Potential Anti-Inflammatory Constituents from Aesculus wilsonii Seeds
Abstract
1. Introduction
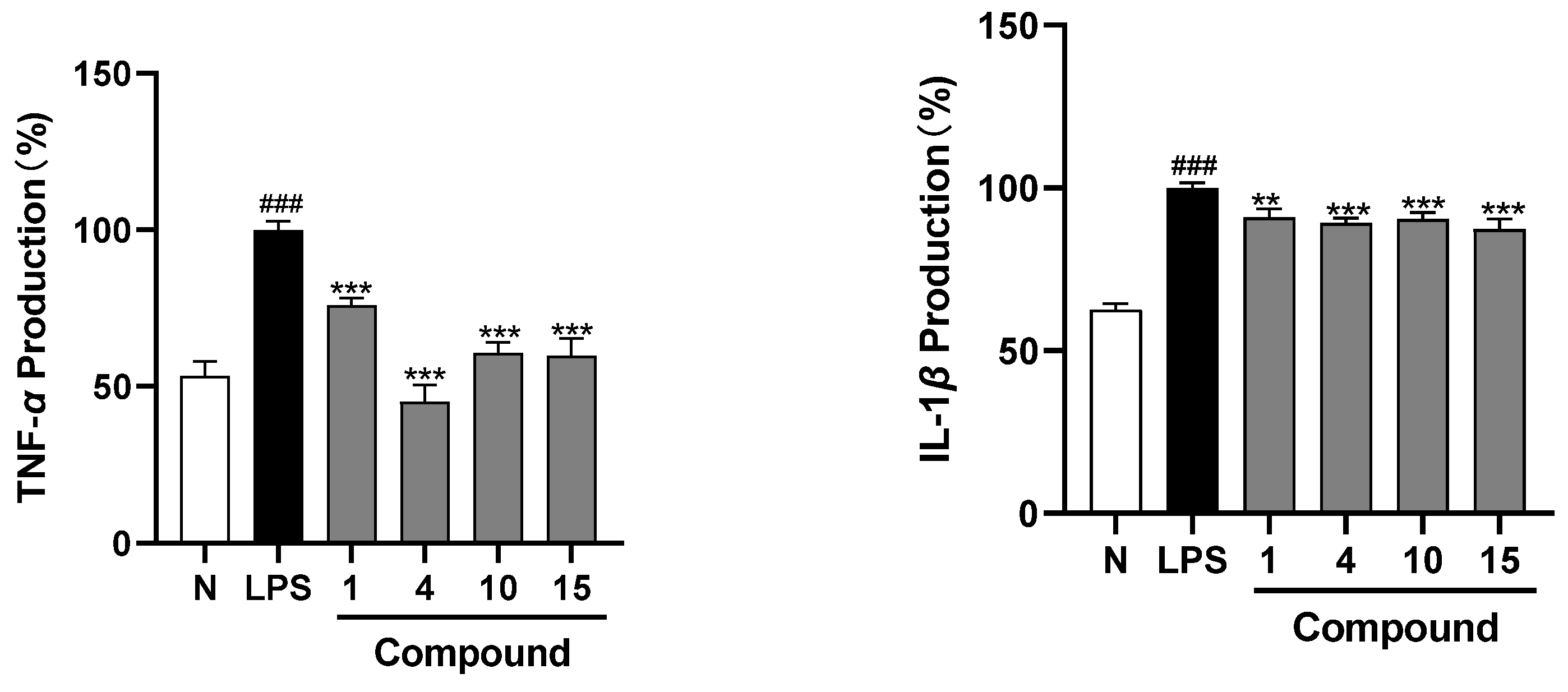
2. Results
3. Discussion
4. Conclusions
5. Experimental Section
5.1. General Experimental Procedures
5.2. Plant Material
5.3. Extraction and Isolation
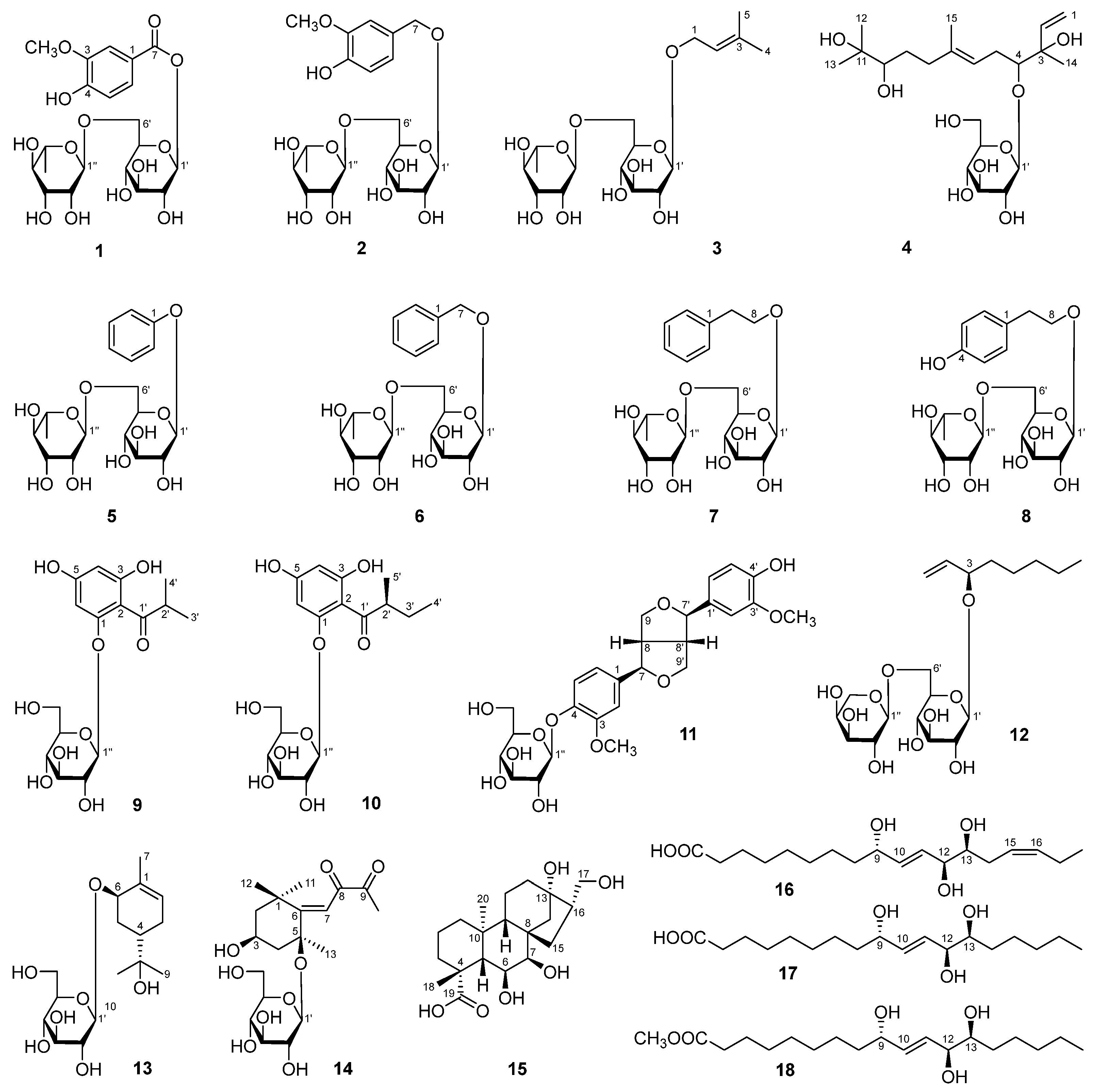
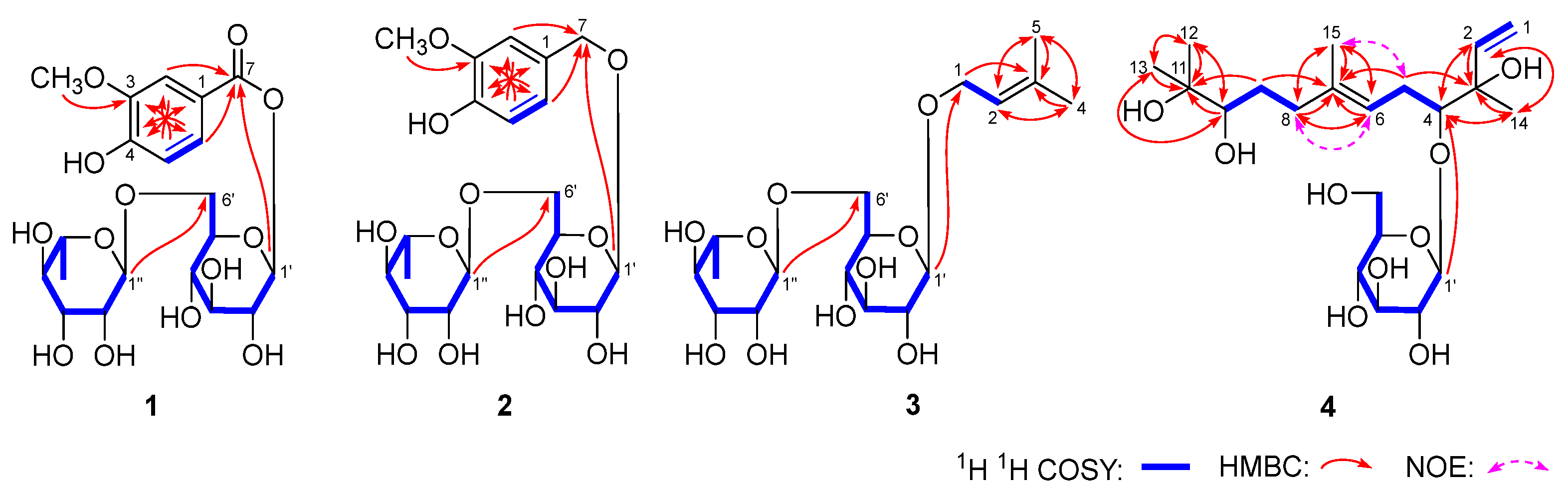
5.4. Spectral Data of 1–18
5.4.1. Aeswiloside I (1)
5.4.2. Aeswiloside II (2)
5.4.3. Aeswiloside III (3)
5.4.4. Aeswiloside IV (4)
5.4.5. Phenyl-O-α-l-Rhamnopyranosyl(1→6)-β-d-Glucopyranoside (5)
5.4.6. Benzyl-O-α-l-Rhamnopyranosyl(1→6)-β-d-Glucopyranoside (6)
5.4.7. 2-Phenethyl-O-α-l-Rhamnopyranosyl(1→6)-β-d-Glucopyranoside (7)
5.4.8. Asechipuroside A (8)
5.4.9. 1-[(2-Methylpropanoyl)Phloroglucinyl]-β-d-Glucopyranoside (9)
5.4.10. 1-(2-Methylbutyryl)Phloroglucinyl-Glucopyranoside (10)
5.4.11. (−)-Pinoresinol 4-O-β-d-Glucoside (11)
5.4.12. 3-O-[α-l-Arabinopyranosyl(1→6)-β-d-Glucopyranosyl]oct-1-ene-3-ol (12)
5.4.13. Myrseguinoside A (13)
5.4.14. Lippianoside E (14)
5.4.15. Pisuminic Acid (15)
5.4.16. Fulgidic Acid (16)
5.4.17. (9S,10E,12S,13S)-9,12,13-Trihydroxy-10-Octadecenoic Acid (17)
5.4.18. Methyl (9S,10E,12S,13S)-9,12,13-Trihydroxy-10-Octadecenoate (18)
5.5. Acid Hydrolysis of Compounds 1–4
5.6. Scifinder Searching
5.7. Bioassays
5.8. Supplementary Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roe, K. An inflammation classification system using cytokine parameters. Scand J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- Lane, N.E.; Ivanova, J.; Emir, B.; Mobasheri, A.; Jensen, M.G. Characterization