Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability
Abstract
1. Introduction
2. Results and Discussion
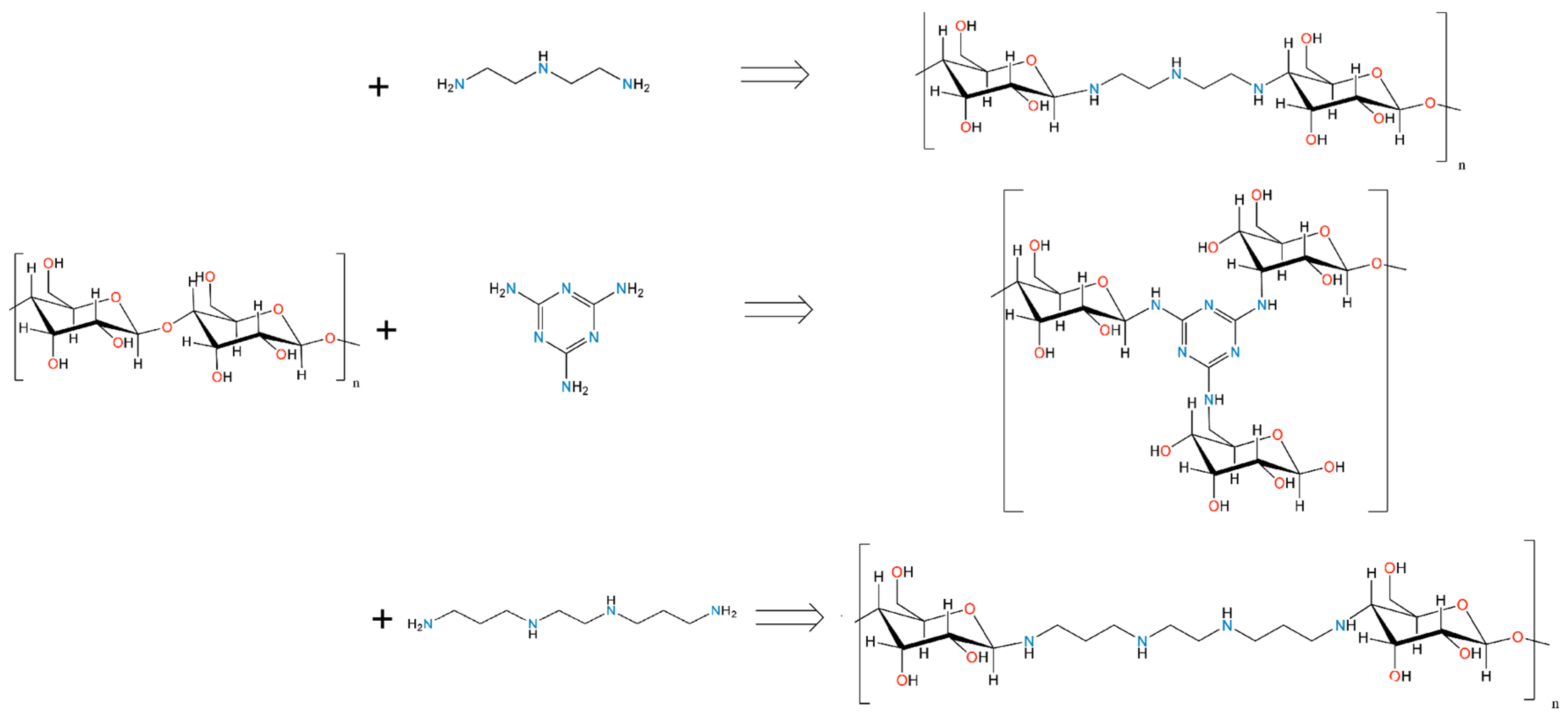
2.1. Composition and Structural Characterisation
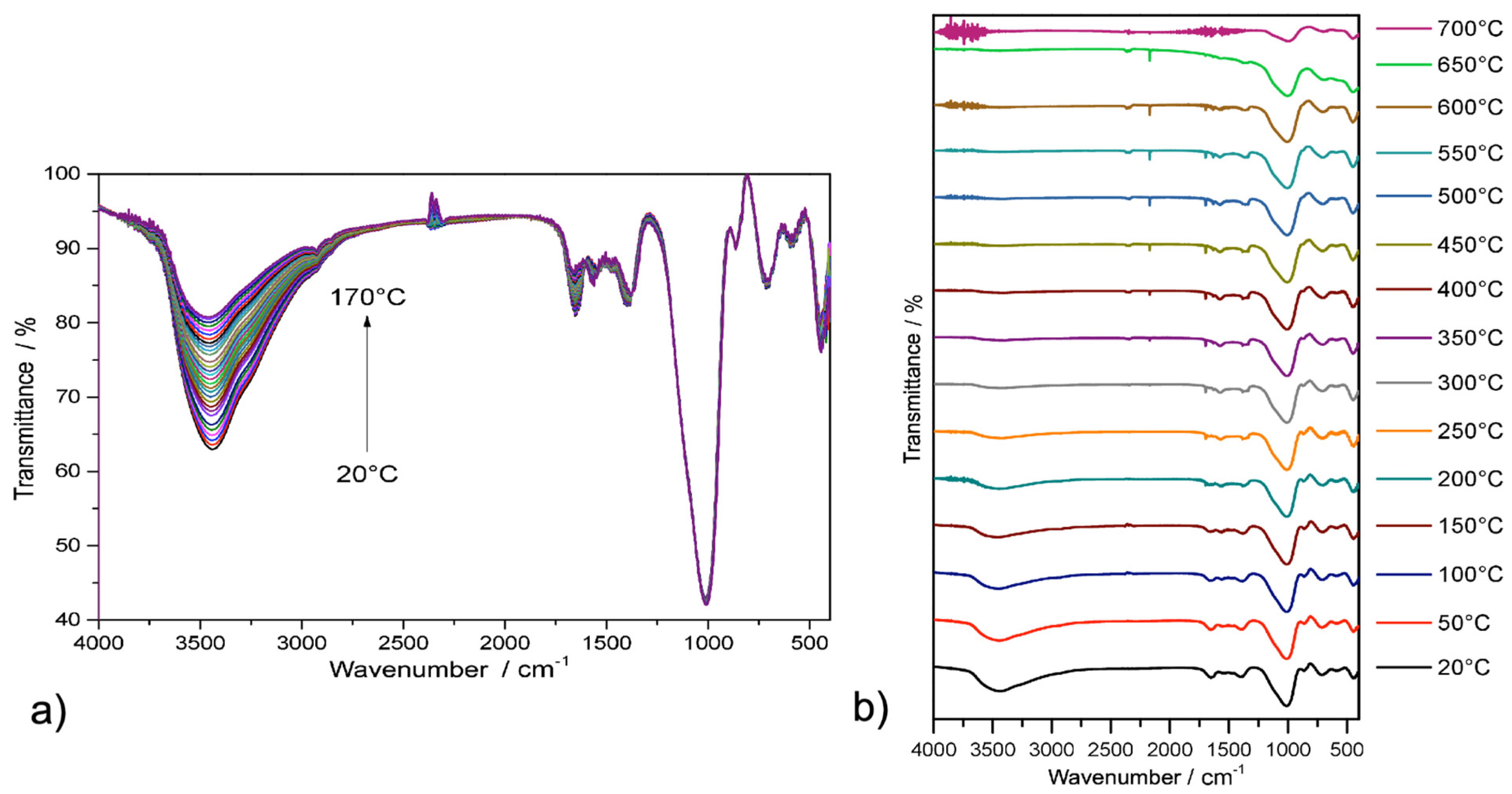
2.2. Spectroscopic Analysis
2.3. Thermal Stability
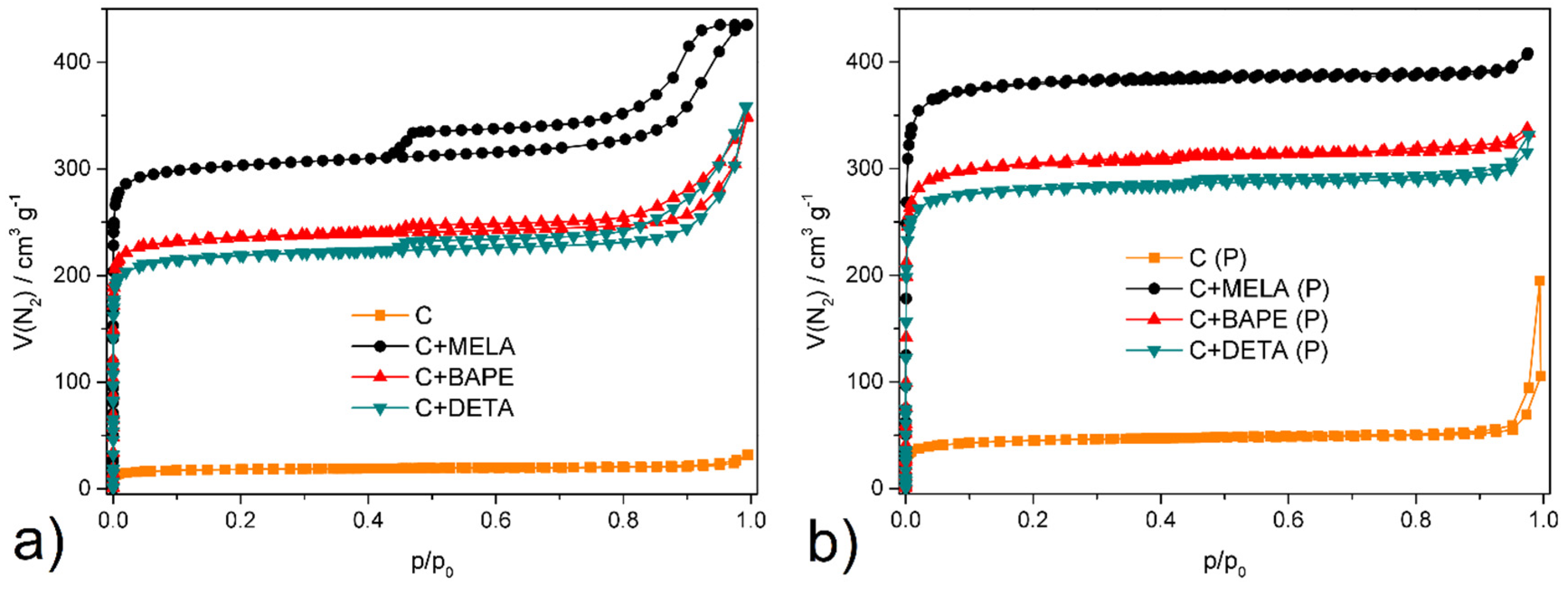
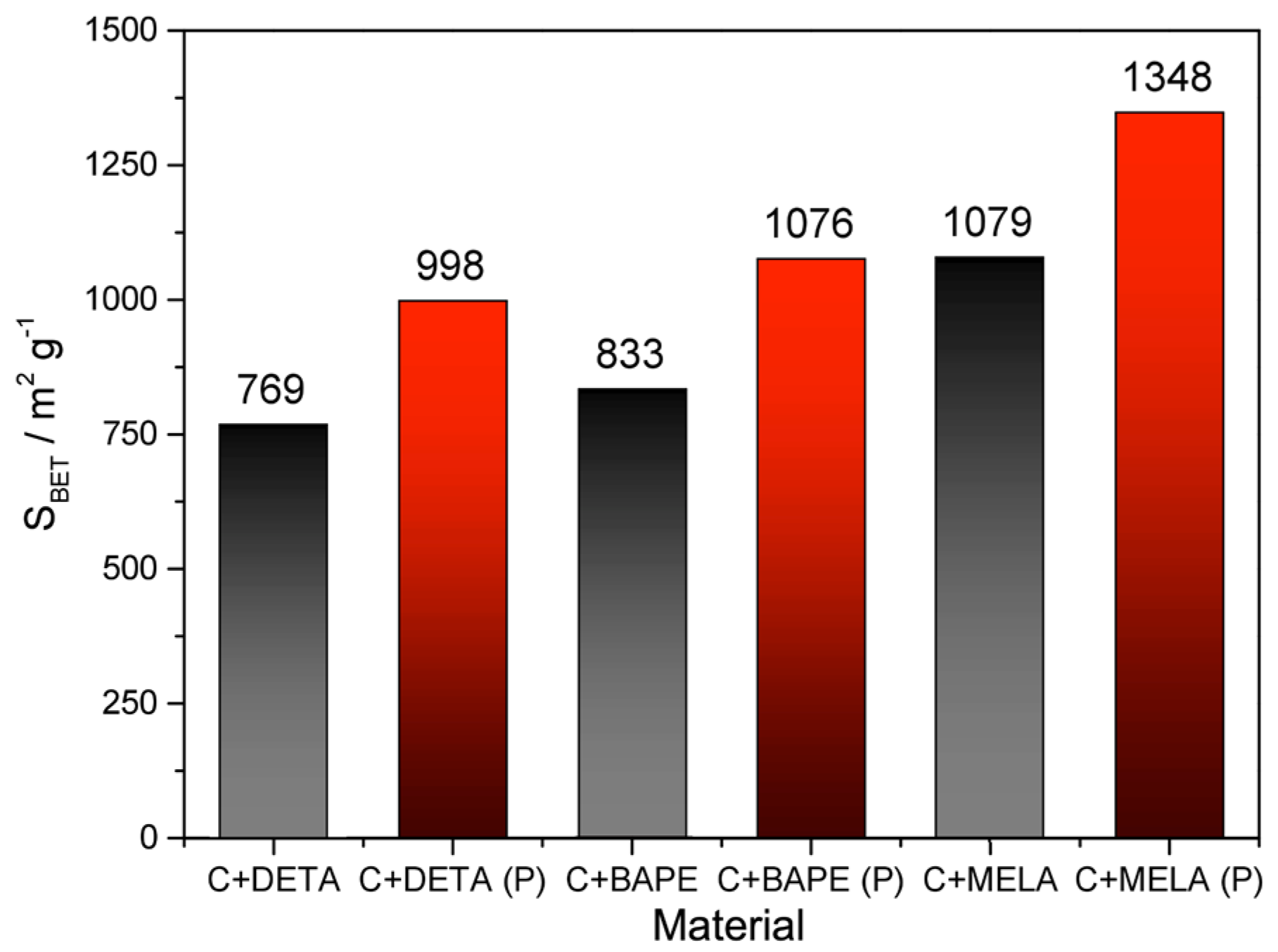
2.4. Textural Properties
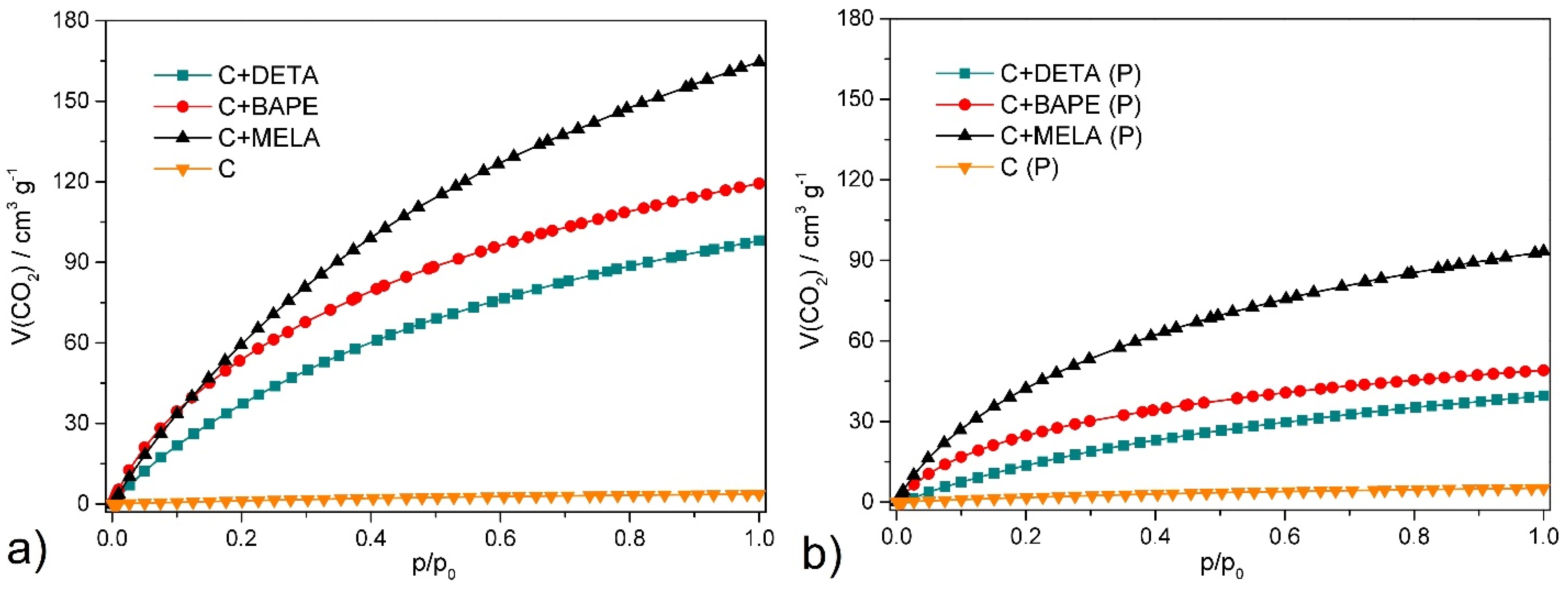
2.5. CO2 Adsorption
| Precursor of APCs | Activation | Activating Agent | Activation Method | SBET (m2 g−1) | Adsorption Capacity at 0 °C and 1 Bar (mmol) | References |
|---|---|---|---|---|---|---|
| Peanut shell char | chemical | KOH | homogenisation | 1713 | 7.25 | [60] |
| Black locust | chemical | KOH | impregnation | 2064 | 7.19 | [61] |
| Fern Leaves | chemical | KOH/CO2 | impregnation | 937 | 6.77 | [62] |
| Pine Cone | chemical | KOH | impregnation | 1787 | 6.57 | [63] |
| Arundo donax | chemical | KOH | homogenisation | 1122 | 6.30 | [64] |
| Pomegranate peels | chemical | KOH | impregnation | 585 | 6.03 | [65] |
| Rice husk | chemical | KOH | homogenisation | 1486 | 5.83 | [66] |
| Coconut Shell | chemical | CO2 | impregnation | 1327 | 5.60 | [67] |
| Sugarcane bagasse | chemical | LiOH | homogenisation | 1149 | 5.50 | [68] |
| Palm sheath | chemical | KOH | homogenisation | 1052 | 5.28 | [69] |
2.6. Recyclability
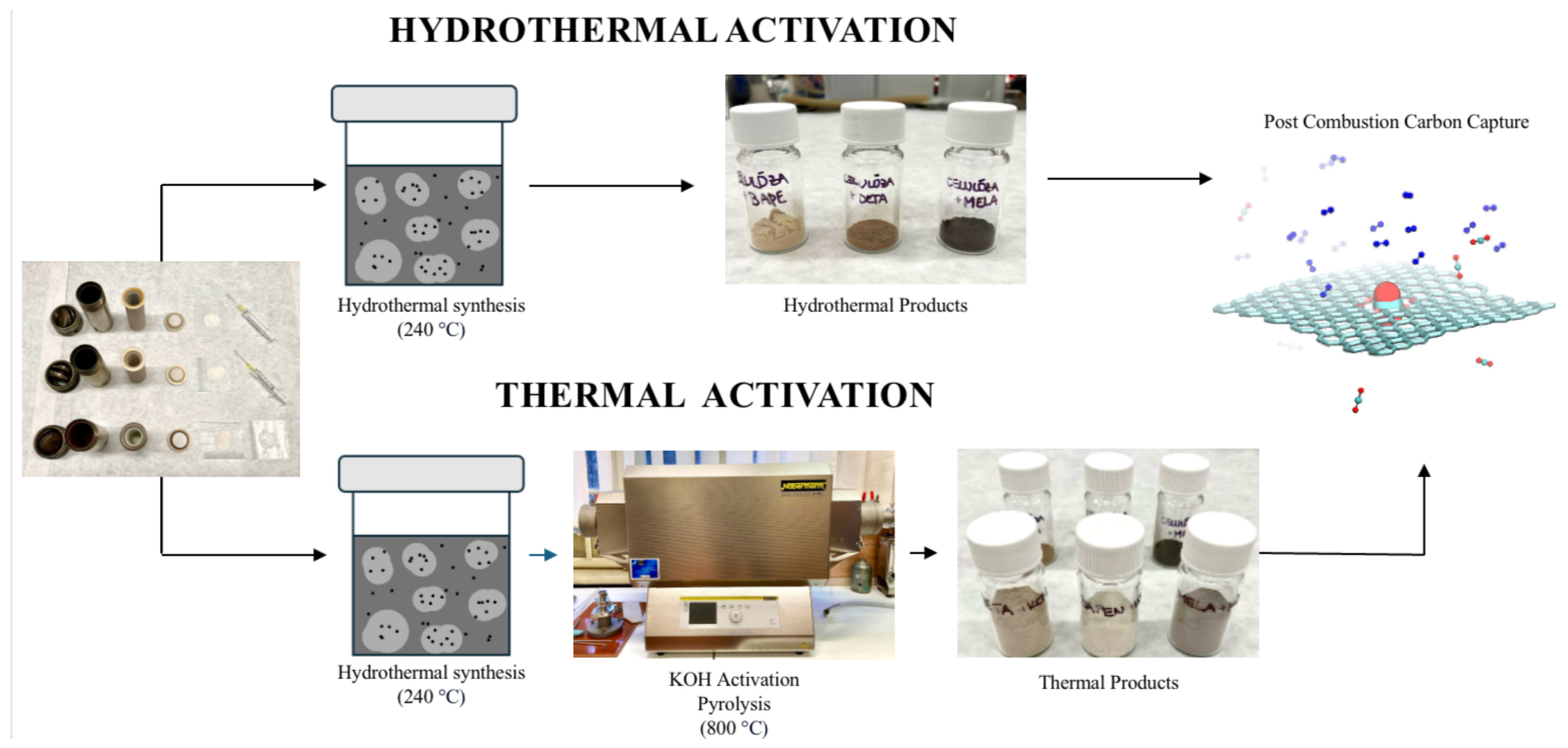
3. Experimental
3.1. Materials
3.2. Preparation of Hydrothermal Activated Carbons
3.3. Preparation of Pyrolysed Activated Carbons
3.4. Characterisation of Activated Porous Carbons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langsdorf, S.; Löschke, S.; Möller, V.; Okem, A.; Rama, B.; Belling, D.; Dieck, W.; Götze, S.; Kersher, T.; Mangele, P.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability; Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022; Volume 1. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.; Péan, C.; Chen, Y.; Goldfarb, L.; Gomis, M.; Matthews, J.; Berger, S.; et al. The Physical Science Basis Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Edited By. In Climate Change 2021 The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021; Volume 2. [Google Scholar] [CrossRef]
