Immunomodulatory, Antioxidant, and Potential Anticancer Activity of the Polysaccharides of the Fungus Fomitiporia chilensis
Abstract
1. Introduction
2. Results
2.1. Authentication of Basidiocarps
2.2. Polysaccharides Characterization/Chemical Assessment
2.2.1. Total Carbon (C), Total Hydrogen (H), Total Nitrogen (N), Total Sulfur (S), and Total Protein
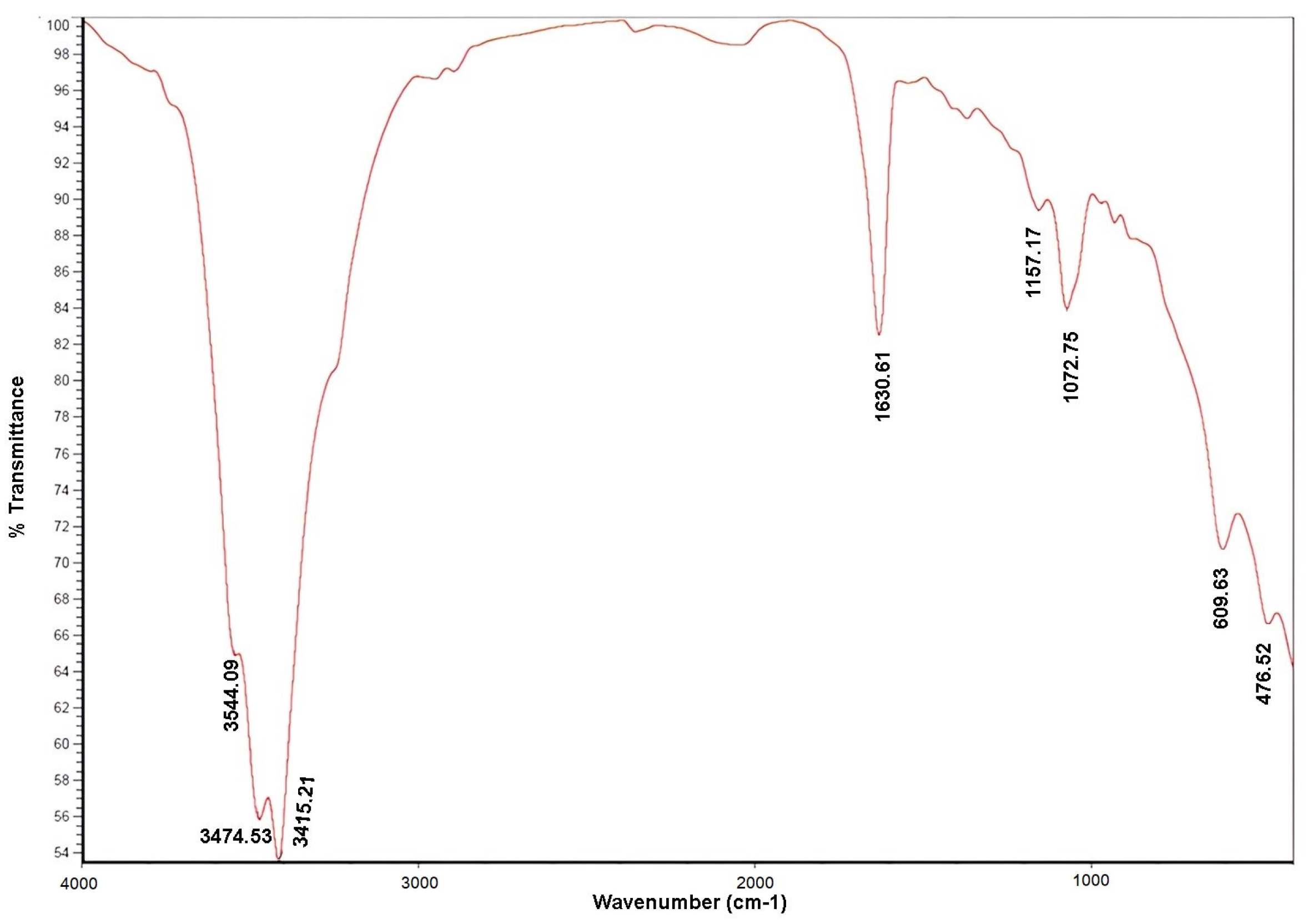
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
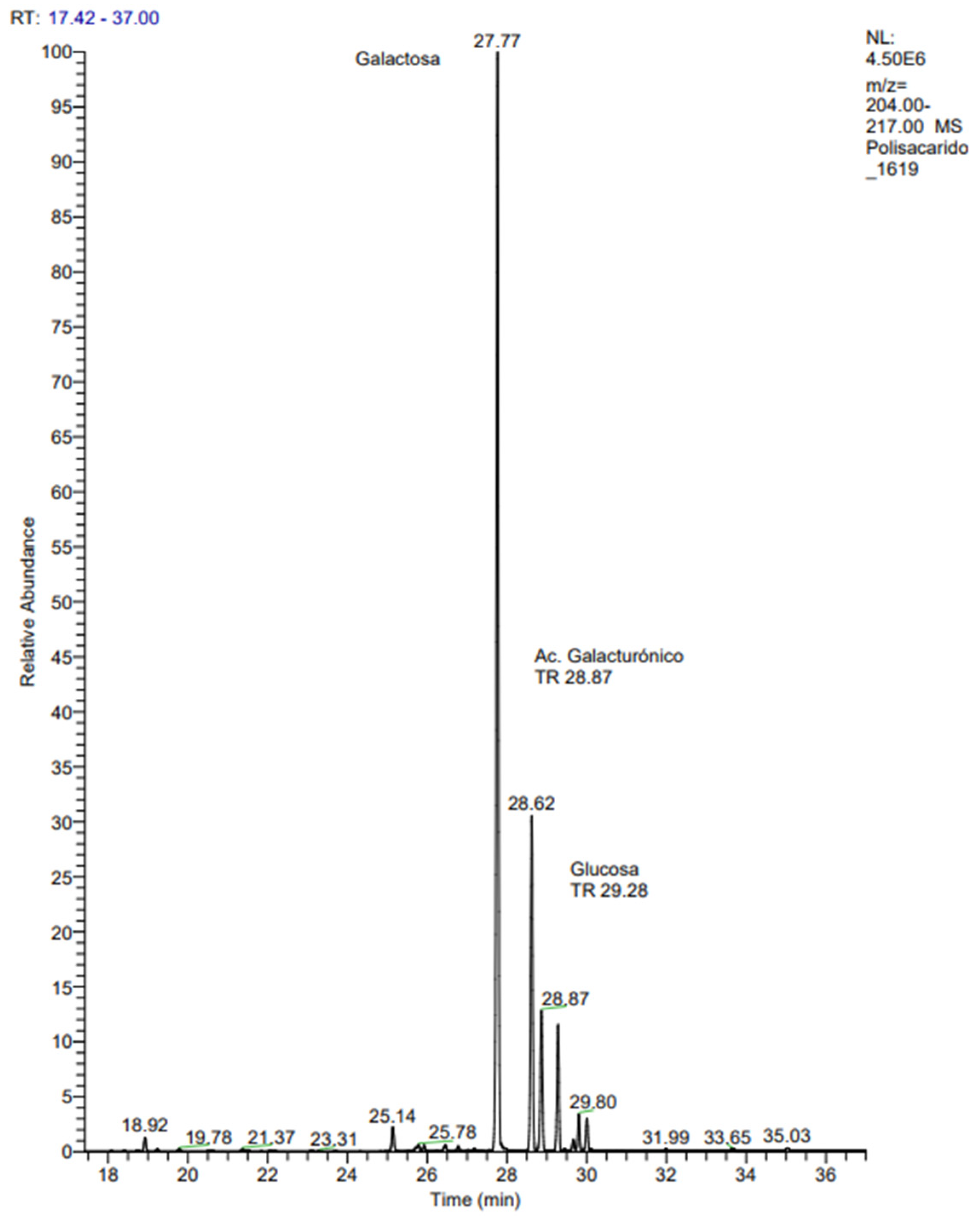
2.2.3. Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Biological Assessment
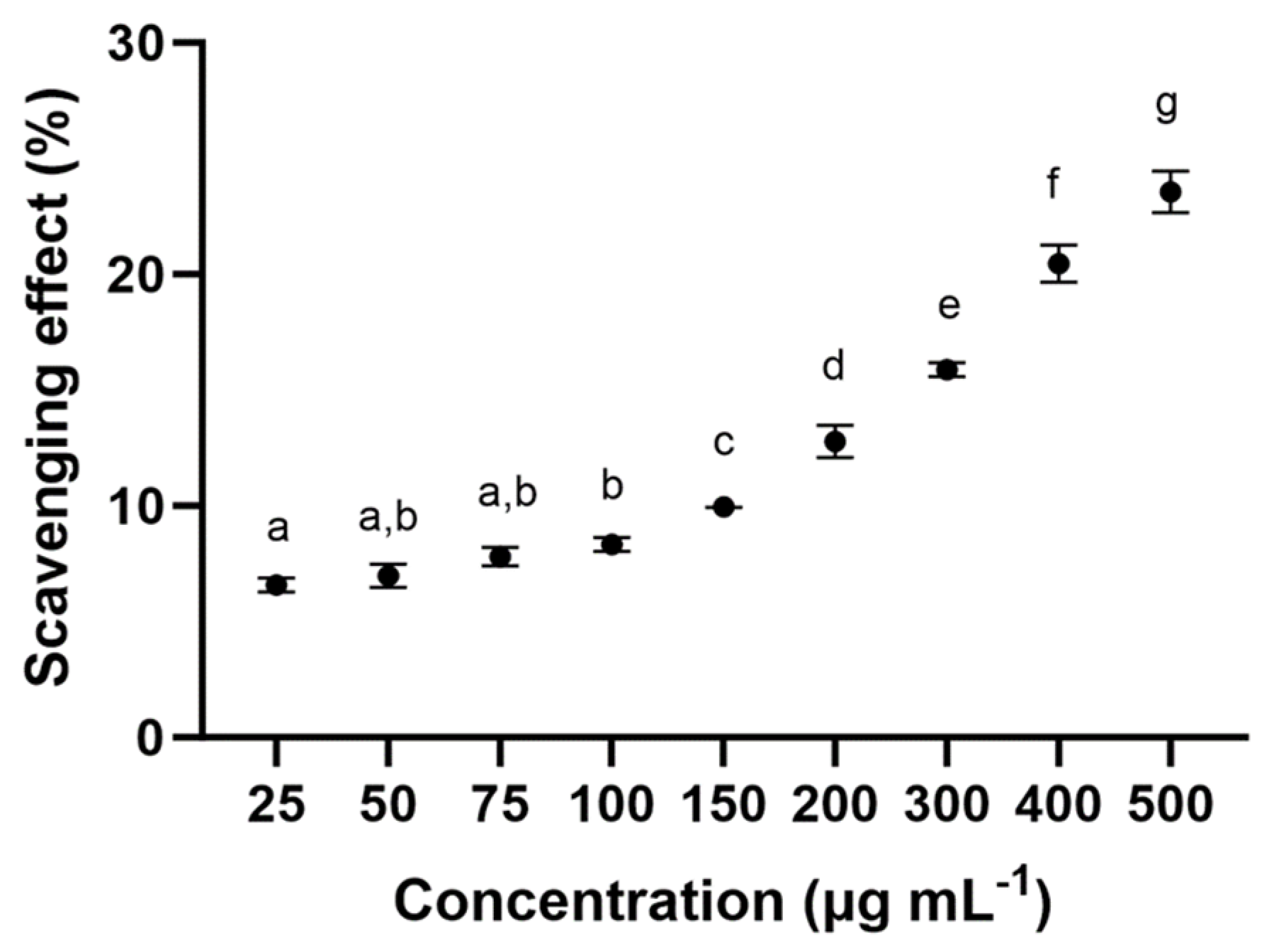
2.3.1. Antioxidant Activity (ABTS Method)
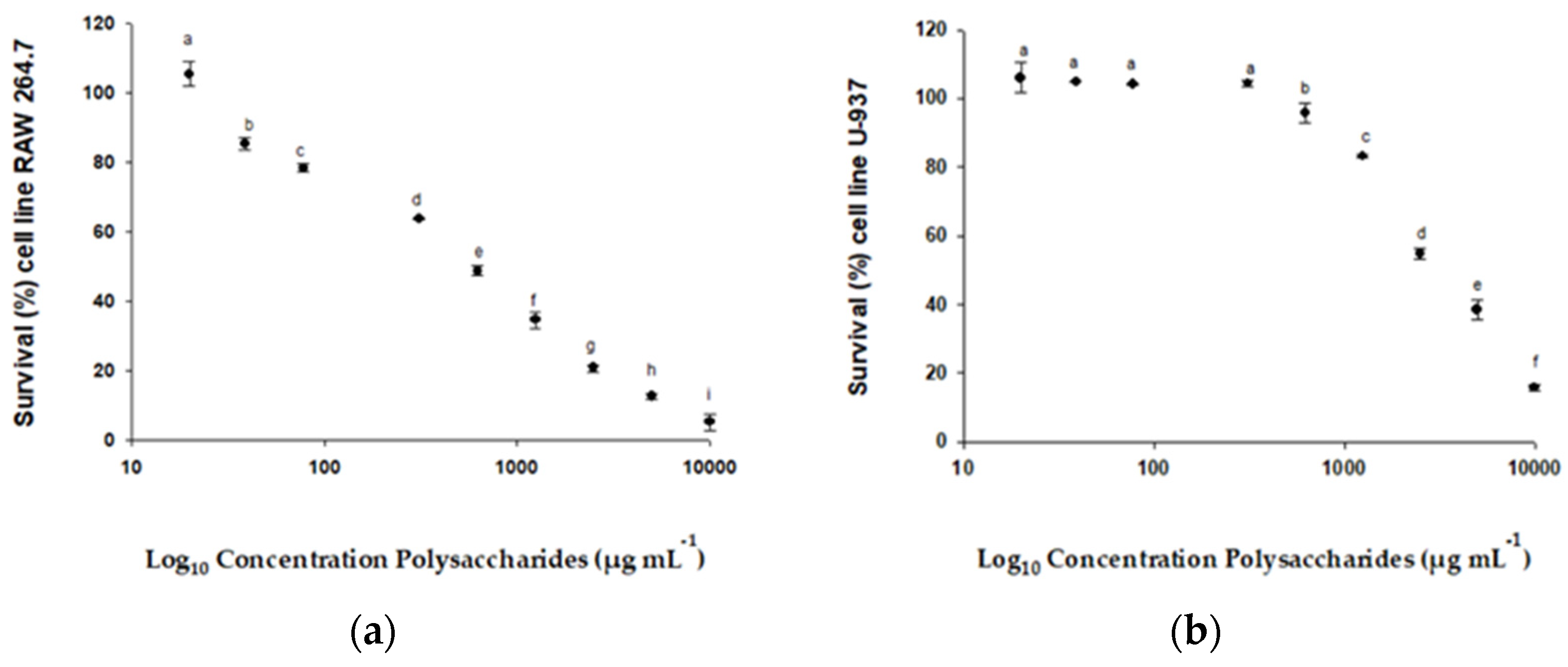
2.3.2. Cell Viability of Lines RAW 264.7, U-937, HTC-116, and HGF-1
2.3.3. Determination of Cytokines (IL-6 and TNF-α) in RAW 264.7 and THP-1 Cell Line
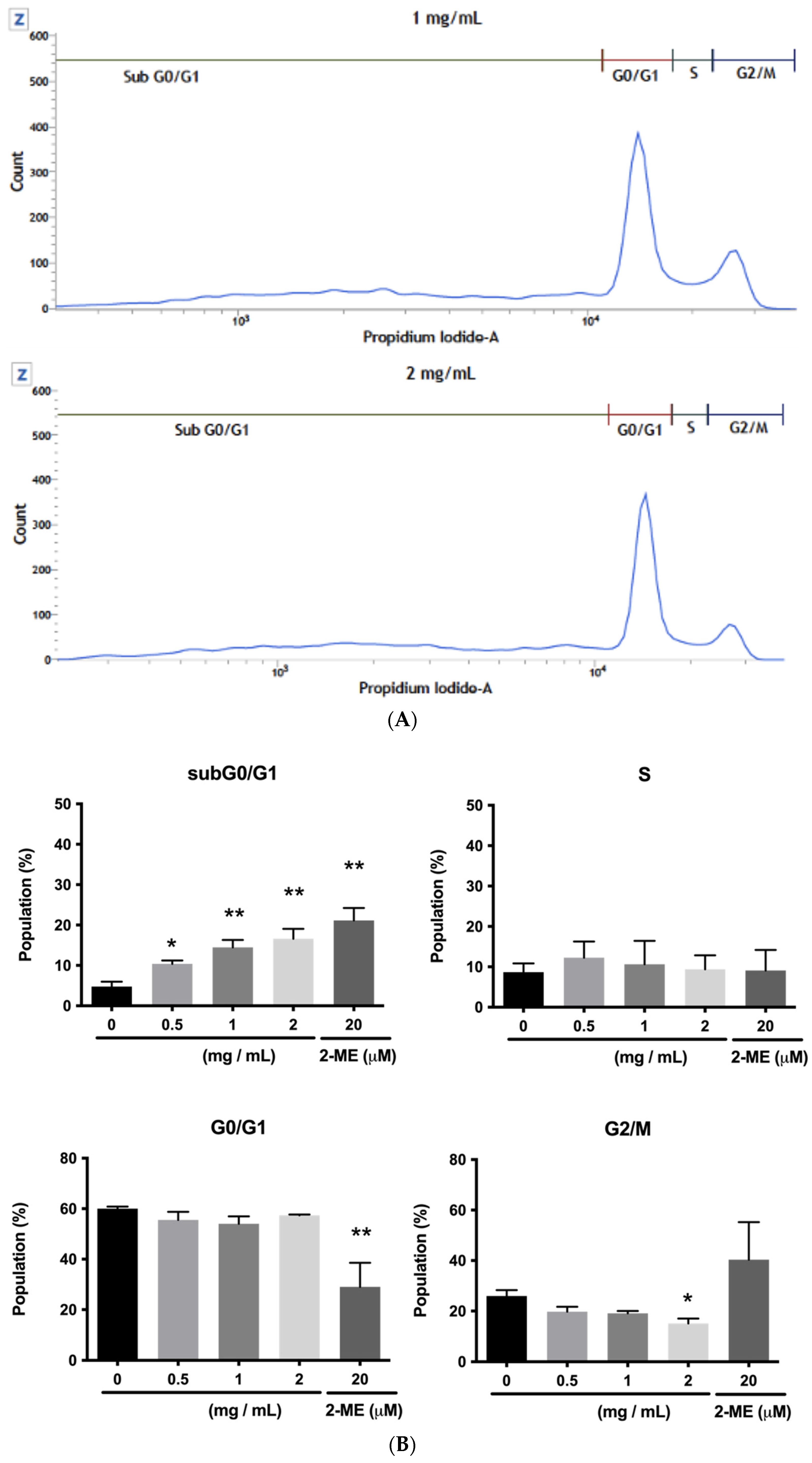
2.3.4. HCT116 Cell Cycle Analysis Using Flow Cytometry
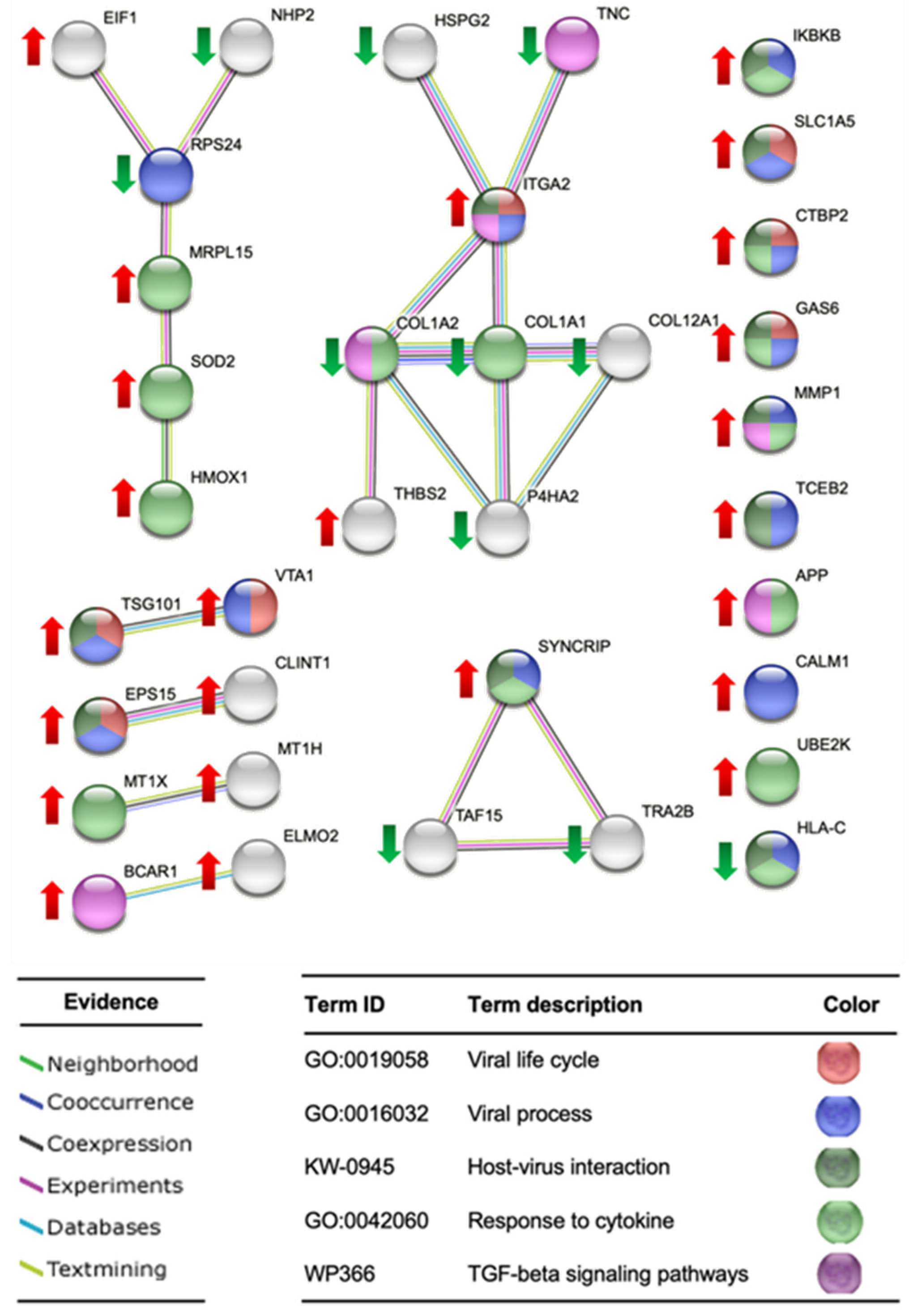
2.3.5. Proteomic Analysis in HGF-1 Cells
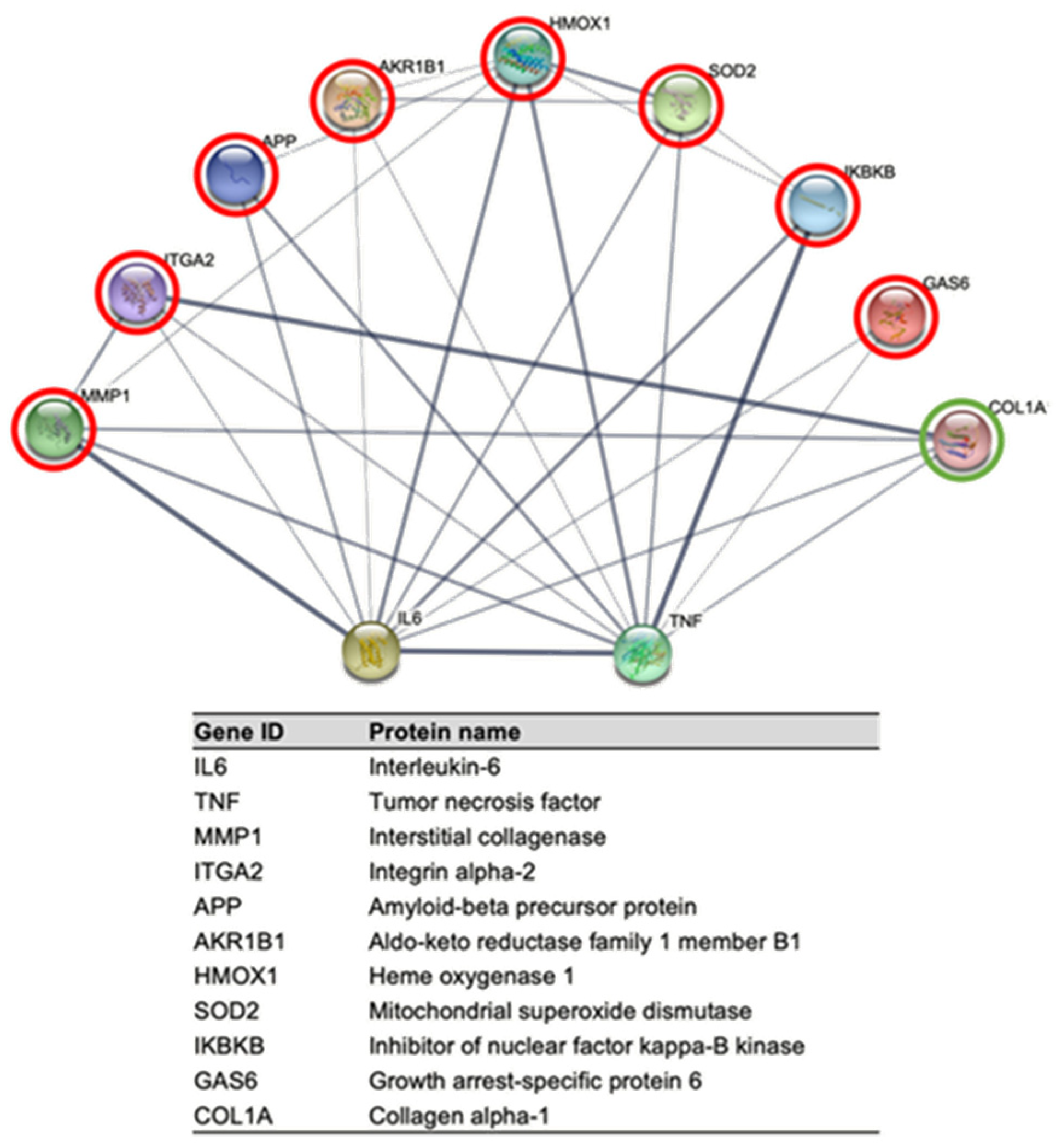
2.4. STRING Analysis of Protein Networks
3. Discussion
3.1. Polysaccharides Characterization
3.1.1. Total Carbon (C), Total Hydrogen (H), Total Nitrogen (N), Total Sulfur (S), and Total Protein
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.1.3. Gas Chromatography Mass Spectrometry (GC-MS)
3.2. Biological Assessment
3.2.1. Antioxidant Activity (ABTS Method)
3.2.2. Cell Viability of Lines RAW 264.7, U-937, HTC-116, and HGF-1
3.2.3. Determination of Cytokines (IL-6 and TNF-α) in RAW 264.7 and THP-1 Cell Line
3.2.4. HCT116 Cell Cycle Analysis Using Flow Cytometry
3.2.5. Proteomic Analysis in HGF-1 Cells
3.3. STRING Analysis of Protein Networks
4. Materials and Methods
4.1. Materials and Reagents
4.2. Collection, Authentication of Basidiocarps and Mycelial Culture
4.3. Extraction of Polysaccharides
4.4. Total Carbon (C), Hydrogen (H), Nitrogen (N), and Sulfur (S), and Protein Content
4.5. Fourier Transform Infrared Spectroscopy (FT-IR)
4.6. Gas Chromatography-Mass Spectrometry (GC-MS)
4.6.1. Derivatization of Polysaccharides
4.6.2. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis
4.7. Antioxidant Activity (ABTS) Free-Radical Method in Biomass from F. chilensis
4.8. In Vitro Immunomodulatory Activity Assay
4.8.1. Cell Culture
4.8.2. Cell Viability Assay of Lines Raw 264.7, U-937, HTC-116, and HGF-1
4.8.3. Cell Cycle Analysis by Flow Cytometry (HCT-116 Human Cancer Cell)
4.8.4. Determination of Cytokines with RAW 264.7 Cell Line
4.8.5. Determination of Cytokines with THP-1 Cell Line
4.9. Proteomics Analysis (UHPLC-HRMS Analysis for Differential Protein Expression Detection in Treated HGF-1 Cells)
4.9.1. Cell Treatment and Protein Extraction
4.9.2. Liquid Chromatography High-Resolution Mass Spectrometry
4.9.3. Data Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajchenberg, M.; Pildain, M.B.; Madriaga, D.C.; de Errasti, A.; Riquelme, C.; Becerra, J. New Poroid Hymenochaetaceae (Basidiomycota, Hymenochaetales) from Chile. Mycol. Prog. 2019, 18, 865–877. [Google Scholar] [CrossRef]
