadat-Hosseini, M.; Moghbeli, M.; Farajpour, M. Phytochemical components, total phenol and mineral contents and antioxidant activity of six major medicinal plants from Rayen, Iran. Nat. Prod. Res. 2018, 32, 564–567. [Google Scholar] [PubMed]
- Mohammadhosseini, M. Hydrodistilled volatile oil from stems of Eryngium creticum Lam. in the marginal brackish regions of Semnan province by using gas chromatography combined with mass spectrometry. Asian J. Chem. 2013, 25, 390–392. [Google Scholar] [CrossRef]
- Çelik, A.; Aydınlık, N.; Arslan, I. Phytochemical constituents and inhibitory activity towards methicillin-resistant Staphylococcus aureus strains of Eryngium species (Apiaceae). Chem. Biodivers. 2011, 8, 454–459. [Google Scholar] [CrossRef]
- Sepanlou, M.G.; Ardakani, M.M.; Hajimahmoodi, M.; Sadrai, S.; Amin, G.R.; Sadeghi, N.; Lamardi, S.N.S. Ethnobotanical and traditional uses, phytochemical constituents and biological activities of Eryngium species growing in Iran. Tradit. Med. Res. 2019, 4, 148. [Google Scholar]
- Trautwein, E.A.; Demonty, I. Phytosterols: Natural compounds with established and emerging health benefits. Oléagineux Corps Gras Lipides 2007, 14, 259–266. [Google Scholar]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Kim, D.H.; Hong, Y.H.; Yang, H.J.; Chung, I.S.; Kim, S.H.; Kwon, B.M.; Kim, D.K.; Park, M.H.; Baek, N.I. Polyacetylenes and sterols from the aerial parts of Chrysanthemum coronarium L.(Garland). Front. Nat. Prod. Chem. 2005, 1, 163–168. [Google Scholar] [CrossRef]
- Sadia, N.; Baoshan, L.; Kamran, T.; Arifullah, K.; Zia, U.H.K.; Shafiullah, K. Antimicrobial activity of five constituents isolated from Ranunculus muricatus. J. Med. Plants Res. 2013, 7, 3438–3443. [Google Scholar]
- Hussain, H.; Ali, I.; Wang, D.; Mamadalieva, N.Z.; Hussain, W.; Csuk, R.; Loesche, A.; Fischer, L.; Staerk, D.; Anam, S.; et al. 4-Benzyloxylonchocarpin and muracatanes AC from Ranunculus muricatus L. and their biological effects. Biomolecules 2020, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Z.; Zhou, C.X.; Zhang, S.L.; Yao, W.; Zhao, Y. Studies on the chemical constituents in herb of Ranunculus sceleratus. China J. Chin. Mater. Medica 2005, 30, 124–126. [Google Scholar]
- Elsbaey, M.; Ibrahim, M.A.A.; Shawky, A.M.; Miyamoto, T. Eryngium creticum L.: Chemical Characterization, SARS-CoV-2 Inhibitory Activity, and In Silico Study. ACS Omega 2022, 7, 22725–22734. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.d.C.; Cámara, M.; Tardío, J. Fatty acids profiles of some Spanish wild vegetables. Food Sci. Technol. Int. 2012, 18, 281–290. [Google Scholar] [CrossRef]
- Radwan, H.M.; Abdel-Shafeek, K.A. Phytochemical and bioactivity investigation of Chrysanthemum coronarium [var. discolor] durv growing in Egypt. Egypt. J. Pharm. Sci. 2006, 47, 59–72. [Google Scholar]
- Hu, B.; Khutsishvili, M.; Fayvush, G.; Atha, D.; Borris, R.P. Phytochemical investigations and antimicrobial activities of Anchusa azurea. Chem. Nat. Compd. 2020, 56, 119–121. [Google Scholar] [CrossRef]
- Osw, P.; Hussain, F.; Gozzini, D.; Vidari, G. GC-MS determination and identification of eleven fatty acids in triglycerides isolated from the seeds of traditional Kurdish medicinal plant Anchusa azurea Mill. Eurasian J. Sci. Eng. 2017, 3, 230–240. [Google Scholar]
- Ozcan, T. Fatty acid composition of seed oils in some sand dune vegetation species from Turkey. Chem. Nat. Compd. 2014, 50, 804–809. [Google Scholar] [CrossRef]
- Lajnef, H.B.; Pasini, F.; Politowicz, J.; Tlili, N.; Khaldi, A.; Caboni, M.F.; Nasri, N. Lipid characterization of Eryngium maritimum seeds grown in Tunisia. Ind. Crops Prod. 2017, 105, 47–52. [Google Scholar] [CrossRef]
- Zhang, L.; Anjaneya, S.R.; Sundar, R.K.; Sang, C.J.; Narsimha, R.; Paul, T.S.; John, B.; Kirubakaran, S.; Gerald, M.; Ming, J.W. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. [Google Scholar]
- Wan, C.; Li, S.; Liu, L.; Chen, C.; Fan, S. Caffeoylquinic acids from the aerial parts of Chrysanthemum coronarium L. Plants 2017, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Sulas, L.; Petretto, G.L.; Pintore, G.; Piluzza, G. Bioactive compounds and antioxidants from a Mediterranean garland harvested at two stages of maturity. Nat. Prod. Res. 2017, 31, 2941–2944. [Google Scholar] [CrossRef]
- Góngora, L.; Máñez, S.; Giner, R.M.; Recio, M.C.; Gray, A.I.; Ríos, J.-L. Phenolic glycosides from Phagnalon rupestre. Phytochemistry 2002, 59, 857–860. [Google Scholar] [CrossRef]
- Giner, E.; El Alami, M.; Máñez, S.; Recio, M.C.; Ríos, J.-L.; Giner, R.M. Phenolic substances from Phagnalon rupestre protect against 2, 4, 6-trinitrochlorobenzene-induced contact hypersensitivity. J. Nat. Prod. 2011, 74, 1079–1084. [Google Scholar] [CrossRef]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Saturnino, C.; Malzert-Fréon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill. (Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef] [PubMed]
- Kuruüzüm-Uz, A.; Güvenalp, Z.; Kazaz, C.; Demirezer, L.Ö. Phenolic compounds from the roots of Anchusa azurea var. azurea. Turk. J. Pharm. Sci. 2013, 10, 177–184. [Google Scholar]
- Hou, Y.; Chen, K.; Deng, X.; Fu, Z.; Chen, D.; Wang, Q. Anti-complementary constituents of Anchusa italica. Nat. Prod. Res. 2017, 31, 2572–2574. [Google Scholar] [CrossRef] [PubMed]
