Research Progress in Nanoparticle Inhibitors for Crude Oil Asphaltene Deposition
Abstract
1. Introduction
2. Analysis of Nanoparticles Inhibiting Asphaltene Deposition
2.1. Asphaltene Deposition Mechanism
2.1.1. The Existing State and Influencing Factors of Asphaltenes
2.1.2. Accumulation and Deposition of Asphaltenes
2.2. Mechanism of Nano-Asphalt Inhibitor
3. Asphaltene Nano-Inhibitors
3.1. Metal Oxide Nanoparticles
3.2. Organic Nanoparticles
3.3. Inorganic Nonmetal Nanoparticles
3.4. Inhibition Effects of Different Nanoparticles
4. Numerical Simulations of Asphaltene Nano-Inhibitors
5. Field Application of Nano-Asphaltene Deposition Inhibitor
6. Conclusions
- (1)
- The main factors that affect asphaltene precipitation are temperature, pressure, and composition. Temperature and oil composition are the main parameters that affect the stability of asphaltene. At the same time, it is believed that the colloid in the oil component mainly affects the stability of asphaltene. When the resin content is high, asphaltene is not easy to precipitate. When the resin content is low, the asphaltene becomes unstable and easy to precipitate. From the mechanism of action of the inhibitor, the nanoparticles cannot aggregate by adsorbing asphaltene molecules to inhibit precipitation; at the same time, it was shown that the grafted nanoparticles can also promote the dispersion stability of asphaltene molecules in crude oil.
- (2)
- At present, the inhibitors with future development prospects are nano-inhibitors. These inhibitors are mainly divided into metal oxide nanoparticles, organic nanoparticles, and inorganic nonmetal nanoparticles.
- Metal oxide nanoparticles mainly include CaO, Co3O4, and Fe3O4. These nanoparticles have attracted extensive attention due to their superparamagnetism, stability, and easy surface functionalization.
- In the study of organic nanoparticles, the surface modification of nanoparticles is mainly used. A long organic chain containing a polar group is introduced, and it has dual properties by grafting. This not only retains the characteristics of the nanoparticles themselves, but also has a strong polarity and can form a strong interaction or steric hindrance with asphaltenes. At the same time, quantum dot nanoparticles also showed an excellent inhibitory effect on asphaltene deposition.
- In the investigation of inorganic nonmetal nanoparticles, particularly SiO2 nanoparticles, they exhibit not only adsorption capability toward asphaltenes but also enable surface functionalization through grafting, thereby impeding asphaltene deposition by promoting dispersion.
- (3)
- Nanoparticles have a larger specific surface area and adsorption capacity, have better suspension and catalytic performances, and are less likely to cause reservoir damage than chemical agents. Therefore, they have incomparable advantages over conventional organic asphaltene inhibitors in solving the problem of asphaltene deposition. In the face of asphaltene deposition in special reservoirs, such as volatile reservoirs, it also shows a more excellent inhibition effect. However, the existing nanoparticles also show the characteristics of low stability. Due to the strong interaction, the nanoparticles are easy to aggregate, resulting in a larger size and losing the expected effect. Confirming the optimal concentration of nanoparticles will reduce the key to the precipitation and deposition of asphaltenes. Excessive concentration will not only increase the treatment cost but also reduce the ability of nanoparticles to stabilize asphaltenes, resulting in an increase in asphaltene deposition and precipitation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, H.J.; Dong, J. Research on Asphaltene Inhibitors in Halahatang Oilfield. Petrochem. Technol. 2016, 23, 88–90. [Google Scholar]
- Lian, P.Q.; Ma, C.Y.; Gao, M.; Feng, R.Q. Research progress in asphaltene deposition characteristics and control strategies. Sci. Technol. Eng. 2015, 15, 101–107+116. [Google Scholar]
- Muneer, R.; Hashmet, M.R.; Pourafshary, P. Fine Migration Control in Sandstones: Surface Force Analysis and Application of DLVO Theory. ACS Omega 2020, 5, 31624–31639. [Google Scholar] [CrossRef]
- Karambeigi, M.A.; Fallah, N.; Nikazar, M.; Kharrat, R. A novel test method for evaluate asphaltene inhibitor efficiency on damage permeability. Pet. Sci. Technol. 2019, 37, 2146–2149. [Google Scholar] [CrossRef]
