A Comparative Study of New Fluorescent Anthraquinone and Benzanthrone α-Aminophosphonates: Synthesis, Spectroscopy, Toxicology, X-ray Crystallography, and Microscopy of Opisthorchis felineus
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Spectroscopy
2.2.1. Structure
2.2.2. Photophysical Properties
2.3. Toxicity Assessment
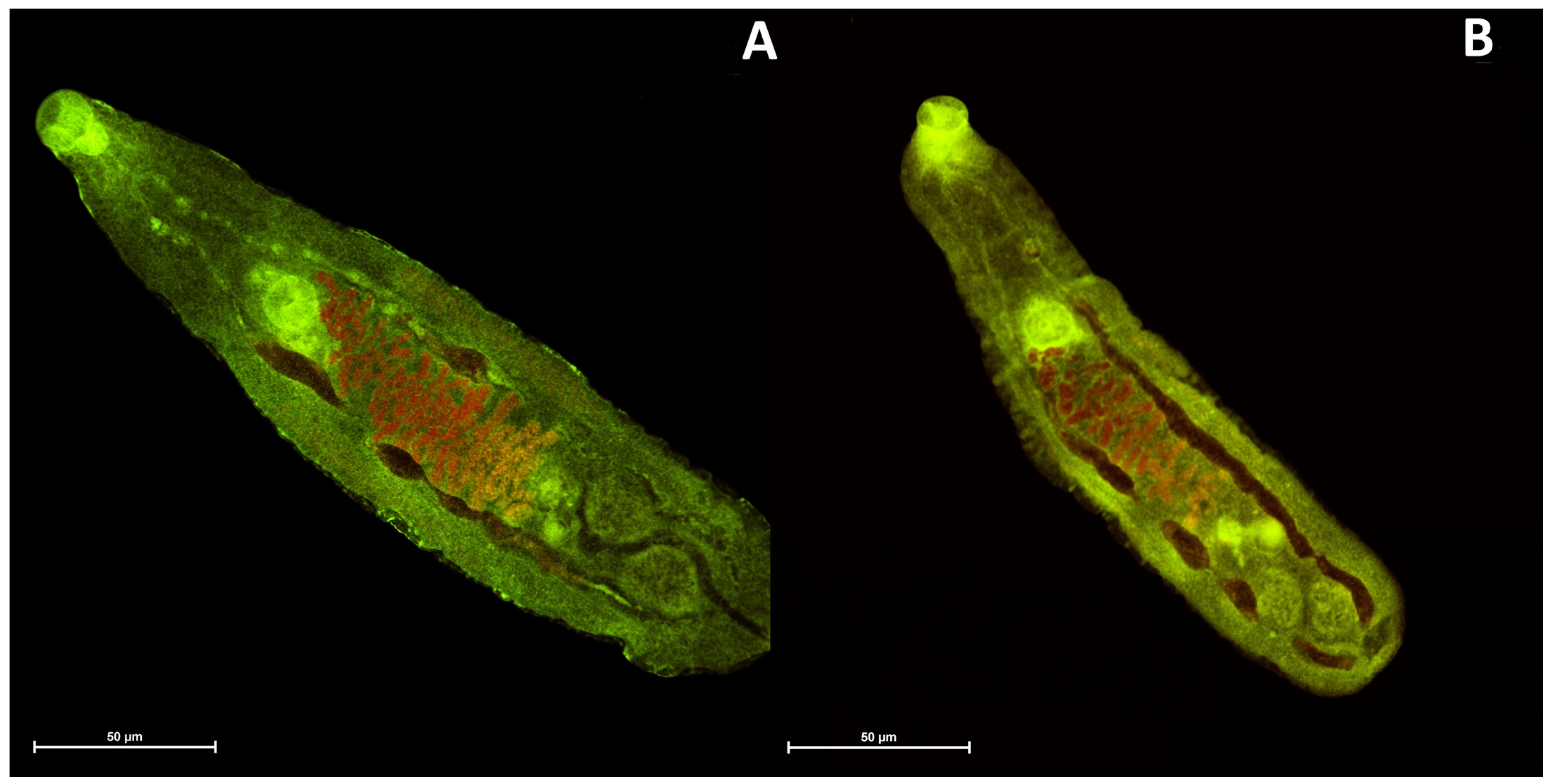
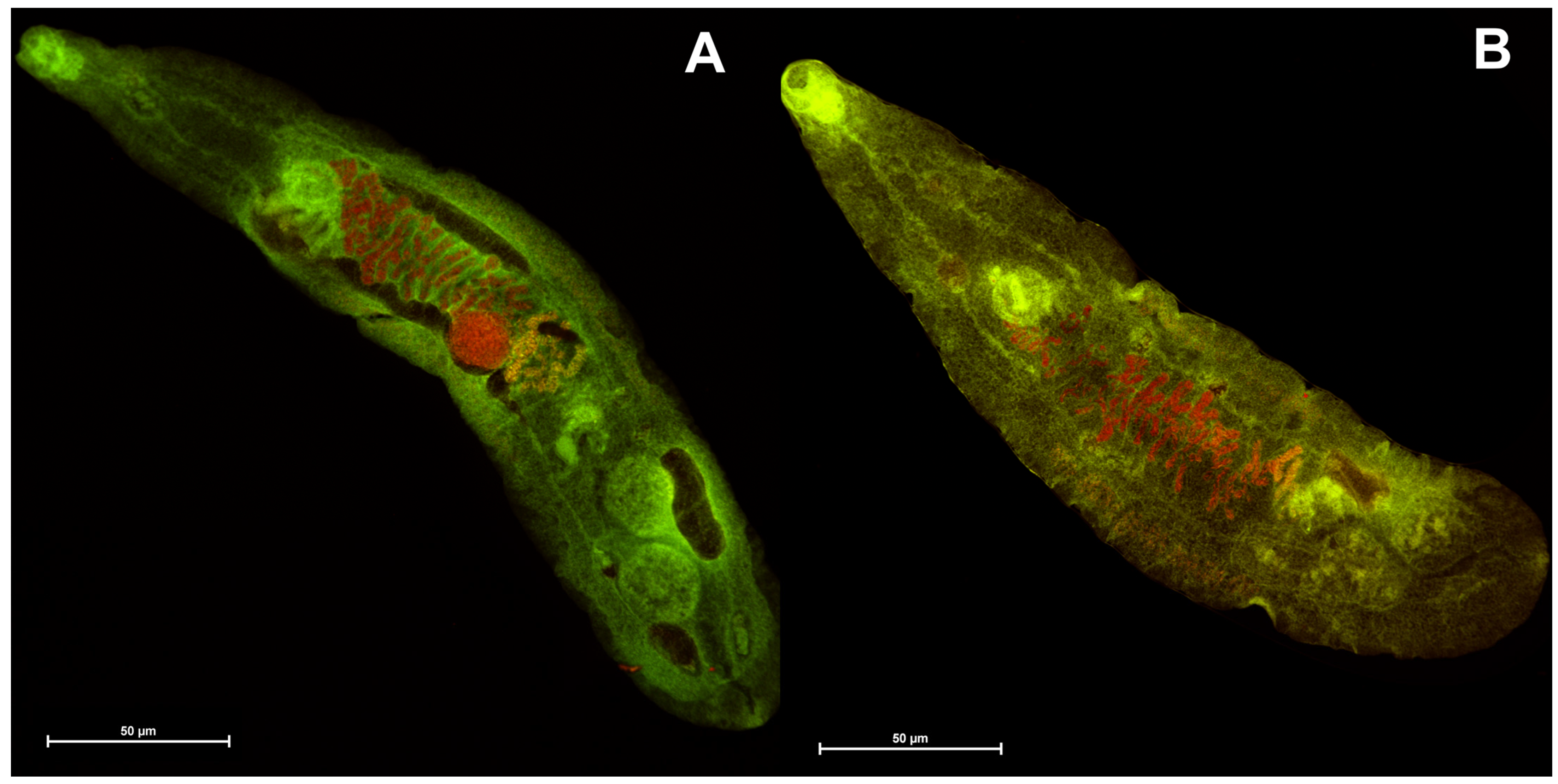
2.4. Confocal Laser Scanning Microscopy Imaging of Trematode Opisthorchis Felineus
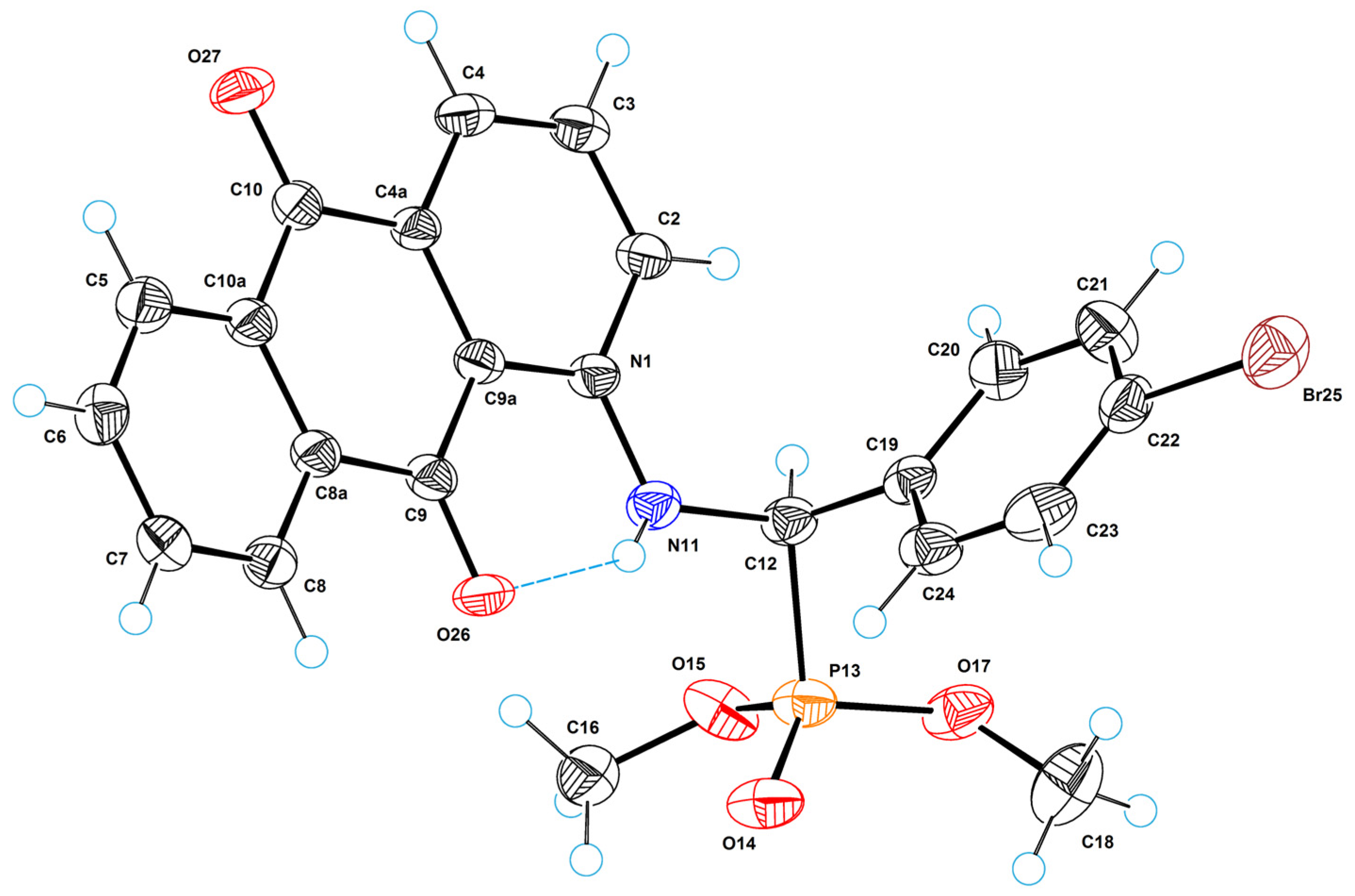
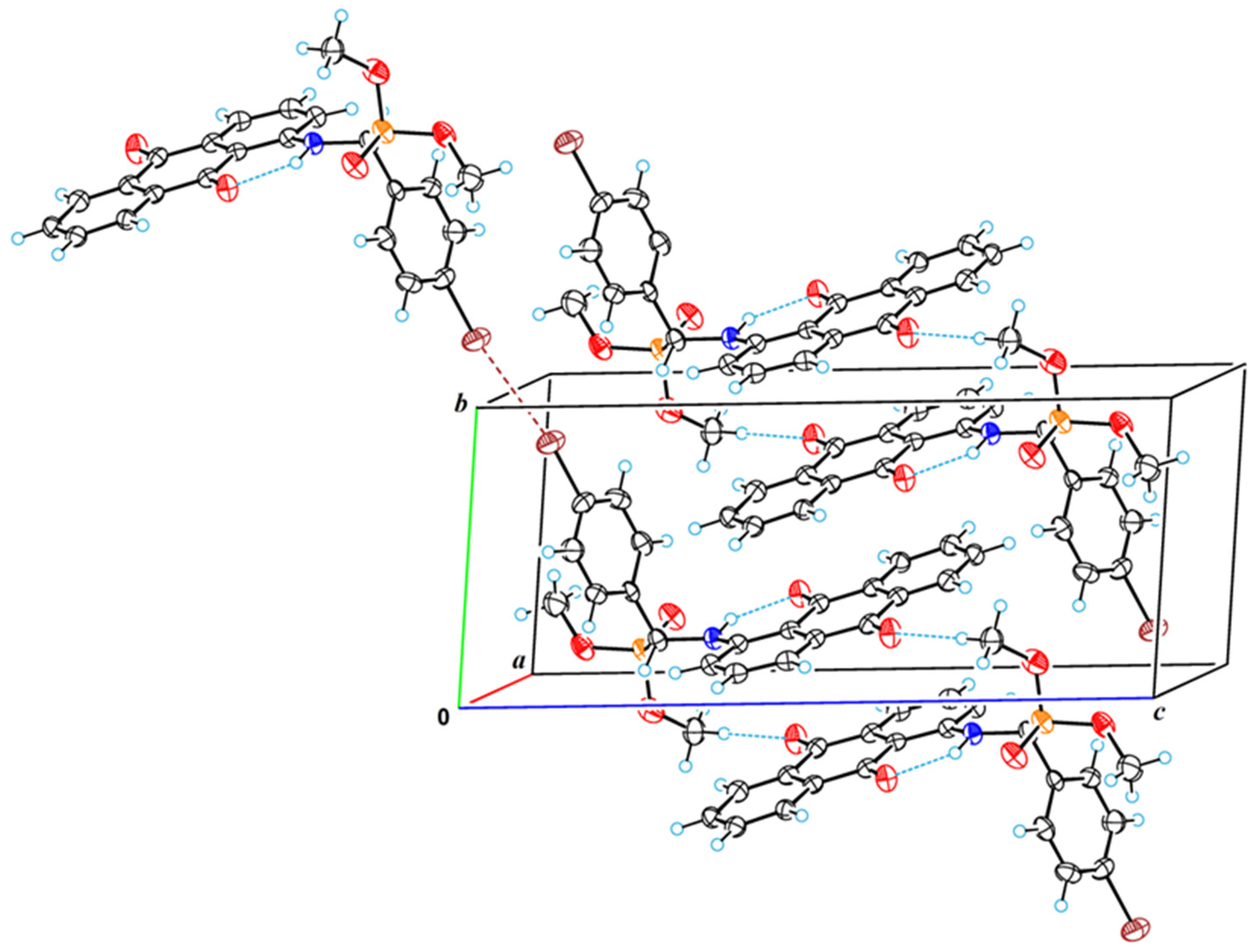
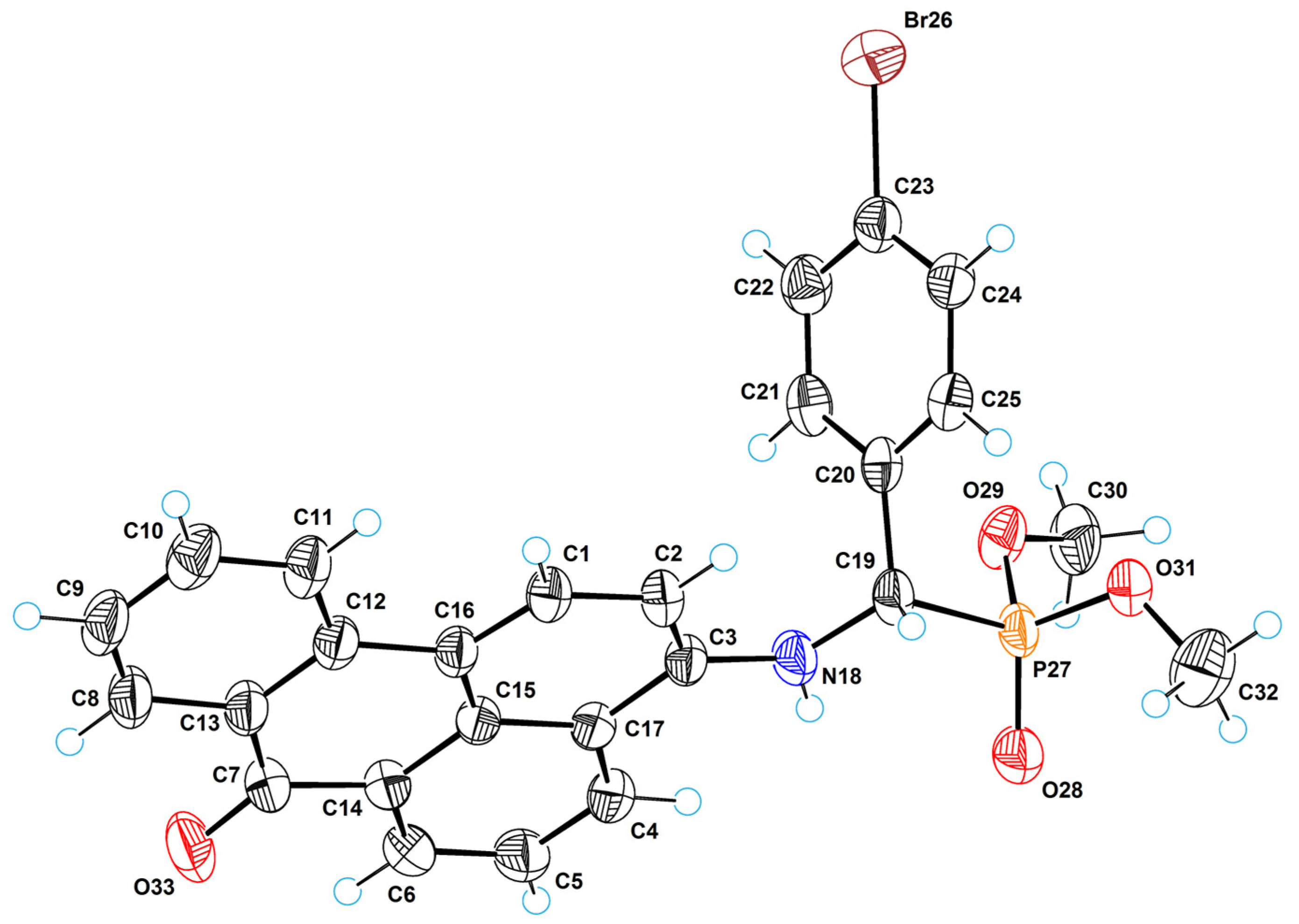
2.5. X-ray Crystallographic Study
3. Materials and Methods
3.1. Materials and Measurments
3.2. Synthesis and Characterization
- In a 10 mL round-bottom flask fitted with a magnetic stirrer bar, 2 mmol of an amine, 6 mmol of an aldehyde, and 5 mL of dimethylphosphonate were combined. The mixture was stirred at 120 °C for 1 to 3 h, with progress monitored using TLC. After the completion of the reaction, the mixture was poured into 100 mL of concentrated sodium bicarbonate (NaHCO3) solution and left overnight while being stirred until a solid product was formed. The precipitate was then filtered, thoroughly washed with water, and dried. Purification of the product was achieved through multi-solvent recrystallization using xylenes and hexane.