- Alves-Silva, G.; Drechsler-Santos, E.R.; da Silveira, R.M.B. Bambusicolous fomitiporia Revisited: Multilocus Phylogeny Reveals a Clade of Host-Exclusive Species. Mycologia 2020, 112, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, G.; Reck, M.A.; da Silveira, R.M.B.; Bittencourt, F.; Robledo, G.L.; Góes-Neto, A.; Drechsler-Santos, E.R. The Neotropical Fomitiporia (Hymenochaetales, Basidiomycota): The Redefinition of F. Apiahyna s.s. Allows Revealing a High Hidden Species Diversity. Mycol. Prog. 2020, 19, 769–790. [Google Scholar] [CrossRef]
- Zan, L.F.; Bao, H.Y.; Bau, T.; Li, Y. A New Antioxidant Pyrano[4,3-c]Benzopyran-1,6-Dione Derivative from the Medicinal Mushroom Fomitiporia ellipsoidea. Nat. Prod. Commun. 2015, 10, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Chepkirui, C.; Sum, W.; Cheng, T.; Matasyoh, J.; Decock, C.; Stadler, M. Aethiopinolones A–E, New Pregnenolone Type Steroids from the East African Basidiomycete Fomitiporia aethiopica. Molecules 2018, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Zhang, K.; Wang, Y.; Zhou, R.; Zeng, Y.; Han, Y.; Ng, T.B. A Novel Polysaccharide with Antioxi-dant, HIV Protease Inhibiting and HIV Integrase Inhibiting Activities from Fomitiporia punctata (P. Karst.) Murrill (Basidiomycota, Hymenochaetales). Int. J. Biol. Macromol. 2017, 97, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Doskocil, I.; Havlik, J.; Verlotta, R.; Tauchen, J.; Vesela, L.; Macakova, K.; Opletal, L.; Kokoska, L.; Rada, V. In Vitro Immunomodulatory Activity, Cytotoxicity and Chemistry of Some Central European Polypores. Pharm. Biol. 2016, 54, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, C.; Rajchenberg, M. Aphyllophoroid Fungi (Basidiomycota) of Chile: An Annotated Checklist. Mycotaxon 2021, 136, 691. [Google Scholar] [CrossRef]
- Chuensun, T.; Chewonarin, T.; Laopajon, W.; Kawee-ai, A.; Pinpart, P.; Utama-ang, N. Comparative Evaluation of Physicochemical Properties of Lingzhi (Ganoderma lucidum) as Affected by Drying Conditions and Extraction Methods. Int. J. Food Sci. Technol. 2021, 56, 2751–2759. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Hou, T.; An, S.; Guo, B.; Liu, C.; Hu, L.; Huang, Y.; Zhang, S.; Song, M.; et al. Extraction Kinetics, Physicochemical Properties and Immunomodulatory Activity of the Novel Continuous Phase Transition Extraction of Polysaccharides from Ganoderma lucidum. Food Funct. 2021, 12, 9708–9718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; He, F.; Fu, L.; Zhang, Y. Polysaccharide from Rubescens: Extraction, Optimization, Characterization and Antioxidant Activities. RSC Adv. 2021, 11, 18974–18983. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Shen, M.; Zhang, Y.; Yu, H.; Xie, J. Food Polysaccharides and Proteins: Processing, Characterization, and Health Benefits. Foods 2024, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Sulfated Modification, Characterization and Bioactivities of an Acidic Polysaccharide Fraction from an Edible Mushroom Pleurotus eous (Berk.) Sacc. Heliyon 2021, 7, e05964. [Google Scholar] [CrossRef] [PubMed]
- Baeva, E.; Bleha, R.; Sedliaková, M.; Sushytskyi, L.; Švec, I.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Evaluation of the Cultivated Mushroom Pleurotus ostreatus Basidiocarps Using Vibration Spectroscopy and Chemometrics. Appl. Sci. 2020, 10, 8156. [Google Scholar] [CrossRef]