- Ghalib, S.A.; Kadhim, E.J. The Investigation of Some Phytochemical Compounds Found in Anchusa strigosa L. Grown Naturally in Iraq. Iraqi J. Pharm. Sci. 2021, 30, 179–188. [Google Scholar] [CrossRef]
- Li, H.; Zhou, C.X.; Pan, Y.; Gao, X.; Wu, X.; Bai, H.; Zhou, L.; Chen, Z.; Zhang, S.; Shi, S.; et al. Evaluation of antiviral activity of compounds isolated from Ranunculus sieboldii and Ranunculus sceleratus. Planta Med. 2005, 71, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qin, F.; Zhou, G. Studies on chemical constituents of Ranunculus muricatus Linn. Nat. Prod. Res. Dev. 2013, 25, 736–741. [Google Scholar]
- Mei, H.; Zuo, S.; Ye, L.; Wang, J.; Ma, S. Review of the application of the traditional Chinese medicinal herb, Ranunculus sceleratus Linn. J. Med. Plant Res. 2012, 6, 1821–1826. [Google Scholar]
- Mejri, H.; Tir, M.; Feriani, A.; Ghazouani, L.; Allagui, M.S.; Saidani-Tounsi, M. Does Eryngium maritimum seeds extract protect against CCl4 and cisplatin induced toxicity in rats: Preliminary phytochemical screening and assessment of its in vitro and in vivo antioxidant activity and antifibrotic effect. J. Funct. Foods 2017, 37, 363–372. [Google Scholar] [CrossRef]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The Evaluation of phenolic acids and flavonoids content and antiprotozoal activity of Eryngium Species biomass produced by biotechnological methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef]
- Conea, S.; Vlase, L.; Chirila, I. Comparative study on the polyphenols and pectin of three Eryngium species and their antimicrobial activity. Cellul. Chem. Technol. 2016, 50, 473–481. [Google Scholar]
- Guven, H.; Arici, A.; Simsek, O. Flavonoids in our foods: A short review. J. Basic. Clin. Heal. Sci. 2019, 3, 96–106. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.C. An Up-To-Date Review of Phytochemicals and Biological Activities in Chrysanthemum Spp. Biosci. Biotechnol. Res. Asia 2016, 13, 615–623. [Google Scholar] [CrossRef]
- Robertson, J.; Stevens, K. Pyrrolizidine alkaloids. Nat. Prod. Rep. 2014, 31, 1721–1788. [Google Scholar] [CrossRef]
- Pereira, C.G.; Locatelli, M.; Innosa, D.; Cacciagrano, F.; Polesná, L.; Santos, T.F.; Rodrigues, M.J.; Custódio, L. Unravelling the potential of the medicinal halophyte Eryngium maritimum L.: In vitro inhibition of diabetes-related enzymes, antioxidant potential, polyphenolic profile and mineral composition. S. Afr. J. Bot. 2019, 120, 204–212. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant alkaloids: Structures and bioactive properties. In Plant-derived Bioactives; Swamy, M., Ed.; Springer: Singapore, 2020; pp. 85–117. [Google Scholar] [CrossRef]
- Zandavar, H.; Babazad, M.A. Secondary metabolites: Alkaloids and flavonoids in medicinal plants. In Herbs and Spices-New Advances; IntechOpen: London, UK, 2023. [Google Scholar]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine alkaloids: Chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Siciliano, T.; De Leo, M.; Bader, A.; De Tommasi, N.; Vrieling, K.; Braca, A.; Morelli, I. Pyrrolizidine alkaloids from Anchusa strigosa and their antifeedant activity. Phytochemistry 2005, 66, 1593–1600. [Google Scholar] [CrossRef]
- Barbakadze, V.; Gogilashvili, L.; Amiranashvili, L.; Merlani, M.; Mulkijanyan, K.; Churadze, M.; Salgado, A.; Chankvetadze, B. Poly [3-(3, 4-dihydroxyphenyl) glyceric acid] from Anchusa italica roots. Nat. Prod. Commun. 2010, 5, 1934578X1000500722. [Google Scholar] [CrossRef]
- Azam, F.; Ahmad, B.; Uzair, M.; Qadir, M.I. Leishmanicidal Activity of Aerial Parts of Ranunculus muricatus and Isolation of Stigmasterol and beta-Sitosterol as Active Constituents. Lat. Am. J. Pharm. 2018, 37, 1905–1908. [Google Scholar]
- Barakat, S.; Burns, D.; Sloul, Z. New Compounds in Eryngium creticum Lam & Biological Activities Evaluation. Jordan J. Pharm. Sci. 2017, 10, 1–12. [Google Scholar]






| Genus | Species Present in Jordan | Common Name | Reference |
|---|---|---|---|
| Chrysanthemum L. | Ch. segetum L. | Common chrysanthemum | [6] |
| Ch. coronarium L. | Corn marigold | ||
| Onopordum Vaill. ex L. | O. alexandrinum Boiss. | Cotton thistle and artichoke Cotton thistle | [8] |
| O. carduiforme Boiss. | |||
| O. cynarocephalum Boiss & Blanche. | |||
| O. heretacanthum C.A.Mey. | |||
| O. palaestinum Eig. | |||
| Phagnalon Cass. | Ph. rupestre L. | African Fleabane | [10] |
| Senecio L. | S. vulgaris L. S. glaucus L. subsp. coronopofolius C. Alexander. S. flavus sch.Bip. S. leucanthemifolius subsp. vernalis Poir. | Common groundsel Decaisne groundsel Bucks horn groundsel | [10] |
| Clematis L. | C. cirrhosa L. | Evergreen Virgin’s Bower | [14] |
| C. flammula L. | Fragrant Bower | ||
| Ranunculus L. | R. arvensis L. | Corn buttercup | [14] |
| R. asiaticus L. | Turban buttercup | ||
| R. cornutus DC. | Evil memedotu | ||
| R. chius DC. | Buttercup | ||
| R. sceleratus L. | Cerely-leaved crowfoot | ||
| R. muricatus L. | Spiny-fruited buttercup | ||
| R. paludusus Poir. | Fine-leaved crowfoot | ||
| Anchusa L. | A. undulate L., | Common alkanet | [10] |
| A. strigosa Banks & Sol, | Prickly alkanet | ||
| A. azurea Mill., | Italian bugloss | ||