- Dimethyl ((4-bromophenyl)((9,10-dioxo-9,10-dihydroanthracen-1-yl)amino)methyl)phosphonate (2a) was obtained as an orange compound in a 71% yield with a m.p. of 166 °C. Rf = 0.72 (hexane-acetone, v/v 1:2). 1H NMR (500 MHz, Chloroform-d) δ 10.64 (dd, J = 10.4, 7.4 Hz, 1H, NH), 8.31 (dd, J = 7.7, 1.6 Hz, 1HAr), 8.18 (dd, J = 7.6, 1.6 Hz, 1HAr), 7.73 (td, J = 7.5, 1.5 Hz, 1HAr), 7.68 (td, J = 7.5, 1.5 Hz, 1HAr), 7.59 (d, J = 7.4 Hz, 1HAr), 7.44 (d, J = 8.1 Hz, 2HAr), 7.38 (t, J = 8.0 Hz, 1HAr), 7.36–7.30 (m, 2HAr), 6.75 (d, J = 8.6 Hz, 1HAr), 4.92 (dd, J = 24.0, 7.3 Hz, 1H, NCH), 3.70 (ddd, J = 18.9, 10.7, 1.4 Hz, 6H, OCH3). 13C NMR (126 MHz, Chloroform-d) δ 185.86 (C=O), 183.41 (C=O), 149.92 (d, J = 13.5 Hz, C), 135.41 (CH), 134.82 (C), 134.67 (C), 134.12 (CH), 134.01 (d, J = 3.7 Hz, C), 133.44 (CH), 132.98 (C), 132.12 (d, J = 2.8 Hz, CH), 129.37 (d, J = 5.2 Hz, CH), 127.13 (CH), 126.85 (CH), 122.46 (d, J = 4.2 Hz, C), 118.24 (CH), 117.16 (CH), 114.82 (C), 54.60 (d, J = 152.5 Hz, NCH), 54.39 (d, J = 7.2 Hz, OCH3), 54.09 (d, J = 7.1 Hz, OCH3). 31P NMR (202 MHz, Chloroform-d) δ 22.38. FTIR (neat): 1631 and 1668 (C=O); 2852, 2905, 2952 and 3067 (CH); 3225 (NH). ESI-FTMS: calculated for [C23H19BrNO5P]: 500.0257, found: 500.0254.