- Rousset, N. The Impact of Climate Change, Water Security and the Implications for Agriculture. China Perspect. 2007, 2007, 30–38. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Ofori, D.O.; Bandauko, E.; Kutor, S.K.; Odoi, A.; Asare, A.B.; Akyea, T.; Arku, G. A Systematic Review of International and Internal Climate-Induced Migration in Africa. Sustainability 2023, 15, 16105. [Google Scholar] [CrossRef]
- Hasaba, S. The Impact of Climate Change in the Developing World: Where Developing Countries Are Positioned in Climate Change Discussions. Int. J. Clim. Chang. Impacts Responses 2012, 3, 41–52. [Google Scholar] [CrossRef]
- Alo, A.O.; Baines, R.; Conway, J.; Cannon, N. The Impacts of Climate Change on Agriculture in Developing Countries: A Case Study of Oyo State, Nigeria. Int. J. Clim. Chang. Impacts Responses 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Chinowsky, P.; Hayles, C.; Schweikert, A.; Strzepek, N.; Strzepek, K.; Schlosser, C.A. Climate Change: Comparative Impact on Developing and Developed Countries. Eng. Proj. Organ. J. 2011, 1, 67–80. [Google Scholar] [CrossRef]
- Bandilla, K.W. Carbon Capture and Storage. Future Energy 2020, 669–692. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-Combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Gyepes, R.; Zauška, L.; Bourrelly, S. A Series of Four Novel Alkaline Earth Metal-Organic Frameworks Constructed of Ca(ii), Sr(ii), Ba(ii) Ions and Tetrahedral MTB Linker: Structural Diversity, Stability Study and Low/High-Pressure Gas Adsorption Properties. RSC Adv. 2020, 10, 32323–32334. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.Z.M.; Buthiyappan, A.; Raman, A.A.A.; Patah, M.F.A.; Sufian, S. Recent Advances in Biomass Based Activated Carbon for Carbon Dioxide Capture—A Review. J. Ind. Eng. Chem. 2022, 116, 1–20. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research Progress of Aqueous Amine Solution for CO2 Capture: A Review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Rochelle, G.T. Thermal Degradation of Amines for CO2 Capture. Curr. Opin. Chem. Eng. 2012, 1, 183–190. [Google Scholar] [CrossRef]
- Kim, C.; Talapaneni, S.N.; Dai, L. Porous Carbon Materials for CO2 Capture, Storage and Electrochemical Conversion. Mater. Rep. Energy 2023, 3, 100199. [Google Scholar] [CrossRef]
- Zelenková, G.; Zelenka, T.; Almáši, M.; Soldánová, M. Graphene as a Promising Additive to Hierarchically Porous Carbon Monoliths for Enhanced H2 and CO2 Sorption. J. CO2 Util. 2023, 68, 102371. [Google Scholar] [CrossRef]
