Facile One-Pot Synthesis and Anti-Microbial Activity of Novel 1,4-Dihydropyridine Derivatives in Aqueous Micellar Solution under Microwave Irradiation
Abstract
1. Introduction
2. Result and Discussion
2.1. Characterization of Aluminum Dodecyl Sulfate, Al(DS)3
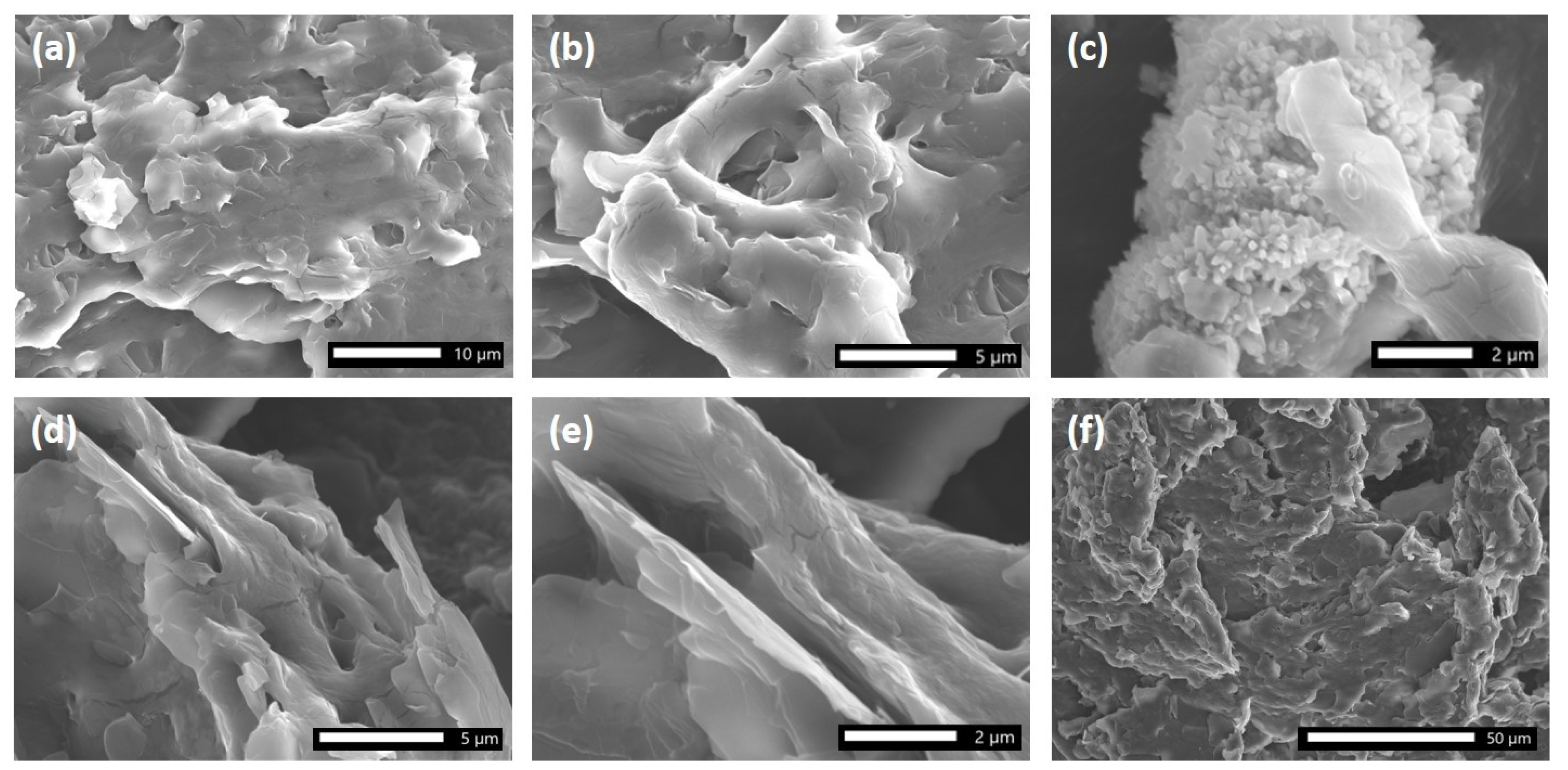
2.1.1. SEM Characterization
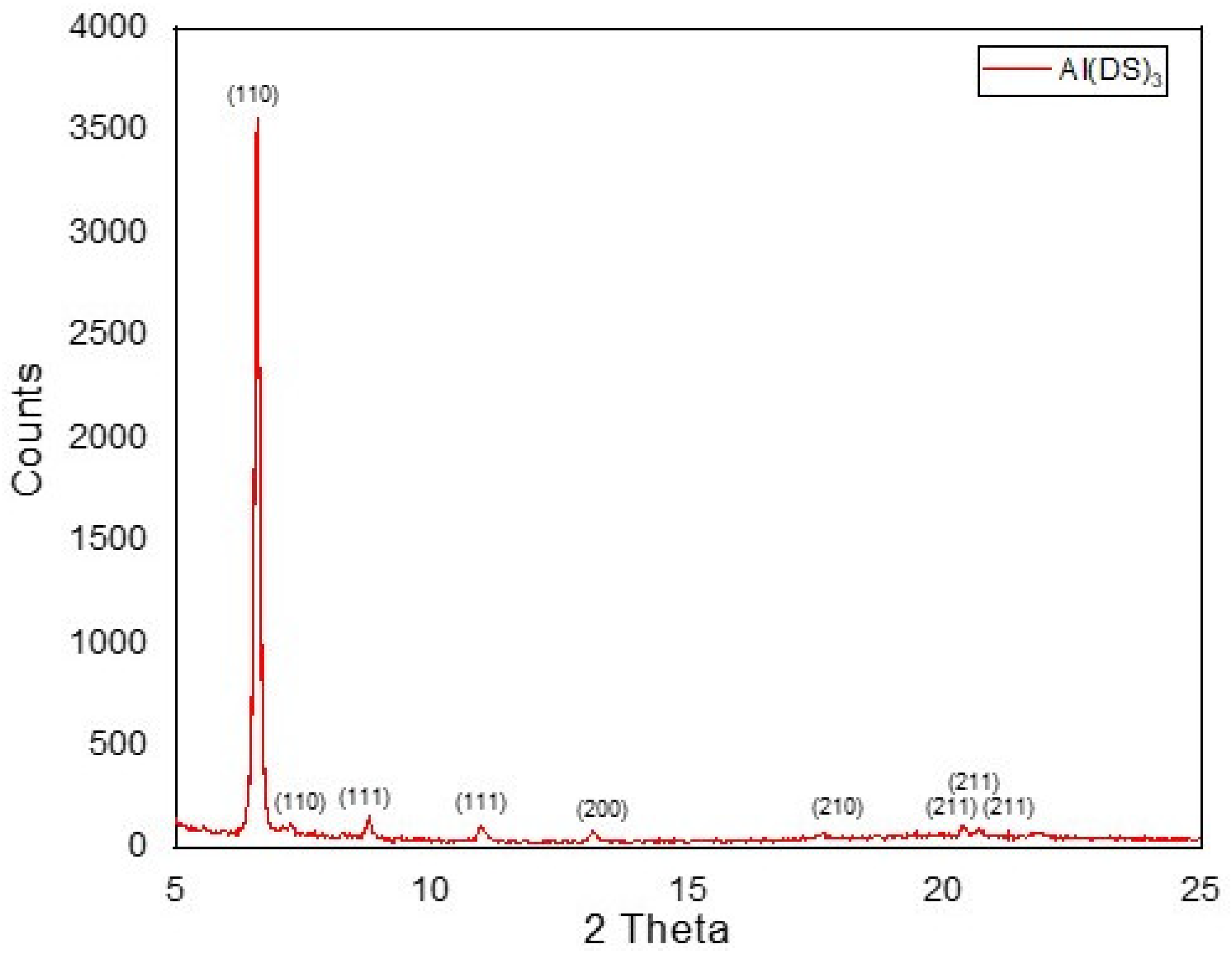
2.1.2. XRD Characterization
2.2. Preparation of [1,4-DHPs] 10-Amino-3,3,6,6-Tetramethyl-9-Aryl-3,4,6,7,9,10-Hexa-Hydroacridine-1,8(2H,5H)-Dione Derivatives (5a–n) Using Al(DS)3
2.3. Characterization of Diethyl 6-Amino-5-Cyano-4-Phenyl-1,4-Dihydropyridine-2,3-Dicarboxylate (5a) Using Al(DS)3
2.4. Anti-Microbial Activity
3. Experimental Section
3.1. Materials and Methods
3.1.1. Chemicals
3.1.2. Analytical Instruments
3.2. Synthesis of Aluminum Dodecyl Sulfate
3.3. Synthesis of 1,4-DHPs Derivatives (5a–n)
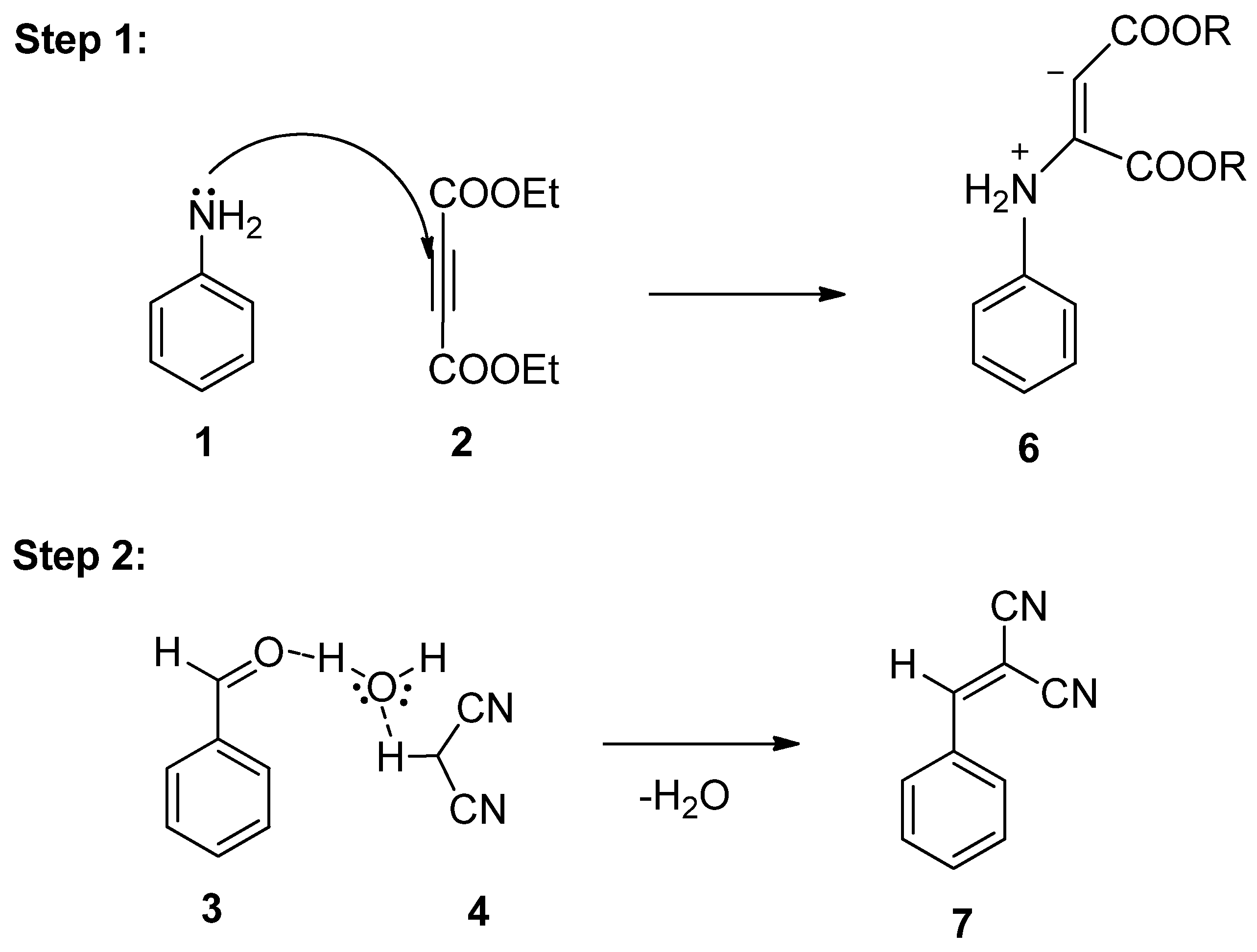
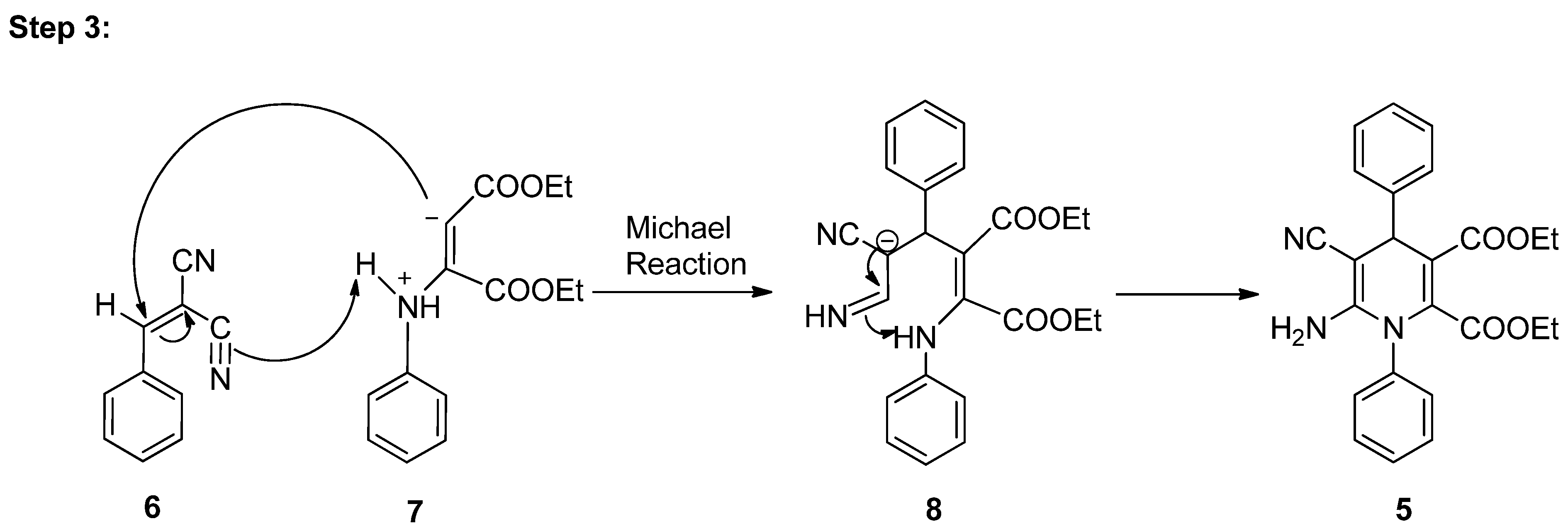
3.4. Plausible Mechanism
3.5. Anti-Microbial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirisha, K.; Achaiah, G.; Reddy, V.M. Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines. Arch. Pharm. 2010, 343, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Dodiya, D.; Dholariya, B.; Kataria, V.; Bhuva, V.; Shah, V. Synthesis and biological evaluation of some novel 1,4-dihydropyridines as potential antitubercular agents. Chem. Biol. Drug Des. 2011, 78, 881–886. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A.; Sharma, S.; Kumar, M.; Bhatia, G. Synthesis and biological evaluation of N-aryl-1,4-dihydropyridines as novel antidyslipidemic and antioxidant agents. Eur. J. Med. Chem. 2010, 45, 501–509. