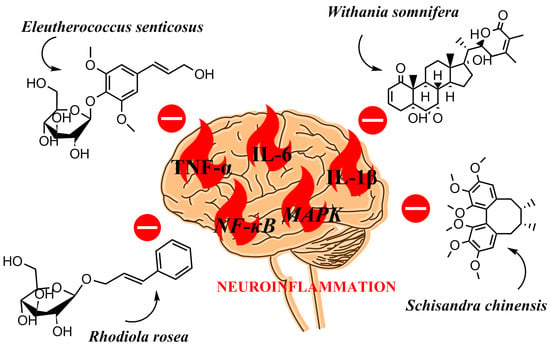
Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review
Abstract
1. Introduction
2. Molecular Basis of Neuroinflammation
3. Anti-Neuroinflammatory Activity of the Selected Adaptogens‘
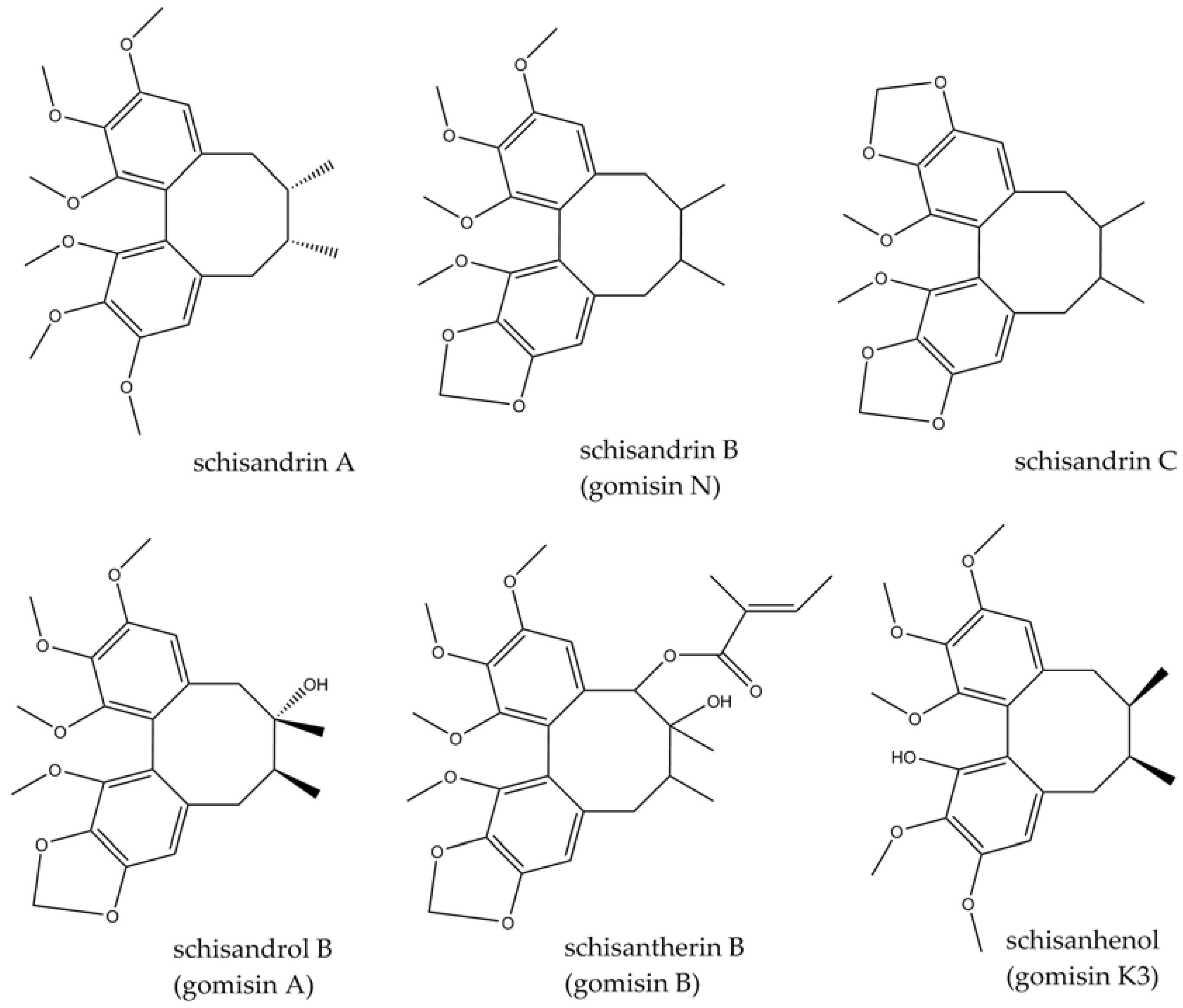
3.1. Schisandra chinensis (Turcz.) Baill.
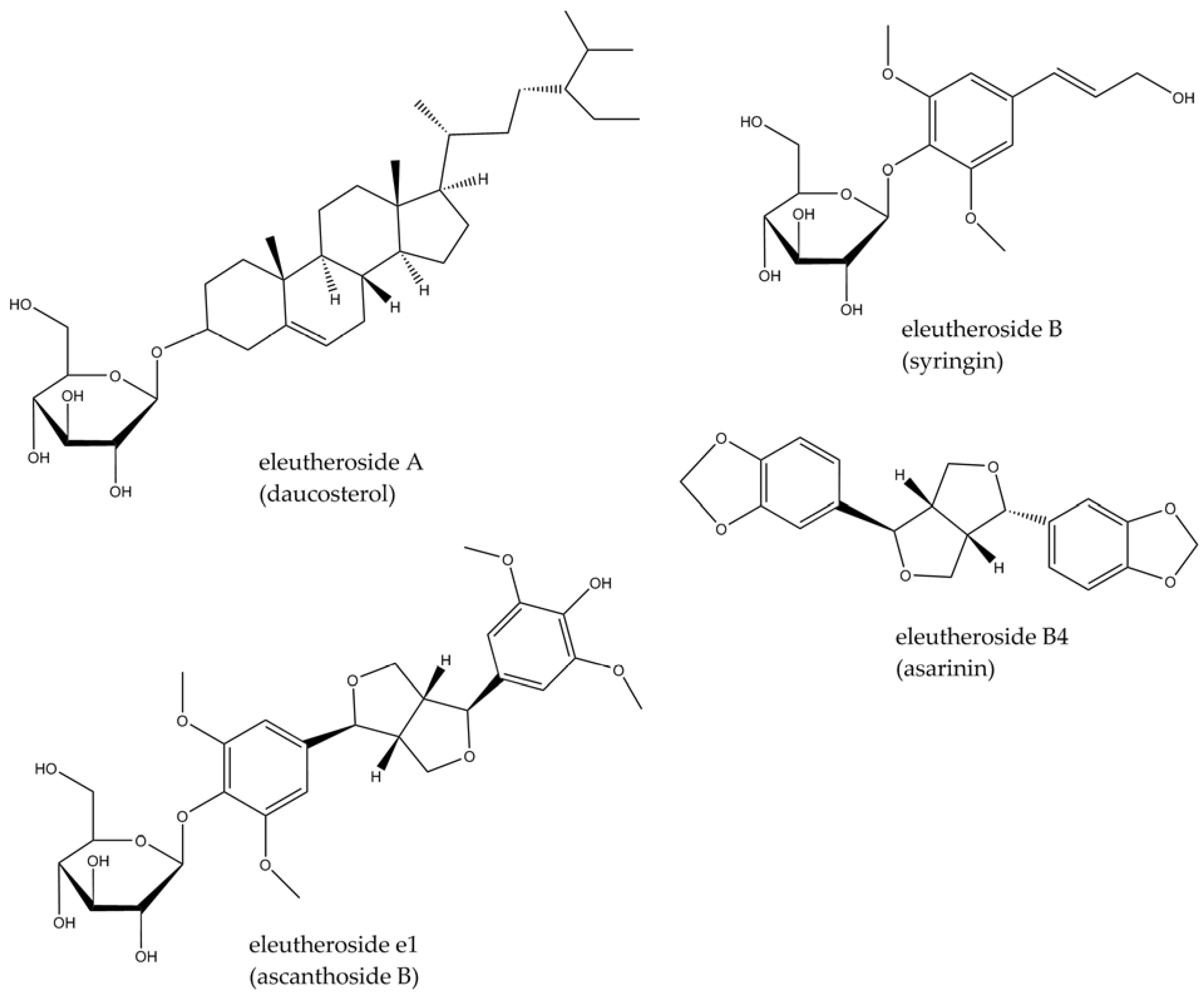
3.2. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.