- Gao, Y.; He, X.; Mao, K.; Russell, C.K.; Toan, S.; Wang, A.; Chien, T.; Cheng, F.; Russell, A.G.; Zeng, X.C.; et al. Catalytic CO2 Capture via Ultrasonically Activating Dually Functionalised Carbon Nanotubes. ACS Nano 2023, 17, 8345–8354. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.C.; De Souza Figueiredo, K.C. Asymmetric Oxygen-functionalised Carbon Nanotubes Dispersed in Polysulfone for CO2 Separation. J. Appl. Polym. Sci. 2022, 140, e53303. [Google Scholar] [CrossRef]
- Li, H.; Dilipkumar, A.; Abubakar, S.; Zhao, D. Covalent Organic Frameworks for CO2 Capture: From Laboratory Curiosity to Industry Implementation. Chem. Soc. Rev. 2023, 52, 6294–6329. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Paul, R.; Chatterjee, R.; Shinde, D.B.; Lai, Z.; Bhaumik, A.; Mondal, J. Tuning of Microenvironment in Covalent Organic Framework via Fluorination Strategy promotes Selective CO2 Capture. Chem. Asian J. 2023, 18, e202200970. [Google Scholar] [CrossRef]
- Garg, A.; Almáši, M.; Bednarčík, J.; Sharma, R.; Rao, V.S.; Panchal, P.; Jain, A.; Sharma, A. Gd(III) Metal-organic Framework as an Effective Humidity Sensor and its Hydrogen Adsorption Properties. Chemosphere 2022, 305, 135467. [Google Scholar] [CrossRef] [PubMed]
- Almáši, M.; Zeleňák, V.; Gyepes, R.; Zukal, A.; Čejka, J. Synthesis, Characterisation and Sorption Properties of Zinc(II) Metal–organic Framework Containing Methanetetrabenzoate Ligand. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 101–107. [Google Scholar] [CrossRef]
- Király, N.; Capková, D.; Gyepes, R.; Vargová, N.; Kazda, T.; Bednarčík, J.; Yudina, D.; Zelenka, T.; Čudek, P.; Zeleňák, V.; et al. Sr(II) and Ba(II) Alkaline Earth Metal–Organic Frameworks (AE-MOFs) for Selective Gas Adsorption, Energy Storage, and Environmental Application. Nanomaterials 2023, 13, 234. [Google Scholar] [CrossRef]
- Capková, D.; Kazda, T.; Čech, O.; Király, N.; Zelenka, T.; Čudek, P.; Sharma, A.; Hornebecq, V.; Straková Fedorková, A.; Almáši, M. Influence of Metal-Organic Framework MOF-76(Gd) Activation/Carbonisation on the Cycle Performance Stability in Li-S Battery. J. Energy Storage 2022, 51, 104419. [Google Scholar] [CrossRef]