| A. milleri Lam.ex Spreng, | Miller’s alkanet | ||
| A. aegyptiaca (L.) A.DC. | Egyptian alkanet | ||
| Eryngium L. | E. creticum Lam., | Field Eryngo | [10,14] |
| E. glomeratum Lam., | |||
| E. falcatum F. Delaroche | Eryngo | ||
| E. maritimum |
| Plant | Extracts/Plant Parts Used | Method Used | Results | Reference |
|---|---|---|---|---|
| Ch.segetum | Ethanol extract of the flower chloroform, ethyl acetate and n-butanol fractions | DPPH CUPRAC | The EtOAc extract demonstrated the highest antioxidant capacity in all assays, with IC50 values of 23.58 µg/mL for DPPH activity and 14.85 µg/mL for CUPRAC capacity A050. | [26] |
| O. alexandrinum | Lipid and essential oil from the seed and aerial parts | DPPH | The unsaponifiable fractions of the plant’s seed and aerial parts, volatile oil, exhibited strong antioxidant activity, with a radical scavenging effect of 79.18%, 82.83%, and 81.65%. | [27] |
| O. alexandrinum | Methanol extract of the flowers n-hexane, chloroform, ethyl acetate and n-butanol fractions | DPPH | The ethanolic extract’s IC50 value for its ability to scavenge free radicals was 200 μg/mL. EtOAc fraction displayed the highest activity (IC50 of 65 μg/mL), followed by the n-butanol fraction (IC50 of 150 μg/mL). The n-hexane and chloroform fractions had negligible activity. | [28] |
| C. cirrhosa | Essential oil from the aerial parts | TAC DPPH. ABTS•+ FRAP CUPRAC | The plant displayed strong antioxidant activity in TAC, FRAP, and ABTS tests, with values of 291.36 mg AAE/g, 119.71 mg TE/g, and 128.91 µg TE/mg, respectively. Moderate antioxidant activity in CUPRAC and DPPH tests, with an IC50 value of 5.10 mg/mL. | [29] |
| C. flammula | (100%) methanol and (70%) methanol extracts of the leaves | DPPH FRAP TAC | The total antioxidant capacity of C. flammula leaf methanol/water extract is 642 mg α-tocopherol/g extract. | [30] |
| C. cirrhosa | (100%) methanol and (70%) methanol extracts of aerial parts | DPPH. ABTS•+ FRAP CUPRAC | The methanol extract had higher TAC activity (138.64 mg AAE/g) than the hydromethanol extract (75.00 mg AAE/g). The difference in ferric ion reducing antioxidant power was slight, with values of 212.42 mg TE/g and 205.15 mg TE/g for methanol and hydromethanol extracts, respectively. Both C. cirrhosa extracts showed significant cupric reducing capacity, the hydromethanol extract had slightly higher ABTS•+ scavenging capacity (237.80 0.24 µg/mg TE) than the methanol extract. | [31] |
| R. sceleratus | Chloroform, ethyl acetate, n-butanol and aqueous fractions | TEAC FRAP DPPH |
The soluble fraction of ethyl acetate inhibited the DPPH radical by 80.9%. Total antioxidant activity (1.04) and FRAP value (238.5TE μM/mL). | [32] |
| R. sceleratus | Hydroalcohol and glycerol-ethanol extracts of aerial part | DPPH, TEAC FRAP CUPRAC SNP | IC50 of hydro alcohol by different methods: 872.1 μL, 186.7 μL, 103, 61, 297 μM ET/100 mL extract. IC50 of glycerol-ethanol by different methods: 988.4 μL, 250.7 μL, 60, 49, 161, 297 μM ET/100 mL extract. | [33] |
| R. sceleratus | Ethanol, chloroform, methanol extract of the root | DPPH ABTS H2O2 | The ethanol extract of R. sceleratus exhibited the highest H2O2 scavenging activity and displayed optimal ABTS and DPPH radical scavenging activity. | [34] |
| R. sceleratus | Methanol extract of Shoot and roots | DPPH | The antioxidant activity of the R. sceleratus has an IC50 value of 0.37 mg/mL and 0.34 mg/mL for the shoot and root, respectively | [35] |
| A. undulata | Methanol extract of aerial parts | DPPH ABTS FRAP CUPRAC β-carotene-linoleic acid method Phosphomolybdenum method Hydroxyl radical scavenging activity Superoxide anion scavenging activity Nitric oxide radical scavenging activity | The methanol extract exhibited high inhibition values for linoleic acid oxidation and had a total antioxidant capacity of 1.531 mmol AAEs/g extract, DPPH scavenging activity of 2.086 mmolTEs/g extract, ABTS assay of 0.112 mmol TEs/g extract, hydroxyl radical scavenging activity of 0.208 mmol MEs/g extract, and NO scavenging activity of 3.866 mmol TEs/g extract. The extract displayed concentration-dependent chelating activity and reduction of Cu(II) ability, with 0.081 mmol TEs/g extract. The reducing power assays showed values of 0.329 mmol TEs/g extract for potassium ferric cyanide and 0.425 mmol TEs/g extract for FRAP. | [36] |
| A. undulata L. subsp. hybrida | Methanol extract of roots and aerial parts | ABTS DPPH | IC50 values for DPPH were 239.47 and 292.04 µg/mL and for ABTS were calculated as 41.15 and 32.3 µg/mL for roots and aerial parts respectively. | [37] |
| A. strigosa | Methanol extract of the flower | DPPH β-carotene bleaching assay | IC50 value 43.75 µg/mL against DPPH radical. IC50 value for β-carotene bleaching 425.8 µg/mL | [38] |
| A. italica | Hydro-ethanol extract of the roots |
FRAP DPPH TAC | Root extract displayed strong iron reduction capacity in the FRAP assay (IC50 0.11 µg/mL). IC50 values in the DPPH test were 0.11 µg/mL for root extract and 0.14 µg/mL for leaf extract, lower than those for ascorbic acid (IC50 0.16 µg/mL) and BHT (IC50 0.20 µg/mL). TAC values were 0.51 and 0.98 mg AAE/g extract for the leaf and root extracts, respectively. | [39] |
