Exploring the Effect of Milk Fat on Fermented Milk Flavor Based on Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) and Multivariate Statistical Analysis
Abstract
1. Introduction
2. Results and Discussion
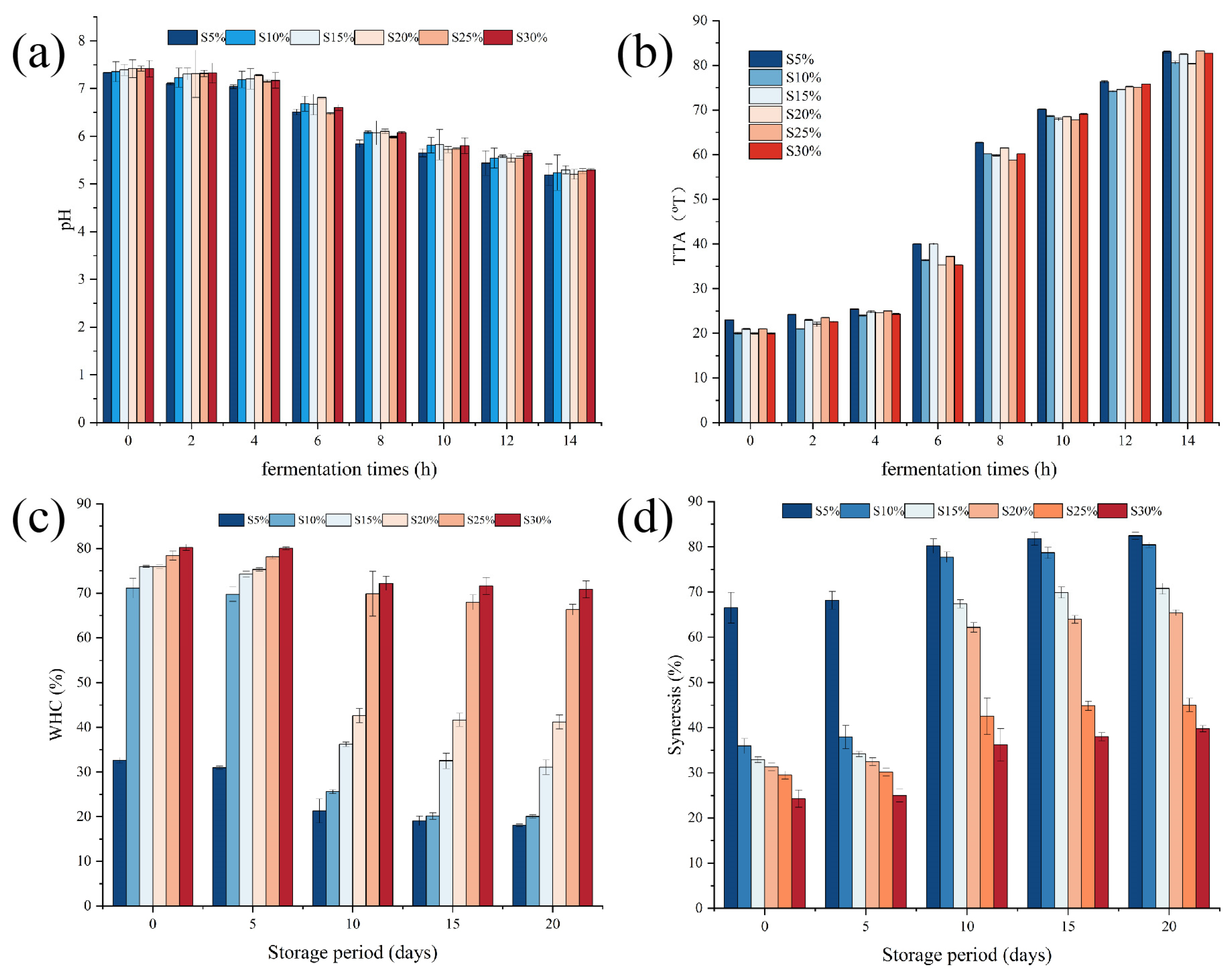
2.1. Effects of Milk Fat on pH and Total Titratable Acidity of Fermented Milk
2.2. Effects of Milk Fat on Water-Holding Capacity, Syneresis, and Color of Fermented Milk
2.3. Effects of Milk Fat on Texture of Fermented Milk
2.4. Results of the Single-Factor Experiment and Response Surface Methodology
2.5. Analysis of E-Nose
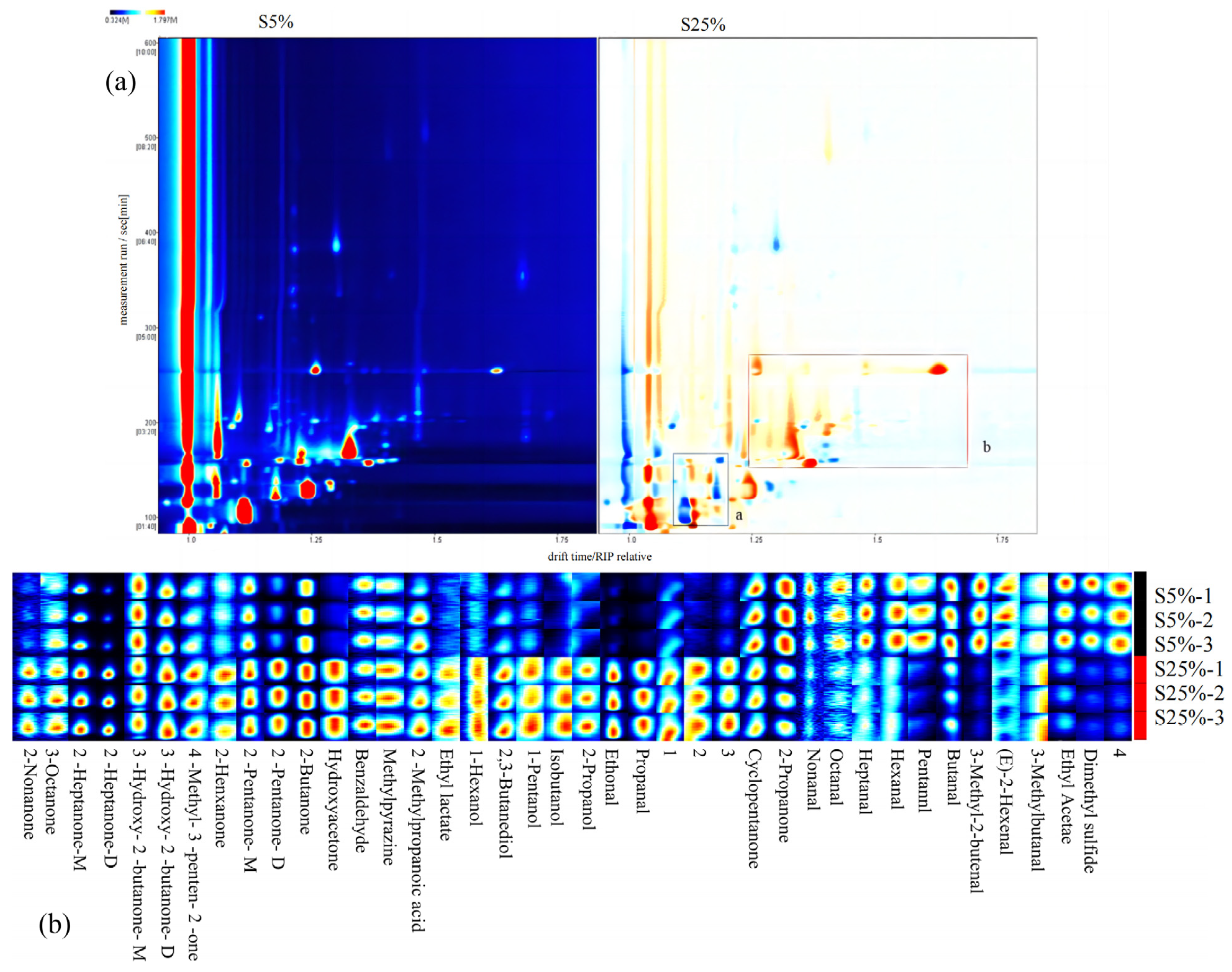
2.6. Analysis of GC-IMS
2.6.1. The Volatile Components in Two Fermented Milks Identified by GC-IMS
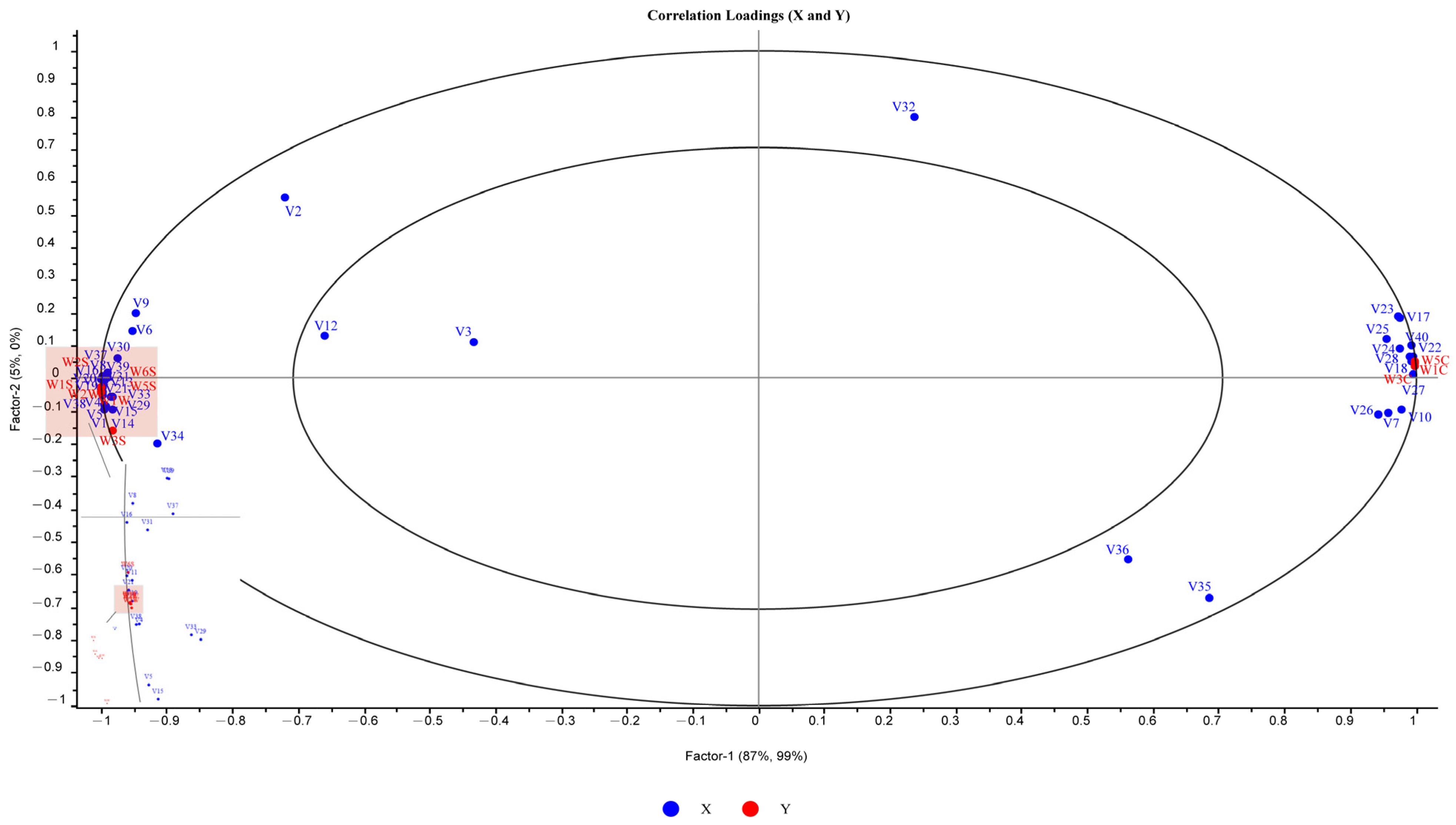
2.6.2. Multivariate Statistical Analysis by GC-IMS
2.6.3. The Key VOC Analysis by ROAV
2.7. Correlation Analysis of VOCs with an E-Nose
3. Materials and Methods
3.1. Materials and Reagents
3.2. Fermented Milk Preparation
3.3. Physicochemical Determinations
3.4. Texture Profile Analysis
3.5. Experimental Design of Single-Factor Experiments and Response Surface Methodology
3.6. Electronic Nose (E-Nose) Analysis
3.7. Analysis of Volatile Compounds (VOCs) by GC-IMS
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Luo, J.; Tian, H.; Li, J.; Zhang, X.; Chen, Z.; Li, M.; Loor, J. Rapid communication: Lipid metabolic gene expression and triacylglycerol accumulation in goat mammary epithelial cells are decreased by inhibition of SREBP-1. J. Anim. Sci. 2018, 96, 2399–2407. [Google Scholar] [CrossRef]