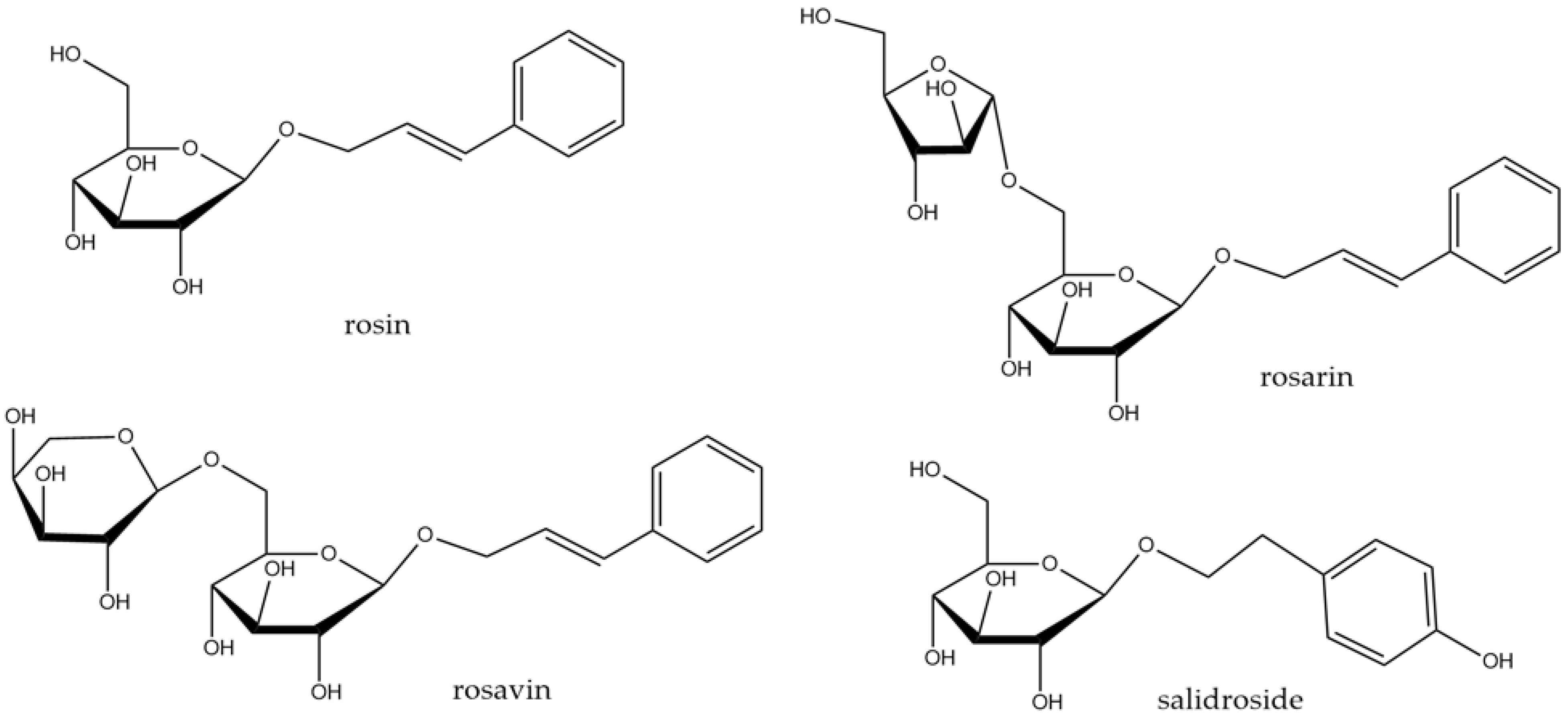
3.3. Rhodiola rosea L.
3.4. Withania somnifera Dunal
4. Discussion
4.1. Adaptogens in Neuroinflammation
4.2. Limitations of Studies
4.3. Safety of Adaptogens
5. Conclusions
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AKT | protein kinase B (PKB) |
| AP-1 | activator protein-1 |
| ATP | adenosine triphosphate |
| BDNF | brain-derived neurotrophic factor |
| COX | cyclooxygenase |
| CNS | central nervous system |
| ERK | extracellular signal-regulated kinase |
| GDNF | glial-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GSK-3 | glycogen synthase kinase-3 |
| HD | Huntington’s disease |
| HO-1 | hemoxygenase-1 |
| IFN-γ | interferon-γ |
| IL | interleukin |
| iNOS | inducible NO synthase |
| JAK/STAT | janus kinase/signal transducer and activator of transcription |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| M2 | anti-inflammatory microglial phenotype |
| MCP-1 | monocyte chemoattractant protein-1 |
| MS | multiple sclerosis |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP | nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain, |
| NO | nitric oxide |
| NOS | NO synthase |
| NPY | neuropeptide Y |
| Nrf-2 | nuclear factor erythroid 2-related factor 2 |
| PGE2 | prostaglandin E2 |
| PI3K/AKT | phosphatidylinositol-3 kinase/protein kinase B |
| PD | Parkinson’s disease |
| PKB | protein kinase B (AKT) |
| PPAR-γ | proliferator-activated receptor γ |
| PRR | pattern recognition receptor |
| TGF-β | transforming growth factor β |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor-α |
| TNFR | tumor necrosis factor receptor |
| TRADD | TNFR1 signal transducer |
| TRAF | TNFR-associated factor |
| VEGF | vascular endothelial growth factor |
References
- Winston, D. Adaptogens: Herbs for Strength, Stamina, and Stress Relief; Simon and Schuster: New York, NY, USA, 2019. [Google Scholar]
- Tewari, N.; Verma, L.; Jawaid, T. Adaptogenic agents: A review. Int. J. Biomed. Res. 2011, 5, 285–304. [Google Scholar]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant adaptogens—History and future perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress—Protective activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Effects of anti-inflammatory and adaptogenic herbal extracts on gene expression of eicosanoids signaling pathways in isolated brain cells. Phytomedicine 2019, 60, 152881. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; Exon Publications: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar]
- Bano, D.; Zanetti, F.; Mende, Y.; Nicotera, P. Neurodegenerative processes in Huntington’s disease. Cell Death Dis. 2011, 2, e228. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. (Yakhteh) 2017, 19, 1–10. [Google Scholar]
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of adult hippocampal neuroplasticity in major depression: Pathogenesis and therapeutic implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef]
- Baldwin, D.; Stein, M.B.; Hermann, R. Generalized Anxiety Disorder in Adults: Epidemiology, Pathogenesis, Clinical Manifestations, Course, Assessment, and Diagnosis; UpToDate: Waltham, MA, USA, 2018; Available online: https://medilib.ir/uptodate/show/496 (accessed on 11 December 2023).