- Cavallo, M.; Dosa, M.; Porcaro, N.G.; Bonino, F.; Piumetti, M.; Crocellà, V. Shaped Natural and Synthetic Zeolites for CO2 Capture in a Wide Temperature Range. J. CO2 Util. 2023, 67, 102335. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Fatemeh Bahmanzadegan; Mahyar Ashourzadeh Pordsari; Ghaemi, A. Improving the Efficiency of 4A-Zeolite Synthesised from Kaolin by Amine Functionalization for CO2 Capture. Sci. Rep. 2023, 13, 12533. [Google Scholar] [CrossRef]
- Borcănescu, S.; Popa, A.; Verdeş, O.; Suba, M. Functionalized Ordered Mesoporous MCM-48 Silica: Synthesis, Characterization and Adsorbent for CO2 capture. Int. J. Mol. Sci. 2023, 24, 10345. [Google Scholar] [CrossRef]
- Bisht, M.; Bhawna, B.; Singh, B.; Pandey, S. Deep Eutectic Solvent-modified Mesoporous Silica for CO2 Capture: A New Generation of Hybrid Sorbents. J. Mol. Liq. 2023, 384, 122203. [Google Scholar] [CrossRef]
- Zeleňák, V.; Badaničová, M.; Halamová, D.; Čejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-Modified Ordered Mesoporous Silica: Effect of Pore Size on Carbon Dioxide Capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar] [CrossRef]
- Krzyżak, A.; Habina-Skrzyniarz, I.; Machowski, G.; Mazur, W. Overcoming the Barriers to the Exploration of Nanoporous Shales Porosity. Microporous Mesoporous Mater. 2020, 298, 110003. [Google Scholar] [CrossRef]
- Pan, C.; Song, Y.; Wang, J.; Zhan, S.; Unluer, C.; Ruan, S. Unlocking the Role of Recycled Aggregates in the Performance Enhancement and CO2 Capture of Reactive Magnesia Cement Formulations. Cem. Concr. Res. 2023, 168, 107148. [Google Scholar] [CrossRef]
- Marques, L.M.; Mota, S.M.; Teixeira, P.; Pinheiro, C.I.C.; Matos, H.A. Ca-Looping Process Using Wastes of Marble Powders and Limestones for CO2 Capture from Real Flue Gas in the Cement Industry. J. CO2 Util. 2023, 71, 102450. [Google Scholar] [CrossRef]
- Lv, Q.; Zhou, T.; Luan, Y.; Zheng, R.; Guo, X.; Wang, X.; Hemmati-Sarapardeh, A. A Green Aqueous Foam Stabilized by Cellulose Nanofibrils and Camellia Saponin for Geological CO2 Sequestration. J. Clean. Prod. 2023, 406, 136980. [Google Scholar] [CrossRef]
- Rahma, N.A.; Kurniasari, A.; Pambudi, Y.D.S.; Bintang, H.M.; Zulfia, A.; Hudaya, C. Characteristics of Corncob-Originated Activated Carbon Using Two Different Chemical Agent. IOP Conf. Ser. Mater. Sci. Eng. 2019, 622, 012030. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Alsultan, M.; Sabah, A.A.; Swiegers, G.F. Carbon Dioxide Adsorption by a High-Surface-Area Activated Charcoal. J. Compos. Sci. 2023, 7, 179. [Google Scholar] [CrossRef]