| E. creticum | Aqueous extract of leaves and stems | ABTS H2O2 | E. creticum leaves and stems (100 g fresh) provide antioxidants equivalent to 78.50 mg and 50.42 mg of vitamin C. Inhibition of H2O2 by 25 mg/mL of E. creticum leaves was 96%. | [40] |
| E. creticum | Ethanol and aqueous extracts from both leaves and stems |
DPPH Ferrozine H2O2 | The EtOH extract of E. creticum leaves and stems exhibited higher antioxidant activity. The IC50 values for the EtOH extract were 0.18 mg for leaves and 3 mg for stems. The EtOH extract also showed better chelating activity than the aqueous extract, with IC50 values of 0.4 mg for EtOH leaves and 0.5 mg for aqueous leaves. The IC50 values for H2O2 were 2.4 mg for leaves and 12 mg for stems. | [41] |
| E. creticum | Ethanol (40%, 80% and 100%) extract of the plant | DPPH Chelating effects on ferrous ions | 40% ethanol extract possessed the highest iron chelating activity (87.92%) while the 80% ethanol extract showed 89.92% DPPH scavenging activity. | [42] |
| E . maritimum | Essential oils from aerial parts | DPPH ABTS | The total essential oil exhibited strong antioxidant activity with IC50 values of 5.9 mg/mL and 0.7 mg/mL for DPPH and ABTS radical-scavenging abilities, respectively. The oxygenated fraction demonstrated the best radical-scavenging effect, with IC50 values of 8.8 mg/mL for DPPH and 1.34 mg/mL for ABTS radical. | [43] |
| E . maritimum | Volatile fraction of the fruits | DPPH ABTS | The volatile extracts displayed significantly higher antioxidant activity than Trolox, with IC50 values for DPPH and ABTS radical-scavenging capacity at least twice lower than those observed for the reference compound. | [44] |
| E. maritimum | Water, methanol acetone, and ethyl acetate extract of the Aerial parts | DPPH ABTS | Different extracts were active using DPPH, the IC50 value ranged from 1.247 to 31.19 mg/mL of solution. ABTS values ranged from 0.109 to 3.36 mg AA/g. | [45] |
| E. maritimum | Conventional reflux extraction (water and ethanol) and alternative techniques for aerial parts | DPPH assay Xanthine oxidase assay | Aqueous extracts prepared by reflux, microwave-assisted, and ultrasound-assisted exhibited the highest antioxidant activity in the DPPH assay (>70%). Reflux 80% ethanol and supercritical fluid extraction showed the best response to the xanthine oxidase assay (105% and 137%). | [46] |
| R. muricatus | Ethyl acetate soluble fraction of methanol extract of the whole plants | DPPH Lipoxygenase inhibition assay Urease inhibition assay | Muricazine exhibited greater effectiveness in scavenging DPPH radical, with an IC50 value of 42.1 μM compared to the positive control. It displayed moderate inhibitory potential against lipoxygenase (65.2 μM) and urease (54.8 μM). | [47] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| Ch. coronarium | Essential oils from flower head | Agar diffusion plate assay | The growth of Alternaria sp., A. flavus, and P. ultimum was significantly reduced by more than 80%. | [52] |
| Ch. coronarium | Essential oils from flower head | Paper-disk diffusion method | Good antibacterial properties against B. subtilis and S. aureus with zone of inhibition (mm) values: 19 and 20. | [53] |
| S. glaucus | Ethyl acetate soluble fraction of methanol extract of the aerial parts | Agar diffusion plate assay | 2,3-dihydro-3bhydroxyeuparin 3-O-glucopyranoside exhibited strong antibacterial properties (MIC = 8 μM) against E. Coli, B. subtilis, and S. aureus. Antifungal activity against C. albicans and C. tropicalis (MIC = 8 μM). | [54] |
| S. vulgaris | Aerial parts methanol extract n-hexane, dichloromethane, ethyl acetate, and n-butanol fractions | Microdilution technique | MICs of 0.5 mg/mL of methanol extract for B. subtilis and 0.125 mg/mL for S. aureus. The methanol extracts exhibited limited efficacy against dermatophytes, displaying MIC values of 0.5 mg/mL. The n-hexane fractions displayed effectiveness against T. tonsurans, with an inhibitory concentration of 0.031 mg/mL. | [55] |
| S.leucanthemifolius | Aerial parts methanol extract n-hexane, dichloromethane, ethyl acetate, and n-butanol fractions | Microdilution technique | The ethyl acetate extract showed the most potent efficacy against S. aureus, with an MIC value of 31.25 mg/mL, and against C. albicans, with an MIC value of 125 mg/mL. n-Hexane extract showed noteworthy efficacy in combating the dermatophytes T. tonsurans and M. gypseum with an MIC value of 125 mg/mL. | [56] |
| C. flammula | Ethanol extract of leaves | Agar diffusion plate assay | Six bacterial species were inhibited: E. faecalis, P. mirabilis, L. monocytogenes, P. aeruginosa, C. jejuni, C. xerosis, growth inhibition zone is given in mm: 10.6, 9.3, 13.5, 10.4, 10.3, respectively. | [57] |
| C. flammula | Ethanol extract of leaves | Broth microdilution method In vitro biofilm inhibition assay Cell surface hydrophobicity assay Germ tube elongation assay | MIC50 against C. albicans strains 164.89 µg/mL. A dose-dependent reduction in cell surface hydrophobicity was observed. Reduction in both germ tube and hyphae. | [58] |
| R. sceleratus | Essential oils | Agar well diffusion method | The extract displayed modest antibacterial efficacy against P. aeruginosa, E. faecalis, and S. aureus; antifungal activity against C. albicans, with MIC values of 8, 8, 8, and 34 µg/mL, respectively | [59] |