of individuals with osteoarthritis in the United States and their use of prescription and over-the-counter supplements. Maturitas 2021, 145, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Fu, T.S.; Lin, Y.C.; Lin, Y.J. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos. Int. 2021, 32, 663–670. [Google Scholar] [CrossRef]
- Lindsay, S.E.; Philipp, T.; Ryu, W.H.A.; Wright, C.; Yoo, J. Nonsteroidal anti-inflammatory drugs in the acute post-operative period are associated with an increased incidence of pseudarthrosis, hardware failure, and revision surgery following single-level spinal fusion. Spine 2023, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, H.; Mao, M.; Liang, C.; Zhang, Y.; Yang, D.; Wei, Z.; Gao, S.; Hu, B.; Wang, L.; et al. Escin increases the survival rate of LPS-induced septic mice through inhibition of HMGB1 release from macrophages. Cell Physiol. Biochem. 2015, 36, 1577–1586. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, N.; Han, B.; Liu, W.; Liu, T.; Fu, F.; Zhao, D. Escin attenuates cerebral edema induced by acute omethoate poisoning. Toxicol. Mech. Methods 2011, 21, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ha, T.K.; Cho, H.; Kim, E.; Shim, S.H.; Yang, J.L.; Oh, W.K. Antiviral escin derivatives from the seeds of Aesculus turbinata Blume (Japanese horse chestnut). Bioorg. Med. Chem. Lett. 2017, 27, 3019–3025. [Google Scholar] [CrossRef]