- Cardozo, F.T.G.S.; Camelini, C.M.; Cordeiro, M.N.S.; Mascarello, A.; Malagoli, B.G.; Larsen, I.V.; Rossi, M.J.; Nunes, R.J.; Braga, F.C.; Brandt, C.R.; et al. Characterization and Cytotoxic Activity of Sulfated Derivatives of Polysaccharides from Agaricus brasiliensis. Int. J. Biol. Macromol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Barros, L.; Fernandes, Â.; Sokovic, M.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. A Natural Food Ingredient Based on Ergosterol: Optimization of the Extraction from Agaricus blazei, Evaluation of Bioactive Properties and Incorporation in Yogurts. Food Funct. 2018, 9, 1465–1474. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Kim, H.J.; Tewolde, H.; Zhang, H. Fourier Transform Infrared Spectral Features of Plant Biomass Components during Cotton Organ Development and Their Biological Implications. J. Cotton Res. 2022, 5, 11. [Google Scholar] [CrossRef]
- Jiang, J.; Kong, F.; Li, N.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Manrique, G.D.; Lajolo, F.M. FT-IR Spectroscopy as a Tool for Measuring Degree of Methyl Esterification in Pectins Isolated from Ripening Papaya Fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Gonzaga, M.L.C.; Menezes, T.M.; de Souza, J.R.R.; Ricardo, N.M.; Soares, S.D.A. Structural Characterization of β Glucans Isolated from Agaricus blazei Murill Using NMR and FTIR Spectroscopy. Bioact. Carbohydr. Diet. Fibre 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Purification, Chemical Characterization and Antioxidant Activities of a Novel Polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural Characterization and Antiviral Activity of Lentinan from Lentinus edodes Mycelia against Infectious Hematopoietic Necrosis Virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.-I.; Su, C.-H.; Lai, M.-N.; Ng, L.-T. Comparative Anti-Inflammatory Characterization of Selected Fungal and Plant Water Soluble Polysaccharides. Food Sci. Technol. Res. 2021, 27, 453–462. [Google Scholar] [CrossRef]
- Chen, S.-K.; Li, Y.-H.; Wang, X.; Guo, Y.-Q.; Song, X.-X.; Nie, S.-P.; Yin, J.-Y. Evaluation of the “Relative Ordered Structure of Hericium erinaceus Polysaccharide” from Different Origins: Based on Similarity and Dissimilarity. J. Agric. Food Chem. 2023, 71, 17886–17898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Alcazar-Magana, A.; Qian, Y.P.; Tao, Y.; Qian, M.C. Isolation, Characterization, and Compositional Analysis of Polysaccharides from Pinot Noir Wines: An Exploratory Study. Molecules 2022, 27, 8330. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, P.; Tang, Y.; Pang, Y.; Sun, J.; Li, J.; Rao, C.; Wu, C.; He, X.; Li, L.; et al. Evaluation of Bioactive Compounds and Bioactivities in Plum (Prunus salicina Lindl.) Wine. Front. Nutr. 2021, 8, 766415. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [PubMed]
- Caldas, L.A.; Santos, P.D.; Carbonero, E.R.; Ionta, M.; Miyazawa, M.; Caixeta, E.S.; Fregnan, A.M.; Nóbrega, B.B.; Di Medeiros, M.C.B.; Menolli, N., Jr.; et al. Immunomodulatory Effect of Polysaccharides from the Mushroom-Forming Basidiomycete Gymnopilus imperialis (Agaricomycetes, Basidiomycota). Pharmaceuticals 2022, 15, 1179. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Lai, M.-N.; Lin, C.-C.; Ng, L.-T. Comparative Characterization of Physicochemical Properties and Bioactivities of Polysaccharides from Selected Medicinal Mushrooms. Appl. Microbiol. Biotechnol. 2016, 100, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural Characterization and Immunomodulatory Activity of Exopolysaccharides from Submerged Culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Reactive Oxygen Species and Antioxidant Properties from Mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Liu, Y.; Yue, F.; Lu, Y.; Qiu, H.; Gao, D.; Gao, Y.; Wu, Y.; Wang, Z.; et al. In Vitro Kinetic Evaluation of the Free Radical Scavenging Ability of Propofol. Anesthesiology 2012, 116, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 273–307. ISBN 9780128241271. [Google Scholar]