| R. sceleratus | Methanol, aqueous and chloroform extracts of the leaves | Agar well diffusion method Broth macro dilution method | Chloroform extract exhibited the maximum activity with a halo of 23 mm diameter inhibition against T. mentagrophytes followed by T. rubrum (22 mm), M. fulvum (21 mm), M. gypseum (18 mm), and T. tonsurans (15 mm). The methanol extracts produced inhibition zones of 21, 16, 17, 17 and 10 mm, respectively for T. mentagrophytes, M. gypseum, T. rubrum, M. fulvum, and T. tonsurans. | [60] |
| R. sceleratus | Aqueous extracts of the leaves | % of inhibition in colony diameter | Mycelial inhibition (%) for A. brassicae and A. brassicicola were 93.42 and 85.16, respectively. | [61] |
| R. sceleratus | Ethanol and methanol extracts of roots | Disc diffusion assay | MIC of the ethanol extract ranged from 21 to 17.67 mg/mL; methanol extract ranged from 13.67 to 14 mg/mL against A. baumannii, A. niger, B. subtilis, P. aeruginosa, S. aureus, and S. cerevisiae. | [62] |
| R. arvensis | Aqueous extract of aerial parts | Diffusion discs methods | Strong antifungal action against C. albicans. The growth inhibition zone measured 21 mm. | [63] |
| R. arvensis | Essential oils | Paper disk diffusion technique | Significant activity against Escherichia coli, S. aureus, Enterobacter sp., and P. vulgaris, maximal inhibition zones ranging from 15 to 21 mm. | [64] |
| R. arvensis | Whole plants methanol extract dichloromethane, ethyl acetate and n-butanol fractions | Agar tube dilution protocol Microplate Alamar Blue assay | The dichloromethane fraction exhibited antibacterial effects against B. subtilis, S. aureus, P. aeruginosa, and S. typhi; fungicidal effects against M. canis and F. solani. | [65] |
| R. muricatus | Fractions from n-hexane, chloroform, ethyl acetate and ethanol extract | Agar well diffusion method Agar dilution method | Ethyl acetate fraction exhibited the most potent antibacterial efficacy against S. aureus with an MIC of 0.119 µg/mL. n-Hexane fraction showed the strongest antifungal efficacy against A. niger. | [66] |
| A. strigosa | n-Hexane extract of flowers | Disc diffusion method | The total lipids showed a notable antibacterial effect at different doses (0.01–10 mg/mL), with greater activity observed in Gram-positive bacteria in the order of P. aeruginosa, S. faecalis, S. aureus, and B. subtilis. The effect on Gram-negative bacteria followed the sequence Proteus sp., E. coli, Enterobacter sp., and Klebsiella sp. | [67] |
| A. strigosa | Essential oil extracted from the flowers, Fixed oil extracted from the flowers | Disc diffusion method | The essential oil had antibacterial efficacy against both Gram-positive and Gram-negative bacteria including P. aeruginosa, Proteus sp., and S. faecalis. Good efficacy was shown by fixed oil against P. aeruginosa, Proteus sp., and Klebsiella sp., particularly at higher concentrations of 500 μg/mL. | [68] |
| A. strigosa | Aqueous and ethanol extract | Agar well diffusion method | The alcohol extract of A. strigosa exhibited a pronounced inhibitory effect on resistant bacteria, S. salivarius and S. pyogenes, with inhibition zone diameters of 27.0 and 26.0 mm, respectively, compared to its aqueous extract. | [69] |
| A. azurea | Aerial parts methanol extract n-hexane, chloroform, ethyl acetate fraction | Beta-latamase inhibition assays | At a concentration of 10 mg/mL, both the crude extract and ethyl acetate extract of A. azurea showed a very high percentage of inhibition, ranging from 58% to 68%. | [70] |
| A. azurea | Ethanol extract of leaves and roots | Agar disk diffusion microdilution assay. | Both the leaves and roots extracts demonstrated inhibitory effects against four strains of E. coli, two strains of K. pneumoniae, and coagulase-negative Staphylococcus, with zone of inhibition diameters ranging from 11.00 to 16.00 mm for the root extract and 11.67 to 14.33 mm for the leaf extract. | [39] |
| E. creticum | Aqueous and ethanol extract of leaves and stem | Broth microdilution assay | The aqueous and ethanol extracts of the leaves were effective against S. epidermidis, with MIC values of 5 mg/mL and an MBC of 10 mg/mL for the ethanol extract. E. faecalis was sensitive to the extracts but showed greater resistance in the second test phase. S. aureus exhibited consistent sensitivity, while E. coli was highly resistant. P. aeruginosa displayed alternate resistance and had higher values in the second period extracts. The MBC and MIC for the leaves were both 244 mg/mL. | [71] |
| E. creticum | Petroleum ether and methanol extracts of leaves | Mycelial growth inhibition Spore germination tests | In the mycelia growth inhibition test, the petroleum ether extracts exhibited antimycotic activity ranging from 33% to 98%, while the methanol extracts ranged from 3% to 75%. Petroleum ether showed high activity against only B. cinerea and F. oxysporum, with more than 95% inhibition of spore germination. | [72] |
| E. glomeratum | Essential oil extracted from aerial parts | Agar dilution method | MIC values of up to 2 μg/mL. | [73] |