- Jaffe, A.E.; Straub, R.E.; Shin, J.H.; Tao, R.; Gao, Y.; Collado-Torres, L.; Weinberger, D.R. Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat. Neurosci. 2018, 21, 1117–1125. [Google Scholar] [CrossRef]
- Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Forte, M.; Volpe, M.; Rubattu, S. Pathogenesis of ischemic stroke: Role of epigenetic mechanisms. Genes 2020, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Afshari, K.; Dehdashtian, A.; Haddadi, N.S.; Haj-Mirzaian, A.; Iranmehr, A.; Ebrahimi, M.A.; Dehpour, A.R. Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: Introduction of an alternative therapy. Spinal Cord 2018, 56, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, W.N.; Matei, N.; Li, X.; Pang, J.W.; Mo, J.; Zhang, J.H. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxidative Med. Cell. Longev. 2020, 2020, 4717258. [Google Scholar] [CrossRef] [PubMed]
- Dionisie, V.; Filip, G.A.; Manea, M.C.; Manea, M.; Riga, S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: A review of human and rodent research studies. Inflammopharmacology 2021, 29, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, H.; Sakharkar, M.K.; Dhawan, U.; Chidambaram, S.B.; Chandra, R. Neuroinflammation mechanisms and phytotherapeutic intervention: A systematic review. ACS Chem. Neurosci. 2020, 11, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Henney, N.C.; Barreto, G.E.; Sahebkar, A. Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases. Neural Regen. Res. 2022, 17, 1675. [Google Scholar]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Park, G.; Kim, H.G.; Ju, M.S.; Ha, S.K.; Park, Y.; Kim, S.Y.; Oh, M.S. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson’s disease models via anti-neuroinflammation. Acta Pharmacol. Sin. 2013, 34, 1131–1139. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, M.; Yao, W.; Du, K.; He, M.; Jin, X.; Wei, M. Epigallocatechin-3-gallate attenuates microglial inflammation and neurotoxicity by suppressing the activation of canonical and noncanonical inflammasome via TLR4/NF-κB pathway. Mol. Nutr. Food Res. 2019, 63, 1801230. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wu, Q.; Lu, Y.; Nie, J.; Xie, X.; Shi, J. Resveratrol protects cortical neurons against microglia-mediated euroinflammation. Phytother. Res. 2013, 27, 344–349. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.H.; Yao, X.L.; Zhang, Y.; Zhang, S.F.; Hu, J.C. Luteolin could improve cognitive dysfunction by inhibiting neuroinflammation. Neurochem. Res. 2018, 43, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Kim, M.E.; Cho, J.H.; Lee, Y.; Lee, J.; Park, Y.D.; Lee, J.S. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways. Arch. Pharmacal Res. 2019, 42, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Xie, T.; Zhu, D.; Shan, J.; Di, L.; Zheng, X. Suppressive effect of ginsenoside Rg3 against lipopolysaccharide-induced depression-like behavior and neuroinflammation in mice. J. Agric. Food Chem. 2017, 65, 6861–6869. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jing, H.; Yang, H.; Liu, Z.; Guo, H.; Chai, L.; Hu, L. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson׳ s disease. J. Ethnopharmacol. 2015, 164, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Li, F.; An, L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 2016, 38, 426–433. [Google Scholar] [CrossRef]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the neuroinflammation and its association with neurological disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Aïd, S.; Bosetti, F. Targeting cyclooxygenases-1 and-2 in neuroinflammation: Therapeutic implications. Biochimie 2011, 93, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M.O. Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J. Clin. Med. 2019, 8, 680. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Liesz, A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Global Biodiversity Information Facility. Available online: https://www.gbif.org/species/search (accessed on 30 November 2023).
- Wang, X.; Wang, X.; Yao, H.; Shen, C.; Geng, K.; Xie, H. A comprehensive review on Schisandrin and its pharmacological features. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 397, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, L.; Yang, H. Schisandra chinensis fructus and its active ingredients as promising resources for the treatment of neurological diseases. Int. J. Mol. Sci. 2018, 19, 1970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Men, L.; Sun, Y.; Wei, M.; Fan, X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz.(Baill.), a current review. Eur. J. Pharmacol. 2021, 892, 173796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total lignans of Schisandra chinensis ameliorates Aβ1-42-induced neurodegeneration with cognitive impairment in mice and primary mouse neuronal cells. PLoS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, S.J.; Park, T.G.; Rajasekar, S.; Lee, S.J.; Choi, Y.W. Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. Int. Immunopharmacol. 2013, 17, 415–426. [Google Scholar] [CrossRef]
- Joo, S.S.; Yoo, Y.M.; Won, T.J.; Kim, M.J.; Lee, S.G.; Hwang, K.W.; Lee, D.I. Regulation of Inflammatory Repertoires and NF-κB Signal Transduction by DDB, an Active Compound from Schizandra Chinensis Baillon. Immune Netw. 2006, 6, 27–32. [Google Scholar] [CrossRef][Green Version]