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Ragno, G. 1,4-dihydropyridine antihypertensive drugs: Recent advances in photostabilization strategies. Pharmaceutics 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.; Darweesh, A.F.; Elwahy, A.H.M.; Abdelhamid, I.A. Synthesis, characterization and antitumor activity of novel tetrapodal 1,4-dihydropyridines: P53 induction, cell cycle arrest and low damage effect on normal cells induced by genotoxic factor H2O2. RSC Adv. 2016, 6, 40900–40910. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Alinezhad, H.; Norouzi, M.; Baghery, S.; Akbari, M. Protic pyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water. J. Mol. Liq. 2013, 177, 44–48. [Google Scholar] [CrossRef]
- Mathur, R.; Negi, K.S.; Shrivastava, R.; Nair, R. Recent developments in the nanomaterial-catalyzed green synthesis of structurally diverse 1,4-dihydropyridines. RSC Adv. 2021, 11, 1376–1393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; A Johnson, B.; Ait-Daoud, N.; Wells, L.T. Effects of isradipine, a dihydropyridine-class calcium channel antagonist, on D-methamphetamine-induced cognitive and physiological changes in humans. Neuropsychopharmacology 2000, 22, 504–512. [Google Scholar] [CrossRef]
- Huai, Y.; Wang, H.; Zhao, L.; Zhao, L.; Pei, J. Lacidipine inhibits endoplasmic reticulum stress and myocardial remodeling induced by pressure overload in rat heart. Eur. J. Pharmacol. 2013, 718, 441–447. [Google Scholar] [CrossRef]
