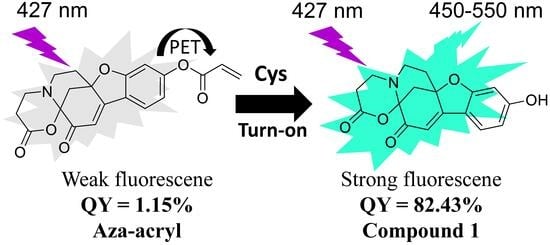
A Novel Fluorescence Probe Based on Azamonardine for Detecting and Imaging Cysteine in Cells and Zebrafish with High Selectivity and Sensitivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Design of Aza-acryl
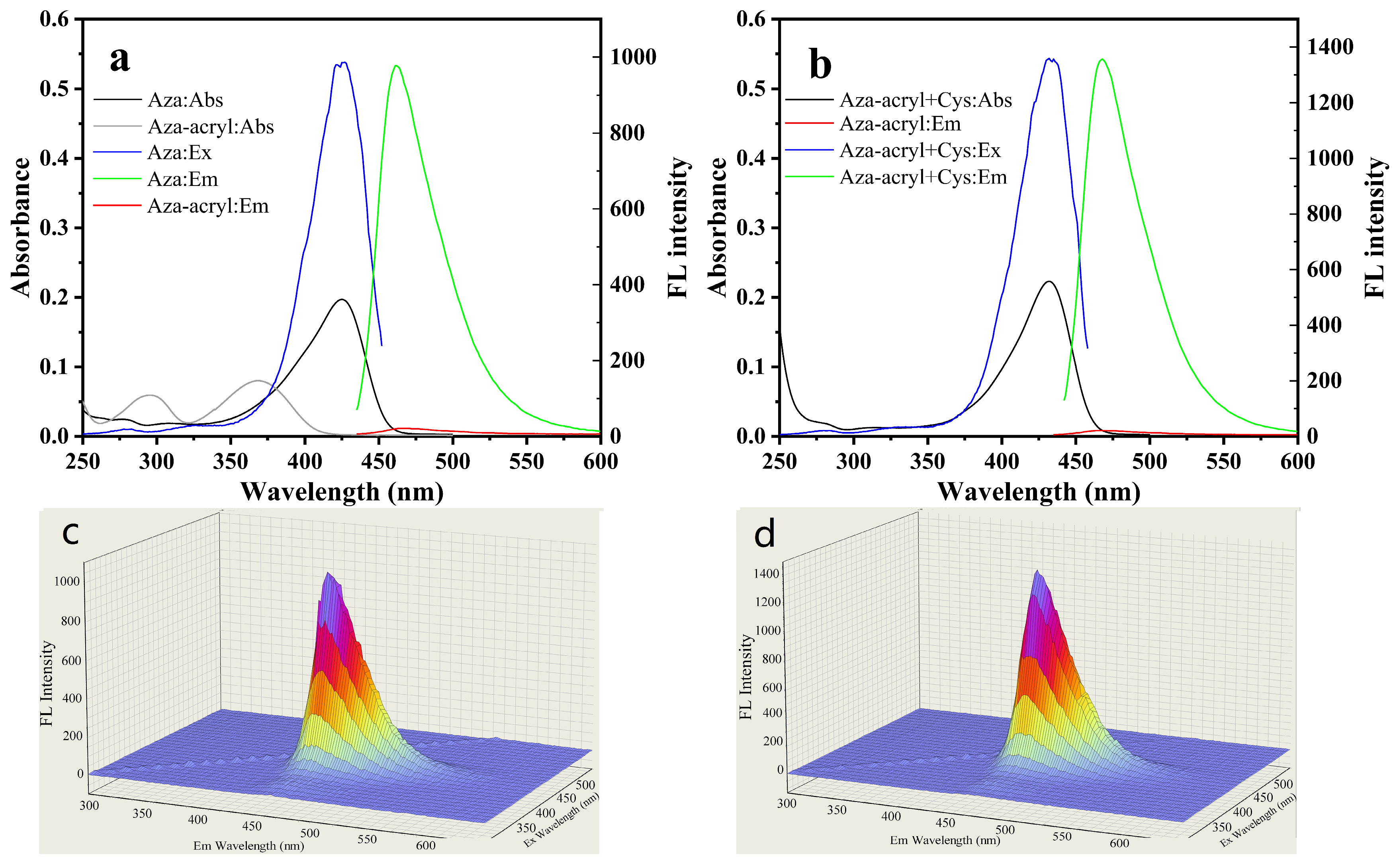
2.2. Spectra and Fluorescence Response
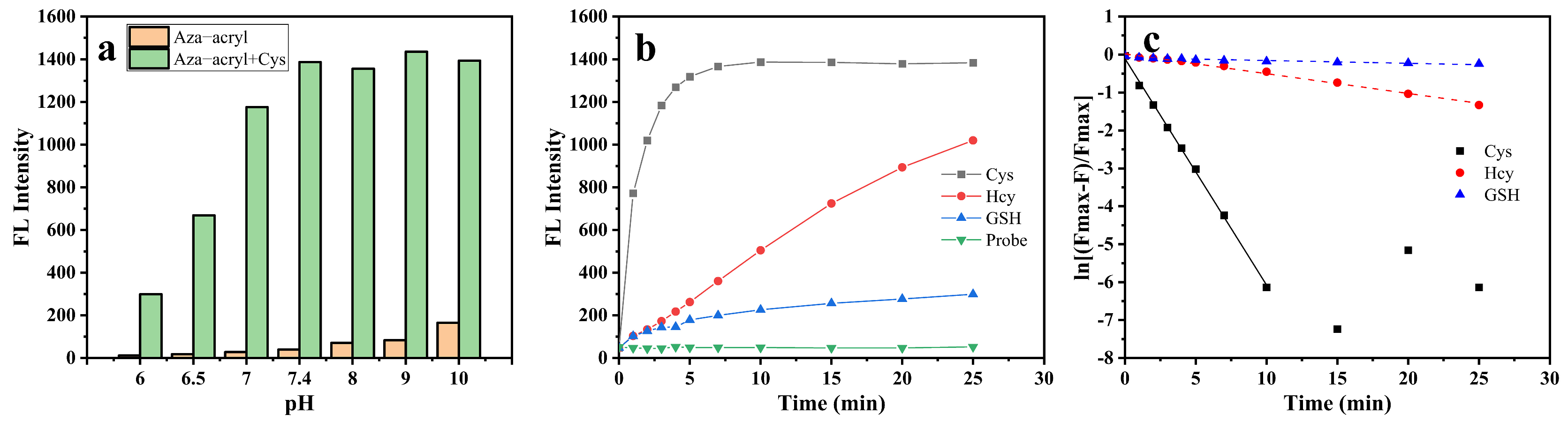
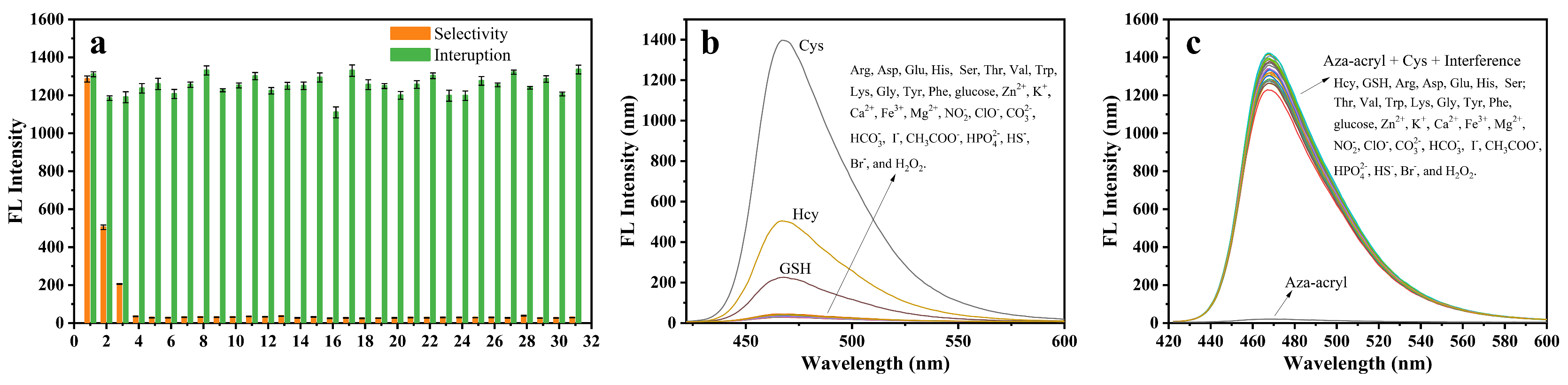
2.3. Feasibility, Selectivity, and Sensitivity of Aza-acryl to Cys
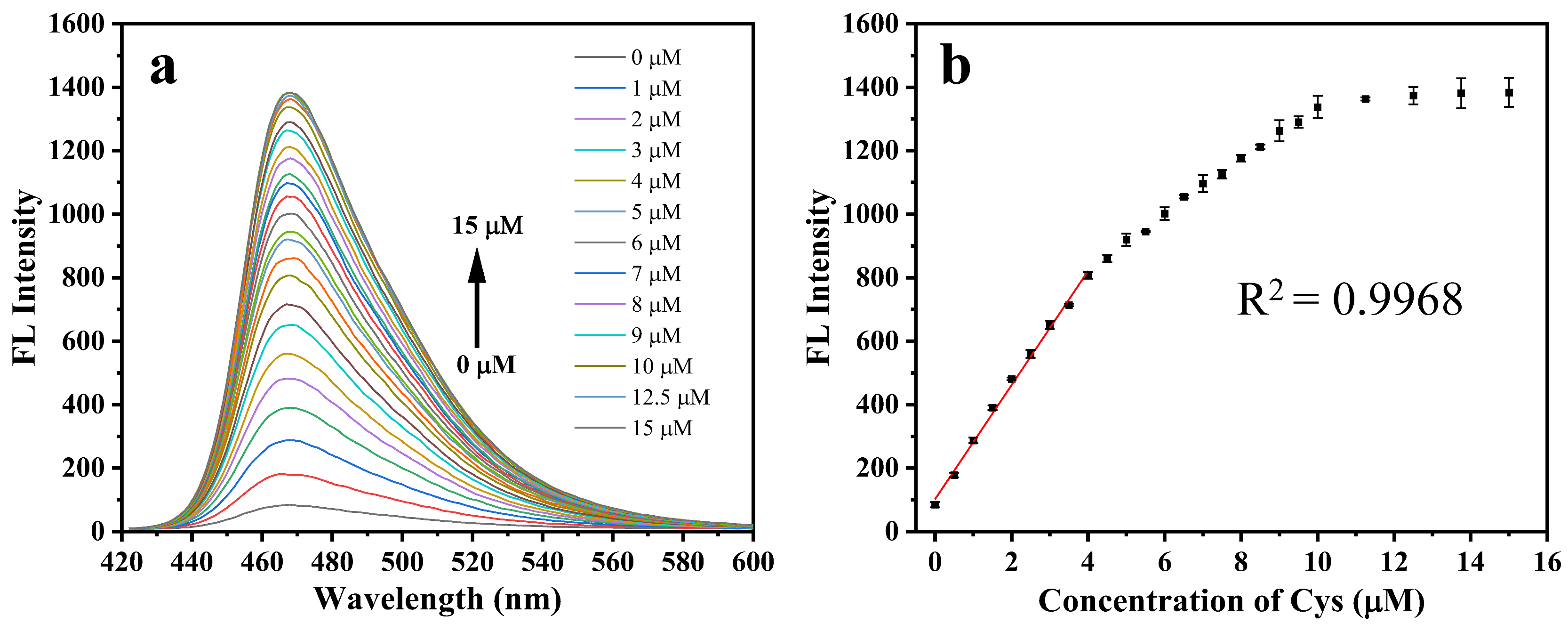
2.4. Cys Titration Experiment
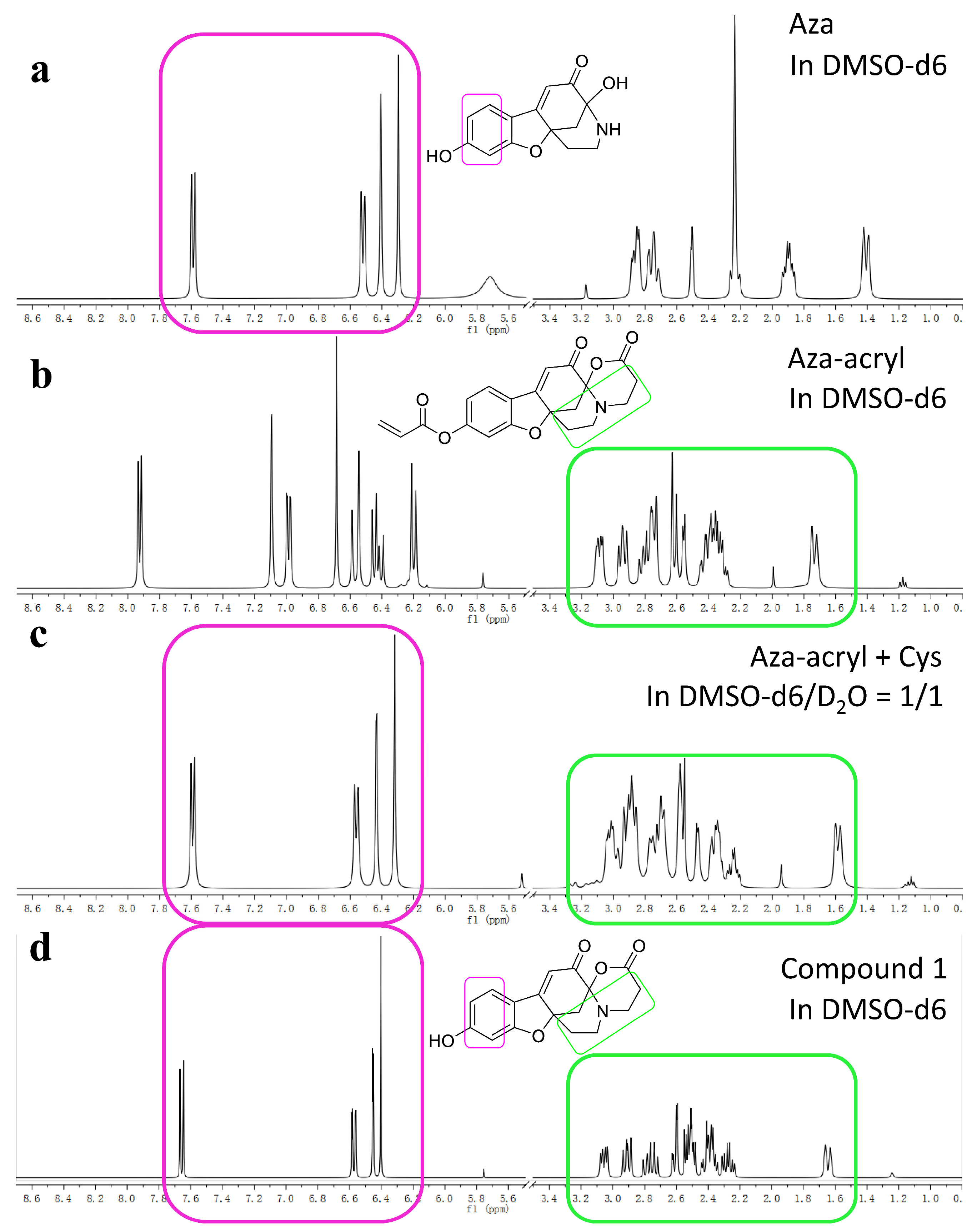
2.5. The Reaction Mechanism of Aza-acryl with the Thiols
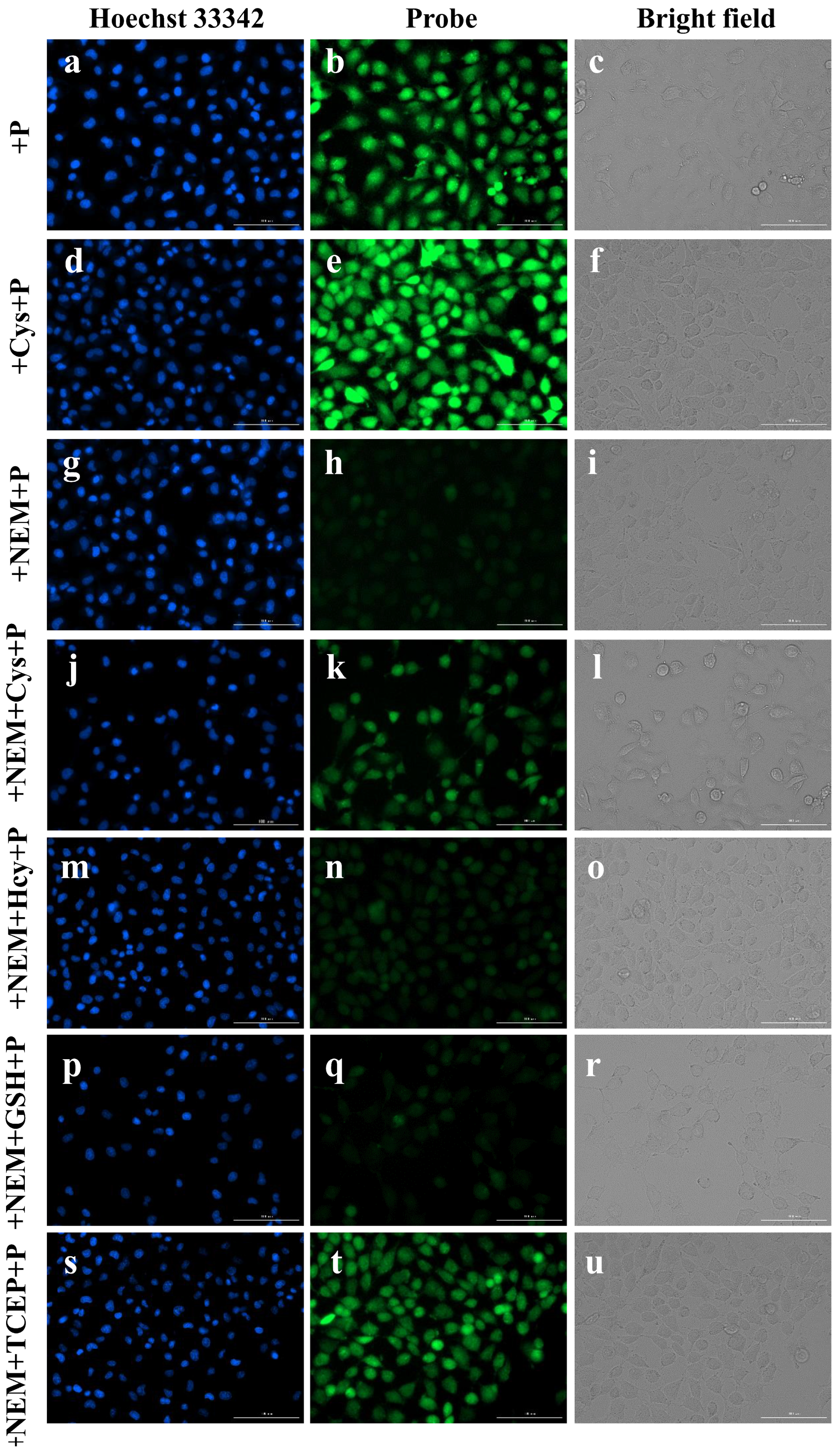
2.6. Imaging of Cys in Live Cells
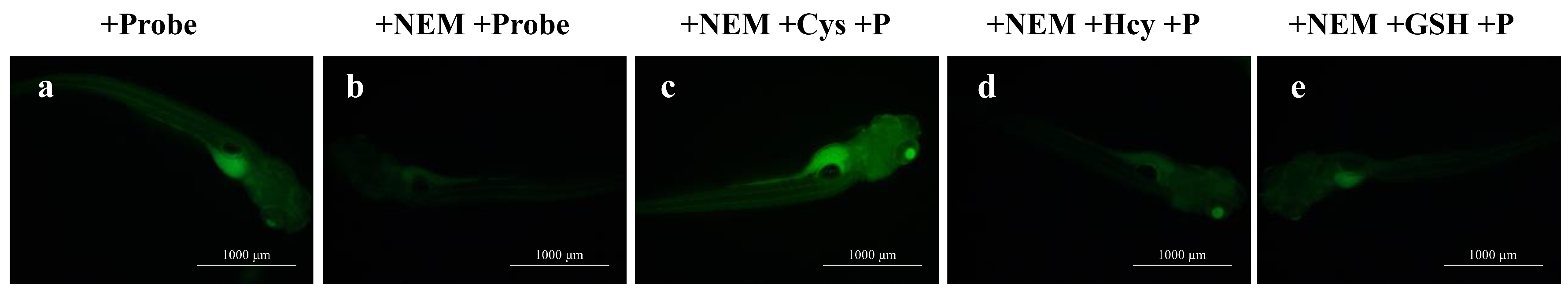
2.7. Imaging of Cys in Zebrafish
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis
3.3.1. Synthesis of 4,9-Dihydroxy-1,2,3,4-tetrahydro-5H-4,11a-methanobenzofuro[2,3-d]azocin-5-one (Aza)
3.3.2. Synthesis of 2,14-Dioxo-3,4,6,7-tetrahydro-2H,14H-7a,14a-methanobenzofuro[3,2-e][1,3]oxazino[3,2-a]azocin-10-ylacrylate (Aza-acryl)
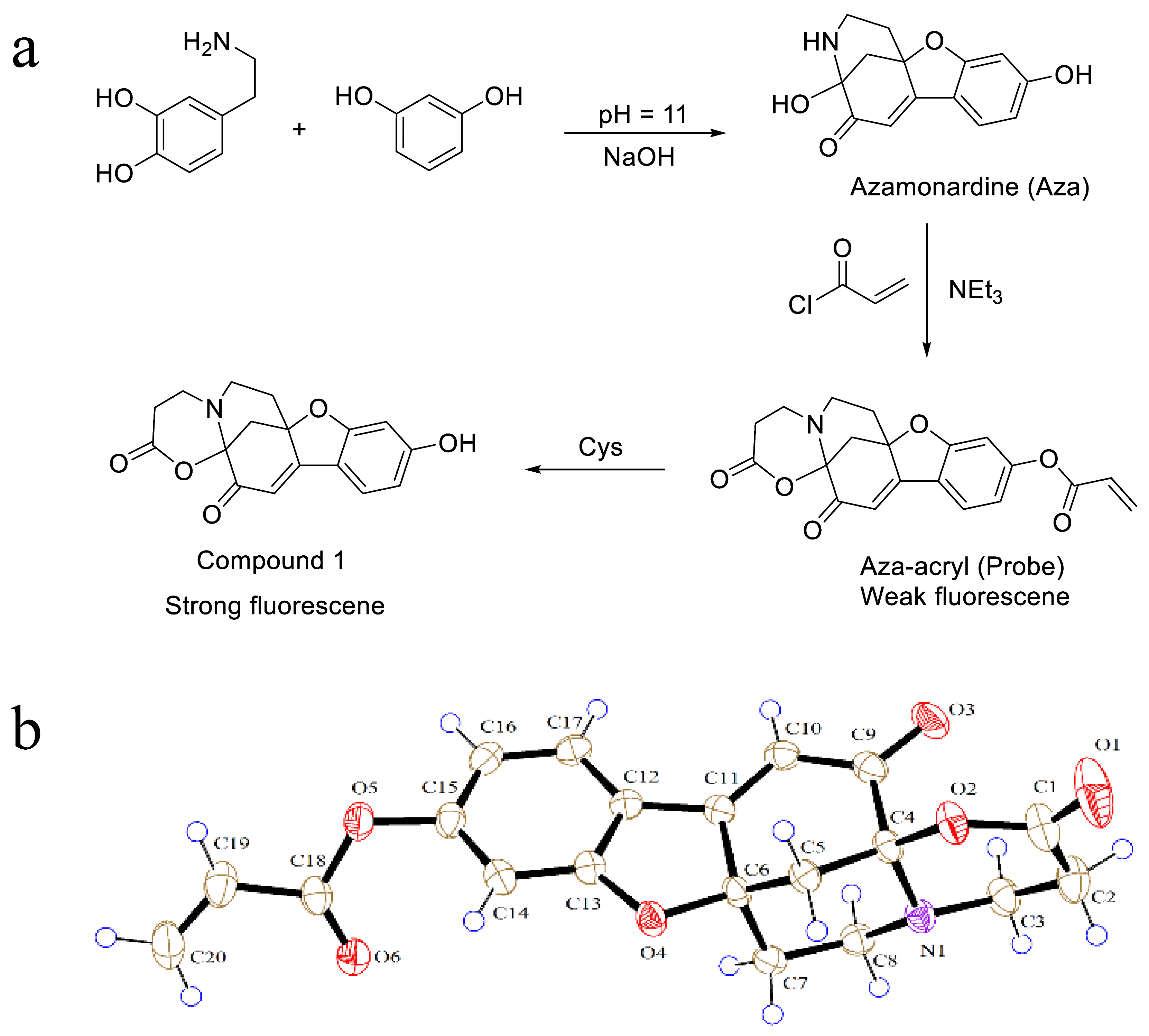
3.4. Crystal Structure Analysis
3.5. General Spectral Analysis
3.6. Imaging
3.6.1. Cell Imaging
3.6.2. Zebrafish Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Niu, L.Y.; Chen, Y.Z.; Zheng, H.R.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef]
- Xie, W.Z.; Jiang, J.Y.; Shu, D.J.; Zhang, Y.J.; Yang, S.; Zhang, K. Recent Progress in the Rational Design of Biothiol-Responsive Fluorescent Probes. Molecules 2023, 28, 4252. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, Z.; Jiang, Z.; Yang, Y.; Liu, G.; Cheng, X.; Jiang, Z.; Zhang, G.; Tong, L.; Tang, B. Detection of Cysteine Sulfenic Acid on E. coli Proteins with a Biotin-Benzoboroxole Probe. ACS Chem. Biol. 2023, 18, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Huang, Y.; Li, B.; Zeng, F.; Wu, S. Real-time monitoring of endogenous cysteine levels in vivo by near-infrared turn-on fluorescent probe with large stokes shift. Anal. Chem. 2018, 90, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Kubalczyk, P.; Bald, E.; Furmaniak, P.; Głowacki, R. Simultaneous determination of total homocysteine and cysteine in human plasma by capillary zone electrophoresis with pH-mediated sample stacking. Anal. Methods 2014, 6, 4138–4143. [Google Scholar] [CrossRef]