- Dimethyl (((9,10-dioxo-9,10-dihydroanthracen-1-yl)amino)(4-(methylthio)phenyl)methyl)phosphonate (2b) was obtained as an orange compound in 63% yield with a m.p. of 154 °C. Rf = 0.69 (hexane-acetone, v/v 1:2). 1H NMR (500 MHz, Chloroform-d) δ 10.63 (dd, J = 10.2, 7.4 Hz, 1H, NH), 8.31 (dd, J = 7.6, 1.6 Hz, 1HAr), 8.18 (dd, J = 7.6, 1.6 Hz, 1HAr), 7.72 (td, J = 7.5, 1.6 Hz, 1HAr), 7.67 (td, J = 8.2, 6.7 Hz, 1HAr), 7.58 (d, J = 7.4 Hz, 1HAr), 7.41–7.33 (m, 3HAr), 7.17 (d, J = 8.4 Hz, 2HAr), 6.80 (d, J = 8.5 Hz, 1HAr), 4.92 (dd, J = 23.6, 7.5 Hz, 1H, NCH), 3.71 (d, J = 10.6 Hz, 2H, OCH3), 3.66 (d, J = 10.7 Hz, 2H, OCH3), 2.39 (s, 3H, SCH3), 1.56 (d, J = 3.5 Hz, 2H, OCH3). 13C NMR (126 MHz, Chloroform-d) δ 185.75 (C=O), 183.49 (C=O), 150.16 (C), 150.06 (C), 138.99 (d, J = 3.8 Hz, C), 135.34 (CH), 134.75 (d, J = 3.6 Hz, C), 134.09 (CH), 133.36 (CH), 132.99 (C), 131.33 (d, J = 4.0 Hz, C), 128.14 (d, J = 5.3 Hz, CH), 127.12 (CH), 126.81 (CH), 126.67 (d, J = 2.7 Hz, CH), 118.44 (CH), 117.00 (CH), 114.70 (C), 54.65 (d, J = 153.0 Hz, NCH), 54.33 (d, J = 7.1 Hz, OCH3), 54.05 (d, J = 7.1 Hz, OCH3), 15.50 (SCH3). 31P NMR (202 MHz, Chloroform-d) δ 22.97. FTIR (neat): 1630 and 1669 (C=O); 2848, 2950, 2998 and 3077 (CH); 3237 (NH). ESI-FTMS: calculated for [C24H22SNO5P+H+]: 468.1029, found: 468.1017.