- Amenta, F.; Tomassoni, D.; Traini, E.; Mignini, F.; Veglio, F. Nicardipine: A hypotensive dihydropyridine-type calcium antagonist with a peculiar cerebrovascular profile. Clin. Exp. Hypertens. 2008, 30, 808–826. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, L.; Cai, L.L.; Zhang, Y. Catalyzing synthesis of chiral nitrendipine. Adv. Mater. Res. 2012, 518–523, 3943–3946. [Google Scholar] [CrossRef]
- Affeldt, R.F.; Benvenutti, E.V.; Russowsky, D. A new In-SiO2 composite catalyst in the solvent-free multicomponent synthesis of Ca2+ channel blockers nifedipine and nemadipine B. New J. Chem. 2012, 36, 1502–1511. [Google Scholar] [CrossRef]
- Hantzsch, A. Ueber die Synthese pyridinartiger Verbindungen aus Acetessigäther und Aldehydammoniak. Justus Liebigs Ann. Chem. 1882, 215, 1–82. [Google Scholar] [CrossRef]
- Jassem, A.M.; Almashal, F.A.K.; Mohammed, M.Q.; Jabir, H.A.S. A catalytic and green method for one-pot synthesis of new Hantzsch 1,4-dihydropyridines. SN Appl. Sci. 2020, 2, 359. [Google Scholar] [CrossRef]
- Machado, I.V.; dos Santos, J.R.; Januario, M.A.; Corrêa, A.G. Greener organic synthetic methods: Sonochemistry and heterogeneous catalysis promoted multicomponent reactions. Ultrason. Sonochem. 2021, 78, 105704. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, J.; Ghahremani, M.; Najafi, O. Synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives via Hantzsch reaction using nicotinic acid as a green and reusable catalyst. J. Mol. Struct. 2019, 1177, 525–535. [Google Scholar] [CrossRef]