- Song, F.; Zeng, K.; Liao, L.; Yu, Q.; Tu, P.; Wang, X. Schizandrin A inhibits microglia-mediated neuroninflammation through inhibiting TRAF6-NF-κB and Jak2-Stat3 signaling pathways. PLoS ONE 2016, 11, e0149991. [Google Scholar] [CrossRef]
- Liu, N.; Zheng, J.X.; Zhuang, Y.S.; Zhou, Z.K.; Zhao, J.H.; Yang, L. Anti-inflammatory effects of schisandrin B on LPS-stimulated BV2 microglia via activating PPAR-γ. Inflammation 2017, 40, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Hiraki, Y.; Nishida, S.; Inatomi, Y.; Yabe, T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J. Pharmacol. Sci. 2016, 132, 138–144. [Google Scholar] [CrossRef]
- Fan, X.; Elkin, K.; Shi, Y.; Zhang, Z.; Cheng, Y.; Gu, J.; Ji, X. Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-κB signaling pathway inhibition. Neurol. Res. 2020, 42, 693–702. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y.H.; Shi, S.S.; Tu, X.K. Schisandrin B inhibits NLRP3 inflammasome pathway and attenuates early brain injury in rats of subarachnoid hemorrhage. Chin. J. Integr. Med. 2022, 28, 594–602. [Google Scholar] [CrossRef]
- Lam, H.Y.P.; Liang, T.R.; Jiang, S.J.; Peng, S.Y. Albendazole-schisandrin B co-therapy on Angiostrongylus cantonensis-induced meningoencephalitis in mice. Biomolecules 2020, 10, 1001. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Guan, H.; Zhou, Y.; Liu, L. Schisandrin inhibits NLRP1 inflammasome-mediated neuronal pyroptosis in mouse models of Alzheimer’s disease. In Neuropsychiatric Disease and Treatment; Taylor & Francis: Abingdon, UK, 2021; pp. 261–268. [Google Scholar]
- Song, L.; Piao, Z.; Yao, L.; Zhang, L.; Lu, Y. Schisandrin ameliorates cognitive deficits, endoplasmic reticulum stress and neuroinflammation in streptozotocin (STZ)-induced Alzheimer’s disease rats. Exp. Anim. 2020, 69, 363–373. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Yang, Y.; Zhang, X.; Shi, K.; Zhang, X.; Jia, Y. Schisandra chinensis Lignans Exert Antidepressant Effects by Promoting BV2 Microglia Polarization toward the M2 Phenotype through the Activation of the Cannabinoid Receptor Type-2–Signal Transducer and Activator of Transcription 6 Pathway. J. Agric. Food Chem. 2022, 70, 14157–14169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, D.; Zhang, L.; Lian, G.; Zhao, S.; Wang, C.; Yang, J. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway. Food Chem. Toxicol. 2014, 63, 119–127. [Google Scholar] [CrossRef]
- Xu, X.L.; Li, S.; Zhang, R.; Le, W.D. Neuroprotective effects of naturally sourced bioactive polysaccharides: An update. Neural Regen. Res. 2022, 17, 1907. [Google Scholar] [PubMed]
- Fu, J.; Li, J.; Sun, Y.; Liu, S.; Song, F.; Liu, Z. An integrated study on the comprehensive mechanism of Schisandra chinensis polysaccharides mitigating Alzheimer’s disease in rats using a UPLC-Q-TOF-MS based serum and urine metabolomics strategy. Food Funct. 2023, 14, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, X.; Ren, F.; Yan, T.; Wu, B.; Bi, K.; Jia, Y. Essential oil of Schisandra chinensis ameliorates cognitive decline in mice by alleviating inflammation. Food Funct. 2019, 10, 5827–5842. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Choi, J.H.; Jang, D.S.; Cho, I.H. Micrandilactone C, a Nortriterpenoid Isolated from Roots of Schisandra chinensis, Ameliorates Huntington’s Disease by Inhibiting Microglial STAT3 Pathways. Cells 2023, 12, 786. [Google Scholar] [CrossRef]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmacol. 2000, 72, 345–393. [Google Scholar]
- Solomonova, E.; Trusov, N.; Nozdrina, T. Opportunities for using of eleutherococcuses fruits as a new food raw material. In Proceedings of the 1st International Symposium Innovations in Life Sciences (ISILS 2019), Belgorod, Russia, 10–11 October 2019; Atlantis Press: Cambridge, MA, USA, 2019; pp. 24–28. [Google Scholar]
- Huang, Y.H.; Li, J.T.; Zan, K.; Wang, J.; Fu, Q. The traditional uses, secondary metabolites, and pharmacology of Eleutherococcus species. Phytochem. Rev. 2022, 21, 1081–1184. [Google Scholar] [CrossRef]
- Li, X.T.; Zhou, J.C.; Zhou, Y.; Ren, Y.S.; Huang, Y.H.; Wang, S.M.; Ge, Y.W. Pharmacological effects of Eleutherococcus senticosus on the neurological disorders. Phytother. Res. 2022, 36, 3490–3504. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Schmiech, M.; El Gaafary, M.; Zhang, X.; Syrovets, T.; Simmet, T. A comparative study on root and bark extracts of Eleutherococcus senticosus and their effects on human macrophages. Phytomedicine 2020, 68, 153181. [Google Scholar] [CrossRef]
- Graczyk, F.; Orzechowska, B.; Franz, D.; Strzemski, M.; Verpoorte, R.; Załuski, D. The intractum from the Eleutherococcus senticosus fruits affects the innate immunity in human leukocytes: From the ethnomedicinal use to contemporary evidence-based research. J. Ethnopharmacol. 2021, 268, 113636. [Google Scholar] [CrossRef]
- Ahmed, S.; Moni, D.A.; Sonawane, K.D.; Paek, K.Y.; Shohael, A.M. A comprehensive in silico exploration of pharmacological properties, bioactivities and COX-2 inhibitory potential of eleutheroside B from Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. J. Biomol. Struct. Dyn. 2021, 39, 6553–6566. [Google Scholar] [PubMed]