- Dimethyl ((3-cyano-4-fluorophenyl)((9,10-dioxo-9,10-dihydroanthracen-1-yl)amino)methyl)phosphonate (2c) was obtained as an orange solid in a 59% yield with a m.p. of 205 °C. Rf = 0.71 (hexane-acetone, v/v 1:2). 1H NMR (500 MHz, Chloroform-d) δ 10.63 (dd, J = 10.7, 7.4 Hz, 1H, NH), 8.31 (d, J = 7.6 Hz, 1HAr), 8.20 (d, J = 7.5 Hz, 1HAr), 7.78–7.69 (m, 2HAr), 7.69 (t, J = 4.0 Hz, 2HAr), 7.64 (d, J = 7.4 Hz, 1HAr), 7.42 (t, J = 8.0 Hz, 1HAr), 7.16 (d, J = 8.4 Hz, 1HAr), 6.68 (d, J = 8.5 Hz, 1HAr), 4.95 (dd, J = 24.3, 7.3 Hz, 1H, NCH), 3.76 (dd, J = 12.9, 10.8 Hz, 6H, OCH3). 13C NMR (126 MHz, Chloroform-d) δ 186.14 (C=O), 183.23 (C=O), 163.94 (d, J = 3.3 Hz, C), 161.85 (C), 149.42 (d, J = 13.4 Hz, C), 135.59 (CH), 135.00 (C), 134.52 (C), 134.30 (dd, J = 8.5, 4.7 Hz, CH), 134.22 (CH), 133.66 (CH), 132.94 (C), 132.60 (C), 132.46 (d, J = 5.3 Hz, CH), 127.18 (CH), 126.93 (CH), 117.72 (CH), 117.58 (CH), 117.15 (dd, J = 20.0, 2.5 Hz, CH), 115.13 (C), 113.51 (C), 54.53 (d, J = 7.2 Hz, OCH3), 54.20 (d, J = 7.0 Hz, OCH3), 53.80 (d, J = 153.0 Hz, NCH). 31P NMR (202 MHz, Chloroform-d) δ 21.54 (d, J = 7.4 Hz). FTIR (neat): 1630 and 1667 (C=O); 2236 (C≡N); 2857, 2904, 2960 and 3046 (CH); 3235 (NH). ESI-FTMS: calculated for [C24H22SNO5P+H+]: 465.1010, found: 465.0996.
- Dimethyl ((4-bromophenyl)((7-oxo-7H-benzo[de]anthracen-3-yl)amino)methyl)phosphonate (4a) was obtained as a red solid in a 75% yield with a m.p. of 207 °C. Rf = 0.68 (hexane-acetone, v/v 1:2). 1H NMR (500 MHz, Chloroform-d) δ 8.77 (dd, J = 7.3, 1.2 Hz, 1HAr), 8.37 (td, J = 7.9, 1.4 Hz, 2HAr), 8.09 (d, J = 8.3 Hz, 1HAr), 8.04 (d, J = 8.2 Hz, 1HAr), 7.75 (t, J = 7.8 Hz, 1HAr), 7.61–7.54 (m, 1HAr), 7.45 (d, J = 8.5 Hz, 2HAr), 7.39–7.32 (m, 3HAr), 6.42 (d, J = 8.3 Hz, 1HAr), 5.87 (dd, J = 10.5, 6.7 Hz, 1H, NH), 4.93 (dd, J = 24.0, 6.6 Hz, 1H, NCH), 3.77 (d, J = 10.8 Hz, 3H, OCH3), 3.53 (d, J = 10.7 Hz, 3H, OCH3). 13C NMR (126 MHz, Chloroform-d) δ 184.01 (C=O), 143.65 (d, J = 14.3 Hz, C), 136.80 (C), 133.87 (d, J = 3.9 Hz, C), 133.25 (CH), 132.22 (d, J = 2.8 Hz, CH), 130.13 (CH), 129.67 (C), 129.23 (CH), 129.19 (CH), 129.16 (C), 128.75 (C), 127.98 (CH), 126.67 (CH), 126.05 (CH), 125.58 (CH), 123.40 (C), 122.53 (d, J = 4.2 Hz, C), 122.05 (CH), 117.48 (C), 106.92 (CH), 55.23 (d, J = 151.1 Hz, NCH), 54.28 (d, J = 7.0 Hz, OCH3), 54.08 (d, J = 7.0 Hz, OCH3). 31P NMR (202 MHz, Chloroform-d) δ 23.60. FTIR (neat): 1638 (C=O); 2849, 2952 and 3050 (CH); 3401 (NH). ESI-FTMS: calculated for [C26H21BrNO4P–H+]: 520.0318, found: 521.0320.
- Dimethyl ((4-(methylthio)phenyl)((7-oxo-7H-benzo[de]anthracen-3-yl)amino)methyl)phosphonate (4b) was obtained as a red solid in a 68% yield with a m.p. of 123 °C. Rf = 0.77 (hexane-acetone, v/v 1:2). 1H NMR (500 MHz, Chloroform-d) δ 8.77 (dd, J = 7.1, 0.8 Hz, 1HAr), 8.41–8.35 (m, 2HAr), 8.10 (d, J = 8.3 Hz, 1HAr), 8.05 (d, J = 8.2 Hz, 1HAr), 7.75 (t, J = 7.8 Hz, 1HAr), 7.61–7.54 (m, 1HAr), 7.42–7.32 (m, 3HAr), 7.18 (d, J = 7.8 Hz, 2HAr), 6.48 (d, J = 8.3 Hz, 1HAr), 5.90 (s, 1H, NH), 4.94 (d, J = 23.8 Hz, 1H, NCH), 3.76 (d, J = 10.8 Hz, 3H, OCH3), 3.50 (d, J = 10.6 Hz, 3H, OCH3), 2.39 (s, 3H, SCH3). 13C NMR (126 MHz, Chloroform-d) δ 184.04 (C=O), 143.94 (d, J = 14.4 Hz, C), 139.20 (C), 136.89 (C), 133.22 (CH), 131.15 (d, J = 3.9 Hz, C), 130.10 (CH), 129.64 (C), 129.12 (C), 128.74 (C), 128.02 (CH), 127.97 (d, J = 1.6 Hz, CH), 126.78 (CH), 126.69 (d, J = 2.7 Hz, CH), 126.56 (CH), 126.19 (CH), 125.48 (CH), 123.41 (C), 122.02 (CH), 117.20 (C), 106.91 (CH), 55.24 (d, J = 151.8 Hz, NCH), 54.29 (d, J = 7.0 Hz, OCH3), 53.97 (d, J = 7.1 Hz, OCH3), 15.46 (SCH3). 31P NMR (202 MHz, Chloroform-d) δ 24.16. FTIR (neat): 1630 (C=O); 2851, 2914, 2951, 2982 and 3059 (CH); 3484 (NH). ESI-FTMS: calculated for [C27H24NO4PS+H+]: 490.1236, found: 490.1221.
- Dimethyl ((3-cyano-4-fluorophenyl)((7-oxo-7H-benzo[de]anthracen-3-yl)amino)methyl)phosphonate (4c) was obtained as a red solid in a 55% yield with a m.p. of 120 °C. Rf = 0.66 (hexane-acetone, v/v 1:2). 1H NMR (500 MHz, Chloroform-d) δ 8.79 (d, J = 7.3 Hz, 1HAr), 8.38 (d, J = 7.9 Hz, 1HAr), 8.35 (d, J = 8.3 Hz, 1HAr), 8.10 (d, J = 8.2 Hz, 1HAr), 8.06 (d, J = 8.2 Hz, 1HAr), 7.81–7.70 (m, 2HAr), 7.60 (t, J = 7.7 Hz, 1HAr), 7.38 (t, J = 7.5 Hz, 1HAr), 7.13–7.05 (m, 2HAr), 6.36 (d, J = 8.2 Hz, 1HAr), 5.81 (dd, J = 10.9, 6.3 Hz, 1H, NH), 4.97 (dd, J = 24.0, 5.9 Hz, 1H, NCH), 3.80 (d, J = 10.8 Hz, 3H, OCH3), 3.65 (d, J = 10.8 Hz, 3H, OCH3). 13C NMR (126 MHz, Chloroform-d) δ 183.94 (C=O), 161.89 (C), 159.30 (C), 143.01 (d, J = 13.7 Hz, C), 136.60 (C), 133.98 (dd, J = 8.4, 4.8 Hz, CH), 133.36 (CH), 132.44 (C), 132.43 (d, J = 5.2 Hz, CH), 130.26 (CH), 129.75 (C), 129.24 (C), 129.05 (CH), 128.83 (C), 128.24 (CH), 128.03 (CH), 126.72 (d, J = 55.9 Hz, CH), 125.80 (d, J = 16.5 Hz, CH), 123.42 (C), 122.12 (CH), 118.21 (C), 117.26 (dd, J = 20.0, 2.5 Hz, CH), 113.46 (C), 106.88 (CH), 54.56 (d, J = 151.5 Hz, NCH), 54.39 (d, J = 7.1 Hz, OCH3), 54.21 (d, J = 7.0 Hz, OCH3). 31P NMR (202 MHz, Chloroform-d) δ 22.74 (d, J = 5.2 Hz). FTIR (neat): 1648 (C=O); 2236 (C≡N); 2851, 2962, 3023 and 3067 (CH); 3297 (NH). ESI-FTMS: calculated for [C27H20FN2O4P+H+]: 487.1218, found: 487.1200.