- Li, G.T. Shallow talk about the prevention and control measures of asphaltene precipitation in Iran Yada Oilfield. Sci. Technol. Innov. 2018, 6, 3. [Google Scholar]
- Zhang, W.X.; Shu, F.C.; Yin, Y.C.; Yao, P.Y. Evaluation of inhibition effect of asphaltene deposition inhibitor by transmittance method. Petrochem. Appl. 2018, 37, 5. [Google Scholar]
- Gu, Y.C.; Yuan, L.G.; Cao, G.; Yin, C.Y. Study on the mechanism and inhibitor of mixed caking of heavy oil and condensate in Tahe Oilfield Pipeline Technology and Equipment. Pipeline Tech. Equip. 2020, 1, 8–11. [Google Scholar]
- Shu, F.C.; Zhang, W.X. Simulation Experiment on Asphalt Deposition of Iran BA Crude Oil and Inhibition of High Pressure. Sci. Technol. Eng. 2018, 18, 87–92. [Google Scholar] [CrossRef]
- Papa, A.L.; Ali, T.; Benmadi, M. Experimental study on the influences of pressure and flow rates in the deposition of asphaltenes in a sandstone core sample. Fuel 2022, 310, 122420. [Google Scholar]
- Jin, H. Evaluation and Analysis of Asphalt Deposition Inhibitors in Iran Y Oilfield. Chem. Manag. 2016, 27, 57. [Google Scholar]
- Qin, B.; Zhao, L.; Jiang, J.L. Research progress of crude asphaltene deposition inhibition and blockage removal technology. Pet. Refin. Chem. Eng. 2020, 51, 7–15. [Google Scholar]
- Zeng, H.; Tessarolo, N.; Gonzalez, D.; Gramin, P.; Wicking, C.; Pietrobon, M. ”Sticky molecules” on rock surface might play an important role in formation damage due to asphaltene deposition. Fuel 2020, 277, 117983. [Google Scholar] [CrossRef]
- He, H.J. Prediction and Prevention of Asphaltene Deposition in Crude Oil. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2016. [Google Scholar]
- Kuang, J.; Yarbrough, J.; Enayat, S.; Edward, N.; Wang, J.; Vargas, F.M. Evaluation of solvents for in-situ asphaltene deposition remediation. Fuel 2019, 241, 1076–1084. [Google Scholar] [CrossRef]
- Khormali, A.; Sharifov, A.R.; Torba, D.I. The Control of Asphaltene Precipitation in Oil Wells. Pet. Sci. Technol. 2018, 36, 443–449. [Google Scholar] [CrossRef]
- Li, Y.; Di, Q.; Hua, S.; Zhang, J.; Ye, F.; Wang, W. Research progress of reservoirs wettability alteration by using nanofluids for enhancing oil recovery. Chem. Ind. Eng. Prog. 2019, 38, 3612–3620. [Google Scholar]
- Lei, Q.; Luo, J.; Peng, B.; Wang, X.; Xiao, P.; Wang, P.; He, L.; Ding, B.; Geng, X. Mechanism of Expanding Swept Volume by Nano-Sized Oil-Displacement Agent. Pet. Explor. Dev. 2019, 46, 991–997. [Google Scholar] [CrossRef]
- Fletcher, A.; Davis, J. How EOR Can be Transformed by Nanotechnology. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar] [CrossRef]
- Udoh, T.H. Improved Insight on the Application of Nanoparticles in Enhanced Oil Recovery Process. Sci. Afr. 2021, 13, e00873. [Google Scholar] [CrossRef]
- Li, Y.; Di, Q.F.; Hua, S.; Zhang, J.N.; Ye, F.; Wang, W.C. Research progress in improving oil recovery by inversion of reservoir wettability with nanofluids. Progress. Chem. Ind. 2019, 38, 3612–3620. [Google Scholar] [CrossRef]
- Qu, H.Y.; Liu, Q.; Peng, B.; Luo, D.; Liu, S.X.; Liu, K.W. Effect of nanoparticles on CO2 Influence of foam system stability. Pet. Geol. Recovery 2019, 26, 120–126. [Google Scholar] [CrossRef]
- Qin, B.; Zhao, L.; Jiang, J. Advance of Inhibition and Plug Removal Technology for Asphaltene Deposition. Pet. Process. Petrochem. 2020, 51, 7–15. [Google Scholar]
- Alrashidi, H.; Afra, S.; Nasr-El-Din, H.A. Application of Natural Fatty Acids as Asphaltenes Solvents with Inhibition and Dispersion Effects: A Mechanistic Study. J. Pet. Sci. Eng. 2019, 172, 724–730. [Google Scholar] [CrossRef]
- Saeedi Dehaghani, A.H.; Badizad, M.H. Inhibiting Asphaltene Precipitation from Iranian Crude Oil Using Various Dispersants: Experimental Investigation through Viscometry and Thermodynamic Modelling. Fluid. Phase Equilibria 2017, 442, 104–118. [Google Scholar] [CrossRef]
- Rocha Junior, L.C.; Ferreira, M.S.; da Silva Ramos, A.C. Inhibition of Asphaltene Precipitation in Brazilian Crude Oils Using New Oil Soluble Amphiphiles. J. Pet. Sci. Eng. 2006, 51, 26–36. [Google Scholar] [CrossRef]
- Ghosh, B.; Sulemana, N.; Banat, F.; Mathew, N. Ionic Liquid in Stabilizing Asphaltenes during Miscible CO2 Injection in High Pressure Oil Reservoir. J. Pet. Sci. Eng. 2019, 180, 1046–1057. [Google Scholar] [CrossRef]