- Phothong, K.; Tangsathitkulchai, C.; Lawtae, P. The Analysis of Pore Development and Formation of Surface Functional Groups in Bamboo-Based Activated Carbon during CO2 Activation. Molecules 2021, 26, 5641. [Google Scholar] [CrossRef] [PubMed]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A Review on Surface Modification of Activated Carbon for Carbon Dioxide Adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kiełbasa, K. Advances in Modification of Commercial Activated Carbon for Enhancement of CO2 Capture. Appl. Surf. Sci. 2019, 494, 137–151. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R.M. Activated Carbons from Biomass-Based Sources for CO2 Capture Applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef] [PubMed]
- Hack, J.; Maeda, N.; Meier, D.M. Review on CO2 Capture Using Amine-Functionalised Materials. ACS Omega 2022, 7, 39520–39530. [Google Scholar] [CrossRef] [PubMed]
- Zaker, A.; Hammouda, S.B.; Sun, J.; Wang, X.; Li, X.; Chen, Z. Carbon-based Materials for CO2 Capture: Their Production, Modification and Performance. J. Environ. Chem. Eng. 2023, 11, 109741. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ebrahium, S.M.; Hammam, A.S.; Kuo, S.-W.; Aly, K.I. Enhanced CO2 Capture in Nitrogen-Enriched Microporous Carbons Derived from Polybenzoxazines Containing Azobenzene and Carboxylic Acid Units. J. Polym. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Shang, S.; Tao, Z.; Yang, C.; Hanif, A.; Li, L.; Tsang, D.C.; Gu, Q.; Shang, J. Facile Synthesis of CuBTC and its Graphene Oxide Composites as Efficient Adsorbents for CO2 Capture. Chem. Eng. J. 2020, 393, 124666. [Google Scholar] [CrossRef]
- Wang, Z.; Goyal, N.; Liu, L.; Tsang, D.C.; Shang, J.; Liu, W.; Li, G. N-doped porous carbon derived from polypyrrole for CO2 capture from humid flue gases. Chem. Eng. J. 2020, 396, 125376. [Google Scholar] [CrossRef]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective Preparation of Biomass-derived Porous Carbon with Controllable Pore Sizes Toward Highly Efficient CO2 Capture. Chem. Eng. J. 2019, 360, 250–259. [Google Scholar] [CrossRef]
- Byrne, J.F.; Marsh, H. Porosity in Carbons: Characterisation and Applications; CDIAC, Carbon Dioxide Information Analysis Center: Bethel, PA, USA, 1995. [Google Scholar]