- Lee, D.; Park, J.; Yoon, J.; Kim, M.Y.; Choi, H.Y.; Kim, H. Neuroprotective effects of Eleutherococcus senticosus bark on transient global cerebral ischemia in rats. J. Ethnopharmacol. 2012, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, S.; Liu, T.; Wu, J.; Zhang, H.; Sun, Z.; Liu, Z. Mass spectrometry-based serum lipidomics strategy to explore the mechanism of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. Leaves Treat. Ischemic Stroke. Food Funct. 2021, 12, 4519–4534. [Google Scholar]
- Wang, R.; Sun, Y.; Wang, M.; Li, H.; Liu, S.; Liu, Z. Therapeutic effect of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. Leaves Ischemic Stroke Via Microbiota–Gut–Brain Axis. Phytother. Res. 2023, 37, 4801–4818. [Google Scholar]
- Huang, Y.H.; Ding, W.L.; Li, X.T.; Cai, M.T.; Li, H.L.; Yang, Z.Y.; Ge, Y.W. Memory enhancement effect of saponins from Eleutherococcus senticosus leaves and blood–brain barrier-permeated saponins profiling using a pseudotargeted monitoring strategy. Food Funct. 2022, 13, 3603–3620. [Google Scholar] [CrossRef]
- Jagtap, P.N.; Mhetre, O.S.; Malavdkar, P.R. A Review Article on Rhodiola Rosea: An Adaptogen Having Multiple Benefits. Int. J. Pharmacogn 2020, 7, 62–69. [Google Scholar]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic compounds of Rhodiola rosea L. as the potential alternative therapy in the treatment of chronic diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, J.C.; Jang, S.; Kim, J.; Ali, Z.; Khan, I.A.; Oh, S. Anti-inflammatory and neuroprotective effects of constituents isolated from Rhodiola rosea. Evid. Based Complement. Altern. Med. 2013, 2013, 514049. [Google Scholar]
- Borgonetti, V.; Governa, P.; Biagi, M.; Dalia, P.; Corsi, L. Rhodiola rosea L. modulates inflammatory processes in a CRH-activated BV2 cell model. Phytomedicine 2020, 68, 153143. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, Y.; Ma, L.; Ma, X.; Chen, Z.; Chen, H.; Chen, X. Amelioration of experimental autoimmune encephalomyelitis by Rhodiola rosea, a natural adaptogen. Biomed. Pharmacother. 2020, 125, 109960. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, M.; Mao, G.X.; Shu, Q.F.; Liu, X.B.; Liu, X.L. Preclinical evidence and possible mechanisms of Rhodiola rosea L. and its components for ischemic stroke: A systematic review and meta-analysis. Front. Pharmacol. 2021, 12, 736198. [Google Scholar] [CrossRef]
- El Maadawi, Z.M. Conditioned medium derived from salidroside-pretreated mesenchymal stem cell culture ameliorates mouse lipopolysaccharide-induced cerebral neuroinflammation-histological and immunohistochemical study. Int. J. Stem Cells 2017, 10, 60. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, W.; Ying, X.; Xu, L.; Chu, K.; Brown, J.; Hong, G. Salidroside reduces inflammation and brain injury after permanent middle cerebral artery occlusion in rats by regulating PI3K/PKB/Nrf2/NFκB signaling rather than complement C3 activity. Inflammation 2019, 42, 1830–1842. [Google Scholar] [CrossRef]
- Xu, N.; Huang, F.; Jian, C.; Qin, L.; Lu, F.; Wang, Y.; Zhang, Q. Neuroprotective effect of salidroside against central nervous system inflammation-induced cognitive deficits: A pivotal role of sirtuin 1-dependent N rf-2/HO-1/NF-κ B pathway. Phytother. Res. 2019, 33, 1438–1447. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Foo, N.X.; Tan, W.J.; Chan, H.W.; Lim, D.Y.; Yeo, W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2020, 34, 583–590. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on stress and the stress-related neuropsychiatric disorders anxiety, depression, and insomnia. Curr. Neuropharmacol. 2021, 19, 1468. [Google Scholar] [CrossRef] [PubMed]
- Elhadidy, M.E.; Sawie, H.G.; Meguid, N.A.; Khadrawy, Y.A. Protective effect of ashwagandha (Withania somnifera) against neurotoxicity induced by aluminum chloride in rats. Asian Pac. J. Trop. Biomed. 2018, 8, 59–66. [Google Scholar]
- Pandey, A.; Bani, S.; Dutt, P.; Satti, N.K.; Suri, K.A.; Qazi, G.N. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine 2018, 102, 211–221. [Google Scholar] [CrossRef]
- Zhu, J.; Park, S.; Jeong, K.H.; Kim, W.J. Withanolide-A treatment exerts a neuroprotective effect via inhibiting neuroinflammation in the hippocampus after pilocarpine-induced status epilepticus. Epilepsy Res. 2020, 165, 106394. [Google Scholar] [CrossRef] [PubMed]
- Atluri, V.S.R.; Tiwari, S.; Rodriguez, M.; Kaushik, A.; Yndart, A.; Kolishetti, N.; Yatham, M.; Nair, M. Inhibition of Amyloid-Beta production, associated neuroinflammation, and Histone Deacetylase 2-mediated epigenetic modifications prevent neuropathology in Alzheimer’s disease in vitro Model. Front. Aging Neurosci. 2020, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.M.; Eliwa, H.A.; El-Shiekh, R.A.; Al-Mokaddem, A.K.; Hassan, M.; Tawfek, A.M.; El-Maadawy, W.H. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2021, 277, 114141. [Google Scholar] [CrossRef]
- Epuri, V.; Prathap, L.; Reddy, V.; Krishnan, M. Anti oxidative/neuro-inflammation properties of Withania somnifera root extract on rotenone induced stress in rat brain. Bioinformation 2023, 19, 729. [Google Scholar]
- Kaur, T.; Kaur, G. Withania somnifera as a potential candidate to ameliorate high fat diet-induced anxiety and neuroinflammation. J. Neuroinflamm. 2017, 14, 201. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Withania somnifera as a potential anxiolytic and anti-inflammatory candidate against systemic lipopolysaccharide-induced neuroinflammation. Neuromolecular Med. 2018, 20, 343–362. [Google Scholar] [CrossRef]