- Ghanem, A.; Nessim, M.I.; Khalil, N.A.; El-Nagar, R.A. Imidazolium-based ionic liquids as dispersants to improve the stability of asphaltene in Egyptian heavy crude oil. Sci. Rep. 2023, 13, 17158. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, R.A.; Nessim, M.I.; Ismail, D.A.; Mohamed, M.G.; Ghanem, A. Investigation the Effect of Different Ionic Liquids Based-Aryl Imidazole on the Onset Precipitation of Asphaltene. Sci. Rep. 2023, 13, 4054. [Google Scholar] [CrossRef] [PubMed]
- El-hoshoudy, A.N.; Ghanem, A.; Desouky, S.M. Imidazolium-Based Ionic Liquids for Asphaltene Dispersion; Experimental and Computational Studies. J. Mol. Liq. 2021, 324, 114698. [Google Scholar] [CrossRef]
- Guerrero-Martin, C.A.; Montes-Pinzon, D.; Meneses Motta da Silva, M.; Montes-Paez, E.; Guerrero-Martin, L.E.; Salinas-Silva, R.; Camacho-Galindo, S.; Fernandes Lucas, E.; Szklo, A. Asphaltene Precipitation/Deposition Estimation and Inhibition through Nanotechnology: A Comprehensive Review. Energies 2023, 16, 4859. [Google Scholar] [CrossRef]
- Fuentes, J.F.; Montes, D.; Lucas, E.F.; Montes-Páez, E.G.; Szklo, A.; Guerrero-Martin, C.A. Nanotechnology Applied to the Inhibition and Remediation of Formation Damage by Fines Migration and Deposition: A Comprehensive Review. J. Pet. Sci. Eng. 2022, 216, 110767. [Google Scholar] [CrossRef]
- Yen, T.F.; Erdman, J.G.; Pollack, S.S. Investigation of the Structure of Petroleum Asphaltenes by X-ray Diffraction. Anal. Chem. 1961, 33, 1587–1594. [Google Scholar] [CrossRef]
- Murgich, J.; Abanero, J.A.; Strausz, O.P. Molecular Recognition in Aggregates Formed by Asphaltene and Resin Molecules from the Athabasca Oil Sand. Energy Fuels 1999, 13, 278–286. [Google Scholar] [CrossRef]
- Mullins, O.C. The Modified Yen Model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. Chemical Compositions of Improved Model Asphalt Systems for Molecular Simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Huang, L.; You, J.; Li, D. Prevention of asphaltene chokes in Ordovician oil production wells, district X, Tahe oil field. Pet. Geol. Exp. 2016, 38, 52–55. [Google Scholar] [CrossRef]
- Chaisoontornyotin, W.; Haji-Akbari, N.; Fogler, H.S.; Hoepfner, M.P. Combined Asphaltene Aggregation and Deposition Investigation. Energy Fuels 2016, 30, 1979–1986. [Google Scholar] [CrossRef]
- Li, M.X. Literature Review on Asphaltene Deposition. Spec. Oil Gas. Reserv. 1996, 3, 59–62. [Google Scholar]
- Guo, P. Summarization of the research on solid precipitation and gas-liquid-solid phase equilibrium. Spec. Oil Gas. Reserv. 2005, 12, 1–4+8. [Google Scholar]
- Yang, Y.; Chaisoontornyotin, W.; Hoepfner, M.P. Structure of Asphaltenes during Precipitation Investigated by Ultra-Small-Angle X-ray Scattering. Langmuir 2018, 34, 10371–10380. [Google Scholar] [CrossRef] [PubMed]
- Alian, S.S.; Singh, K.; Saidu Mohamed, A.; Ismail, M.Z.; Anwar, M.L. Organic Deposition: From Detection and Laboratory Analysis to Treatment and Removal. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 22–24 October 2013. [Google Scholar]
- Mohammadi, S.; Rashidi, F.; Mousavi-Dehghani, S.A.; Ghazanfari, M.-H. On the Effect of Temperature on Precipitation and Aggregation of Asphaltenes in Light Live Oils. Can. J. Chem. Eng. 2016, 94, 1820–1829. [Google Scholar] [CrossRef]
- Lu, X.; Wang, D.; Wang, Z.; Zheng, J. Research Status of Gum Asphaltene in Crude Oil Pipeline. Stand. Qual. China Pet. Chem. Ind. 2012, 33, 263–264. [Google Scholar]
- Ruan, Z.; Wang, Q. Literature Review of Asphaltene Deposition Mechanism. Contemp. Chem. Ind. 2016, 45, 125–128. [Google Scholar]
- Burke, N.E.; Hobbs, R.E.; Kashou, S.F. Measurement and Modeling of Asphaltene Precipitation (Includes Associated Paper 23831). J. Pet. Technol. 1990, 42, 1440–1446. [Google Scholar] [CrossRef]
- Zanganeh, P.; Ayatollahi, S.; Alamdari, A.; Zolghadr, A.; Dashti, H.; Kord, S. Asphaltene Deposition during CO2 Injection and Pressure Depletion: A Visual Study. Energy Fuels 2012, 26, 1412–1419. [Google Scholar] [CrossRef]
- Pu, W. Asphaltene precipitation in petroleum production. J. Southwest Pet. Inst. 1999, 21, 38–41. [Google Scholar]
- Cruz, A.A.; Amaral, M.; Santos, D.; Palma, A.; Franceschi, E.; Borges, G.R.; Coutinho, J.A.P.; Palácio, J.; Dariva, C. CO2 Influence on Asphaltene Precipitation. J. Supercrit. Fluids 2019, 143, 24–31. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Huang, S.S.; Dong, M. Asphaltene Deposition during CO2 Flooding. SPE Prod. Facil. 1999, 14, 235–245. [Google Scholar] [CrossRef]