3.3. Toxicology
3.3.1. Quantification of Malondialdehyde
3.3.2. Electrolyte Leakage Measurements
3.4. Imaging
3.5. Single-Crystal X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakowicz, J.R. Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 1–26. [Google Scholar]
- Maļeckis, A.; Griškjāns, E.; Cvetinska, M.; Savicka, M.; Belyakov, S.; Kirilova, E. Synthesis, Characterization, Spectroscopic Studies and Evaluation of Toxicological Effect on Growth of Wheat Sprouts (Triticum Aestivum) of New Benzanthrone α-Aryl-α-Aminophosphonates. J. Mol. Struct. 2023, 1277, 134838. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Griškjāns, E.; Mežaraupe, L.; Kirjušina, M.; Pavlova, V.; Kirilova, E. Novel Anthraquinone α-Aryl-α-Aminophosphonates: Synthesis, Spectroscopy and Imaging by Confocal Laser Scanning Microscopy of Trematode Opisthioglyphe Ranae. J. Photochem. Photobiol. A Chem. 2023, 444, 114918. [Google Scholar] [CrossRef]
- Langhals, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments. 3rd Revised Edition. By Heinrich Zollinger. Angew. Chem. Int. Ed. 2004, 43, 5291–5292. [Google Scholar] [CrossRef]
- Christie, R. Colour Chemistry; The Royal Society of Chemistry: London, UK, 2014; ISBN 978-1-84973-328-1. [Google Scholar]
- Huang, B.; Ji, Y.; Li, Z.; Zhou, N.; Jiang, W.; Feng, Y.; Lin, B.; Sun, Y. Simple Aggregation–Induced Delayed Fluorescence Materials Based on Anthraquinone Derivatives for Highly Efficient Solution–Processed Red OLEDs. J. Lumin. 2017, 187, 414–420. [Google Scholar] [CrossRef]
- Yin, C.; Liu, R.; Zhang, D.; Duan, L. Indeno-Anthraquinone Hosts with Thermally Activated Delayed Fluorescence for Deep-Red OLEDs. J. Mater. Chem. C Mater. 2022, 10, 4668–4673. [Google Scholar] [CrossRef]
- Kaur, N.; Gauri. Anthraquinone Appended Chemosensors for Fluorescence Monitoring of Anions and/or Metal Ions. Inorganica Chim. Acta 2022, 536, 120917. [Google Scholar] [CrossRef]
- Ghosh, A.; Jose, D.A.; Kaushik, R. Anthraquinones as Versatile Colorimetric Reagent for Anions. Sens. Actuators B Chem. 2016, 229, 545–560. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, D.; Guo, J.; Li, M.; Wang, T.; Yin, H.; Wang, H.; Liu, J. An Anthraquinone-Based “Turn-on” Fluorescence Probe for Hg2+ Detection and Its Application in Cell Imaging. Inorg. Chem. Commun. 2021, 130, 108753. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, Y.; Cui, F.; Zhang, G. Modeling Techniques and Fluorescence Imaging Investigation of the Interactions of an Anthraquinone Derivative with HSA and CtDNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 572–579. [Google Scholar] [CrossRef]
- Malik, E.M.; Müller, C.E. Anthraquinones as Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Wolarz, E.; Bauman, D. Fluorescent 3-Oxy Benzanthrone Dyes in Liquid Crystalline Media. Dye. Pigment. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I. Synthesis and Properties of Benzanthrone Derivatives as Luminophore Dyes for Liquid Crystals. Dye. Pigment. 1998, 37, 155–164. [Google Scholar] [CrossRef]
- Konstantinova, T.N. The Synthesis of Some Benzanthrone Derivatives for Use as Dyes for Polymeric Materials. Dye. Pigment. 1989, 10, 63–67. [Google Scholar] [CrossRef]
- Maļeckis, A.; Avotiņa, L.; Ķizāne, G.; Pučkins, A.; Osipovs, S.; Kirilova, E. New Fluorescent Heterocyclic Compounds Derived From 3-Cyanobenzanthrone. Polycycl. Aromat. Compd. 2022, 42, 5508–5520. [Google Scholar] [CrossRef]
- Adam, A.M.A.; Altalhi, T.A.; El-Megharbel, S.M.; Saad, H.A.; Refat, M.S.; Grabchev, I.; Althobaiti, R.A. Capturing of Environment Polluting Metal Ions Co2+, Ni2+, Cu2+, and Zn2+ Using a 3-Azomethine Benzanthrone-Based Fluorescent Dye: Its Synthesis, Structural, and Spectroscopic Characterizations. Russ. J. Gen. Chem. 2020, 90, 2394–2399. [Google Scholar] [CrossRef]
- Vus, K.; Trusova, V.; Gorbenko, G.; Kirilova, E.; Kirilov, G.; Kalnina, I.; Kinnunen, P. Novel Aminobenzanthrone Dyes for Amyloid Fibril Detection. Chem. Phys. Lett. 2012, 532, 110–115. [Google Scholar] [CrossRef]
- Rubenina, I.; Gavarane, I.; Kirilova, E.; Mezaraupe, L.; Kirjusina, M. Comparison of the Benzanthrone Luminophores: They Are Not Equal for Rapid Examination of Parafasciolopsis Fasciolaemorpha (Trematoda: Digenea). Biomolecules 2021, 11, 598. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Biological Activity of Aminophosphonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Han, X.; Ge, C.; Zhang, X. Diethyl α-Aminophosphonate Containing Lubricating Additives Synthesized from (3-Aminophenyl)Boronic Acid Pinacol Ester. Inorg. Chem. Commun. 2022, 140, 109477. [Google Scholar] [CrossRef]