- Koukabi, N.; Kolvari, E.; Khazaei, A.; Zolfigol, M.A.; Shirmardi-Shaghasemi, B.; Khavasi, H.R. Hantzsch reaction on free nano-Fe2O3 catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Chem. Commun. 2011, 47, 9230–9232. [Google Scholar] [CrossRef]
- Shaibuna, M.; Sreekumar, K. Dual solvent-catalyst role of deep eutectic solvents in Hantzsch dihydropyridine synthesis. Synth. Commun. 2021, 51, 1742–1753. [Google Scholar] [CrossRef]
- Elumalai, D.; Gnanasekaran, R.; Leelakrishnan, S.; Nachimuthu, G.; Kannan, T.; Paramasivam, T.P.; Jayabal, K. InCl3-Assisted Eco-Friendly Approach for N-Fused 1,4-Dihydropyridine Scaffolds via Ring Opening Michael Addition of Cyclic Nitroketene and Iminocoumarin: Synthesis and DFT Studies. ChemistrySelect 2018, 3, 2070–2079. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, S.K. Synthesis, utility and medicinal importance of 1,2- & 1,4-dihydropyridines. RSC Adv. 2017, 7, 2682–2732. [Google Scholar] [CrossRef]
- Sutarni, Y.D.S.Y.D.; Purwono, B.; Kunarti, E.S.K.E.S.; Mardjan, M.I.D. Fe/ZSM-5-Catalyzed-Synthesis of 1,4-Dihydropyridines under Ultrasound Irradiation and Their Antioxidant Activities. Sains Malays. 2023, 52, 1189–1202. [Google Scholar] [CrossRef]
- Rostamnia, S.; Morsali, A. Basic isoreticular nanoporous metal-organic framework for Biginelli and Hantzsch coupling: IRMOF-3 as a green and recoverable heterogeneous catalyst in solvent-free conditions. RSC Adv. 2014, 4, 10514–10518. [Google Scholar] [CrossRef]