- Yen, A.; Yin, Y.R.; Asomaning, S. Evaluating Asphaltene Inhibitors: Laboratory Tests and Field Studies. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar]
- Zou, C.; Qiu, Z.; Zhang, J.; Li, Z.; Wei, H.; Liu, B.; Zhao, J.; Yang, T.; Zhu, S.; Tao, H.; et al. Unconventional Petroleum Sedimentology: A Key to Understanding Unconventional Hydrocarbon Accumulation. Engineering 2022, 18, 62–78. [Google Scholar] [CrossRef]
- Zhou, X. Investigation of the Asphaltene Deposition Distribution in CO2 Flooding of Low Permeability Reservoir. Master’s Thesis, China University of Petroleum, Beijing, China, 2017. [Google Scholar]
- Roux, J.-N.; Broseta, D.; Demé, B. SANS Study of Asphaltene Aggregation: Concentration and Solvent Quality Effects. Langmuir 2001, 17, 5085–5092. [Google Scholar] [CrossRef]
- Andreatta, G.; Bostrom, N.; Mullins, O.C. High-Q Ultrasonic Determination of the Critical Nanoaggregate Concentration of Asphaltenes and the Critical Micelle Concentration of Standard Surfactants. Langmuir 2005, 21, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Song, Y.-Q.; Johnson, D.L.; Mullins, O.C. Critical Nanoaggregate Concentration of Asphaltenes by Direct-Current (DC) Electrical Conductivity. Energy Fuels 2009, 23, 1201–1208. [Google Scholar] [CrossRef]
- Lisitza, N.V.; Freed, D.E.; Sen, P.N.; Song, Y.-Q. Study of Asphaltene Nanoaggregation by Nuclear Magnetic Resonance (NMR). Energy Fuels 2009, 23, 1189–1193. [Google Scholar] [CrossRef]
- Goual, L.; Sedghi, M.; Zeng, H.; Mostowfi, F.; McFarlane, R.; Mullins, O.C. On the Formation and Properties of Asphaltene Nanoaggregates and Clusters by DC-Conductivity and Centrifugation. Fuel 2011, 90, 2480–2490. [Google Scholar] [CrossRef]
- Mostowfi, F.; Indo, K.; Mullins, O.C.; McFarlane, R. Asphaltene Nanoaggregates Studied by Centrifugation. Energy Fuels 2009, 23, 1194–1200. [Google Scholar] [CrossRef]
- Orbulescu, J.; Mullins, O.C.; Leblanc, R.M. Surface Chemistry and Spectroscopy of UG8 Asphaltene Langmuir Film, Part 2. Langmuir 2010, 26, 15265–15271. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, K.L.; Blankenship, G.A.; Kilpatrick, P.K. Solvent Entrainment in and Flocculation of Asphaltenic Aggregates Probed by Small-Angle Neutron Scattering. Langmuir 2006, 22, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Hortal, A.R.; Martínez-Haya, B.; Lobato, M.D.; Pedrosa, J.M.; Lago, S. On the Determination of Molecular Weight Distributions of Asphaltenes and Their Aggregates in Laser Desorption Ionization Experiments. J. Mass. Spectrom. 2006, 41, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Deo, M.D. Near Infrared Spectroscopy to Study Asphaltene Aggregation in Solvents. In Asphaltenes, Heavy Oils, and Petroleomics; Springer: New York, NY, USA, 2007; pp. 469–488. [Google Scholar]
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in Asphaltene Science and the Yen–Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Li, S.; Liu, N.; Chu, Y.-P.; Park, S.J.; Mansoori, G.A.; Guo, T.-M. Measurement and Corresponding States Modeling of Asphaltene Precipitation in Jilin Reservoir Oils. J. Pet. Sci. Eng. 2004, 41, 169–182. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Li, J.; Liu, B.; Shen, Y.; Yan, Y.; Zhang, J. Study on the Asphaltene Precipitation in CO2 Flooding: A Perspective from Molecular Dynamics Simulation. Ind. Eng. Chem. Res. 2018, 57, 1071–1077. [Google Scholar] [CrossRef]
- Salehzadeh, M.; Husein, M.M.; Ghotbi, C.; Taghikhani, V.; Dabir, B. Investigating the Role of Asphaltenes Structure on Their Aggregation and Adsorption/Deposition Behavior. Geoenergy Sci. Eng. 2023, 230, 212204. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, G.; Jin, N.; Zhang, Q.; Gao, C.; Gao, J. Petroleum asphaltene micro-structure analysis and lightening. Chem. Ind. Eng. Prog. 2019, 38, 1147–1159. [Google Scholar]
- Li, N.; Dong, M.; Li, L.; Wang, Z. Research Advances of Chemical Structure Model of Petroleum Asphaltenes. Pet. Asph. 2014, 28, 40–48. [Google Scholar]
- Ye, H.; Liu, Q.; Peng, B.; Luo, D. Inhibition of nanoparticles on asphaltene deposition during CO2 flooding:A review. Oil Gas. Geol. Recovery 2020, 27, 86–96. [Google Scholar] [CrossRef]