- Zauška, Ľ.; Bova, S.; Beňová, E.; Bednarčík, J.; Baláž, M.; Zeleňák, V.; Hornebecq, V.; Almáši, M. Thermosensitive Drug Delivery System SBA-15-PEI for Controlled Release of Nonsteroidal Anti-Inflammatory Drug Diclofenac Sodium Salt: A Comparative Study. Materials 2021, 14, 1880. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Melouki, R.; Ouadah, A.; Llewellyn, P.L. The CO2 Adsorption Behavior Study on Activated Carbon Synthesised from Olive Waste. J. CO2 Util. 2020, 42, 101292. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Gecit, F.H.; Altay, B.N.; Wang, L.; Hu, X. One-pot Synthesis of Self S-doped Porous Carbon for Efficient CO2 Adsorption. Fuel Process. Technol. 2023, 244, 107700. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterisation of Porous Solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Paryanto; Wibowo, W.A.; Hantoko, D.; Saputro, M.E. Preparation of Activated Carbon from Mangrove Waste by KOH Chemical Activation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012087. [Google Scholar] [CrossRef]
- Plachy, T.; Kutalkova, E.; Skoda, D.; Holcapkova, P. Transformation of Cellulose via Two-Step Carbonization to Conducting Carbonaceous Particles and Their Outstanding Electrorheological Performance. Int. J. Mol. Sci. 2022, 23, 5477. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Spessato, L.; Duarte, V.A.; Fonseca, J.M.; Arroyo, P.A.; Almeida, V.C. Nitrogen-Doped Activated Carbons with High Performances for CO2 Adsorption. J. CO2 Util. 2022, 61, 102013. [Google Scholar] [CrossRef]
- Zelenka, T.; Simanova, K.; Saini, R.; Zelenková, G.; Nehra, S.; Sharma, A.; Almáši, M. Carbon Dioxide and Hydrogen Adsorption Study on Surface-modified HKUST-1 with Diamine/triamine. Sci. Rep. 2022, 12, 17366. [Google Scholar] [CrossRef]
- Almáši, M.; Király, N.; Zeleňák, V.; Vilková, M.; Bourrelly, S. Zinc(ii) and Cadmium(ii) Amorphous Metal-organic Frameworks (aMOFs): Study of Activation Process and High-pressure Adsorption of Greenhouse Gases. RSC Adv. 2021, 11, 20137–20150. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, R. Preparation of a Dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Li, D.; Tian, Y.; Li, L.; Li, J.; Zhang, H. Production of Highly Microporous Carbons with Large CO2 Uptakes at Atmospheric Pressure by KOH Activation of Peanut Shell Char. J. Porous Mater. 2015, 22, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, C.; Song, W.; Ma, Q.; Xie, L.; Zhang, X.; Guo, H. Enhancement of CO2 Capture on Biomass-Based Carbon from Black Locust by KOH Activation and Ammonia Modification. Energy Fuels 2016, 30, 4181–4190. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Vendrell, X.; Kiełbasa, K.; Michalkiewicz, B. Biomass Waste Fern Leaves as a Material for a Sustainable Method of Activated Carbon Production for CO2 Capture. Biomass Bioenergy 2023, 175, 106880. [Google Scholar] [CrossRef]