- Parthiban, A.; Makam, P. 1,4-Dihydropyridine: Synthetic advances, medicinal and insecticidal properties. RSC Adv. 2022, 12, 29253–29290. [Google Scholar] [CrossRef]
- Zonouz, A.M.; Hosseini, S.B. Montmorillonite K10 clay: An efficient catalyst for Hantzsch synthesis of 1,4-dihydropyridine derivatives. Synth. Commun. 2008, 38, 290–296. [Google Scholar] [CrossRef]
- Sadeghi, B.; Namakkoubi, A.; Hassanabadi, A. BF3.SiO2 nanoparticles: A solid phase acidic catalyst for efficient one-pot Hantzsch synthesis of 1,4-dihydropyridines. J. Chem. Res. 2013, 37, 11–13. [Google Scholar] [CrossRef]
- Lee, J.H. Synthesis of Hantsch 1,4-dihydropyridines by fermenting bakers’ yeast. Tetrahedron Lett. 2005, 46, 7329–7330. [Google Scholar] [CrossRef]
- Sivamurugan, V.; Kumar, R.S.; Palanichamy, M.; Murugesan, V. Synthesis of Hantzsch 1,4-dihydropyridines under solvent-free condition using Zn[(L)proline]2 as Lewis acid catalyst. J. Heterocycl. Chem. 2005, 42, 969–974. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Basak, A.K.; Narsaiah, A.V. Three-component coupling reactions in ionic liquids: An improved protocol for the synthesis of 1,4-dihydropyridines. Green Chem. 2003, 5, 60–63. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. A grinding-induced catalyst- and solvent-free synthesis of highly functionalized 1,4-dihydropyridines via a domino multicomponent reaction. Green Chem. 2011, 13, 2017–2020. [Google Scholar] [CrossRef]
- Hesari, Z.; Hadavand, B.S. Deep Eutectic Solvent Based on Choline Chloride/Urea as an Efficient Catalytic System for the One-Pot Synthesis of Highly Functionalized 1,4-Dihydropyridines and Polysubstituted 4H- Chromenes. J. Appl. Chem. Res. 2019, 13, 97–106. [Google Scholar]