- Sun, G.Y.; Li, R.; Cui, J.; Hannink, M.; Gu, Z.; Fritsche, K.L.; Simonyi, A. Withania somnifera and its withanolides attenuate oxidative and inflammatory responses and up-regulate antioxidant responses in BV-2 microglial cells. Neuromolecular Med. 2016, 18, 241–252. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Withania somnifera (L.) Dunal ameliorates neurodegeneration and cognitive impairments associated with systemic in-flammation. BMC Complement. Altern. Med. 2019, 19, 217. [Google Scholar] [CrossRef]
- Chengappa, K.R.; Brar, J.S.; Gannon, J.M.; Schlicht, P.J. Adjunctive use of a standardized extract of Withania somnifera (Ashwagandha) to treat symptom exacerbation in schizophrenia: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2018, 79, 22496. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Patel, P.; Rahimian, R.; Phaneuf, D.; Julien, J.P. Withania somnifera reverses transactive response DNA binding protein 43 proteinopathy in a mouse model of amyotrophic lateral sclerosis/frontotemporal lobar degeneration. Neurotherapeutics 2017, 14, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Phaneuf, D.; Julien, J.P. Withaferin-A Treatment Alleviates TAR DNA-Binding Protein-43 Pathology and Improves Cognitive Function in a Mouse Model of FTLD. Neurotherapeutics 2021, 18, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Zhang, T.; Fu, H.; Liu, G.X.; Wang, X.M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur. J. Pharmacol. 2012, 692, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Heufelder, A.; Zimmermann, A. Therapeutic Effects and Safety of Rhodiola rosea Extract WS® 1375 in Subjects with Life-stress Symptoms–Results of an Open-label Study. Phytother. Res. 2012, 26, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.; Yadav, S.S. Safety and clinical effectiveness of Withania somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol. 2020, 255, 112768. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Thomsen, M.; Kelber, O.; Kraft, K. Myths and facts in herbal medicines: Eleutherococcus senticosus (Siberian ginseng) and its contraindication in hypertensive patients. In Botanics: Targets and Therapy; Taylor & Francis: Abingdon, UK, 2014; pp. 27–32. [Google Scholar]
- Kim, D.S.; Baek, H.I.; Ha, K.C.; Cha, Y.S.; Park, S.J. Efficacy and safety of Omija (Schisandra chinensis) extract mixture on the improvement of hyperglycemia: A randomized, double-blind, and placebo-controlled clinical trial. Nutrients 2022, 14, 3159. [Google Scholar] [CrossRef]


| CNS Disorder | Main Pathogenetic Factor | Ref. |
|---|---|---|
| Alzheimer’s disease (AD) | extracellular Aβ amyloid plaques and intracellular hyperphosphorylated τ-protein neurofibrillary tangles; cholinergic neuron loss; neurodegenerative | [9] |
| Parkinson’s disease (PD) | intracellular aggregates of α-synuclein; dopaminergic neuron loss in substantia nigra pars compacta; neurodegenerative | [10] |
| Huntington’s disease(HD) | aggregates of misfolded huntingtin; shrinkage of brain; neuron loss in striatum; neurodegenerative | [11] |
| Multiple sclerosis (MS) | auto-immune-mediated demyelination of neurons; neurodegenerative | [12] |
| Depression | dysregulated neuroplasticity monoamine neurotransmission; neuroendocrinal function | [13] |
| Anxiety disorders | disturbances of neurotransmittency | [14] |
| Schizophrenia | disturbances of neurotransmittency; altered connectivity and neuroplasticity in neurodevelopmental period | [15] |
| Ischemic stroke | occlusion of cerebral artery, e.g., by thrombus with subsequent regional decrease of oxygen to the brain; | [16] |
| Infections | bacteria (e.g., Escherichia coli, Neisseria meningitidis); viruses (e.g., Ebola virus, HIV); protozoa and helminths (e.g., Toxoplasma gondii); fungi (e.g., Cryptococcus neoformans) | [17] |
| CNS cancer | gene alteration; oxidative damage; environmental causes (e.g., diet); infections | [18] |
| Observed Effect | Substance | Assay/Model | Ref. |
|---|---|---|---|
| ↓TNF-α | schisandrin A schisandrin B (gomisin N) schisandrin C schisandrol B (gomisin A) Schisandra lignans SCP-2 Schisandra essential oil E. senticosus extract salidroside R. rosea extract, rosin, rosarin, salidroside R. rosea extract withanone withanolide A W. somnifera extract | BV-2 microglial cells, mouse primary microglia micorglia–neuron cocultures BV-2 microglial cells forced swim test (amygdala, hypothalamus) BV-2 microglial cells N9 microglial cells forced swim test in mice (hippocampal tissue) LPS-injected mice Aβ1-42-induced dementia in rats transient middle cerebral artery occlusion in rats rats with induced ischemic strokeBV-2 microglial cells LPS-injected mice BV-2 cells streptozotocin-induced dementia in rats pilocarpine-induced status epilepticus in mice AlCl3-induced brain damage in rats (hippocamups, cortex) thioacetamice-induced hepatic encephalopathy in mice | [53] [103] [54] [55] [51] [62] [61] [63] [65] [75] [76] [85] [80] [81] [90] [91] [89] [94] |
| ↓IL-1β | schisandrin B schisandrol B (gomisin A) Schisandra lignans Schisandra essential oil E. senticosus extract R. rosea extract, rosin, rosarin, salidroside withanone withanolide A W. somnifera extract | micorglia-neuron cocultures BV-2 microglial cells forced swim test (amygdala, hypothalamus) subarachnoid hemorrhage in rats N9 microglial cells BV-2 microglial cells forced swim test in mice (hippocamp tissue) Aβ1-42-induced dementia in rats rats with induced ischemic stroke BV-2 microglial cells streptozotocin-induced dementia in rats pilocarpine-induced status epilepticus in mice transgenic mice with ALS | [103] [54] [55] [57] [62] [61] [65] [76] [80] [90] [91] [101] |