| E . maritimum | Essential oils extracted from fruits and leaves | Broth microdilution assay | The essential oils from both the leaves and fruits were effective against T. mentagrophytes, with MIC values of 1.56 mg/mL and 7.5 mg/mL, respectively as well as S. aureus, with MIC values of 12.5 mg/mL and 60 mg/mL, respectively. The basal leaf essential oil exhibited moderate antibacterial activity against C. albicans and E. coli, with MIC values of 12.5 mg/mL and 25 mg/mL, respectively. | [74] |
| E . maritimum | Roots methanol extract Acetone, ethyl acetate and n-butanol fractions | Disc diffusion method | Inhibition was shown by all extracts against B. cereus, S. aureus, E. coli, and L. monocytogenes. Methanol and n-butanol extracts were effective against P. aeruginosa. The ethyl acetate extract exhibited the maximum activity against all test fungi, with A. flavus showing the greatest inhibition zone of 10 mm at a concentration of 50 mg/mL. | [75] |
| E. maritimum | Ethanol extracts from leaves and roots | Method of series dilutions | The antifungal activity of E. Maritimum was most prominent against T. mentagrophytes dermatophyte strains, with MIC values ranging from 40 to 100 mg/mL. | [76] |
| E. maritimum | Conventional reflux extraction (water and ethanol) and alternative techniques for aerial parts | Agar dilution method | The extracts demonstrated antibacterial activity against five species including P. acnes, Streptococcus bovis, S. pyogenes, S. dysgalactiae, and S. pneumoniae. The supercritical fluid extraction was effective in inhibiting all strains of P. acnes at 400 mg/L, while the reflux ethanol extract was able to inhibit the clinical strain N896 of P. acnes. | [46] |
| E. falcatum | Aqueous, methanol, and ethyl acetate extracts of the root and aerial parts Essential oils | Microbroth dilution technique | E. Maritimum essential oil had moderate antimicrobial potential, with K. pneumoniae and P. mirabilis being sensitive, S. pyogenes was resistant. Acetone extract was the most efficient, followed by the EtOAc and MeOH extracts, while the H2O extract had no activity. E. falcatum aerial and root parts had moderate antibacterial activity against Gram-positive strains. | [77] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| Ch. coronarium | Essential oils of flower head | MTT cell proliferation assay | The essential oil inhibited the growth of various tumor cell lines (Caco-2, T47D, MCF-7, HeLa), with LD50 values ranging from 43 to 110 µg/mL. | [53] |
| Ch. coronarium | The methanol extract of aerial parts | MTT cell proliferation assay | The IC50 values of the Ch. coronarium extract against WM1361A, CACO-2, HRT18, MCF-7, T47D, and A375.S2 ranged from 75.8 to 138.5 μg/mL. | [80] |
| O. cynarocephalum | Water extract of aerial parts | MTT cell proliferation assay Flow cytometry analysis TUNEL assay Western blot analysis | The extract dose-dependently inhibited HCT-116 cell growth (IC50 0.18 mg/mL) more effectively than HT-29 cells (IC50 1.8 mg/mL). It upregulated p53 and Bax while suppressing Bcl-2, inducing apoptosis. | [81] |
| O. cynarocephalum | Acetone and chloroform extracts of the aerial parts | MTT cell proliferation assay DNA analysis by COMET assay Caspase colorimetric assay Western blot analysis | Acetone and chloroform extracts inhibited the M14, A2058, and A375 cell lines, with higher potency against A375. IC50 values on A375 cells were 21.32 µg/mL (acetone) and 10.12 µg/mL (chloroform). Both extracts increased caspase-3 activity, inducing apoptosis via PTEN inhibition and Hsp70 downregulation. | [82] |
| S. vulgaris | Aerial parts methanol extract (n-hexane, dichloromethane and ethyl acetate) fractions | Sulforodamine B assay | Caco-2 exhibited the highest sensitivity to the methanolic and CH2Cl2 extracts of S. vulgaris, with IC50 values of 34 mg/mL and 5 mg/mL, respectively. | [83] |
| S. leucanthemifolius | Aerial parts methanol extract (n-hexane, dichloromethane, and ethyl acetate, n-butanol) fractions | Sulforhodamine B assay | The large cell carcinoma (IC50 20.1 μg/mL) and colorectal adenocarcinoma (IC50 36.37 μg/mL) were inhibited by dichloromethane extracts, the n-hexane extract demonstrated notable activity against hepatocellular carcinoma (IC50 value 30.88 μg/mL). | [84] |
| C. flammula | Ethanol extract of leaves | MTT cell proliferation assay | Cytotoxic potential on CHL and PLC with IC50 values of 58.5 and 47.3 µg/mL, respectively). | [85] |
| E. creticum | Aqueous, methanol, and ethyl acetate from fresh leaves and stems | XTT cell viability assay | The methanol extracts of the two plant parts inhibited MCF7 cell growth by 72% and 68% after 48 h of treatment. The aqueous and ethyl acetate extracts (at 2.5 mg/mL) of the leaves and stems showed no cytotoxicity. | [86] |
| E. creticum | Ethanol extracts of leaves, stems, roots, and whole plant | Neutral red assay DNA fragmentation assay | All plant parts inhibited HeLa cell viability (0–250 M). The ethanol extract of the second harvest leaves displayed the strongest potency with an IC50 value of 47.24 μg/mL at 48 h. | [87] |
| E. glomeratum | Petroleum ether extracts of the aerial parts | MTT cell proliferation assay | Cytotoxicity of E. glomeratum against cancer macrophage-like cell lines (J774) was 1.11 μg/mL, with selectivity indices of 2.1. | [88] |