- Yang, X.; Ji, H.; Feng, Y.; Yu, J.; Liu, A. Structural Characterization and Antitumor Activity of Polysaccharides from Kaempferia galanga L. Oxidative Med. Cell. Longev. 2018, 2018, 9579262. [Google Scholar] [CrossRef]
- Albornoz, V.; Casas-Arrojo, V.; Figueroa, F.; Hernández, V.; Pérez, C.; Rajchenberg, M.; Smith, C.T.; Becerra, J.; Cabrera-Pardo, J.R.; Campos, V.L.; et al. Evaluation of cytotoxic effect against tumour cells of the acidic polysaccharides of the fungus Nothophellinus andinopatagonicus. J. Chil. Chem. Soc. 2022, 67, 5418–5424. [Google Scholar] [CrossRef]
- Del Cornò, M.; Gessani, S.; Conti, L. Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers 2020, 12, 155. [Google Scholar] [CrossRef]
- Castro-Varela, P.; Rubilar, M.; Rodrigues, B.; Pacheco, M.J.; Caneda-Santiago, C.T.; Marí-Beffa, M.; Figueroa, F.L.; Abdala-Díaz, R. A Sequential Recovery Extraction and Biological Activity of Water-Soluble Sulfated Polysaccharides from the Polar Red Macroalgae Sarcopeltis skottsbergii. Algal Res. 2023, 73, 103160. [Google Scholar] [CrossRef]
- Snarr, B.; Qureshi, S.; Sheppard, D. Immune Recognition of Fungal Polysaccharides. J. Fungi 2017, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.-W. Mushroom Polysaccharide-Assisted Anticarcinogenic Mycotherapy: Reviewing Its Clinical Trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef]
- Ehlers, S. Role of Tumour Necrosis Factor (TNF) in Host Defence against Tuberculosis: Implications for Immunotherapies Targeting TNF. Ann. Rheum. Dis. 2003, 62, ii37–ii42. [Google Scholar] [CrossRef]
- Johnson, L.L. A Protective Role for Endogenous Tumor Necrosis Factor in Toxoplasma Gondii Infection. Infect. Immun. 1992, 60, 1979–1983. [Google Scholar] [CrossRef]
- Tun, M.M.N.; Aoki, K.; Senba, M.; Buerano, C.C.; Shirai, K.; Suzuki, R.; Morita, K.; Hayasaka, D. Protective Role of TNF-α, IL-10 and IL-2 in Mice Infected with the Oshima Strain of Tick-Borne Encephalitis Virus. Sci. Rep. 2014, 4, 5344. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Saito, K.; Kanda, T.; Kobayashi, I.; Fujii, H.; Fujigaki, S.; Maekawa, N.; Takatsu, H.; Fujiwara, H.; Sekikawa, K.; et al. Tumor Necrosis Factor-α (TNF-α) Plays a Protective Role in Acute Viral Myocarditis in Mice: A Study Using Mice Lacking TNF-α. Circulation 2001, 103, 743–749. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, S.; Wang, H.; Jia, Q.; Jiang, W.; Zhang, X.; Zhang, A.; Liu, J.; Ni, L. Absent in Melanoma 2 (AIM2) in Rat Dental Pulp Mediates the Inflammatory Response during Pulpitis. J. Endod. 2013, 39, 1390–1394. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Mac Nair, C.E.; Fernandes, K.A.; Schlamp, C.L.; Libby, R.T.; Nickells, R.W. Tumor Necrosis Factor Alpha Has an Early Protective Effect on Retinal Ganglion Cells after Optic Nerve Crush. J. Neuroinflamm. 2014, 11, 194. [Google Scholar] [CrossRef]