- Yang, Y.; Long, L.; Zhang, X.; Song, K.; Wang, D.; Xiong, X.; Gao, H.; Sha, L. 16-Tigloyl linked barrigenol-like triterpenoid from Semen aesculi and its anti-tumor activity in vivo and in vitro. RSC. Adv. 2019, 9, 31758–31772. [Google Scholar] [CrossRef]
- Cao, H.; Ruan, J.; Cao, X.; Zhang, Y.; Hao, J.; Wu, Y.; Zhang, Y.; Wang, T. Nitrogenous compounds from Aesculus wilsonii seeds. Fitoterapia 2023, 172, e105783. [Google Scholar] [CrossRef]
- Li, H.; Cao, H.; Ruan, J.; Wu, Y.; Yang, D.; Gao, Q.; Wang, D.; Chen, Q.; Zhang, Y.; Wang, T. Saponins from Aesculus wilsonii seeds exert anti-inflammatory activity through the suppression of NF-κB and NLRP3 pathway. Arab. J. Chem. 2023, 16, e105077. [Google Scholar] [CrossRef]
- Cao, H.N.; Ruan, J.Y.; Han, Y.; Zhao, W.; Zhang, Y.; Gao, C.; Wu, H.H.; Ma, L.; Gao, X.M.; Zhang, Y.; et al. NO release inhibitory activity of flavonoids from Aesculus wilsonii seeds through MAPK (P38), NF-κB, and STAT3 cross-talk signaling pathways. Planta Med. 2023, 89, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Y.; Cao, H.N.; Jiang, H.Y.; Li, H.M.; Hao, M.M.; Zhao, W.; Zhang, Y.; Han, Y.; Zhang, Y.; Wang, T. Structural characterization of phenolic constituents from the rhizome of Imperata cylindrica var. major and their anti-inflammatory activity. Phytochemistry 2022, 196, e113076. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Ilg, K.; Mashaghi, A.; Textor, M.; Priem, B.; Aebi, M.; Reimhult, E. Supported lipopolysaccharide bilayers. Langmuir 2012, 28, 12199–12208. [Google Scholar] [CrossRef]