- Cortés, F.B.; Mejía, J.M.; Ruiz, M.A.; Benjumea, P.; Riffel, D.B. Sorption of Asphaltenes onto Nanoparticles of Nickel Oxide Supported on Nanoparticulated Silica Gel. Energy Fuels 2012, 26, 1725–1730. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T.; Ma, M. Enhanced Oil Recovery by Flooding with Hydrophilic Nanoparticles. China Particuology 2006, 4, 41–46. [Google Scholar] [CrossRef]
- Mohammadi, M.; Akbari, M.; Fakhroueian, Z.; Bahramian, A.; Azin, R.; Arya, S. Inhibition of Asphaltene Precipitation by TiO2, SiO2, and ZrO2 Nanofluids. Energy Fuels 2011, 25, 3150–3156. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Carbognani, L.; Lopez-Linares, F.; Pereira-Almao, P. Iron Oxide Nanoparticles for Rapid Adsorption and Enhanced Catalytic Oxidation of Thermally Cracked Asphaltenes. Fuel 2012, 95, 257–262. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Balabin, R.M.; Syunyaev, R.Z.; Schmid, T.; Stadler, J.; Lomakina, E.I.; Zenobi, R. Asphaltene Adsorption onto an Iron Surface: Combined Near-Infrared (NIR), Raman, and AFM Study of the Kinetics, Thermodynamics, and Layer Structure. Energy Fuels 2011, 25, 189–196. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of the Particle Size on Asphaltene Adsorption and Catalytic Oxidation onto Alumina Particles. Energy Fuels 2011, 25, 3961–3965. [Google Scholar] [CrossRef]
- Setoodeh, N.; Darvishi, P.; Lashanizadegan, A. A Comparative Study to Evaluate the Performance of Coated Fe3O4 Nanoparticles for Adsorption of Asphaltene from Crude Oil in Bench Scale. J. Dispers. Sci. Technol. 2018, 39, 711–720. [Google Scholar] [CrossRef]
- Vollath, D. Nanomaterials: An introduction to synthesis, properties and application. Environ. Eng. Manag. J. 2008, 7, 865–870. [Google Scholar]
- Lines, M.G. Nanomaterials for Practical Functional Uses. J. Alloys Compd. 2008, 449, 242–245. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Application of Nanotechnology for Enhancing Oil Recovery—A Review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Sviridenko, N.N.; Akimov, A.S. Characteristics of Products of Thermal and Catalytic Cracking of Heavy Oil Asphaltenes under Supercritical Water Conditions. J. Supercrit. Fluids 2023, 192, 105784. [Google Scholar] [CrossRef]
- Urazov, K.K.; Sviridenko, N.N. NiO Based Catalysts Obtained “In-Situ” for Heavy Crude Oil Upgrading: Effect of NiO Precursor on the Catalytic Cracking Products Composition. J. Taiwan Inst. Chem. Eng. 2021, 127, 151–156. [Google Scholar] [CrossRef]
- Li, K.; Zhao, F.; Li, X.; Wang, L.; Zhang, Y. Review on Application of Nanotechnology in Enhancing Oil Recovery. Contemp. Chem. Ind. 2018, 47, 1753–1756. [Google Scholar]
- Fabio Mercado, D.; Akimushkina, L.; Rivera-Quintero, P.A.; Valderrama-Zapata, R.; Guerrero-Amaya, H.; Ballesteros-Rueda, L.M. Comprehensive Analysis of the Transition Metal Oxide Nanomaterials Role as Catalysts in the Low-Temperature Oxidation of Adsorbed nC7-Asphaltenes. Fuel 2022, 327, 125179. [Google Scholar] [CrossRef]
- Mohammadi, M.; Dadvar, M.; Dabir, B. TiO2/SiO2 Nanofluids as Novel Inhibitors for the Stability of Asphaltene Particles in Crude Oil: Mechanistic Understanding, Screening, Modeling, and Optimization. J. Mol. Liq. 2017, 238, 326–340. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Fan, W.; Zhang, X.; Lv, Q. Nanoparticles for Inhibition of Asphaltenes Deposition during CO2 Flooding. Ind. Eng. Chem. Res. 2016, 55, 6723–6733. [Google Scholar] [CrossRef]
- Shojaati, F.; Riazi, M.; Mousavi, S.H.; Derikvand, Z. Experimental Investigation of the Inhibitory Behavior of Metal Oxides Nanoparticles on Asphaltene Precipitation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 99–110. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, Y.; Malayeri, M.R.; Riazi, M.; Parsaei, R. Impact of Fe3O4 Nanoparticles on Asphaltene Precipitation during CO2 Injection. J. Nat. Gas. Sci. Eng. 2015, 22, 227–234. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative Oxidation of Adsorbed Asphaltenes onto Transition Metal Oxide Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Sedighi, M.; Mohammadi, M.; Sedighi, M. Green SAPO-5 supported NiO nanoparticles as a novel adsorbent for removal of petroleum asphaltenes: Financial assessment. J. Pet. Sci. Eng. 2018, 171, 1433–1442. [Google Scholar] [CrossRef]