- Fryer, A.D.; Jacoby, D.B.; Wicher, S.A. Protective Role of Eosinophils and TNFa after Ozone Inhalation; Research Report; Health Effects Institute: Boston, MA, USA, 2017; pp. 1–41. [Google Scholar]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An In Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. Β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Β-glucan Recognition by the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and Its Receptors: A Highly Regulated and Dynamic System. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, Z.; Zhang, Y.; Zheng, Z.; Li, Q.; Wang, K. Apoptosis induction activity of polysaccharide from Lentinus edodes in H22-bearing mice through ROS-mediated mitochondrial pathway and inhibition of tubulin polymerization. Food Nutr. Res. 2020, 64, 4364. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-tumor Biomacromolecules—A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef]
- Li, W.-J.; Chen, Y.; Nie, S.-P.; Xie, M.-Y.; He, M.; Zhang, S.-S.; Zhu, K.-X. Ganoderma atrum Polysaccharide Induces Anti-tumor Activity via the Mitochondrial Apoptotic Pathway Related to Activation of Host Immune Response. J. Cell. Biochem. 2011, 112, 860–871. [Google Scholar] [CrossRef]
- Xie, P.; Fujii, I.; Zhao, J.; Shinohara, M.; Matsukura, M. A Novel Polysaccharide Derived from Algae Extract Induces Apoptosis and Cell Cycle Arrest in Human Gastric Carcinoma MKN45 Cells via ROS/JNK Signaling Pathway. Int. J. Oncol. 2016, 49, 1561–1568. [Google Scholar] [CrossRef]
- M Ahmed, O. Anti-Proliferative and Apoptotic Efficacies of Ulvan Polysaccharides against Different Types of Carcinoma Cells In Vitro and In Vivo. J. Cancer Sci. Ther. 2014, 6, 202–208. [Google Scholar] [CrossRef]
- Wang, K.-P.; Zhang, Q.-L.; Liu, Y.; Wang, J.; Cheng, Y.; Zhang, Y. Structure and Inducing Tumor Cell Apoptosis Activity of Polysaccharides Isolated from Lentinus edodes. J. Agric. Food Chem. 2013, 61, 9849–9858. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.-X.; Wang, J.; Hu, J.; Lou, G.-H.; Xiong, H.-J.; Peng, C.-Y.; Huang, Q.-W. Modulation of Apoptosis by Plant Polysaccharides for Exerting Anti-Cancer Effects: A Review. Front. Pharmacol. 2020, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Asbun, J.; Manso, A.M.; Villarreal, F.J. Profibrotic Influence of High Glucose Concentration on Cardiac Fibroblast Functions: Effects of Losartan and Vitamin E. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H227–H234. [Google Scholar] [CrossRef]
- Bauters, C.; Lamblin, N.; Mc Fadden, E.P.; Van Belle, E.; Millaire, A.; de Groote, P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc. Diabetol. 2003, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Ambler, S.K.; Mitchell, M.D.; Long, C.S. The Cardiac Fibroblast: Therapeutic Target in Myocardial Remodeling and Failure. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 657–687. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Asemu, G.; Aroutiounova, N.; Dhalla, N.S. Defective Sarcolemmal Phospholipase C Signaling in Diabetic Cardiomyopathy. Mol. Cell. Biochem. 2004, 261, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Beuckmann, C.T.; Fujimori, K.; Urade, Y.; Hayaishi, O. Identification of Mu-Class Glutathione Transferases M2-2 and M3-3 as Cytosolic Prostaglandin E Synthases in the Human Brain. Neurochem. Res. 2000, 25, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tan, Y.; Zhao, P.; Guo, Y.; Chen, S.; Wu, J.; Ren, Z. Glutathione S-Transferase Mu 2 Inhibits Hepatic Steatosis via ASK1 Suppression. Commun. Biol. 2022, 5, 326. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Yeh, H.-Y.; Chao, C.-T.; Chiang, C.-K. Superoxide Dismutase 2 (SOD2) in Vascular Calcification: A Focus on Vascular Smooth Muscle Cells, Calcification Pathogenesis, and Therapeutic Strategies. Oxidative Med. Cell. Longev. 2021, 2021, 6675548. [Google Scholar] [CrossRef]
- Kandasamy, M. NF-κB Signalling as a Pharmacological Target in COVID-19: Potential Roles for IKKβ Inhibitors. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 561–567. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF- B Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Parages, M.L.; Rico, R.M.; Abdala-Díaz, R.T.; Chabrillón, M.; Sotiroudis, T.G.; Jiménez, C. Acidic Polysaccharides of Arthrospira (Spirulina) platensis Induce the Synthesis of TNF-α in RAW Macrophages. J. Appl. Phycol. 2012, 24, 1537–1546. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium Cruentum. (S.f.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.T.A.; Chabrillón, M.; Cabello-Pasini, A.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Characterization of Polysaccharides from Hypnea spinella (Gigartinales) and Halopithys incurva (Ceramiales) and Their Effect on RAW 264.7 Macrophage Activity. J. Appl. Phycol. 2011, 23, 523–528. [Google Scholar] [CrossRef]
- Ojeda-Pérez, B.; Campos-Sandoval, J.A.; García-Bonilla, M.; Cárdenas-García, C.; Páez-González, P.; Jiménez, A.J. Identification of Key Molecular Biomarkers Involved in Reactive and Neurodegenerative Processes Present in Inherited Congenital Hydrocephalus. Fluids Barriers CNS 2021, 18, 30. [Google Scholar] [CrossRef]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An Approach to Correlate Tandem Mass Spectral Data of Peptides with Amino Acid Sequences in a Protein Database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-Supervised Learning for Peptide Identification from Shotgun Proteomics Datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]




| Extracted Polysaccharides (%) | |
|---|---|
| Carbon | 40.03 |
| Hydrogen | 6.08 |
| Nitrogen | 2.14 |
| Sulfur | 0.97 |
| Protein (dry weight, D.W., %) | 18.24 |
| Header | Retention Time (min) | Monosaccharide | Peak Area | % Mass |
|---|---|---|---|---|
| 1 | 27.77 | Galactose (Gal) | 21.099.271 | 53.02 |
| 2 | 28.62 | Galacturonic acid (GalA) | 6.198.261 | 16.02 |
| 3 | 28.87 | Glucose (Glc α) | 2.772.784 | 6.83 |
| 4 | 29.28 | Glucose (Glc β) | 2.406.072 | 6.27 |
| Gene Symbol | Description | Sum PEP Score (1) | Abundance Ratio: Treatment/Control | Abundance Ratio p-Value |
|---|---|---|---|---|
| THBS2 | Thrombospondin-2 | 12.61 | (2) | 1.00 × 10−17 |
| IKBKB | Inhibitor of nuclear factor kappa-B kinase subunit beta | 2.88 | 6.16 | 1.00 × 10−17 |
| APP | Amyloid-beta A4 protein | 2.92 | 4.08 | 1.00 × 10−17 |
| VTA1 | Vacuolar protein sorting-associated protein VTA1 homolog | 3.55 | 3.92 | 1.00 × 10−17 |
| MMP1 | Interstitial collagenase | 3.22 | 3.26 | 1.00 × 10−17 |
| CLINT1 | Clathrin interactor 1 | 3.77 | 2.96 | 1.00 × 10−17 |
| HMOX1 | heme oxygenase 1 | 2.88 | 2.51 | 1.00 × 10−17 |
| GSTM2 | Glutathione S-transferase Mu 2 | 5.85 | 2.07 | 3.96 × 10−8 |