- Reddy, N.B.; Sundar, C.S.; Krishna, B.S.; Santhisudha, S.; Sreelakshmi, P.; Nayak, S.K.; Reddy, C.S. Cellulose-SO3H Catalyzed Synthesis of Bis(α-Aminophosphonates) and Their Antioxidant Activity. Org. Commun. 2017, 10, 46–55. [Google Scholar] [CrossRef]
- Rashad, M.M.; El-Sayed, I.E.; Galhoum, A.A.; Abdeen, M.M.; Mira, H.I.; Elshehy, E.A.; Zhang, S.; Lu, X.; Xin, J.; Guibal, E. Synthesis of α-Aminophosphonate Based Sorbents—Influence of Inserted Groups (Carboxylic vs. Amine) on Uranyl Sorption. Chem. Eng. J. 2021, 421, 127830. [Google Scholar] [CrossRef]
- Neiber, R.R.; Galhoum, A.A.; El-Tantawy El Sayed, I.; Guibal, E.; Xin, J.; Lu, X. Selective Lead (II) Sorption Using Aminophosphonate-Based Sorbents: Effect of Amine Linker, Characterization and Sorption Performance. Chem. Eng. J. 2022, 442, 136300. [Google Scholar] [CrossRef]
- Moumeni, O.; Mehri, M.; Kerkour, R.; Boublia, A.; Mihoub, F.; Rebai, K.; Khan, A.A.; Erto, A.; Darwish, A.S.; Lemaoui, T.; et al. Experimental and Detailed DFT/MD Simulation of α-Aminophosphonates as Promising Corrosion Inhibitor for XC48 Carbon Steel in HCl Environment. J. Taiwan Inst. Chem. Eng. 2023, 147, 104918. [Google Scholar] [CrossRef]
- Deyab, M.A.; Abdeen, M.M.; Hussien, M.; El-Sayed, I.E.; Galhoum, A.; El-Shamy, O.A.A.; Abd Elfattah, M. Novel Corrosion Inhibitor for Carbon Steel in Acidic Solutions Based on α-Aminophosphonate (Chemical, Electrochemical, and Quantum Studies). Molecules 2023, 28, 4962. [Google Scholar] [CrossRef]
- Górny vel Górniak, M.; Kafarski, P. Preparation of the Library of Fluorescent Aromatic Aminophosphonate Phenyl and Benzyl Esters. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 511–519. [Google Scholar] [CrossRef]
- Kuśnierz, A.; Chmielewska, E. Synthesis of Fluorescent Aminophosphonates by Green Chemistry Procedures. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 700–705. [Google Scholar] [CrossRef]
- vel Górniak, M.G.; Czernicka, A.; Młynarz, P.; Balcerzak, W.; Kafarski, P. Synthesis of Fluorescent (Benzyloxycarbonylamino)(Aryl)Methylphosphonates. Beilstein J. Org. Chem. 2014, 10, 741–745. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Gao, W.; Song, J.-L.; Liu, Y.; Qi, H.; Tang, X.-H. Synthesis, X-ray Crystallographic Analysis and BSA Interaction of a New α-Aminophosphonate. J. Appl. Spectrosc. 2016, 83, 703–709. [Google Scholar] [CrossRef]
- Lewkowski, J.; Rodriguez Moya, M.; Wrona-Piotrowicz, A.; Zakrzewski, J.; Kontek, R.; Gajek, G. Synthesis, Fluorescence Properties and the Promising Cytotoxicity of Pyrene–Derived Aminophosphonates. Beilstein J. Org. Chem. 2016, 12, 1229–1235. [Google Scholar] [CrossRef]
- Kraicheva, I.; Vodenicharova, E.; Tashev, E.; Tosheva, T.; Tsacheva, I.; Troev, K. Synthesis and NMR Characterization of Two Novel Anthracene-Derived BIS-Aminophosphonates. Basic Hydrolysis of Some Aminophosphonate Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 660–667. [Google Scholar] [CrossRef]
- Amira, A.; Aouf, Z.; K’tir, H.; Chemam, Y.; Ghodbane, R.; Zerrouki, R.; Aouf, N. Recent Advances in the Synthesis of A-Aminophosphonates: A Review. ChemistrySelect 2021, 6, 6137–6149. [Google Scholar] [CrossRef]
- Sravya, G.; Balakrishna, A.; Zyryanov, G.V.; Mohan, G.; Reddy, C.S.; Bakthavatchala Reddy, N. Synthesis of α-Aminophosphonates by the Kabachnik-Fields Reaction. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 353–381. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields Reaction: Mechanism and Synthetic Use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef]
- Varga, P.R.; Keglevich, G. Synthesis of α-Aminophosphonates and Related Derivatives; The Last Decade of the Kabachnik–Fields Reaction. Molecules 2021, 26, 2511. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Vaidyanathan, A. The 1H NMR Spectrum of Benzanthrone. Spectrochim. Acta A 1981, 37, 145–146. [Google Scholar] [CrossRef]
- Vaidyanathan, A. The Carbon-13 NMR Spectra of Benzanthrone and Its Derivatives. Dye. Pigment. 1982, 3, 243–248. [Google Scholar] [CrossRef]
- Francis, G.W.; Aksnes, D.W.; Holt, Ø. Assignment of the 1H and 13C NMR Spectra of Anthraquinone Glycosides from Rhamnus Frangula. Magn. Reson. Chem. 1998, 36, 769–772. [Google Scholar] [CrossRef]
- Balakrishna, A.; Suresh Reddy, C.; Naik, S.K.; Manjunath, M.; Naga Raju, C. Synthesis, Characterization and Bio-Activity of Some New Aminophosphonates. Bull. Chem. Soc. Ethiop. 2009, 23, 69–75. [Google Scholar] [CrossRef]