- Yang, S.; Sun, F.; Ruan, J.; Yan, J.; Huang, P.; Wang, J.; Han, L.; Zhang, Y.; Wang, T. Anti-inflammatory constituents from Cortex Dictamni. Fitoterapia 2019, 134, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, R.; Sun, S.; Liu, X.; Liu, X.; Wei, W.; Okada, Y.; Wei, W. Chemical constituents from the leaves of Cistus parviflorus. J. Chin. Pharm. Sci. 2018, 27, 40–50. [Google Scholar]
- Wang, J.; Qin, B.H.; Yuan, Q.Y.; Han, H.Y.; Liu, X.Q. Chemical constituents of Caulophyllum robustum and its antitumor activities. Chin. Tradit. Herb. Drugs 2018, 49, 5242–5246. [Google Scholar]
- Bohr, G.; Gerhäuser, C.; Knauft, J.; Zapp, J.; Becker, H. Anti-inflammatory acylphloroglucinol derivatives from Hops (Humulus lupulus). J. Nat. Prod. 2005, 68, 1545–1548. [Google Scholar] [CrossRef]
- Kosasi, S.; Sluis, W.; Labadie, R. Multifidol and multifidol glucoside from the latex of Jatropha multifida. Phytochemistry 1989, 28, 2439–2441. [Google Scholar] [CrossRef]
- Shao, S.Y.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. An efficient method for determining the relative configuration of furofuran lignans by 1H NMR spectroscopy. J. Nat. Prod. 2018, 81, 1023–1028. [Google Scholar] [CrossRef]
- Xu, X.Y.; Xie, H.H.; Wei, X.Y. Five glycosides from the seeds of Litchi chinensis. J. Trop. Subtrop. Bot. 2012, 20, 206–208. [Google Scholar]
- Simonet, A.M.; Stochmal, A.; Oleszek, W.; Macias, F.A. Saponins and polar compounds from Trifolium resupinatum. Phytochemistry 1999, 51, 1065–1067. [Google Scholar] [CrossRef]