- Sedighi, M.; Mohammadi, M.; Sedighi, M.; Ghasemi, M. Biobased cadaverine as a green template in the synthesis of NiO/ZSM-5 nanocomposites for removal of petroleum asphaltenes: Financial analysis, isotherms, and kinetics study. Energy Fuels 2018, 32, 7412–7422. [Google Scholar] [CrossRef]
- Setoodeh, N.; Darvishi, P.; Esmaeilzadeh, F. Adsorption of Asphaltene from Crude Oil by Applying Polythiophene Coating on Fe3O4 Nanoparticles. J. Dispers. Sci. Technol. 2018, 39, 578–588. [Google Scholar] [CrossRef]
- Bagherpour, S.; Riazi, M.; Riazi, M.; Cortés, F.B.; Mousavi, S.H. Investigating the Performance of Carboxylate-Alumoxane Nanoparticles as a Novel Chemically Functionalized Inhibitor on Asphaltene Precipitation. ACS Omega 2020, 5, 16149–16164. [Google Scholar] [CrossRef]
- Tazikeh, S.; Sayyad Amin, J.; Zendehboudi, S.; Dejam, M.; Chatzis, I. Bi-Fractal and Bi-Gaussian Theories to Evaluate Impact of Polythiophene-Coated Fe3O4 Nanoparticles on Asphaltene Precipitation and Surface Topography. Fuel 2020, 272, 117535. [Google Scholar] [CrossRef]
- Rezvani, H.; Kazemzadeh, Y.; Sharifi, M.; Riazi, M.; Shojaei, S. A New Insight into Fe3O4-Based Nanocomposites for Adsorption of Asphaltene at the Oil/Water Interface: An Experimental Interfacial Study. J. Pet. Sci. Eng. 2019, 177, 786–797. [Google Scholar] [CrossRef]
- Hassanpour, S.; Malayeri, M.R.; Riazi, M. Utilization of Co3O4 Nanoparticles for Reducing Precipitation of Asphaltene during CO2 Injection. J. Nat. Gas. Sci. Eng. 2016, 31, 39–47. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, X.; Zhang, Z.; Yuan, X.; Zhou, L.; Zheng, Z.; Farhadian, A.; Hu, J. Asphaltene Adsorption of Co3O4 Nanoparticles Modified by SiO2 Film. Appl. Surf. Sci. 2022, 602, 154267. [Google Scholar] [CrossRef]
- Gandomkar, A.; Reza Nasriani, H. The Role of Direct Asphaltene Inhibitors on Asphaltene Stabilization during Gas Injection. Fuel 2020, 282, 118827. [Google Scholar] [CrossRef]
- Induchoodan, G.; Jansson, H.; Swenson, J. Influence of Graphene Oxide on Asphaltene Nanoaggregates. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127614. [Google Scholar] [CrossRef]
- Thao, V.N.; Zhang, Z.Q.; Wang, J.X.; Aparna, S.; Yogesh, K.; Tanguy, T.; Sibani, L.B. Inhibiting Asphaltene Deposition Using Polymer-Functionalized Nanoparticles in Microfluidic Porous Media. Energy Fuels. 2023, 37, 19461–19471. [Google Scholar] [CrossRef]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2013, 46, 171–180. [Google Scholar] [CrossRef]
- Dan, Y.; Ping, W.; Li, J.Y.; Jesse, L.Q.; Ya, P.S. Carbon “quantum” dots for bioapplications. Exp. Biol. Med. 2022, 247, 300–309. [Google Scholar]
- Wu, Y.; Cao, M.; Zhao, Q.; Wu, X.; Guo, F.; Tang, L.; Tan, X.; Wu, W.; Shi, Y.; Dai, C. Novel High-Hydrophilic Carbon Dots from Petroleum Coke for Boosting Injection Pressure Reduction and Enhancing Oil Recovery. Carbon 2021, 184, 186–194. [Google Scholar] [CrossRef]
- Zhao, M.; Song, X.; Lv, W.; Wu, Y.; Dai, C. The Preparation and Spontaneous Imbibition of Carbon-Based Nanofluid for Enhanced Oil Recovery in Tight Reservoirs. J. Mol. Liq. 2020, 313, 113564. [Google Scholar] [CrossRef]
- Baragau, I.-A.; Lu, Z.; Power, N.P.; Morgan, D.J.; Bowen, J.; Diaz, P.; Kellici, S. Continuous Hydrothermal Flow Synthesis of S-Functionalised Carbon Quantum Dots for Enhanced Oil Recovery. Chem. Eng. J. 2021, 405, 126631. [Google Scholar] [CrossRef]
- Mahmoudi Alemi, F.; Mohammadi, S.; Mousavi Dehghani, S.A.; Rashidi, A.; Hosseinpour, N.; Seif, A. Experimental and DFT Studies on the Effect of Carbon Nanoparticles on Asphaltene Precipitation and Aggregation Phenomena. Chem. Eng. J. 2021, 422, 130030. [Google Scholar] [CrossRef]
- Ye, H. Investigation of Quantum Dot-Based Nano-Fluid System for Inhibiting Asphaltene Deposition during CO2 Flooding. Master’s Thesis, China University of Petroleum, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.R.; Sharifi, M. Stabilizing Silica Nanoparticles in High Saline Water by Using Polyvinylpyrrolidone for Reduction of Asphaltene Precipitation Damage under Dynamic Condition. Chin. J. Chem. Eng. 2019, 27, 1021–1029. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Sharifi, M.; Riazi, M.; Rezvani, H.; Tabaei, M. Potential Effects of Metal Oxide/SiO2 Nanocomposites in EOR Processes at Different Pressures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 372–384. [Google Scholar] [CrossRef]