- Aleksandra, W.; Joanna, B.; Olga, B.; Kamila, S.; Jolanta, S.; Katarzyna, K.; Emilia, P.; Monika, A.Z.; Jadwiga, H.; Gabriela, O. Lipid Profile, Lipase Bioactivity, and Lipophilic Antioxidant Content in High Pressure Processed Donor Human Milk. Nutrients 2019, 11, 1972. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, M.; Zhang, L.; Zan, L.; Yang, W. Bta-miR-34b controls milk fat biosynthesis via the Akt/mTOR signaling pathway by targeting RAI14 in bovine mammary epithelial cells. J. Anim. Sci. Biotechnol. 2021, 12, 83. [Google Scholar] [CrossRef]
- Miles, E.; Calder, P. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, H.; Cao, J.; Ye, B.; Zhao, Y.; Liu, L. Effect of milk fat and its main fatty acids on oxidation and glycation level of milk. J. Food Sci. Technol. 2023, 60, 720–731. [Google Scholar] [CrossRef]
- Wang, M.; Fan, M.; Zheng, A.; Wei, C.; Liu, D.; Thaku, K.; Wei, Z. Characterization of a fermented dairy, sour cream: Lipolysis and the release profile of flavor compounds. Food Chem. 2023, 423, 136299. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, P.; Ehsani, M.; Mizani, M.; Givianrad, M. Characterization of a traditional ripened cheese, Kurdish Kope: Lipolysis, lactate metabolism, the release profile of volatile compounds, and correlations with sensory characteristics. J. Food Sci. 2021, 86, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, H.; Wu, Z.; Zhao, L.; Huang, W.; Shi, B.; He, J.; Wang, S.; Zhong, K. Sensory Description and Consumer Hedonic Perception of Ultra-High Temperature (UHT) Milk. Foods 2022, 11, 1350. [Google Scholar] [CrossRef]