| ↓IL-6 | schisandrin A schisandrin B schisandrin C schisandrol B (gomisin A) Schisandra lignans E. senticosus extract salidroside R. rosea extract, rosin, rosarin, salidroside R. rosea extract R. rosea extract (standardized for salidroside) withanone W. somnifera extract | BV-2 microglial cells, mouse primary microglia micorglia–neuron cocultures BV-2 microglial cells forced swim test (amygdala, hypothalamus) BV-2 microglial cells N9 microglial cells forced swim test in mice (hippocampal tissue) rats with induced ischemic stroke transient middle cerebral artery occlusion in rats BV-2 microglial cells BV-2 cells autoimmune encephalomyelitis in mice streptozotocin-induced dementia in rats transgenic mice with ALS | [53] [103] [54] [55] [51] [62] [61] [76] [85] [80] [81] [82] [90] [101] |
| ↓IFN-γ | R. rosea extract (standardized for salidroside) withanone W. somnifera extract | autoimmune encephalomyelitis in mice streptozotocin-induced dementia in rats transgenic mice with ALS | [82] [90] [101] |
| ↓iNOS | schisandrin B schisandrin C Schisandra lignans R. rosea extract, rosin, rosarin, salidroside E. senticosus extract | BV-2 microglial cells forced swim test (amygdala, hypothalamus) BV-2 microglial cells forced swim test in mice (hippocampal tissue) BV-2 microglial cells rats with induced ischemic stroke | [54] [55] [51] [61] [80] [75] |
| ↓NO | schisandrin A schisandrin B withanolide A withaferin A W. somnifera extract | BV-2 microglial cells, mouse primary microglia micorglia–neuron cocultures BV-2 microglial cells BV-2 microglial cells BV-2 microglial cells | [53] [103] [98] [98] [98] |
| ↓COX-2 | schisandrin B schisandrin C Schisandra lignans E. senticosus extract | BV-2 microglial cells forced swim test (amygdala, hypothalamus) BV-2 microglial cells forced swim test in mice (hippocamp) rats with induced global cerebral ischemia rats with induced ischemic stroke | [54] [55] [51] [61] [74] [75] |
| ↓PGE2 | schisandrin B schisandrin C | micorglia–neuron cocultures BV-2 microglial cells BV-2 microglial cells | [103] [54] [51] |
| ↓NF-κB | schisandrin A schisandrin B schisandrin C schisandrol B (gomisin A) SCP-2 salidroside R. rosea extract withanolide A withaferin A W. somnifera extract | BV-2 microglial cells, mouse primary microglia micorglia–neuron cocultures BV-2 microglial cells middle cerebral artery occlusion and reperfusion in rats subarachnoid hemorrhage in rats BV-2 microglial cells N9 microglial cells LPS-injected mice transient middle cerebral artery occlusion in rats LPS-injected rats BV-2 cells AD in mice transgenic mice with FTLD thioacetamice-induced hepatic encephalopathy LPS-injected rats transgenic mice with ALS | [53] [103] [54] [56] [57] [51] [62] [63] [85] [86] [81] [92] [101] [94] [99] [101] |
| ↓MAPK | schisandrin C SCP-2 R. rosea extract R. rosea extract W. somnifera extract | BV-2 microglial cells LPS-injected mice LPS-injected mice BV-2 cells thioacetamice-induced hepatic encephalopathy LPS-injected rats | [51] [63] [80] [81] [94] [99] |
| ↓JAK/STAT activation | schisandrin A schisandrin C Schisandra lignans micrandilactone C R. rosea extract (standardized for salidroside) | BV-2 microglial cells, mouse primary microglia BV-2 microglial cells forced swim test in mice (hippocampal tissue) 3-NPA-induced HD in mice autoimmune encephalomyelitis in mice | [53] [51] [61] [66] [82] |
| ↓inflammasome formation | schisandrin B withanolide A | subarachnoid hemorrhage in rats AD in mice | [57] [92] |
| ↓MCP-1 | withanone W. somnifera extract | streptozotocin-induced dementia in rats transgenic mice with ALS | [90] [101] |
| ↑Nrf-2 | schisandrin C salidroside withanolide A withaferin A W. somnifera extract | BV-2 microglial cells transient middle cerebral artery occlusion in rats LPS-injected rats BV-2 microglial cells BV-2 microglial cells thioacetamice-induced hepatic encephalopathy BV-2 microglial cells | [51] [85] [86] [98 [98] [94] [98] |
| ↑HO-1 | salidroside withanolide A withaferin A W. somnifera extract | transient middle cerebral artery occlusion in rats LPS-injected rats BV-2 microglial cells BV-2 microglial cells thioacetamice-induced hepatic encephalopathy BV-2 microglial cells | [85] [87] [98] [98] [94] [98] |
| ↑IL-4 | E. senticosus extract R. rosea extract (standardized for salidroside) W. somnifera extract | rats with induced ischemic stroke autoimmune encephalomyelitis in mice transgenic mice with ALS | [75] [82] [101] |
| ↑IL-10 | E. senticosus extract | rats with induced ischemic stroke | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Biedrawa, D.; Podolak, I. Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review. Molecules 2024, 29, 866. https://doi.org/10.3390/molecules29040866
Wróbel-Biedrawa D, Podolak I. Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review. Molecules. 2024; 29(4):866. https://doi.org/10.3390/molecules29040866
Chicago/Turabian StyleWróbel-Biedrawa, Dagmara, and Irma Podolak. 2024. "Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review" Molecules 29, no. 4: 866. https://doi.org/10.3390/molecules29040866
APA StyleWróbel-Biedrawa, D., & Podolak, I. (2024). Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review. Molecules, 29(4), 866. https://doi.org/10.3390/molecules29040866