| NDRG1 | Protein NDRG1 | 6.02 | 2.03 | 3.17 × 10−11 |
| MT1X | metallothionein-1X | 6.31 | 2.03 | 2.29 × 10−13 |
| CALM1, 2, 3 | Calmodulin | 21.48 | 2.03 | 2.76 × 10−13 |
| MT1H | MX10tallothionX10in-1H | 3.84 | 1.92 | 1.47 × 10−8 |
| PLCB3 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-3 | 2.59 | 1.88 | 1.37 × 10−7 |
| AKR1B1 | aldose reductase | 25.63 | 1.85 | 2.75 × 10−10 |
| MRPL15 | 39S ribosomal protein L15, mitochondrial | 3.39 | 1.85 | 2.67 × 10−8 |
| LOXL2 | Lysyl oxidase homolog 2 | 3.66 | 1.79 | 2.39 × 10−6 |
| DSP | Desmoplakin | 2.85 | 1.79 | 3.61 × 10−9 |
| AKR1C1 | Aldo-keto reductase family 1 member C1 | 20.00 | 1.79 | 3.10 × 10−9 |
| SKIV2L | Helicase SKI2W | 2.87 | 1.74 | 2.86 × 10−5 |
| ELMO2 | Engulfment and cell motility protein 2 | 5.25 | 1.71 | 2.74 × 10−5 |
| HPCA | Neuron-specific calcium-binding protein hippocalcin | 6.12 | 1.67 | 3.25 × 10−6 |
| SOD2 | Superoxide dismutase [Mn], mitochondrial | 3.40 | 1.66 | 1.66 × 10−4 |
| METAP2 | Methionine aminopeptidase 2 | 2.70 | 1.64 | 2.10 × 10−4 |
| BCAR1 | Breast cancer anti-estrogen resistance protein 1 | 2.79 | 1.62 | 3.33 × 10−4 |
| AEBP1 | Adipocyte enhancer-binding protein 1 | 4.84 | 1.60 | 6.06 × 10−6 |
| TM9SF2 | Transmembrane 9 superfamily member 2 | 3.64 | 1.59 | 7.08 × 10−5 |
| ALDH3A1 | Aldehyde dehydrogenase, dimeric NADP-preferring | 30.54 | 1.59 | 3.96 × 10−6 |
| EIF1 | Eukaryotic translation initiation factor 1 | 3.51 | 1.58 | 5.59 × 10−4 |
| FAM160B1 | Protein FAM160B1 | 2.62 | 1.56 | 4.92 × 10−4 |
| MAPRE2 | Microtubule-associated protein RP/EB family member 2 | 3.10 | 1.55 | 1.37 × 10−3 |
| ITGA2 | Integrin alpha-2 | 61.27 | 1.53 | 2.81 × 10−5 |
| EPS15 | Epidermal growth factor receptor substrate 15 | 3.45 | 1.51 | 1.20 × 10−3 |
| TCEB2; ELOB | Elongin-B | 2.68 | 1.50 | 2.54 × 10−3 |
| SUB1 | Activated RNA polymerase II transcriptional coactivator p15 | 6.69 | 1.49 | 4.80 × 10−4 |
| GAS6 | Growth arrest-specific protein 6 | 3.57 | 1.49 | 9.31 × 10−4 |
| LEPROT | Leptin receptor gene-related protein | 4.86 | 1.48 | 1.21 × 10−3 |
| PRCP | lysosomal Pro-X carboxypeptidase | 3.25 | 1.47 | 7.48 × 10−4 |
| TSG101 | tumor susceptibility gene 101 protein | 4.34 | 1.47 | 1.31 × 10−3 |
| SLC1A5 | Neutral amino acid transporter B (0) | 22.90 | 1.46 | 2.29 × 10−4 |
| FAM21C; WASHC2C | WASH complex subunit 2C | 3.93 | 1.46 | 4.97 × 10−3 |
| CTBP2 | c-terminal-binding protein 2 | 4.67 | 1.46 | 5.77 × 10−3 |
| ACTR1B | Beta-centractin | 14.01 | 1.45 | 8.24 × 10−4 |
| EXOC5 | Exocyst complex component 5 | 2.62 | 1.45 | 4.09 × 10−3 |
| UBE2K | Ubiquitin-conjugating enzyme E2 K | 5.00 | 1.45 | 2.27 × 10−3 |
| SH3PXD2B | SH3 and P.X. domain-containing protein 2B | 5.85 | 1.45 | 1.77 × 10−3 |
| SYNCRIP | Heterogeneous nuclear ribonucleoprotein Q | 34.56 | 1.45 | 3.65 × 10−4 |
| RETSAT | all-trans-retinol 13,14-reductase | 3.86 | 1.44 | 7.73 × 10−3 |
| TNC | Isoform 4 of Tenascin | 137.79 | 0.71 | 7.03 × 10−4 |
| TPM1 | Isoform 3 of Tropomyosin alpha-1 chain | 42.56 | 0.70 | 6.01 × 10−6 |
| PPAP2B; PLPP3 | Phospholipid phosphatase 3 | 14.13 | 0.69 | 4.17 × 10−6 |
| MPST | 3-mercaptopyruvate sulfurtransferase | 3.58 | 0.69 | 6.19 × 10−4 |
| HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein | 22.22 | 0.68 | 2.86 × 10−5 |
| TPM2 | Isoform 2 of Tropomyosin beta chain | 59.61 | 0.68 | 1.96 × 10−6 |