- Litim, B.; Djahoudi, A.; Meliani, S.; Boukhari, A. Synthesis and Potential Antimicrobial Activity of Novel α-Aminophosphonates Derivatives Bearing Substituted Quinoline or Quinolone and Thiazole Moieties. Med. Chem. Res. 2022, 31, 60–74. [Google Scholar] [CrossRef]
- Serigne Abdou Khadir, F.; Boukhssas, S.; Achamlale, S.; Aouine, Y.; Nakkabi, A.; Faraj, H.; Alami, A. Synthesis and Characterization of the Structure of Diethyl [(4-{(1H-Benzo[d]Imidazol-1-Yl)Methyl}-1H-1,2,3-Triazol-1-Yl)(Benzamido)Methyl]Phosphonate Using 1D and 2D NMR Experiments. Eur. J. Adv. Chem. Res. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Griškjāns, E.; Dmitrijevs, K.; Traskovskis, K.; Belyakov, S.; Kirilova, E. Benzanthrone Sulfides: Synthesis, Solvatochromism Characterization and Analysis of Experimental Photophysical Parameters and Theoretical Calculations. Dye. Pigment. 2023, 219, 111599. [Google Scholar] [CrossRef]
- Gonta, S.; Utinans, M.; Kirilov, G.; Belyakov, S.; Ivanova, I.; Fleisher, M.; Savenkov, V.; Kirilova, E. Fluorescent Substituted Amidines of Benzanthrone: Synthesis, Spectroscopy and Quantum Chemical Calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 325–334. [Google Scholar] [CrossRef]
- Olipova, M.; Maleckis, A.; Puckins, A.; Kirilova, A.; Romanovska, E.; Kirilova, E. Spectroscopic Investigation of New Benzanthrone Luminescent Dyes. Bulg. Chem. Commun. 2022, 54, 253–257. [Google Scholar]
- Grabchev, I.; Bojinov, V.; Moneva, I. The Synthesis and Application of Fluorescent Dyes Based on 3-Amino Benzanthrone. Dye. Pigment. 2001, 48, 143–150. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Puckins, A.; Osipovs, S.; Sirokova, J.; Belyakov, S.; Kirilova, E. Synthesis and Properties of New 3-Heterylamino-Substituted 9-Nitrobenzanthrone Derivatives. Molecules 2023, 28, 5171. [Google Scholar] [CrossRef]
- Zamyatnina, V.A.; Bakeeva, L.E.; Aleksandrushkina, N.I.; Vanyushin, B.F. Apoptosis in the Initial Leaf of Etiolated Wheat Seedlings: Influence of the Antioxidant Ionol (BHT) and Peroxides. Biochemistry 2002, 67, 212–221. [Google Scholar] [CrossRef]
- Kishikawa, N.; El-Maghrabey, M.; Nagamune, Y.; Nagai, K.; Ohyama, K.; Kuroda, N. A Smart Advanced Chemiluminescence-Sensing Platform for Determination and Imaging of the Tissue Distribution of Natural Antioxidants. Anal. Chem. 2020, 92, 6984–6992. [Google Scholar] [CrossRef]
- Bogitsh, B.J.; Carter, C.E.; Oeltmann, T.N. Visceral Flukes. In Human Parasitology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–191. [Google Scholar]
- Chai, J.-Y.; Jung, B.-K. Fishborne Zoonotic Heterophyid Infections: An Update. Food Waterborne Parasitol. 2017, 8–9, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Armignacco, O.; Ferri, F.; Gomez Morales, M.A. Opisthorchis Felineus, an Emerging Infection in Italy and Its Implication for the European Union. Acta Trop. 2013, 126, 54–62. [Google Scholar] [CrossRef]
- Pozio, E. Epidemiology and Control Prospects of Foodborne Parasitic Zoonoses in the European Union. Parassitologia 2008, 50, 17–24. [Google Scholar]
- Keiser, J.; Utzinger, J. Food-Borne Trematodiases. Clin. Microbiol. Rev. 2009, 22, 466–483. [Google Scholar] [CrossRef]
- Mordvinov, V.A.; Furman, D.P. The Digenea Parasite Opisthorchis Felineus: A Target for the Discovery and Development of Novel Drugs. Infect. Disord. Drug Targets 2010, 10, 385–401. [Google Scholar] [CrossRef]
- Sripa, B.; Tangkawattana, S.; Laha, T.; Kaewkes, S.; Mallory, F.F.; Smith, J.F.; Wilcox, B.A. Toward Integrated Opisthorchiasis Control in Northeast Thailand: The Lawa Project. Acta Trop. 2015, 141, 361–367. [Google Scholar] [CrossRef]
- Fedorova, O.S.; Fedotova, M.M.; Zvonareva, O.I.; Mazeina, S.V.; Kovshirina, Y.V.; Sokolova, T.S.; Golovach, E.A.; Kovshirina, A.E.; Konovalova, U.V.; Kolomeets, I.L.; et al. Opisthorchis Felineus Infection, Risks, and Morbidity in Rural Western Siberia, Russian Federation. PLoS Negl. Trop. Dis. 2020, 14, e0008421. [Google Scholar] [CrossRef]
- Kitaigorodsky, A. Molecular Crystals and Molecules; Academic Press: Cambridge, MA, USA, 1973. [Google Scholar]
- Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of Light Intensities on Antioxidant Enzymes and Malondialdehyde Content during Short-Term Acclimatization on Micropropagated Phalaenopsis Plantlet. Environ. Exp. Bot. 2005, 54, 109–120. [Google Scholar] [CrossRef]
- Guo, Z.; Ou, W.; Lu, S.; Zhong, Q. Differential Responses of Antioxidative System to Chilling and Drought in Four Rice Cultivars Differing in Sensitivity. Plant Physiol. Biochem. 2006, 44, 828–836. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A Suite of Computer Programs for Crystal Structure Solution of Proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]