- Olagunju, A.; Omoba, O.; Enujiugha, V.; Alashi, A.; Aluko, R. Technological Properties of Acetylated Pigeon Pea Starch and Its Stabilized Set-Type Yoghurt. Foods 2020, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, S.; He, Q.; Hu, J.; Zheng, J. Changes in Proteolysis in Fermented Milk Produced by Streptococcus thermophilus in Co-Culture with Lactobacillus plantarum or Bifidobacterium animalis subsp. lactis during Refrigerated Storage. Molecules 2019, 24, 3699. [Google Scholar] [CrossRef]
- Yadav, V.; Gupta, V.K.; Meena, G.S. Effect of culture levels, ultrafiltered retentate addition, total solid levels and heat treatments on quality improvement of buffalo milk plain set yoghurt. J. Food Sci. Technol. 2018, 55, 1648–1655. [Google Scholar] [CrossRef]
- Trieu, K.; Bhat, S.; Dai, Z.; Leander, K.; Gigante, B.; Qian, F.; Korat, A.; Sun, Q.; Pan, X.; Laguzzi, F.; et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, R.; Ren, F.; Mao, X. Addition of buttermilk improves the flavor and volatile compound profiles of low-fat yogurt. LWT 2018, 98, 9–17. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, H.; Zheng, F. Taste distinguishing of yoghurts based on electronic tongue. Sci. Technol. Food Ind. 2015, 36.04, 49–52+56. [Google Scholar] [CrossRef]