- Matsunami, K.; Otsuka, H.; Takeda, Y. Myrseguinosides A-E, five new glycosides from the fruits of Myrsine seguinii. Chem. Pharm. Bull. 2011, 59, 1274–1280. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, S.; Dong, Y.; Wang, T.; Qu, L.; Li, N.; Wang, T. Bioactive constituents from the aerial parts of Lippia triphylla. Molecules 2015, 20, 21946–21959. [Google Scholar] [CrossRef]
- Murakami, T.; Kohno, K.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXII. Structures of oleanane-type triterpene oligoglycosides, pisumsaponins I and II, and kaurane-type diterpene oligoglycosides, pisumosides A and B, from green peas, the immature seeds of Pisum sativum L. Chem. Pharm. Bull. 2001, 49, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Hong, Y.; Lee, H.H.; Ryu, B.; Cho, Y.W.; Kim, N.J.; Jang, D.S.; Lee, K.T. Fulgidic acid isolated from the rhizomes of Cyperus rotundus suppresses LPS-induced iNOS, COX-2, TNF-α, and IL-6 expression by AP-1 inactivation in RAW264.7 macrophages. Biol. Pharm. Bull. 2015, 38, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Kuwahara, S. A concise synthesis of pinellic acid using a cross-metathesis approach. Tetrahedron 2009, 65, 3364–3368. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, S.; Cao, S.; Zhang, Q.; Kang, N.; Ding, L.; Qiu, F. Bioactive triterpenoid saponins from the seeds of Aesculus chinensis Bge. var. chekiangensis. Front Chem. 2020, 7, e908. [Google Scholar] [CrossRef]
- Lu, Q.; Shi, X.; Hu, H.; Zhou, J.; Cui, X. Research progress on the chemical constituents and bioactivity of Aesculuschinensis Bunge var. chinensis. Northwest Pharm. J. 2016, 31, 651–654. [Google Scholar]