- Kim, C.-H.; Lee, S.-Y.; Park, S.-J. Efficient Micropore Sizes for Carbon Dioxide Physisorption of Pine Cone-Based Carbonaceous Materials at Different Temperatures. J. CO2 Util. 2021, 54, 101770. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. Heteroatom Functionalized Activated Porous Biocarbons and Their Excellent Performance for CO2 Capture at High Pressure. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 21196–21204. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly Microporous Activated Carbons from Biomass for CO2 Capture and Effective Micropores at Different Conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile Preparation of N-Doped Activated Carbon Produced from Rice Husk for CO2 Capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Ello, A.S.; de Souza, L.K.C.; Trokourey, A.; Jaroniec, M. Coconut Shell-Based Microporous Carbons for CO2 Capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous Activated Carbons Derived from Waste Sugarcane Bagasse for CO2 Adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Liu, X.; Liu, F.; Yan, Z.; Zhou, S.; Wang, J.; Deng, Q. Synthesis of Palm Sheath Derived-Porous Carbon for Selective CO2 Adsorption. RSC Adv. 2022, 12, 8592–8599. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Zelenka, T.; Horikawa, T.; Do, D.D. Artifacts and Misinterpretations in Gas Physisorption Measurements and Characterisation of Porous Solids. Adv. Colloid Interface Sci. 2023, 311, 102831. [Google Scholar] [CrossRef]
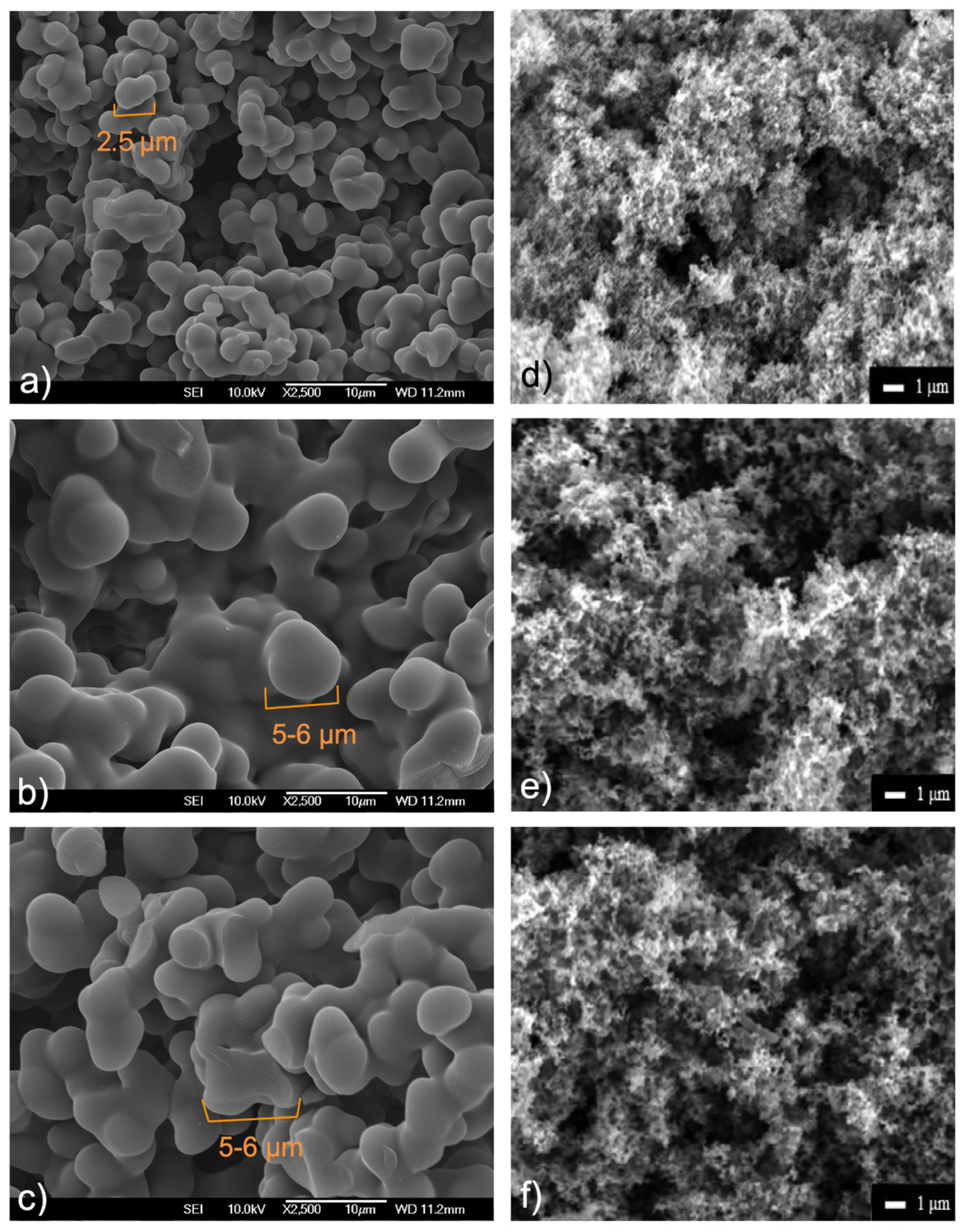




| NN * | C/% | H/% | N/% | O **/% | |
|---|---|---|---|---|---|
| C+DETA | 3 | 41.05 | 7.67 | 6.53 | 44.75 |
| C+BAPE | 4 | 43.69 | 8.18 | 7.84 | 40.29 |
| C+MELA | 6 | 37.84 | 6.35 | 12.61 | 43.20 |
| C+DETA (P) | 3 | 88.56 | 0.96 | 0.27 | 10.21 |
| C+BAPE (P) | 4 | 91.15 | 1.15 | 0.42 | 7.28 |
| C+MELA (P) | 6 | 84.98 | 0.73 | 0.67 | 13.62 |
| C | MELA | DETA | BAPE | C+MELA | C+DETA | C+BAPE | C+MELA (P) | C+DETA (P) | C+BAPE (P) | |
|---|---|---|---|---|---|---|---|---|---|---|