- Guo, B.; Pan, X.; Liu, Y.; Nie, L.; Zhao, H.; Liu, Y.; Jing, J.; Zhang, X. A reversible water-soluble naphthalimide-based chemosensor for imaging of cellular copper (II) ion and cysteine. Sens. Actuators B Chem. 2018, 256, 632–638. [Google Scholar] [CrossRef]
- Badaloo, A.; Reid, M.; Forrester, T.; Heird, W.C.; Jahoor, F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am. J. Clin. Nutr. 2002, 76, 646–652. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, H.; Liu, W.; Wang, J.; Fang, D. A novel dual-excitation and dual-emission fluorescent probe (CQDs-O-NBD) based on carbon quantum dots for detection and discrimination of Cys/Hcy and GSH/H2S in living cells. Dye. Pigment. 2021, 193, 109554. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, Z.R.; Ren, M.G.; Kong, X.Q.; Lin, W.Y. A fast-response two-photon fluorescent probe for the detection of Cys over GSH/Hcy with a large turn-on signal and its application in living tissues. J. Mater. Chem. B. 2017, 5, 134–138. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 780–785. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Wang, J.; Fang, D. A red-emitting Europium (III) complex as a luminescent probe with large Stokes shift for the sequential determination of Cu2+ and biothiols in real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 282, 121663. [Google Scholar] [CrossRef]
- Stabler, S.P.; Marcell, P.D.; Podell, E.R.; Allen, R.H. Quantitation of total homocysteine, total cysteine, and methionine in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 1987, 162, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierek, K.; Głowacki, R.; Bald, E. Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography. Anal. Bioanal. Chem. 2006, 385, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Slowinski, D.; Swierczynska, M.; Romanski, J.; Podsiadly, R. HPLC Study of Product Formed in the Reaction of NBD-Derived Fluorescent Probe with Hydrogen Sulfide, Cysteine, N-acetylcysteine, and Glutathione. Molecules 2022, 27, 8305. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Hoffman, B.; Dwivedi, C.; Matthees, D.P. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homocysteine and their disulfides in biological samples. J. Pharm. Biomed. Anal. 2003, 31, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, Z.; Chen, L.; Qian, K.; Xie, J. L-cysteine-assisted synthesis of hierarchical NiS2 hollow spheres supported carbon nitride as photocatalysts with enhanced lifetime. Nanotechnology 2017, 28, 115708. [Google Scholar] [CrossRef]
- Arvand, M.; Mirroshandel, A.A. Fluorescent nanochemosensor for direct optosensing of butachlor based on l-cysteine-capped ZnS quantum dots. J. Iran. Chem. Soc. 2017, 14, 2287–2297. [Google Scholar] [CrossRef]
- Yan, H.; Huo, F.; Yue, Y.; Chao, J.; Yin, C. Rapid reaction, slow dissociation aggregation, and synergetic multicolor emission for imaging the restriction and regulation of biosynthesis of Cys and GSH. J. Am. Chem. Soc. 2020, 143, 318–325. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Huo, F.G.; Liu, Y.; Yin, C. Dual-channel red fluorescent probe for detection of Cys/Hcy and GSH in plants. Sens. Actuators B-Chem. 2019, 301, 127123. [Google Scholar] [CrossRef]
- Zhang, D.A.W.; Zhang, F.R.; Liao, Y.H.; Wang, F.H.; Liu, H.L. Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off-On” High-Sensitivity Detection of Fe3+ and L-Cysteine. Molecules 2022, 27, 4099. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, X.; Li, C.; Xie, Y. A novel p-aminophenylthio- and cyano- substituted BODIPY as a fluorescence turn-on probe for distinguishing cysteine and homocysteine from glutathione. Dye. Pigment. 2018, 148, 212–218. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Shu, Y.; Wang, J.; Qiu, H. A turn-on fluorescent probe via substitution-rearrangement for highly sensitive and discriminative detection of cysteine and its imaging in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120409. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Liu, R.; Chu, R.; Wang, X.; Xu, K.; Tang, B. A highly sensitive near-infrared fluorescent probe for cysteine and homocysteine in living cells. Chem. Commun. 2013, 49, 9176–9178. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Q.; Yao, Y.; Fan, X.; Zhang, W.; Qian, J. Highly sensitive detection of cysteine over glutathione and homo-cysteine: New insight into the Michael addition of mercapto group to maleimide. Biosens. Bioelectron. 2017, 91, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, S.; Meng, Q.; Zhou, J.; Jin, R.; Long, X.; Tang, Y.-P.; Guo, H. A two-photon multi-emissive fluorescent probe for discrimination of Cys and Hcy/GSH via an aromatic substitution-rearrangement. Talanta 2021, 224, 121833. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, B.; Bai, M.; Dong, M.; Tang, X. Fluorescent probe for highly selective detection of cysteine in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122523. [Google Scholar] [CrossRef]
- Zhao, Q.; Qin, J.; Kong, F.; Wang, D.; Guo, Y.; Li, Y. A novel red-emission fluorescent probe for the detection of cysteine in vitro and in vivo. J. Photochem. Photobiol. A Chem. 2023, 436, 114383. [Google Scholar] [CrossRef]
- Qiao, L.; Yang, Y.; Cai, J.; Lv, X.; Hao, J.; Li, Y. Long wavelength emission fluorescent probe for highly selective detection of cysteine in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120247. [Google Scholar] [CrossRef]
- Yang, X.Z.; Wei, X.R.; Sun, R.; Xu, Y.J.; Ge, J.F. A novel xanthylene-based effective mitochondria-targeting ratiometric cysteine probe and its bioimaging in living cells. Talanta 2020, 209, 120580. [Google Scholar] [CrossRef]
- Wei, L.F.; Thirumalaivasan, N.; Liao, Y.C.; Wu, S.P. Fluorescent coumarin-based probe for cysteine and homocysteine with live cell application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 204–208. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Xu, X.; Jiang, Y.; Wang, B. A novel fluorescent probe for highly sensitive and selective detection of cysteine and its application in cell imaging. Sens. Actuators B Chem. 2017, 251, 902–908. [Google Scholar] [CrossRef]