| E. maritimum | Aqueous extract of aerial and root parts | MTT cell proliferation assay | E. maritimum showed IC50 values of 32.42 µg/mL and 35.01 µg/mL for the aerial and root parts, respectively, in HepG2 cells. In contrast, the IC50 values were 50.00 µg/mL and 30.25 µg/mL for the aerial and root parts, respectively, in Hep2 cells. The LDH method yielded the lowest IC50 values for Hep2 cells, which were 51.67 µg/mL and 34.32 µg/mL for the aerial and root parts, respectively. | [89] |
| Ch. coronarium | Ethyl acetate fraction of methanol extracts of aerial parts | XTT cell viability assay | Campesterol exhibited a concentration-dependent inhibition of bFGF-induced proliferation and tube formation in HUVECs. Campesterol was effective in disrupting bFGF-induced neovascularization in chick chorioallantoic membrane (CAM) in vivo. | [90] |
| S. glaucus | Ethyl acetate fraction of the aerial parts | MTT cell proliferation assay | Cytotoxic activity against PANC-1 cancer cell lines was (IC50 7.5 μM). | [54] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| Ch. coronarium and Ch. segetum | Essential oil of flowerheads | Phosrithong and Nuchtavorn method | IC50 values of Ch. coronarium and Ch. segetum against the 5-lipoxygenase enzyme were 0.151 and 0.017 mg/mL, respectively. | [94] |
| O. cynarocephalum | Water extracts of the aerial part | In vitro model of ET-induced inflammation in SCp2 cells. In vivo model of ET-induced paw edema | The extract was found to inhibit ET-induced IL-6, gelatinases, and NF-B activation in SCp2 mammary epithelial cells. Extract demonstrated a significant reduction in paw edema in a model of ET-induced edema. | [95] |
| C. flammula | Ethanol extract of leaves | Assessment of the mucus content in the stomach wall using the indomethacin ulcer model Inhibition of H+/K+-ATPase Determination of myeloperoxidase activity | The proton pump and MPO activities were significantly inhibited by the ethanol extract, with decreases of 90% and 99%, respectively. | [96] |
| R. sceleratus | Ethanol extract of aerial parts and roots | Nitrite concentration in LPS-stimulated RAW 264.7 macrophage cell line | An inhibitory effect was seen in relation to concentration in all extracts; the aerial parts extract showed the most potent inhibition (IC50 = 22.08 ± 1.32 g/mL), even surpassing indomethacin. | [97] |
| R. muricatus | Aqueous and methanol extract of whole plant | Carrageenan and egg albumin induced paw edema in mice Acetic acid induced writhing Formalin induced paw licking in mice models | At 150 mg/kg, the maximum dosage, the extract reduced paw edema caused by carrageenan and egg albumin. The extract at the same dosage significantly reduced formalin-induced paw licking and acetic acid-induced abdominal constrictions and hind limb stretching. | [98] |
| A. strigosa | Aqueous and methanol extracts of whole plant | Complete Freund’s adjuvant (CFA)-induced | Both methanol and aqueous extracts were found to significantly reduce paw edema, arthritis index, and body weight loss. The extracts decreased the elevated levels of serum WBC in rats induced with CFA. | [99] |
| A. azurea | Methanol extracts of aerial parts and the roots fractionated with n-hexane, n-butanol | Carrageenan-induced paw edema in rats | The methanol extract of aerial parts displayed 30% anti-inflammatory activity at a 200 mg/kg bw dose. n-Butanol fraction of the methanol extract exhibited the highest anti-inflammatory activity with a 42% reduction at the same dosage. | [100] |
| E. maritimum | Ethanol extract of aerial parts | Turpentine oil-induced acute inflammation model in rats | The extract exhibited anti-inflammatory effects by decreasing the proliferation and activity of total leukocytes and neutrophils. The extract significantly reduced the synthesis of NO. | [101] |
| E. maritimum | Methanol extract of leaves | Cyclooxygenase-1 assay | Anti-acetylcholinesterase activity was 65.34%. | [102] |
| E. maritimum | Ethanol extracts from the aerial parts and roots | p-Benzoquinone-induced writhing test Carrageenan-induced paw edema TPA-induced ear edema tests | The ethanol extract of aerial portions significantly reduced ear edema induced by TPA with a 58.8% inhibitory ratio. Inhibitory ratio of aerial parts against p-benzoquinone-induced writhings in mice was 55.8%. Aerial parts had a 36.1% inhibitory ratio against carrageenan-induced paw oedema in mice. | [22] |
| A. azurea | Rosmarinic acid isolated from n-butanol fraction | Carrageenan-induced paw edema in rats | At a dosage of 50 mg/kg bw, the anti-inflammatory effects of rosmarinic acid were found to be similar to those of indomethacin. | [100] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| S.leucanthemifolius | Methanol extract of aerial parts fractionated with n-hexane, dichloromethane, ethyl acetate n-butanol | α-Amylase inhibition assay | At 0.05 mg mL−1, the dichloromethane extract inhibited α-amylase by 56.6%; the n-butanol extract inhibited α-amylase by 89.2%. | [56] |
| C. cirrhosa | 100%methanol and 70%methanol of aerial parts | α-Amylase inhibition assay α-Glucosidase inhibition assay | The methanol extract had a greater inhibitory effect on α-amylase (36.63%) compared to the hydromethanol extract (24.70%). The hydromethanol extract had a significantly higher α-glucosidase inhibition rate (40.93%) compared to the methanol extract (14.03%). | [31] |