| PAFAH1B2 | platelet-activating factor acetylhydrolase I.B. subunit beta | 3.24 | 0.68 | 1.06 × 10−4 |
| HLA-C | HLA class I histocompatibility antigen, Cw-6 alpha chain | 30.81 | 0.65 | 5.63 × 10−5 |
| KDELR1 | ER lumen protein-retaining receptor 1 | 3.22 | 0.62 | 1.58 × 10−5 |
| P4HA2 | Prolyl 4-hydroxylase subunit alpha-2 | 25.64 | 0.61 | 3.40 × 10−9 |
| TRA2B | Transformer-2 protein homolog beta | 9.60 | 0.61 | 1.90 × 10−6 |
| HIST2H2AC | Histone H2A type 2-C | 19.00 | 0.60 | 1.01 × 10−9 |
| NDUFA10 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10, mitochondrial | 2.71 | 0.59 | 4.62 × 10−6 |
| NHP2 | H/ACA ribonucleoprotein complex subunit 2 | 2.80 | 0.58 | 2.24 × 10−7 |
| COL12A1 | Collagen alpha-1 (XII) chain | 97.95 | 0.57 | 9.99 × 10−12 |
| HIST3H2A | Histone H2A type 3 | 18.81 | 0.52 | 7.02 × 10−11 |
| RPS24 | 40S ribosomal protein S24 | 3.17 | 0.51 | 2.25 × 10−9 |
| COL1A2 | Collagen alpha-2 (I) chain | 87.06 | 0.49 | 1.00 × 10−17 |
| TAF15 | TATA-binding protein-associated factor 2N | 11.93 | 0.47 | 6.74 × 10−11 |
| TUBA1A | Tubulin alpha-1A chain | 188.76 | 0.41 | 2.22 × 10−16 |
| SLC35E1 | Solute carrier family 35 member E1 | 3.85 | 0.40 | 4.44 × 10−16 |
| COL1A1 | Collagen alpha-1 (I) chain | 76.45 | 0.40 | 1.00 × 10−17 |
| ANKMY1 | Ankyrin repeat and MYND domain-containing protein 1 | 2.66 | 0.07 | 1.00 × 10−17 |
| SLC39A7 | Zinc transporter SLC39A7 | 4.18 | 0.01 | 1.00 × 10−17 |
| Carbon (%) | Hydrogen (%) | Nitrogen (%) | Sulfur (%) | Protein (Dry Weight, D.W., %) | |
|---|---|---|---|---|---|
| Fomitiporia chilensis | 40.03 | 6.08 | 2.14 | 0.97 | 18.24 |
| Pleurotus eus | 41.08 | 6.25 | 4.99 | 2.79 | - |
| Pleurotus ostreatus | 39.7–41.7 | 6.7–6.8 | 1.6–4.4 | 0.1–0.2 | 17.06 |
| Agaricus brasiliensis (fruiting body) | 34.42 | 5.56 | 2.50 | 0 | 28.9–39.2 |
| Agaricus brasiliensis (mycelial) | 32.07 | 4.36 | 1.63 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdala-Díaz, R.T.; Casas-Arrojo, V.; Castro-Varela, P.; Riquelme, C.; Carrillo, P.; Medina, M.Á.; Cárdenas, C.; Becerra, J.; Pérez Manríquez, C. Immunomodulatory, Antioxidant, and Potential Anticancer Activity of the Polysaccharides of the Fungus Fomitiporia chilensis. Molecules 2024, 29, 3628. https://doi.org/10.3390/molecules29153628
Abdala-Díaz RT, Casas-Arrojo V, Castro-Varela P, Riquelme C, Carrillo P, Medina MÁ, Cárdenas C, Becerra J, Pérez Manríquez C. Immunomodulatory, Antioxidant, and Potential Anticancer Activity of the Polysaccharides of the Fungus Fomitiporia chilensis. Molecules. 2024; 29(15):3628. https://doi.org/10.3390/molecules29153628
Chicago/Turabian StyleAbdala-Díaz, Roberto T., Virginia Casas-Arrojo, Pablo Castro-Varela, Cristian Riquelme, Paloma Carrillo, Miguel Ángel Medina, Casimiro Cárdenas, José Becerra, and Claudia Pérez Manríquez. 2024. "Immunomodulatory, Antioxidant, and Potential Anticancer Activity of the Polysaccharides of the Fungus Fomitiporia chilensis" Molecules 29, no. 15: 3628. https://doi.org/10.3390/molecules29153628
APA StyleAbdala-Díaz, R. T., Casas-Arrojo, V., Castro-Varela, P., Riquelme, C., Carrillo, P., Medina, M. Á., Cárdenas, C., Becerra, J., & Pérez Manríquez, C. (2024). Immunomodulatory, Antioxidant, and Potential Anticancer Activity of the Polysaccharides of the Fungus Fomitiporia chilensis. Molecules, 29(15), 3628. https://doi.org/10.3390/molecules29153628