- Liu, H.-B.; Xu, H.; Guo, X.; Xiao, J.; Cai, Z.-H.; Wang, Y.-W.; Peng, Y. A novel near-infrared fluorescent probe based on isophorone for the bioassay of endogenous cysteine. Org. Biomol. Chem. 2021, 19, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Xia, J.; Zhang, L.; Chen, Q.; Guo, J.; Cui, H.; Kafuti, Y.S.; Wang, J.; Peng, X. Rationally modifying the dicyanoisophorone fluorophore for sensing cysteine in living cells and mice. Sens. Actuators B Chem. 2020, 321, 128441. [Google Scholar] [CrossRef]
- Zhang, J.; Abdulkhaleq, A.M.A.; Wang, J.; Zhou, X. Rational design of a novel acryl-modified CQDs fluorescent probe for highly selective detection and imaging of cysteine in vitro and in vivo. Microchim. Acta 2023, 190, 331. [Google Scholar] [CrossRef]
- Acuña, A.U.; Álvarez-Pérez, M.; Liras, M.; Coto, P.B.; Amat-Guerri, F. Synthesis and photophysics of novel biocompatible fluorescent oxocines and azocines in aqueous solution. PCCP 2013, 15, 16704–16712. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Crescenzi, O.; Napolitano, A.; d’Ischia, M. Nature-Inspired Functional Chromophores from Biomimetic o-Quinone Chemistry. Eur. J. Org. Chem. 2021, 2021, 2982–2989. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Li, X.; Guo, X.; Zhang, B.; Jia, X.; Dai, B. A simple, fast and low-cost turn-on fluorescence method for dopamine detection using in situ reaction. Anal. Chim. Acta 2016, 944, 51–56. [Google Scholar] [CrossRef]
- Zhao, J.H.; Liu, G.Y.; Wang, S.; Lu, S.S.; Sun, J.; Yang, X.R. In Situ Specific Chromogenic and Fluorogenic Reaction for Straight forward and Dual-Modal Dopamine Detection. Chin. J. Anal. Chem. 2020, 48, e20081–e20088. [Google Scholar] [CrossRef]
- Yu, L.; Feng, L.; Xiong, L.; Li, S.; Xu, Q.; Pan, X.; Xiao, Y. Multifunctional nanoscale lanthanide metal–organic framework based ratiometric fluorescence paper microchip for visual dopamine assay. Nanoscale 2021, 13, 11188–11196. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Zhang, J.; Zheng, J.; Yuan, Z.; Lu, C. Catechin-inspired gold nanocluster nanoprobe for selective and ratiometric dopamine detection via forming azamonardine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121142. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, Y.; Liang, W.Y.; Yang, X.P.; Jiang, W.D.; Liu, X.H.; Zhang, W. A facile and sensitive ratiometric fluorescence sensor for rapid visual monitoring of trace resorcinol. Sens. Actuators B Chem. 2021, 330, 129390. [Google Scholar] [CrossRef]
- Long, T.; Cheng, J.; Peng, C.; Xu, W.; Luo, H.; Ouyang, M.; Xu, D.; Lin, Q.; Qu, J.; Huang, X. Highly sensitive and rapid detection of resorcinol by forming fluorescent azamonardine with dopamine. Anal. Biochem. 2022, 642, 114562. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Alfieri, M.L.; Crescenzi, O.; d’Ischia, M.; Napolitano, A. Unimolecular Variant of the Fluorescence Turn-On Oxidative Coupling of Catecholamines with Resorcinols. ACS Omega 2019, 4, 1541–1548. [Google Scholar] [CrossRef]
- Zhao, J.; Bao, X.; Wang, S.; Lu, S.; Sun, J.; Yang, X. In situ fluorogenic and chromogenic reactions for the sensitive dual-readout assay of tyrosinase activity. Anal. Chem. 2017, 89, 10529–10536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, S.; Lu, S.; Bao, X.; Sun, J.; Yang, X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal. Chem. 2018, 90, 7754–7760. [Google Scholar] [CrossRef]
- Cheng, X.; Chai, Y.; Xu, J.; Wang, L.; Wei, F.; Xu, G.; Sun, Y.; Hu, Q.; Cen, Y. Enzyme cascade reaction-based ratiometric fluorescence probe for visual monitoring the activity of alkaline phosphatase. Sens. Actuators B Chem. 2020, 309, 127765. [Google Scholar] [CrossRef]
- Ni, P.; Chen, C.; Jiang, Y.; Zhang, C.; Wang, B.; Lu, Y.; Wang, H. A fluorescent assay for alkaline phosphatase activity based on inner filter effect by in-situ formation of fluorescent azamonardine. Sens. Actuators B Chem. 2020, 302, 127145. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, G.; Sun, J.; Wang, Q.; Li, Z.-J.; Yang, X. Dual-Readout Tyrosinase Activity Assay Facilitated by a Chromo-Fluorogenic Reaction between Catechols and Naphthoresorcin. Anal. Chem. 2020, 92, 2316–2322. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Iacomino, M.; Napolitano, A.; d’Ischia, M. Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis–Resorcinol Coupler. Int. J. Mol. Sci. 2019, 20, 4532. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, Y.; Zhang, X.; Wang, S. Modulating an in situ fluorogenic reaction for the label-free ratiometric detection of biothiols. Analyst 2019, 144, 4520–4525. [Google Scholar] [CrossRef]
- Chen, L.; Du, C.P.; Qi, L.; Wang, Y.; Pei, K.L.; Zhang, R.W.; Qi, W.J. Fluorescent azamonardine probe for “turn-off” detection of chromium (VI) and “turn-on” detection of ascorbic acid based on inner filter effect. Chin. J. Anal. Chem. 2022, 50, 100165. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Yuan, H.; Jia, X.; Dai, B. One-pot synthesis of a natural phenol derived fluorescence sensor for Cu (II) and Hg (II) detection. Dye. Pigment. 2018, 155, 100–106. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Yuan, H.; Guo, X.; Dai, B.; Jia, X. An acid-fluorescence and alkali-colorimetric dual channels sensor for Hg2+ selective detection by different coordination manners in aqueous media. J. Photochem. Photobiol. A Chem. 2019, 373, 12–19. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Strongin, R.M. Conjugate Addition/Cyclization Sequence Enables Selective and Simultaneous Fluorescence Detection of Cysteine and Homocysteine. Angew. Chem. Int. Ed. 2011, 50, 10690–10693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, B.; Liang, Y.; Shi, L.; Cen, X.; Zheng, L.; Liang, E.; Huang, L.; Cheng, K. Near-infrared fluorescent and photoacoustic dual-mode probe for highly sensitive and selective imaging of cysteine in vivo. Anal. Chem. 2022, 94, 10737–10744. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, C.; Jiao, X.; Zhao, L.; Zeng, X. A lysosome-targeted near-infrared fluorescent probe for imaging endogenous cysteine (Cys) in living cells. J. Mater. Chem. B. 2020, 8, 2269–2274. [Google Scholar] [CrossRef]
- Yang, X.Z.; Wei, X.R.; Sun, R.; Xu, Y.J.; Ge, J.F. Benzoxazine-based fluorescent probes with different auxochrome groups for cysteine detection. Spectrochim. Acta Part A 2020, 226, 117582. [Google Scholar] [CrossRef]