- Jeffrey, K.; Elizabeth, R.; Samuel, B.; Shannon, S.; Beata, D.; Bojarski, A.J.; Strekowski, L. Synthesis of 4-Substituted 2-(4-Methylpiperazino)pyrimidines and Quinazoline Analogs as Serotonin 5-HT 2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265. [Google Scholar] [CrossRef]
- Ramesh, R.; Lalitha, A. Facile and Green Chemistry Access to 5-aryl-1,2,4-Triazolidine-3-thiones in Aqueous Medium. ChemistrySelect 2016, 1, 2085–2089. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.H.S.; Maddila, S.N.; Jonnalagadda, S.B. Green synthesis and characterization of novel 1,2,4,5-tetrasubstituted imidazole derivatives with eco-friendly red brick clay as efficacious catalyst. Mol. Divers. 2020, 24, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Maddila, S.; Nagaraju, K.; Chinnam, S.; Jonnalagadda, S.B. Microwave-Assisted Multicomponent Reaction: A Green and Catalyst-Free Method for the Synthesis of Poly-Functionalized 1,4-Dihydropyridines. ChemistrySelect 2019, 4, 9451–9454. [Google Scholar] [CrossRef]
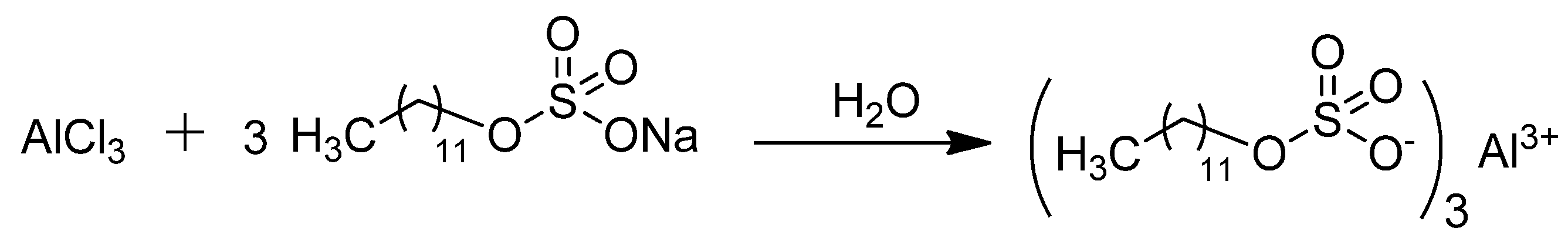

| Solvent | Time (min) | Yield a (%) |
|---|---|---|
| Ethanol | 10 | 87% |
| Glycerol | 8 | 91% |
| p-TSA + Ethanol | 8 | 89% |
| PEG | 9 | 90% |
| Water | 15 | 42% |
| Al(DS)3 + Water | 5 | 97% |
| SDS + Water | 6 | 92% |
| CH3CN | 6 | 95% |
| Ethylene glycol | 8 | 94% |
| Watt | Time (min) | Yield a (%) |
|---|---|---|
| 65 | 8 | 93% |
| 70 | 7 | 93% |
| 75 | 6 | 95% |
| 80 | 5 | 97% |