- Liu, J.; Li, J.; Shin, H.D.; Liu, L.; Du, G.; Chen, J. Protein and metabolic engineering for the production of organic acids. Bioresour. Technol. 2017, 239, 412–421. [Google Scholar] [CrossRef]
- Courdavault, V.; Papon, N. A new path for terpenoid biosynthesis. Trends Biochem. Sci. 2022, 47, 906–908. [Google Scholar] [CrossRef]
- Ju, K.S.; Nair, S.K. Convergent and divergent biosynthetic strategies towards phosphonic acid natural products. Curr. Opin. Chem. Biol. 2022, 71, e102214. [Google Scholar] [CrossRef] [PubMed]
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 121.8 | — | 5′ | 77.8 | 3.56 (m) |
| 2 | 114.0 | 7.62 (d, 1.8) | 6′ | 67.8 | 3.66 (dd, 3.0, 12.0) |
| 3 | 148.8 | — | 3.97 (dd, 1.8, 12.0) | ||
| 4 | 153.4 | — | 1″ | 102.3 | 4.71 (d, 1.2) |
| 5 | 116.0 | 6.86 (d, 8.4) | 2″ | 72.1 | 3.83 (dd, 1.2, 9.6) |
| 6 | 125.8 | 7.65 (dd, 1.8, 8.4) | 3″ | 72.4 | |
| 7 | 166.7 | — | 4″ | 74.0 | 3.33 (dd, 9.6, 9.6) |
| 1′ | 96.1 | 5.65 (d, 8.4) | 5″ | 69.9 | 3.65 (m) |
| 2′ | 74.1 | 3.48 (dd, 8.4, 9.6) | 6″ | 18.0 | 1.19 (d, 6.6) |
| 3′ | 78.1 | 3.49 (dd, 9.6, 9.6) | 3-OCH3 | 56.5 | 3.91 (s) |
| 4′ | 71.2 |
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 130.2 | — | 4′ | 71.8 | 3.27 (dd, 9.0, 9.0) |
| 2 | 113.3 | 7.03 (br. s) | 5′ | 77.0 | 3.37 (m) |
| 3 | 149.0 | — | 6′ | 68.2 | 3.64 (dd, 6.0, 11.0) |
| 4 | 147.4 | — | 4.00 (br. d, ca. 11) | ||
| 5 | 115.8 | 6.75 (d, 8.0) | 1″ | 102.4 | 4.79 (br. s) |
| 6 | 122.6 | 6.82 (br. d, ca. 8) | 2″ | 72.3 | 3.87 (br. d, ca. 3) |
| 7 | 71.2 | 4.54 (d, 11.5) | 3″ | 72.4 | 3.69 (m, overlapped) |
| 4.77 (d, 11.5) | 4″ | 74.1 | 3.38 (dd, 9.0, 9.5) | ||