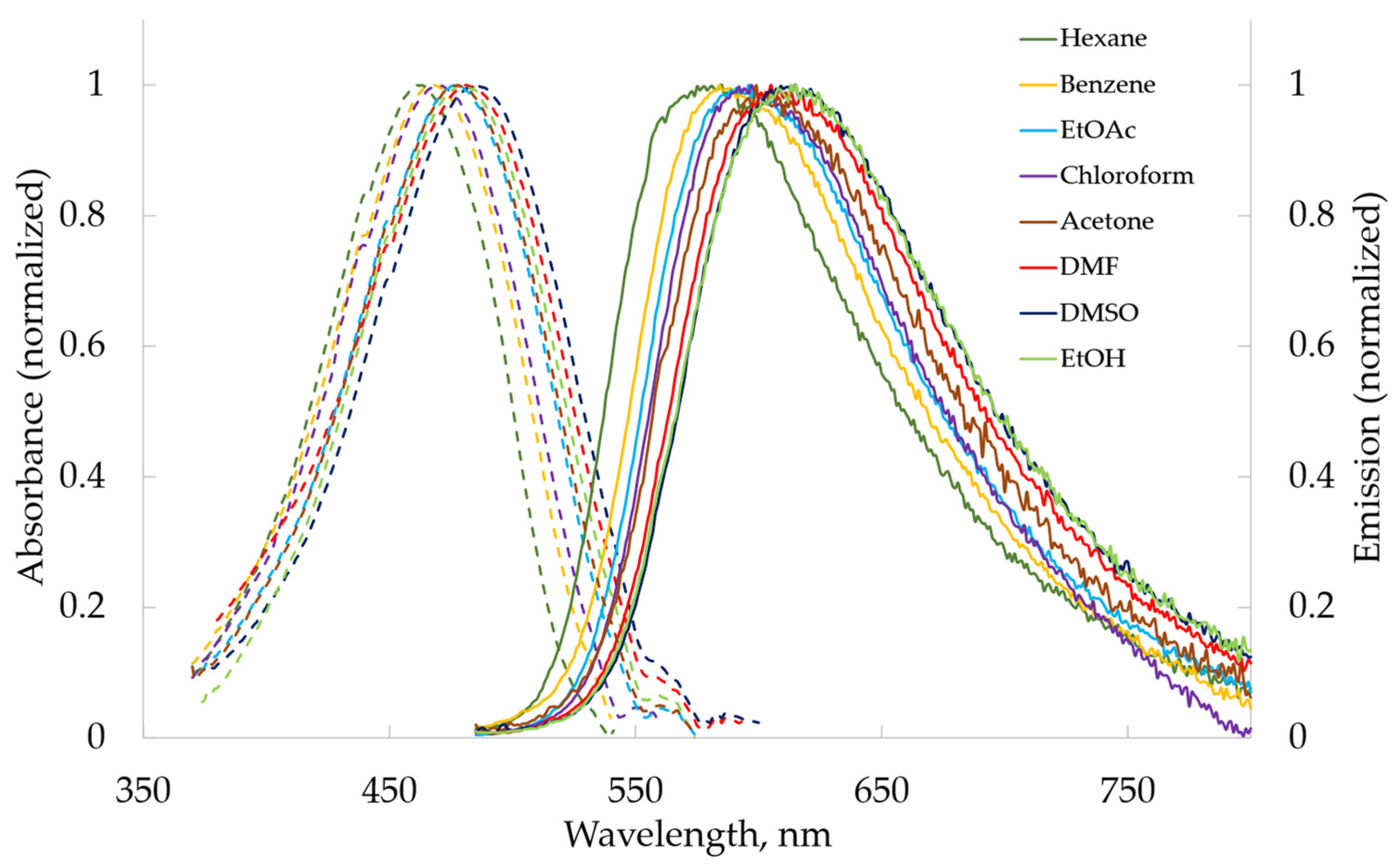
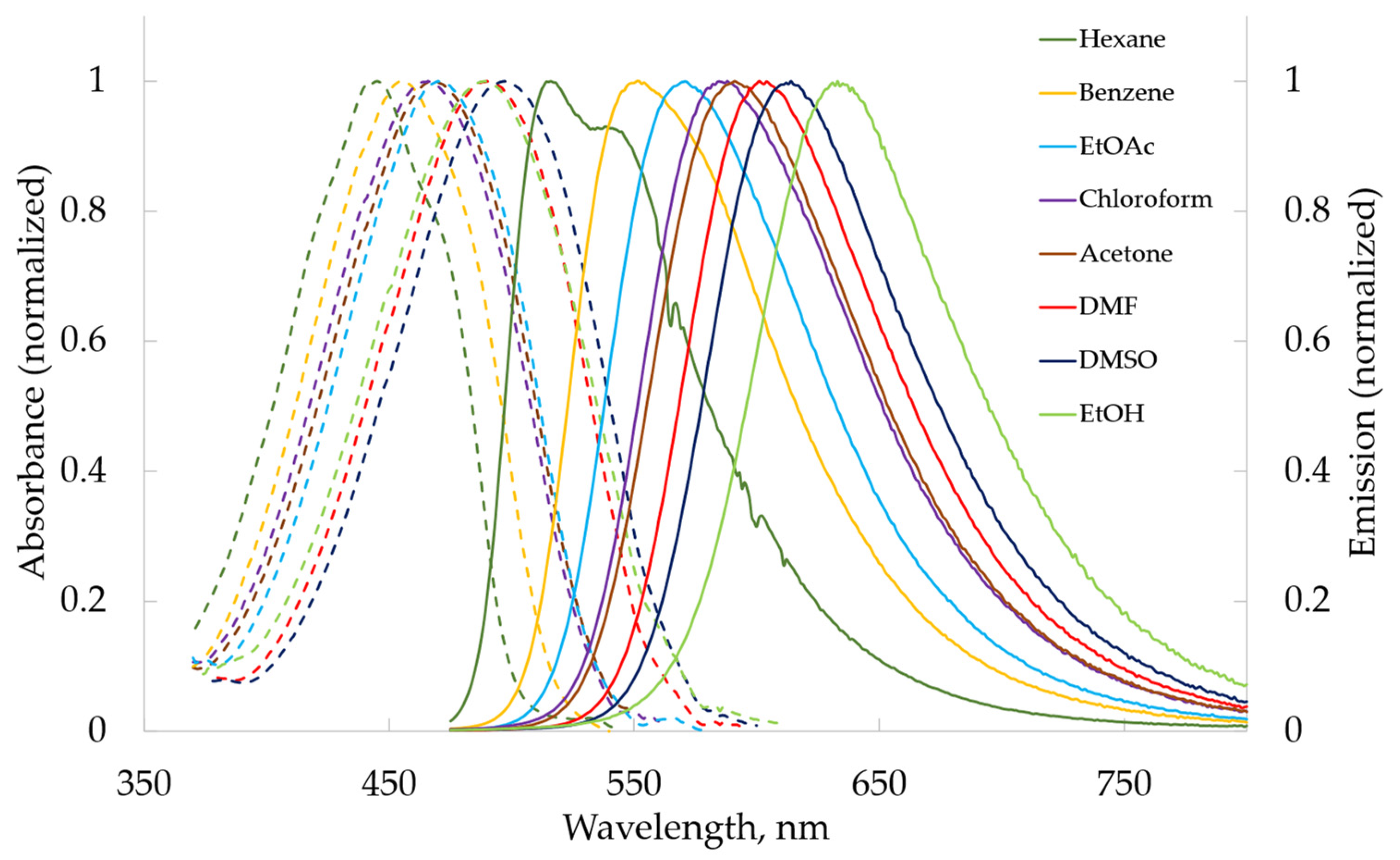
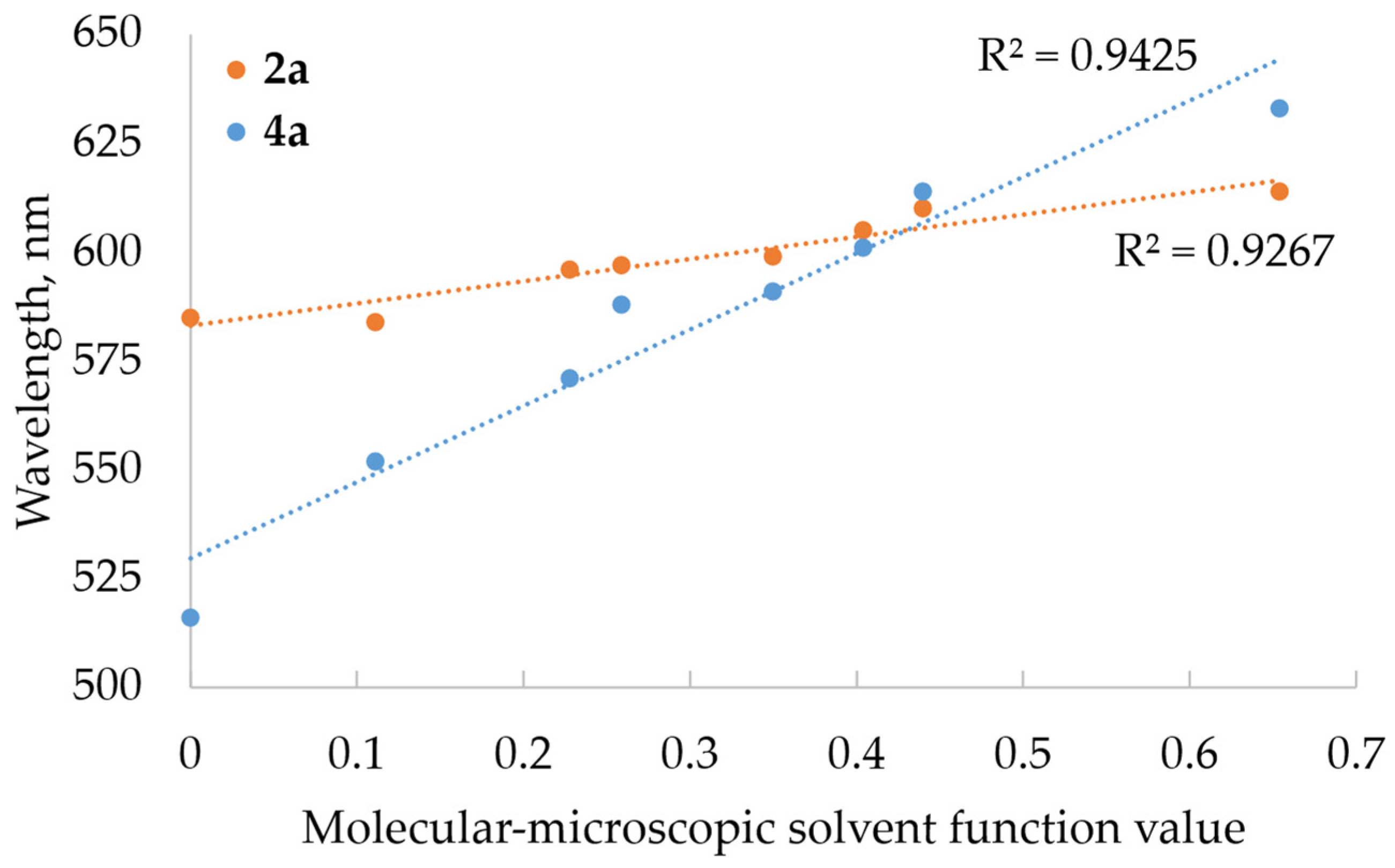



| Solvent | λabs Max, nm (lgε) | |||||
|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 4a | 4b | 4c | |
| Hexane | 471 (3.99) | 475 (3.84) | 465 (3.60) | 454 (4.35) | 457 (4.13) | 447 (3.76) |
| Benzene | 478 (4.00) | 481 (3.89) | 471 (3.87) | 465 (4.38) | 467 (4.27) | 459 (4.00) |
| Chloroform | 480 (3.95) | 483 (3.81) | 448 (3.95) | 476 (4.37) | 467 (4.24) | 465 (4.00) |
| EtOAc | 477 (4.04) | 480 (3.86) | 472 (3.83) | 470 (4.40) | 472 (4.21) | 465 (4.00) |
| Acetone | 478 (4.02) | 478 (3.85) | 472 (3.84) | 477 (4.42) | 478 (4.26) | 476 (4.00) |
| DMF | 481 (4.04) | 486 (3.86) | 478 (3.83) | 490 (4.37) | 491 (4.27) | 486 (4.04) |
| DMSO | 484 (4.03) | 488 (3.88) | 479 (3.85) | 497 (4.40) | 498 (4.25) | 491 (4.08) |
| EtOH | 480 (3.92) | 478 (3.93) | 473 (3.83) | 489 (4.23) | 488 (4.23) | 483 (4.04) |
| Solvent | λem Max, nm | |||||
|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 4a | 4b | 4c | |
| Hexane | 585 (0.05) | - | - | 516 (0.49) | 555 (0.48) | 562 (0.08) |
| Benzene | 584 (0.11) | 580 (0.17) | 572 (0.03) | 552 (0.47) | 555 (0.57) | 542 (0.18) |
| Chloroform | 596 (0.07) | 595 (0.09) | 550 (0.14) | 571 (0.41) | 589 (0.48) | 574 (0.22) |
| EtOAc | 597 (0.08) | 592 (0.07) | 582 (0.02) | 588 (0.48) | 578 (0.53) | 566 (0.18) |
| Acetone | 599 (0.01) | 604 (0.08) | 593 (0.02) | 591 (0.43) | 597 (0.56) | 587 (0.15) |
| DMF | 605 (0.06) | 604 (0.03) | 606 (0.01) | 601 (0.45) | 611 (0.48) | 610 (0.13) |
| DMSO | 610 (0.04) | 617 (0.03) | 616 (<0.01) | 614 (0.36) | 620 (0.42) | 619 (0.12) |
| EtOH | 614 (0.02) | 628 (0.05) | 609 (<0.01) | 633 (0.19) | 636 (0.23) | 629 (0.10) |
| Solvent | νabs − νem, cm–1 | |||||
|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 4a | 4b | 4c | |
| Hexane | 4137 | - | - | 2647 | 3864 | 4578 |
| Benzene | 3797 | 3549 | 3749 | 3389 | 3395 | 3336 |
| Chloroform | 4055 | 3897 | 4140 | 3495 | 4435 | 4084 |
| EtOAc | 4214 | 3941 | 4004 | 4270 | 3885 | 3838 |
| Acetone | 4226 | 4364 | 4323 | 4044 | 4170 | 3973 |
| DMF | 4261 | 4020 | 4419 | 3769 | 4000 | 4183 |
| DMSO | 4268 | 4284 | 4643 | 3834 | 3951 | 4212 |
| EtOH | 4547 | 4997 | 4721 | 4652 | 4769 | 4806 |
| Length of First Leaf (mm) | Length of Coleoptile (mm) | Phytotoxicity on the First Leaf (%) | Phytotoxicity on the Coleoptile (%) | |
|---|---|---|---|---|
| Control | 68.26 | 34.52 | - | - |
| 2a (3 × 10–6 M) | 20.52 | 12.70 | 70 | 63 |
| 4a (3 × 10–6 M) | 24.24 | 14.76 | 64 | 57 |
| Electrolyte Leakage | MDA Assay | |||
|---|---|---|---|---|
| First Leaf | Coleoptile | First Leaf | Coleoptile | |
| Control | 41 | 15 | 31.9 | 15.1 |
| 2a (3 × 10–6 M) | 29 | 23 | 63.8 | 40.9 |
| 4a (3 × 10–6 M) | 20 | 21 | 67.0 | 46.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maļeckis, A.; Cvetinska, M.; Kirjušina, M.; Mežaraupe, L.; Kecko, S.; Gavarāne, I.; Kiyan, V.; Lider, L.; Pavlova, V.; Savicka, M.; et al. A Comparative Study of New Fluorescent Anthraquinone and Benzanthrone α-Aminophosphonates: Synthesis, Spectroscopy, Toxicology, X-ray Crystallography, and Microscopy of Opisthorchis felineus. Molecules 2024, 29, 1143. https://doi.org/10.3390/molecules29051143
Maļeckis A, Cvetinska M, Kirjušina M, Mežaraupe L, Kecko S, Gavarāne I, Kiyan V, Lider L, Pavlova V, Savicka M, et al. A Comparative Study of New Fluorescent Anthraquinone and Benzanthrone α-Aminophosphonates: Synthesis, Spectroscopy, Toxicology, X-ray Crystallography, and Microscopy of Opisthorchis felineus. Molecules. 2024; 29(5):1143. https://doi.org/10.3390/molecules29051143
Chicago/Turabian StyleMaļeckis, Armands, Marija Cvetinska, Muza Kirjušina, Ligita Mežaraupe, Sanita Kecko, Inese Gavarāne, Vladimir Kiyan, Lyudmila Lider, Veronika Pavlova, Marina Savicka, and et al. 2024. "A Comparative Study of New Fluorescent Anthraquinone and Benzanthrone α-Aminophosphonates: Synthesis, Spectroscopy, Toxicology, X-ray Crystallography, and Microscopy of Opisthorchis felineus" Molecules 29, no. 5: 1143. https://doi.org/10.3390/molecules29051143
APA StyleMaļeckis, A., Cvetinska, M., Kirjušina, M., Mežaraupe, L., Kecko, S., Gavarāne, I., Kiyan, V., Lider, L., Pavlova, V., Savicka, M., Belyakov, S., & Kirilova, E. (2024). A Comparative Study of New Fluorescent Anthraquinone and Benzanthrone α-Aminophosphonates: Synthesis, Spectroscopy, Toxicology, X-ray Crystallography, and Microscopy of Opisthorchis felineus. Molecules, 29(5), 1143. https://doi.org/10.3390/molecules29051143