| 85 | 4 | 91% |
| 90 | 3 | 88% |
| 95 | 2 | 85% |
| Compound |  | Rf | Yield (%) | Melting Point (°C) | Literature Melting Point (°C) | |
|---|---|---|---|---|---|---|
| R1 | R2 | |||||
| 5a | H | H | 0.65 | 97 | 227–228 | - |
| 5b | 4-NO2 | H | 0.77 | 96 | 240 | - |
| 5c | 3-NO2 | H | 0.73 | 94 | 242 | - |
| 5d | 4-Cl | H | 0.70 | 94 | 237–239 | - |
| 5e | 4-Br | H | 0.57 | 95 | 267–268 | - |
| 5f | 4-Me | H | 0.61 | 93 | 230–231 | - |
| 5g | 4-OMe | H | 0.63 | 94 | 238–240 | - |
| 5h | H | Ph | 0.66 | 96 | 173–174 | 170–172 [29] |
| 5i | 4-NO2 | Ph | 0.69 | 94 | 171–173 | 172–174 [30] |
| 5j | 3-NO2 | Ph | 0.71 | 95 | 270–272 | - |
| 5k | 4-Cl | Ph | 0.67 | 96 | 186–187 | 188–189 [31] |
| 5l | 4-Br | Ph | 0.60 | 96 | 151–153 | 152–154 [32] |
| 5m | 4-Me | Ph | 0.61 | 93 | 286–287 | - |
| 5n | 4-OMe | Ph | 0.65 | 92 | 253–255 | - |
| Compound | Gram (+ve) Bacteria | Gram (−ve) Bacteria | Fungi | |||||
|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. pyogenes | E. coli | K. pneumonia | S. aureus | A. janus | A. niger | A. sclerotiorum | |
| 5a | 16 | 8 | 8 | 8 | 16 | 8 | 16 | 8 |
| 5b | 4 | 4 | 4 | 8 | 4 | 4 | 4 | 8 |
| 5c | 4 | 8 | 4 | 4 | 8 | 8 | 8 | 32 |
| 5d | 16 | 8 | 16 | 32 | 32 | 16 | 32 | 32 |
| 5e | 16 | 32 | - | 64 | 64 | 32 | 16 | 16 |
| 5f | 32 | 8 | 16 | 8 | 16 | 16 | 16 | 8 |
| 5g | 16 | 16 | 8 | 8 | 16 | 8 | 16 | – |
| 5h | 64 | 16 | 32 | 16 | 32 | 32 | 32 | 16 |
| 5i | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 5j | 4 | 8 | 8 | 4 | 4 | 8 | 4 | 4 |
| 5k | 8 | 16 | 16 | - | – | 16 | 16 | 32 |