| 1′ | 102.7 | 4.29 (d, 8.0) | 5″ | 69.9 | 3.69 (m, overlapped) |
| 2′ | 75.1 | 3.22 (dd, 8.0, 8.5) | 6″ | 18.1 | 1.27 (d, 6.5) |
| 3′ | 78.1 | 3.31 (dd, 8.5, 9.0) | 3-OCH3 | 56.4 | 3.86 (s) |
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 66.1 | 4.21 (dd, 7.8, 11.4) | 5′ | 76.9 | 3.35 (m) |
| 4.25 (dd, 7.8, 11.4) | 6′ | 68.1 | 3.59 (dd, 6.0, 10.8) | ||
| 2 | 121.5 | 5.36 (m) | 3.98 (dd, 1.8, 10.8) | ||
| 3 | 139.0 | — | 1″ | 102.3 | 4.75 (d, 1.2) |
| 4 | 26.0 | 1.76 (s) | 2″ | 72.2 | 3.83 (dd, 1.2, 3.6) |
| 5 | 18.2 | 1.70 (s) | 3″ | 72.4 | 3.65 (dd, 3.6, 9.6) |
| 1′ | 102.5 | 4.26 (d, 7.8) | 4″ | 74.1 | 3.36 (dd, 9.6, 9.6) |
| 2′ | 75.1 | 3.16 (dd, 7.8, 9.0) | 5″ | 69.8 | 3.66 (m) |
| 3′ | 78.2 | 3.33 (dd, 9.0, 9.0) | 6″ | 18.1 | 1.26 (d, 6.0) |
| 4′ | 71.8 | 3.26 (dd, 9.0, 9.6) |
| No. | δC | δH (J in Hz) | No. | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 113.9 | 5.11 (br. d, ca. 11) | 10 | 78.9 | 3.25 (m, overlapped) |
| 5.26 (br. d, ca. 17) | 11 | 73.8 | — | ||
| 2 | 142.8 | 6.05 (dd, 11.0, 17.0) | 12 | 24.9 | 1.12 (s) |
| 3 | 77.2 | — | 13 | 25.9 | 1.16 (s) |
| 4 | 89.9 | 3.53 (dd, 3.5, 9.0) | 14 | 24.5 | 1.27 (s) |
| 5 | 31.2 | 2.16 (ddd, 7.5, 7.5, 15.0) | 15 | 16.5 | 1.60 (s) |
| 2.35 (ddd, 3.5, 7.5, 15.0) | 1′ | 106.2 | 4.45 (d, 8.0) | ||
| 6 | 123.7 | 5.53 (dd, 7.5, 7.5) | 2′ | 75.9 | 3.23 (dd, 8.0, 9.0) |
| 7 | 136.3 | 3.33 (dd, 9.0, 9.0) | 3′ | 78.3 | 3.35 (dd, 9.0, 9.0) |
| 8 | 37.9 | 2.05 (ddd, 8.5, 8.5, 14.0) | 4′ | 71.6 | 3.33 (dd, 9.0, 9.5) |
| 2.24 (ddd, 4.0, 8.5, 14.0) | 5′ | 78.0 | 3.25 (m, overlapped) | ||