- Christos, S.; Franco, B.; Eugenio, A.; Erna, S.; Luca, C.; Tilmann, D.; Flavia, G. PTR-TOF-MS Analysis for Influence of Milk Base Supplementation on Texture and Headspace Concentration of Endogenous Volatile Compounds in Yogurt. Food Bioprocess Technol. 2012, 5, 2085–2097. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Shi, H.; Xue, C.; Wang, Q.; Yu, F.; Xue, Y.; Wang, Y.; Li, Z. The flavor profile changes of Pacific oysters (Crassostrea gigas) in response to salinity during depuration. Food Chem. X 2022, 16, 100485. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Li, G.; Zhao, Y.; Zhang, J.; Niu, C.; Zhang, L.; Zhang, X.; Ying, D.; Li, S. Antioxidant activity of prebiotic ginseng polysaccharides combined with potential probiotic Lactobacillus plantarum C88. Int. J. Food Sci. Technol. 2015, 50, 1673–1682. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Liu, X.; Fan, X.; Li, J.; Zhu, D.; Liu, H. Effects of different probiotic fermentations on the quality, soy isoflavone and equol content of soy protein yogurt made from soy whey and soy embryo powder. LWT 2022, 157, 113096. [Google Scholar] [CrossRef]
- Zeineb, J.; Olfa, O.; Slah, Z.; Touhami, K.; El Halima, H. Co-fermentation process strongly affect the nutritional, texture, syneresis, fatty acids and aromatic compounds of dromedary UF-yogurt. J. Food Sci. Technol. 2020, 58, 1727–1739. [Google Scholar] [CrossRef]
- Ge, X.; Tang, N.; Huang, Y.; Chen, X.; Dong, M.; Rui, X.; Zhang, Q.; Li, W. Fermentative and physicochemical properties of fermented milk supplemented with sea buckthorn (Hippophae eleagnaceae L.). LWT 2022, 153, 112484. [Google Scholar] [CrossRef]