| 5l | 32 | 64 | 128 | 16 | 32 | 16 | 32 | 16 |
| 5m | 8 | 16 | 64 | 32 | 64 | 16 | 16 | 32 |
| 5n | 16 | 8 | 8 | 16 | 8 | 16 | 32 | 16 |
| Amoxicillin | 4 | 4 | 4 | 4 | 4 | – | – | – |
| Fluconazole | – | – | – | – | – | 2 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, A.; Kaur, N.; Kaur, M.; Singh, K.; Sohal, H.S.; Han, H.; Bhowmik, P.K. Facile One-Pot Synthesis and Anti-Microbial Activity of Novel 1,4-Dihydropyridine Derivatives in Aqueous Micellar Solution under Microwave Irradiation. Molecules 2024, 29, 1115. https://doi.org/10.3390/molecules29051115
Goswami A, Kaur N, Kaur M, Singh K, Sohal HS, Han H, Bhowmik PK. Facile One-Pot Synthesis and Anti-Microbial Activity of Novel 1,4-Dihydropyridine Derivatives in Aqueous Micellar Solution under Microwave Irradiation. Molecules. 2024; 29(5):1115. https://doi.org/10.3390/molecules29051115
Chicago/Turabian StyleGoswami, Asmita, Navneet Kaur, Manvinder Kaur, Kishanpal Singh, Harvinder Singh Sohal, Haesook Han, and Pradip K. Bhowmik. 2024. "Facile One-Pot Synthesis and Anti-Microbial Activity of Novel 1,4-Dihydropyridine Derivatives in Aqueous Micellar Solution under Microwave Irradiation" Molecules 29, no. 5: 1115. https://doi.org/10.3390/molecules29051115
APA StyleGoswami, A., Kaur, N., Kaur, M., Singh, K., Sohal, H. S., Han, H., & Bhowmik, P. K. (2024). Facile One-Pot Synthesis and Anti-Microbial Activity of Novel 1,4-Dihydropyridine Derivatives in Aqueous Micellar Solution under Microwave Irradiation. Molecules, 29(5), 1115. https://doi.org/10.3390/molecules29051115