| ν(OH) | 3332 (as) 3281 (s) | X | X | X | X | X | 3403 | X | X | X |
| ν(NH2) | X | 3467 3417 | 3353 3276 | 3353 3276 | 3468 3412 | 3351 3276 | 3331 3284 | X | X | X |
| ν(NH) | X | X | 3184 | 3176 | 3328 | 3184 | 3180 | X | X | X |
| X | 3176 3120 | X | X | 3184 3130 | X | X | X | X | X | |
| ν(CH2)aliph | 2895 | X | 2925, 2879 (as) 2854, 2812 (s) | 2922(as) 2813(s) | 2962, 2931 (as) 2898, 2868 (s) | 2925, 2876 (as) 2851, 2812 (s) | 2915 (as) 2848, 2823 (s) | X | X | X |
| δ(OH) | 1645 | X | X | X | X | X | X | X | X | X |
| δ(NH2) | X | 1626 | 1595 | 1598 | 1649 1624 | 1595 | 1590 | X | X | X |
| ν(CN) | X | 1566 1526 | X | X | 1552 | X | X | X | X | X |
| δ(CH2) | 1428 | X | 1454 | 1456 | 1435 | 1454 | 1455 | X | X | X |
| δ(CH) | 1333 | X | 1351 | 1356 | 1363 | 1351 | 1358 | X | X | X |
| δ(C-OH) | 1314 | X | X | X | 1313 | 1300 | 1314 | X | X | X |
| ν(C-O) | 1053 1028 | X | X | X | 1052 1022 | 1062 1036 | 1055 1031 | X | X | X |
| γ(CCH) | X | X | X | X | X | X | X | 991 | 977 | 992 |
| SBET m2 g−1 | Vp cm3 g−1 | |
|---|---|---|
| C | 32 | 0.014 |
| C+DETA | 769 | 0.378 |
| C+BAPE | 833 | 0.473 |
| C+MELA | 1079 | 0.511 |
| C (P) | 121 | 0.067 |
| C+DETA (P) | 998 | 0.488 |
| C+BAPE (P) | 1076 | 0.502 |
| C+MELA (P) | 1348 | 0.628 |
| V(CO2)cm3 g−1 | n(CO2)mmol g−1 | w(CO2)wt. % | |
|---|---|---|---|
| C | 3.65 | 0.17 | 0.75 |
| C+DETA | 98.08 | 4.38 | 19.28 |
| C+BAPE | 119.35 | 5.33 | 23.46 |
| C+MELA | 164.60 | 7.34 | 32.30 |
| C (P) | 5.18 | 0.23 | 1.01 |
| C+DETA (P) | 39.54 | 1.76 | 7.75 |
| C+BAPE (P) | 49.04 | 2.19 | 9.64 |
| C+MELA (P) | 93.29 | 4.16 | 18.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupšová, S.; Almáši, M. Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability. Molecules 2024, 29, 1158. https://doi.org/10.3390/molecules29051158
Krupšová S, Almáši M. Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability. Molecules. 2024; 29(5):1158. https://doi.org/10.3390/molecules29051158
Chicago/Turabian StyleKrupšová, Sarah, and Miroslav Almáši. 2024. "Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability" Molecules 29, no. 5: 1158. https://doi.org/10.3390/molecules29051158
APA StyleKrupšová, S., & Almáši, M. (2024). Cellulose–Amine Porous Materials: The Effect of Activation Method on Structure, Textural Properties, CO2 Capture, and Recyclability. Molecules, 29(5), 1158. https://doi.org/10.3390/molecules29051158