| 9 | 30.5 | 1.36 (m) | 6′ | 62.8 | 3.71 (dd, 5.0, 12.0) |
| 1.75 (m) | 3.86 (dd, 2.0, 12.0) |
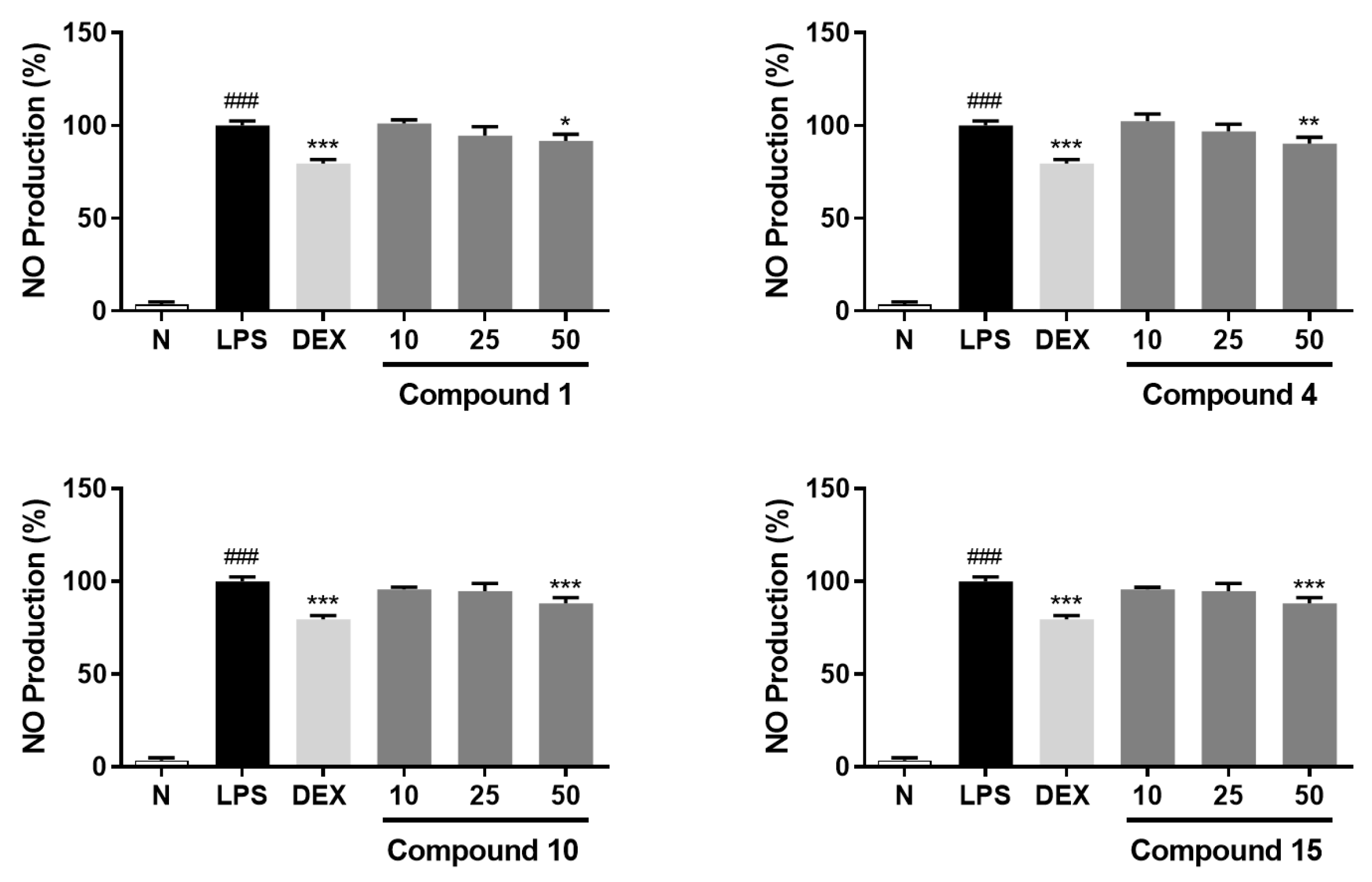
| No. | NRC (%) | No. | NRC (%) | No. | NRC (%) |
|---|---|---|---|---|---|
| Normal | 2.2 ± 0.2 | 5 | 99.8 ± 4.9 | 12 | 95.0 ± 2.3 |
| Control | 100 ± 3.0 | 6 | 93.0 ± 3.9 | 13 | 97.3 ± 3.8 |
| DEX | 76.8 ± 3.8 *** | 7 | 95.0 ± 4.3 | 14 | 98.5 ± 2.2 |
| 1 | 91.7 ± 3.5 * | 8 | 95.6 ± 3.1 | 15 | 91.2 ± 4.9 * |
| 2 | 94.1 ± 3.7 | 9 | 93.9 ± 4.7 | 16 | 94.5 ± 3.4 |
| 3 | 96.7 ± 4.2 | 10 | 88.1 ± 3.2 *** | 17 | 98.1 ± 3.4 |
| 4 | 90.2 ± 3.5 ** | 11 | 93.6 ± 2.8 | 18 | 94.2 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Yu, L.; Cao, H.; Ruan, J.; Li, F.; Wu, L.; Zhang, Y.; Wang, T. Potential Anti-Inflammatory Constituents from Aesculus wilsonii Seeds. Molecules 2024, 29, 1136. https://doi.org/10.3390/molecules29051136
Zhang P, Yu L, Cao H, Ruan J, Li F, Wu L, Zhang Y, Wang T. Potential Anti-Inflammatory Constituents from Aesculus wilsonii Seeds. Molecules. 2024; 29(5):1136. https://doi.org/10.3390/molecules29051136
Chicago/Turabian StyleZhang, Ping, Lequan Yu, Huina Cao, Jingya Ruan, Fei Li, Lijie Wu, Yi Zhang, and Tao Wang. 2024. "Potential Anti-Inflammatory Constituents from Aesculus wilsonii Seeds" Molecules 29, no. 5: 1136. https://doi.org/10.3390/molecules29051136
APA StyleZhang, P., Yu, L., Cao, H., Ruan, J., Li, F., Wu, L., Zhang, Y., & Wang, T. (2024). Potential Anti-Inflammatory Constituents from Aesculus wilsonii Seeds. Molecules, 29(5), 1136. https://doi.org/10.3390/molecules29051136