- Lu, N.; Dong, X.; Chen, Z.; Liu, H.; Zheng, W.; Zhang, B. Effect of solvent on the adsorption behavior of asphaltene on silica surface: A molecular dynamic simulation study. J. Pet. Sci. Eng. 2022, 212, 110212. [Google Scholar] [CrossRef]
- Hosseini-Dastgerdi, Z.; Maleki, A.; Elyassi, E.; Rashidi, A. Silica/Polyacrylamide Nanocomposite for Inhibition of Asphaltene Precipitation from Unstable Crude Oils. Pet. Sci. Technol. 2022, 42, 250–268. [Google Scholar] [CrossRef]
- Maleki, A.; Hosseini-Dastgerdi, Z.; Rashidi, A. Effect of Nanoparticle Modified Polyacrylamide on Wax Deposition, Crystallization and Flow Behavior of Light and Heavy Crude Oils. J. Dispers. Sci. Technol. 2023, 44, 1226–1236. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Ruiz, M.A.; Pereira-Almao, P.; Cortés, F.B. Nanoparticles for Inhibition of Asphaltenes Damage: Adsorption Study and Displacement Test on Porous Media. Energy Fuels 2013, 27, 2899–2907. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene Adsorption onto Alumina Nanoparticles: Kinetics and Thermodynamic Studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Ezeonyeka, N.L.; Hemmati-Sarapardeh, A.; Husein, M.M. Asphaltenes Adsorption onto Metal Oxide Nanoparticles: A Critical Evaluation of Measurement Techniques. Energy Fuels 2018, 32, 2213–2223. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal Oxide Nanoparticles for Asphaltene Adsorption and Oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Abu Tarboush, B.J.; Husein, M.M. Dispersed Fe2O3 Nanoparticles Preparation in Heavy Oil and Their Uptake of Asphaltenes. Fuel Process. Technol. 2015, 113, 120–127. [Google Scholar] [CrossRef]
- Bai, Y.; Sui, H.; Liu, X.; He, L.; Li, X.; Thormann, E. Effects of the N, O, and S Heteroatoms on the Adsorption and Desorption of Asphaltenes on Silica Surface: A Molecular Dynamics Simulation. Fuel 2019, 240, 252–261. [Google Scholar] [CrossRef]
- Mohammed, S.; Gadikota, G. The Role of Calcite and Silica Interfaces on the Aggregation and Transport of Asphaltenes in Confinement. J. Mol. Liq. 2019, 247, 792–800. [Google Scholar] [CrossRef]
- Nassar, N.N.; Betancur, S.; Acevedo, S.; Franco, C.A.; Cortés, F.B. Development of a Population Balance Model to Describe the Influence of Shear and Nanoparticles on the Aggregation and Fragmentation of Asphaltene Aggregates. Ind. Eng. Chem. Res. 2015, 54, 8201–8211. [Google Scholar] [CrossRef]
- Varamesh, A.; Hosseinpour, N. Prediction of Asphaltene Precipitation in Reservoir Model Oils in the Presence of Fe3O4 and NiO Nanoparticles by Cubic Plus Association Equation of State. Ind. Eng. Chem. Res. 2019, 58, 4293–4302. [Google Scholar] [CrossRef]
- Madhi, M.; Bemani, A.; Daryasafar, A.; Khosravi Nikou, M.R. Experimental and Modeling Studies of the Effects of Different Nanoparticles on Asphaltene Adsorption. Pet. Sci. Technol. 2017, 35, 242–248. [Google Scholar] [CrossRef]
- Zabala Romero, R.D.; Acuna, H.M.; Cortes, F.; Patino, J.E.; Cespedes Chavarro, C.; Mora, E.; Botero, O.F.; Guarin, L. Application and Evaluation of a NanoFluid Containing NanoParticles for Asphaltenes Inhibition in Well CPSXL4. In Proceedings of the OTC Brasil, Rio de Janeiro, Brazil, 29–31 October 2013. [Google Scholar]
- Meneses, M.A.; Zabala, R.; Rodríguez, E.; Cortés, F.B. Asphaltenes Inhibition by Using New Generation of Nanofluids in Tenay Field (Russian). In Proceedings of the SPE Russian Petroleum Technology Conference and Exhibition, Moscow, Russia, 24–26 January 2016. [Google Scholar]

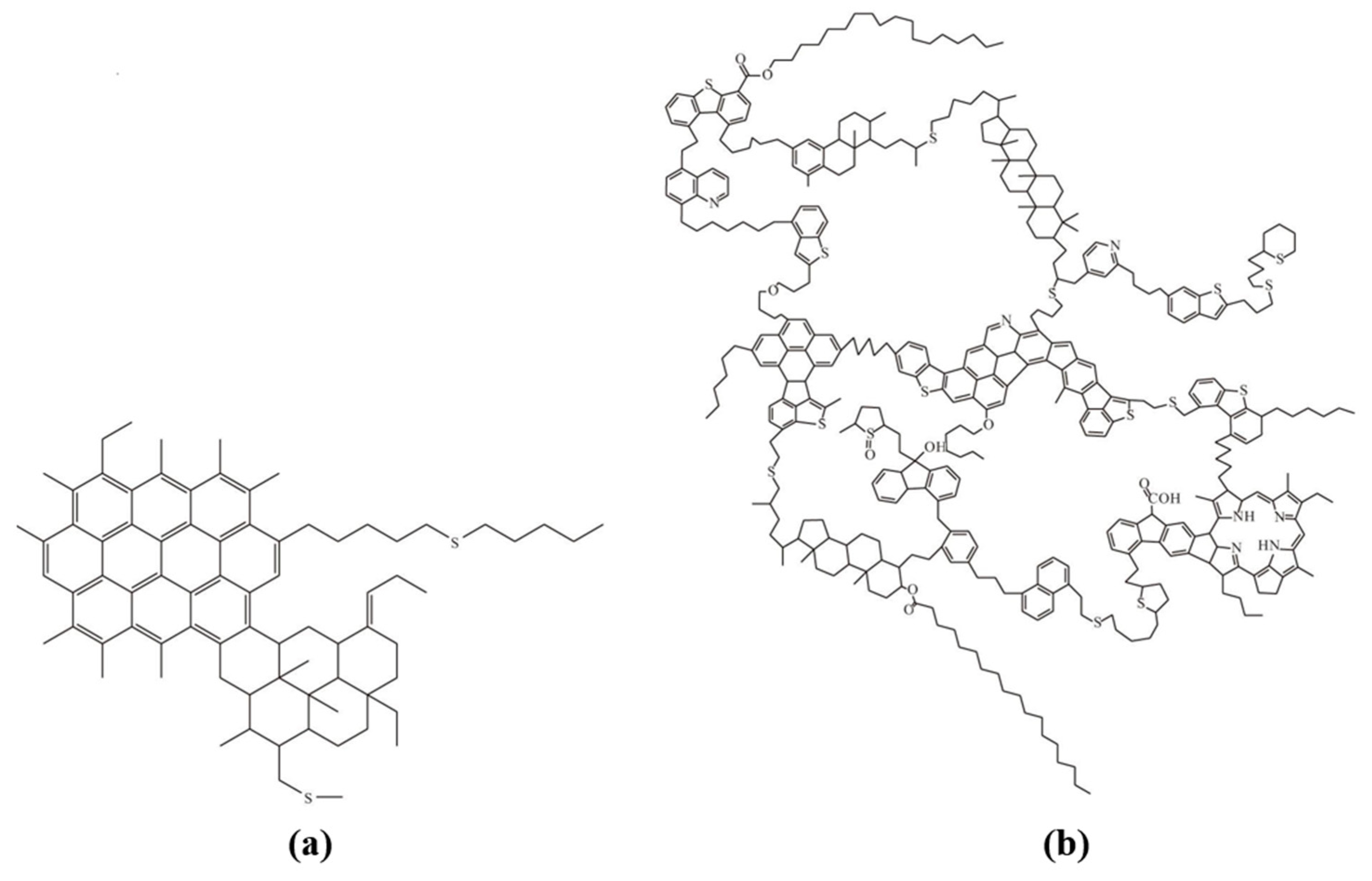

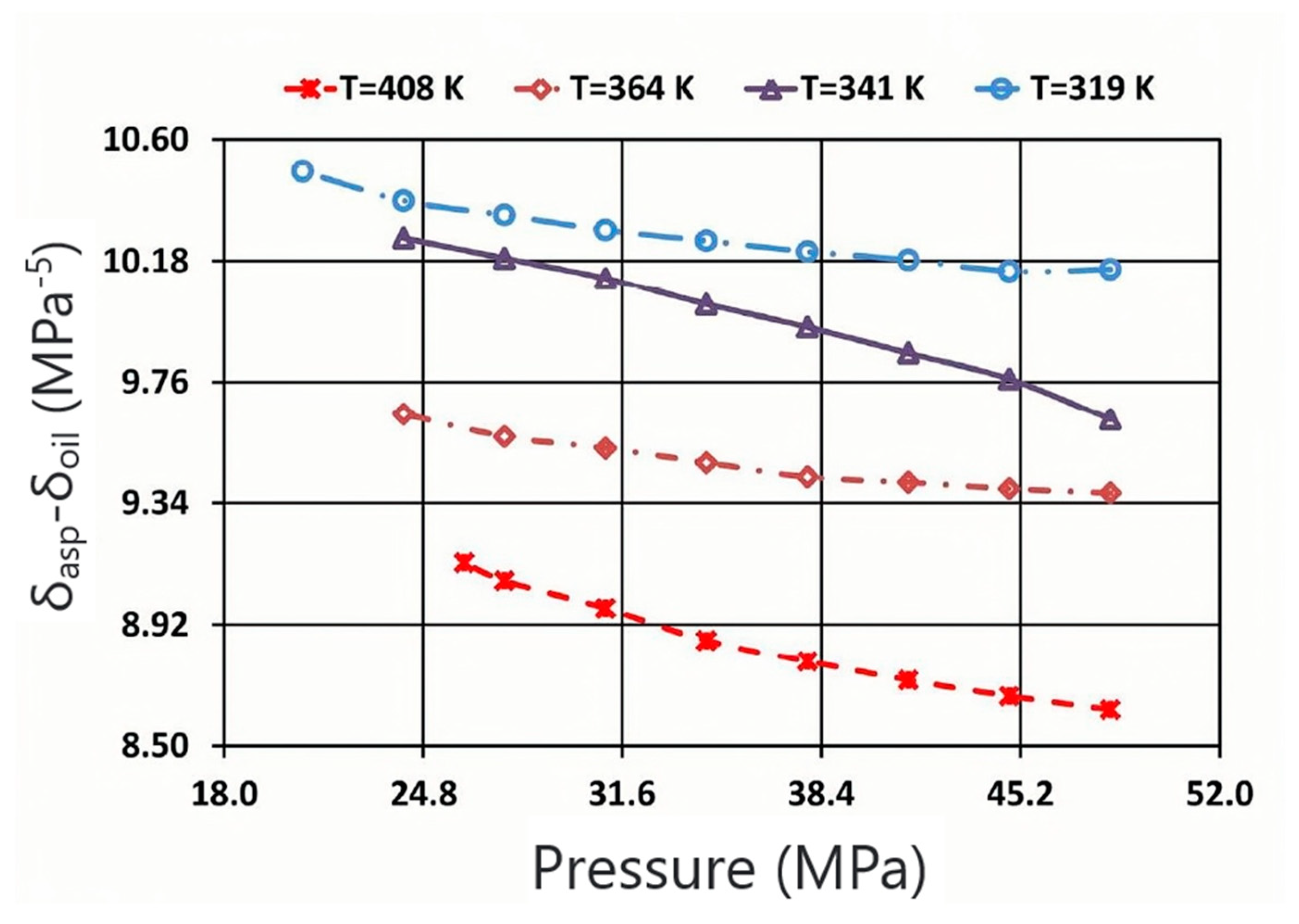

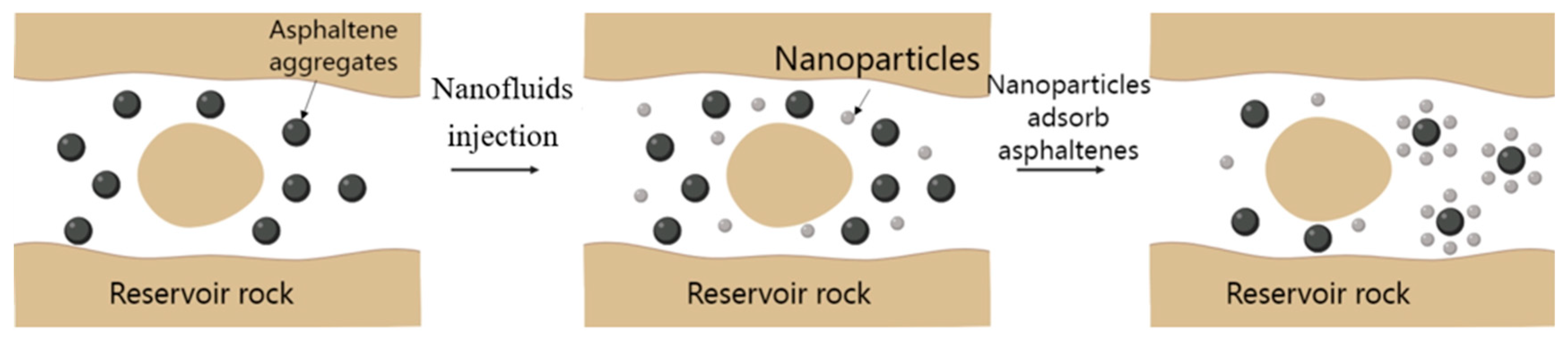
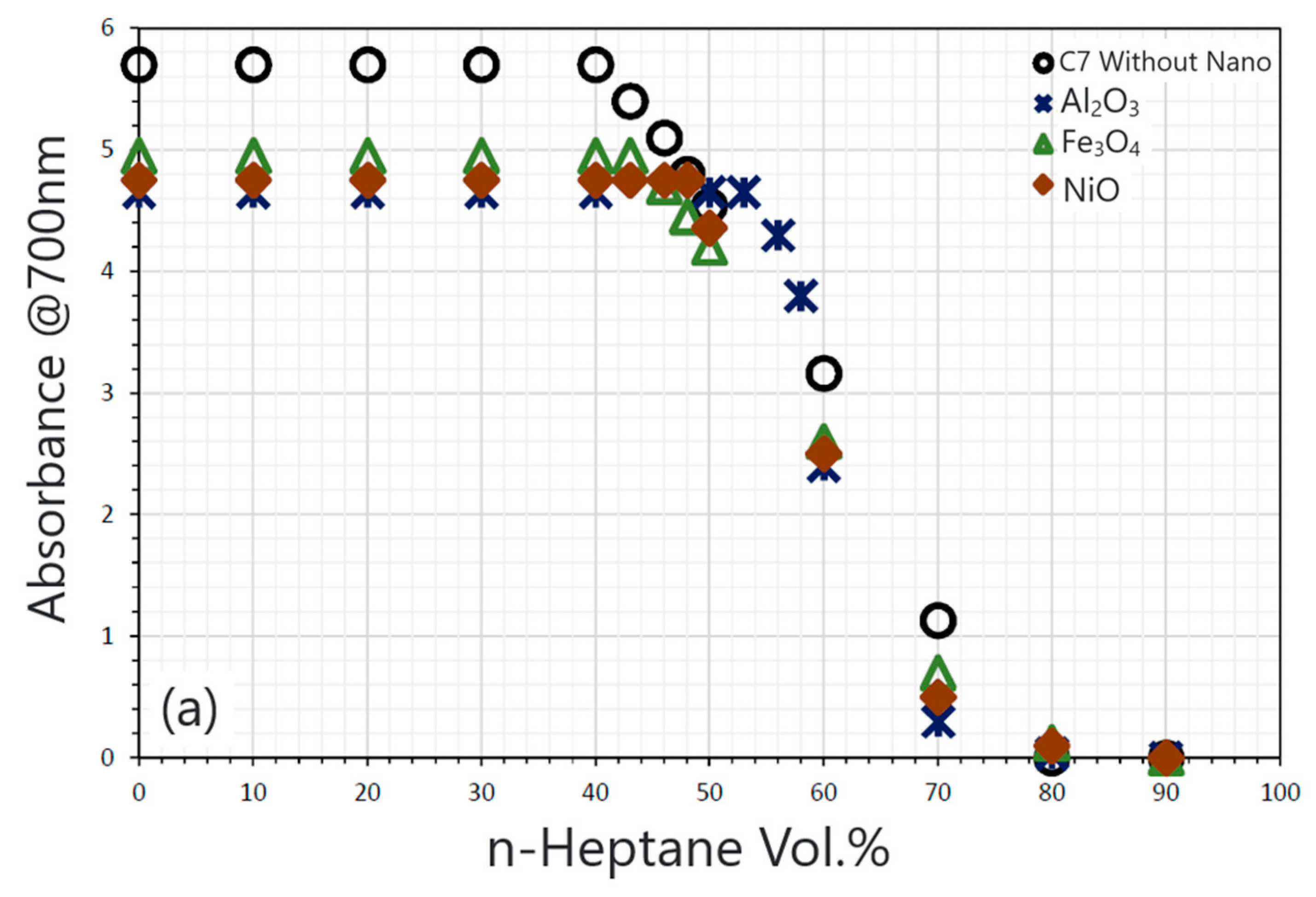
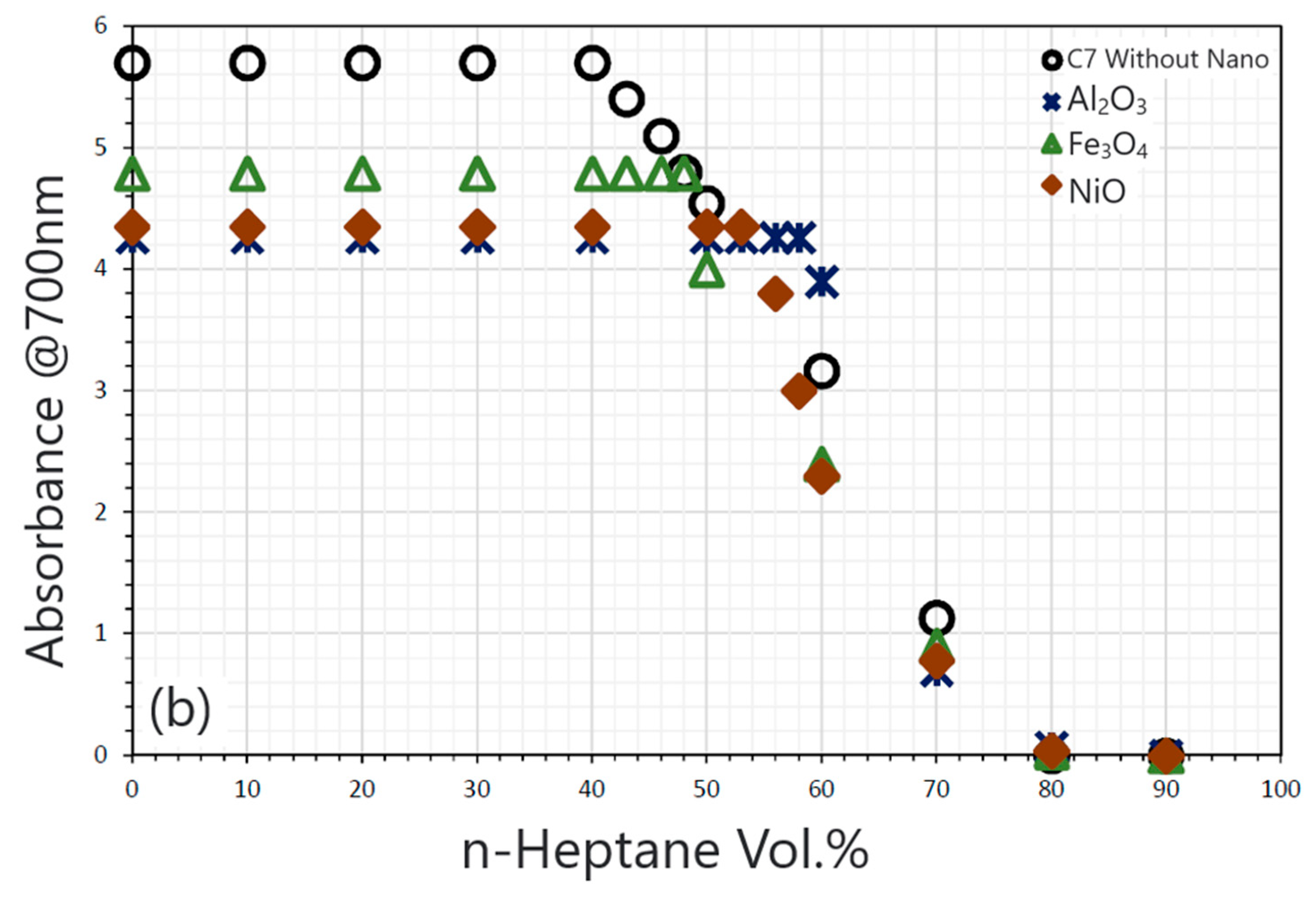
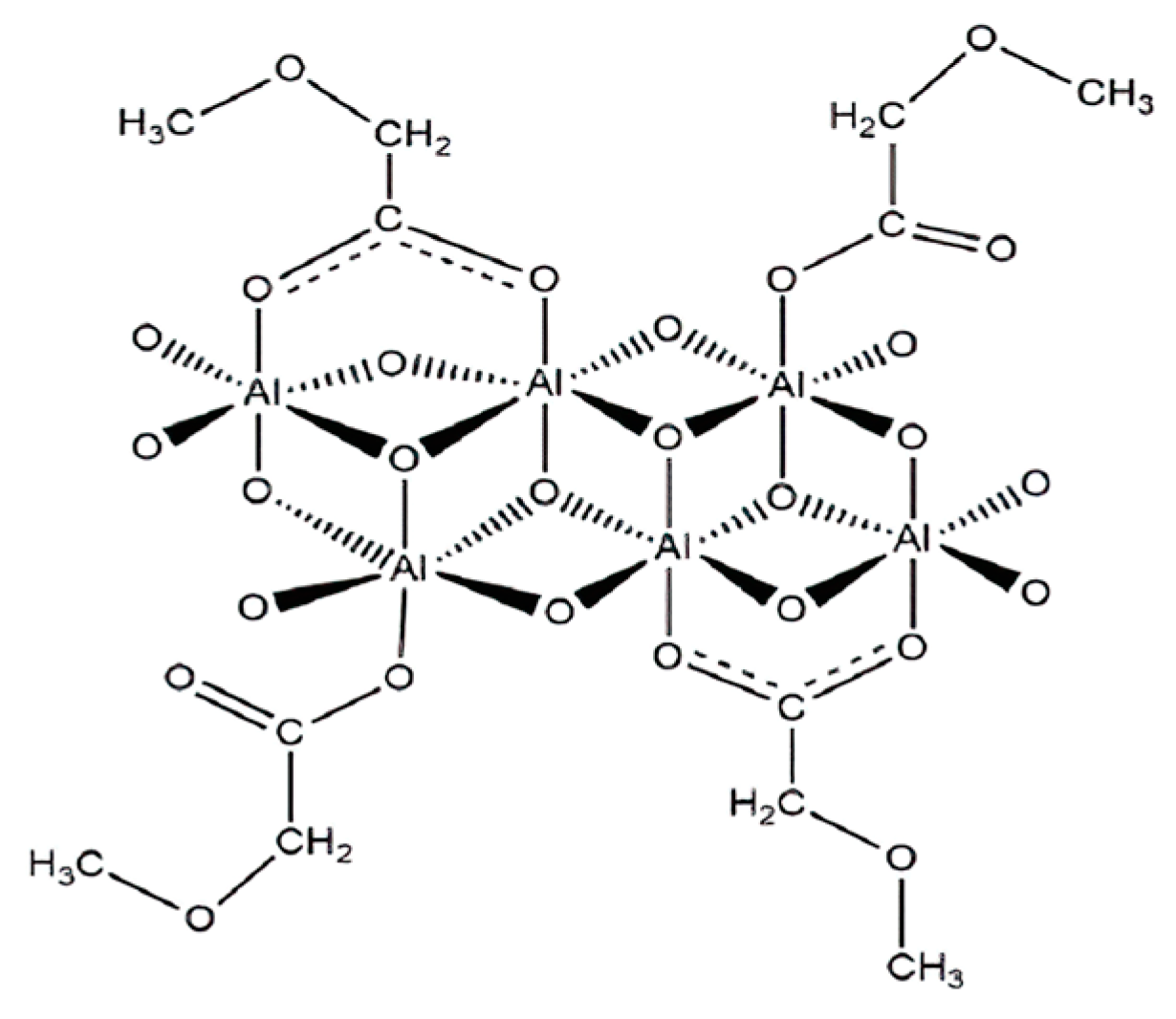
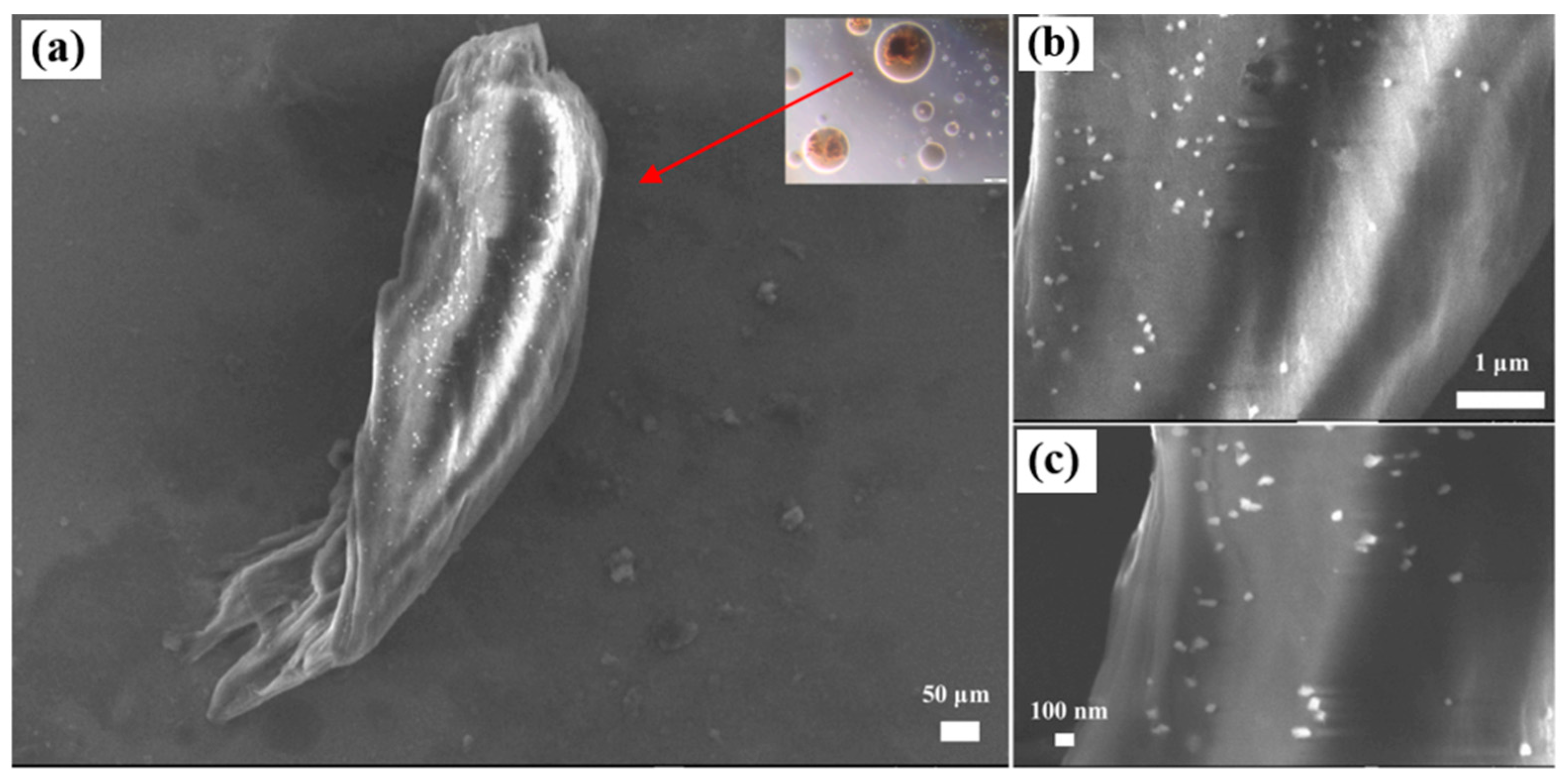

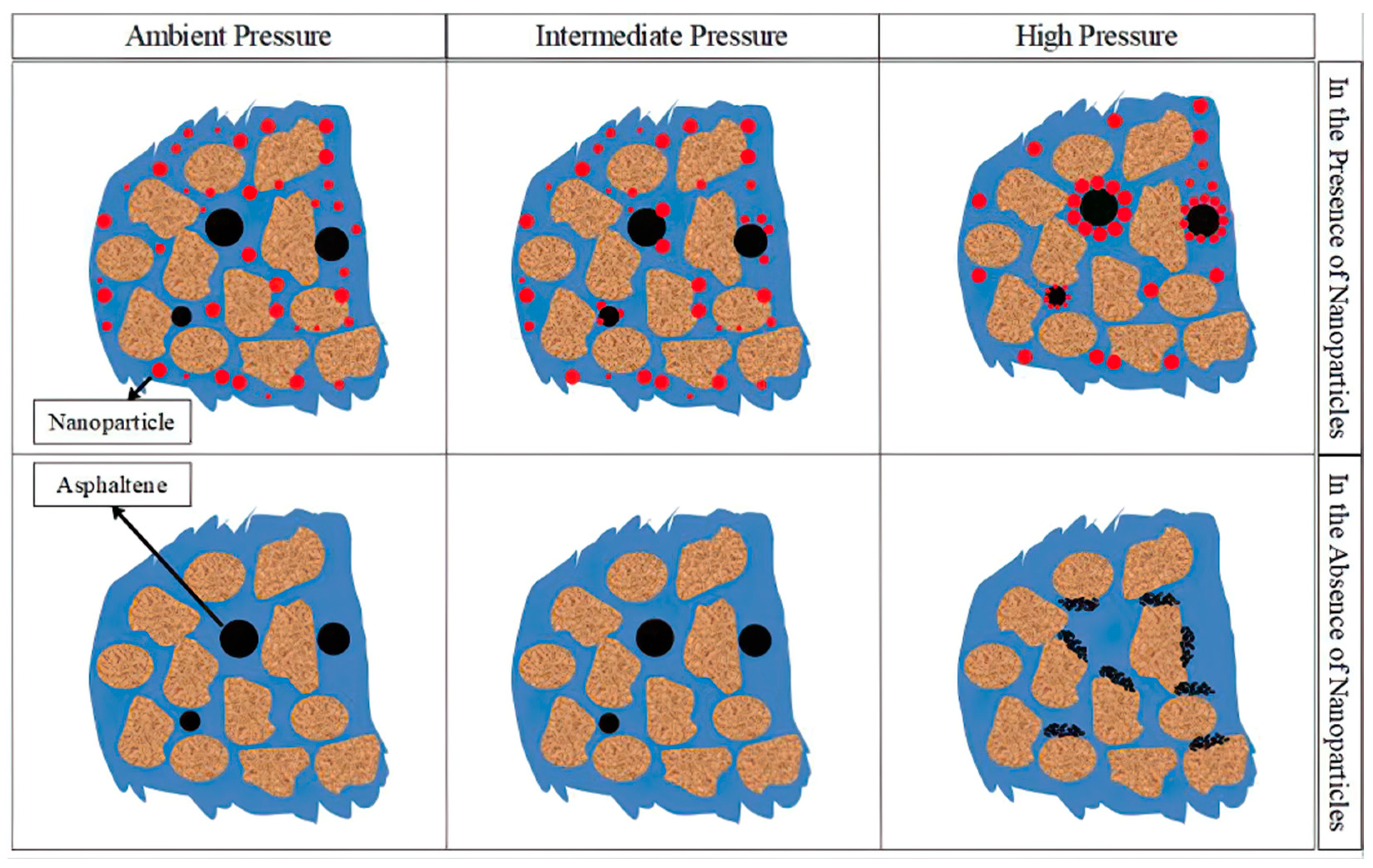
| Nanoparticles | Measured Size (nm) | Specific Surface Area (m2/g) | Chemical Nature |
|---|---|---|---|
| Fe3O4 | 15–20 | 81.98 | Amphoteric |
| γ-Al2O3 | 20 | >138 | Acidic |
| NiO | 10–20 | 50–100 | Acidic |
| Type of Asphaltene | Nanoparticle wt% | Slope of the 1st Region | Slope of the 2nd Region | Ratio of the IFT Slopes of the 2nd to the 1st Regions |
|---|---|---|---|---|
| B-Oil (5 wt%) | 0 | 2.05 | 0.45 | 21.95% |
| 0.5 | 2.03 | 0.83 | 40.89% | |
| N-Oil (5 wt%) | 0 | 2.12 | 0.43 | 20.28% |
| 0.5 | 2.18 | 0.61 | 27.98% |
| DAI | Concentration (ppm) | Asphaltene Precipitation Static Test (wt%) |