| A. undulata | Methanol extract of aerial parts | α-Amylase inhibition assay α-Glucosidase inhibition assay | Compared to α-amylase (0.193 mmol ACEs/g extract), the methanol extract showed stronger α-glucosidase inhibition (0.219 mmol ACEs/g extract). | [36] |
| A. undulata L. subsp. hybrida | Methanol extract of roots and aerial parts | α-Amylase inhibition assay α-Glucosidase inhibition assay | With increasing concentrations, the methanol extract of the roots and aerial parts of herbs inhibited both α-glucosidase and α-amylase. | [37] |
| A. strigosa | Aqueous extract of flowers | Antidiabetic effect in streptozotocin-induced diabetic rats | The aqueous extract had an antidiabetic effect in diabetic rats, reducing the blood glucose levels and improving the serum insulin levels in a dose-dependent manner. Cholesterol and triglyceride levels were significantly lower, and hepatic glycogen levels increased. | [107] |
| E. creticum | Aqueous extracts of aerial parts | MTT cell proliferation assay of β-cell proliferation | Aqueous extracts increased beta-cell proliferation (0.001–1.0 mg mL−1) in a dose-dependent manner | [108] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| A. strigosa | Ethanol extract of the roots partitioned with petroleum ether, chloroform, and n-butanol | Ethanol-induced ulcer model | The petroleum ether-soluble fraction, which provided 91% protection, was the most effective at lowering the ulcer index. The n-butanol-soluble fraction only provided 65% protection; the chloroform-soluble fraction provided 86% protection; the water-soluble portion failed to adequately shield the stomach from the inflammatory agent. | [113] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| A. undulata | Methanol extract of aerial parts | Anticholinesterase activity Anti-butyrylcholinesterase activity | The extract displayed inhibitory effects on both AChE and BChE, with values of 2.238 and 1.239 μmol GALAEs/g extract, respectively. | [36] |
| A. undulata L. subsp. hybrida | Methanol extract of roots and aerial parts | Anticholinesterase activity Anti-butyrylcholinesterase activity | The methanolic extract’s cholinesterase inhibitory effects on AChE and BChE were measured in galantamine equivalents and were found to be 2.238 and 1.239 μmol GALAEs/g extract, respectively. | [37] |
| C. cirrhosa | 100% methanol and 70% methanol of aerial parts | Anticholinesterase activity Anti-butyrylcholinesterase activity | C. cirrhosa extracts showed significant AChE inhibition, with the hydromethanolic extract demonstrating higher activity levels compared to the methanolic extract. The methanol extract exhibited higher BChE inhibition rates; the hydromethanolic extract had a greater overall inhibitory effect on both enzymes. Both extracts were less effective against BChE compared to the positive control galantamine. | [31] |
| Plant | Extract/Part Used | Method Used | Results | Reference |
|---|---|---|---|---|
| R. muricatus | Methanol extract | Langendorff perfused heart apparatus | The methanol extract at doses between 1 ng and 10 mg increased the perfusion pressure and force of contraction. The crude extract also significantly increased the heart rate at doses between 1 ng and 1 μg. | [117] |
| A. italica | Ethanol extract of whole plant | Western blot | The A. italica extract increased post-MI mice survival rates, with 30 mg/kg and 50 mg/kg doses resulting in 73% and 80% survival rates, respectively. Treatment at these doses reduced the infarct size and TNF-, IL-1, and IL-6 levels. Treatment suppressed the activation of the PI3K/Akt/mTOR signaling pathway. | [118] |
| A. italica | 70% ethanol extract of flowers | The ischemia model involved bilateral occlusion of carotid arteries | A. italica’s hydroalcohol extract scavenged the free radicals produced by ischemia/reperfusion. | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruwad, M.I.; Salah El Dine, R.; Gendy, A.M.; Sabry, M.M.; El Hefnawy, H.M. Exploring the Biological and Phytochemical Potential of Jordan’s Flora: A Review and Update of Eight Selected Genera from Mediterranean Region. Molecules 2024, 29, 1160. https://doi.org/10.3390/molecules29051160
Alruwad MI, Salah El Dine R, Gendy AM, Sabry MM, El Hefnawy HM. Exploring the Biological and Phytochemical Potential of Jordan’s Flora: A Review and Update of Eight Selected Genera from Mediterranean Region. Molecules. 2024; 29(5):1160. https://doi.org/10.3390/molecules29051160
Chicago/Turabian StyleAlruwad, Manal I., Riham Salah El Dine, Abdallah M. Gendy, Manal M. Sabry, and Hala M. El Hefnawy. 2024. "Exploring the Biological and Phytochemical Potential of Jordan’s Flora: A Review and Update of Eight Selected Genera from Mediterranean Region" Molecules 29, no. 5: 1160. https://doi.org/10.3390/molecules29051160
APA StyleAlruwad, M. I., Salah El Dine, R., Gendy, A. M., Sabry, M. M., & El Hefnawy, H. M. (2024). Exploring the Biological and Phytochemical Potential of Jordan’s Flora: A Review and Update of Eight Selected Genera from Mediterranean Region. Molecules, 29(5), 1160. https://doi.org/10.3390/molecules29051160