- Xu, Z.; Si, S.; Zhang, Z.; Tan, H.; Qin, T.; Wang, Z.; Wang, D.; Wang, L.; Liu, B. A fluorescent probe with dual acrylate sites for discrimination of different concentration ranges of cysteine in living cells. Anal. Chim. Acta. 2021, 1176, 338763. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.; Wang, H.; Ji, M.; Wang, P. A novel ratiometric fluorescent probe for the detection and imaging of cysteine in living cells. Bioorg. Chem. 2022, 127, 106003. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, W.; Li, F.; Deng, Z.; Hu, Y. A turn-on fluorescent probe for real-time detection of endogenous cysteine in living cells. J. Lumin. 2020, 226, 117506. [Google Scholar] [CrossRef]
- Blondeau, P.; Berse, R.G.C.; Gravel, D. Synthesis of some sTable 7-halo-1, 4-thiazepines. Potential substituted penam precursors. Can. J. Chem. 1971, 49, 3866–3876. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, T.; Abdulkhaleq, A.M.A.; Zuo, Z.; Peng, Y.; Zhou, X. A Novel Fluorescence Probe Based on Azamonardine for Detecting and Imaging Cysteine in Cells and Zebrafish with High Selectivity and Sensitivity. Molecules 2023, 28, 6246. https://doi.org/10.3390/molecules28176246
Zhao Y, Wang T, Abdulkhaleq AMA, Zuo Z, Peng Y, Zhou X. A Novel Fluorescence Probe Based on Azamonardine for Detecting and Imaging Cysteine in Cells and Zebrafish with High Selectivity and Sensitivity. Molecules. 2023; 28(17):6246. https://doi.org/10.3390/molecules28176246
Chicago/Turabian StyleZhao, Yixu, Ting Wang, Ahmed Mohammed Ali Abdulkhaleq, Zhongfu Zuo, Yongjin Peng, and Xibin Zhou. 2023. "A Novel Fluorescence Probe Based on Azamonardine for Detecting and Imaging Cysteine in Cells and Zebrafish with High Selectivity and Sensitivity" Molecules 28, no. 17: 6246. https://doi.org/10.3390/molecules28176246
APA StyleZhao, Y., Wang, T., Abdulkhaleq, A. M. A., Zuo, Z., Peng, Y., & Zhou, X. (2023). A Novel Fluorescence Probe Based on Azamonardine for Detecting and Imaging Cysteine in Cells and Zebrafish with High Selectivity and Sensitivity. Molecules, 28(17), 6246. https://doi.org/10.3390/molecules28176246