|---|---|---|
| Pure CO2 | 5.8 | |
| GO | 50 | 4.0 |
| 100 | 3.1 | |
| 200 | 2.5 | |
| 300 | 2.4 | |
| TiO2 | 500 | 5.0 |
| 1000 | 4.2 | |
| 2000 | 3.8 | |
| 3000 | 3.7 | |
| SiO2 | 500 | 4.8 |
| 1000 | 4.0 | |
| 2000 | 3.5 | |
| 3000 | 3.4 | |
| MgO | 500 | 4.5 |
| 1000 | 3.8 | |
| 2000 | 3.5 | |
| 3000 | 3.5 |
| Nanoparticles | Particles Sources | Particle Size | Adsorption Model | BET Surface Area | Asphaltene Adsorption Capacity | Other Remarks | Reference |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | Commercial | <50 | Langmuir | - | The adsorption capacities of the initial concentrations of 100, 500, and 1000 mg/L were 8.8, 44.6, and 68.0 mg/g. | The absorption of γ-Al2O3 by asphaltenes was spontaneous and exothermic. | [116] |
| Al2O3 | Aladdin Reagents Co. Ltd. (Shanghai, China) | <50 | Consistent with the description of type III isotherms with low adsorbate–adsorbent affinity | 205.7 | The adsorption capacities of the initial concentrations of 500, 1000, and 1500 mg/L were 2965, 6368, and 7857 (mg/m2), respectively. | [87] | |
| 27 ± 1 | Langmuir | 156 | Qm (mg/m2) values for Al2O3 at 410, 550, 700 K, and TGA were 1.4, 1.8, 2.4, and 1.7, respectively. | The shape of Al2O3 was a rod. | [117] | ||
| Alumina | Nano-alumina: 48 ± 3 nm, micro-alumina: <200 μm | Langmuir (nano), Freundlich (micro) | Nano-alumina: 39, micro-alumina: 156 | Qmax (mg/m2): nano-alumina (1.73) > nicro-alumina (0.448). | The surface acidities of micro-alumina and nano-alumina were equivalent. | [77] | |
| Fe3O4 | Commercial | 22 ± 1.5 | Langmuir | 43 | The adsorption capacities of VBASP6, VBASP7, VBASP8, and VBASP9 were 0.047, 0.048, 0.085, and 0.197 mmol/g, respectively. | The adsorption rate increased with the decrease in the molecular weight of asphaltene. | [74] |
| 21 ± 2 | Langmuir | 43 | Qm values (mg/m2) for Fe3O4 at 410, 550, 700 K, and TGA were 1.7, 2.2, 2.9, and 2.1, respectively. | [117] | |||
| 20–30 | Langmuir | 43 | Qm (mg/m2): Fe3O4 (1.7). | [118] | |||
| Fe2O3 | In situ prepared Fe2O3, commercial Fe2O3 | Average diameter in nm: In situ prepared Fe2O3: 35 ± 5, commercial Fe2O3: 20–30 | - | Geometrical surface area for in situ prepared Fe2O3: 25 m2/g | Qm values for in situ prepared Fe2O3 and commercial Fe2O3 were 2.6 and 0.9 g asphaltenes/g NPs, respectively. | In situ NPs adsorbed more asphaltene and had more selectivity for asphaltene. | [119] |
| 29 ± 4 | Langmuir | 37 | Qm values (mg/m2) for Fe2O3 at 410, 550, 700 K, and TGA were 1.7, 2.1, 2.7, and 2.6, respectively. | [117] | |||
| NiO/SAPO-5 composite | Prepared by the sol–gel method | 27.5 ± 7.5 | Langmuir at high temperatures of 342 and 353 K, Freundlich at low temperatures of 298 and 328 K | 304 | Qm values at 298, 325, 342, and 353 K were 111.11, 109.42, 104.53, and 91.74 mg/g, respectively. | - | [92] |
| NiO/ZSM-5 nanocomposite | Prepared by the sol–gel method | - | Langmuir at high temperatures of 342 and 353 K, Freundlich at low temperatures of 298 and 325 K | 348 | Qm values at 298, 325, 342, and 353 K were 117.65, 113.64, 111.28, and 100.00 mg/g, respectively. | [93] | |
| Fe3O4/SiO2; Fe3O4/TiO2; Fe3O4/Chitosan | Mean particle size in nm: Fe3O4/SiO2: 30, Fe3O4/TiO2: 40, Fe3O4/chitosan: 40 | Brunauer–Emmett–Teller (BET) | Qm values for Fe3O4/SiO2, Fe3O4/TiO2, and Fe3O4/ chitosan were 76.50, 74.22, and 100.12 mg/g, respectively. | Nanocomposites could better adsorb asphaltene and stabilize water in oil emulsions. | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Yan, C.; Cai, J.; Pan, Y.; Han, Q. Research Progress in Nanoparticle Inhibitors for Crude Oil Asphaltene Deposition. Molecules 2024, 29, 1135. https://doi.org/10.3390/molecules29051135
Yang S, Yan C, Cai J, Pan Y, Han Q. Research Progress in Nanoparticle Inhibitors for Crude Oil Asphaltene Deposition. Molecules. 2024; 29(5):1135. https://doi.org/10.3390/molecules29051135
Chicago/Turabian StyleYang, Shuangchun, Chenhui Yan, Jiatie Cai, Yi Pan, and Qiuju Han. 2024. "Research Progress in Nanoparticle Inhibitors for Crude Oil Asphaltene Deposition" Molecules 29, no. 5: 1135. https://doi.org/10.3390/molecules29051135
APA StyleYang, S., Yan, C., Cai, J., Pan, Y., & Han, Q. (2024). Research Progress in Nanoparticle Inhibitors for Crude Oil Asphaltene Deposition. Molecules, 29(5), 1135. https://doi.org/10.3390/molecules29051135