- Molaee, P.; Reza, F.; Mortazavian, A.; Sarem, N.; Ali, M.; Akbar, G.; Khorshidian, N. Comparative effects of probiotic and paraprobiotic addition on microbiological, biochemical and physical properties of yogurt. Food Res. Int. 2020, 140, 110030. [Google Scholar] [CrossRef]
- Zhao, X.; Dun, K.; Zhang, Y. Effect of Inulin on the Texture, Rheological Properties, and Microstructure of Synbiotic Yogurt. Sci. Technol. Food Ind. 2023, 44, 72–77. [Google Scholar] [CrossRef]
- Ayyash, M.; Abdalla, A.; Abu-Jdayil, B.; Huppertz, T.; Bhaskaracharya, R.; Al-Mardeai, S.; Mairpady, A.; Ranasinghe, A.; Al-Nabulsi, A. Rheological properties of fermented milk from heated and high pressure-treated camel milk and bovine milk. LWT 2022, 156, 113029. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Sadiq, F.; Cai, Y.; Fan, D.; Zhang, H.; Zhao, J.; Chen, W. Identification of Key Aroma Compounds in Type I Sourdough-Based Chinese Steamed Bread: Application of Untargeted Metabolomics Analysisp. Int. J. Mol. Sci. 2019, 20, 818. [Google Scholar] [CrossRef] [PubMed]
- Aljewicz, M.; Majcher, M.; Nalepa, B. A Comprehensive Study of the Impacts of Oat β-Glucan and Bacterial Curdlan on the Activity of Commercial Starter Culture in Yogurt. Molecules 2020, 25, 5411. [Google Scholar] [CrossRef]
- Huang, J.; Kong, X.; Chen, Y.; Chen, J. Assessment of flavor characteristics in snakehead (Ophiocephalus argus Cantor) surimi gels affected by atmospheric cold plasma treatment using GC-IMS. Front. Nutr. 2023, 9, 1086426. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, T.; Lv, Z.; Liu, L.; Wang, Y.; Li, X.; Fan, Z.; Li, B. Differences between Kazak Cheeses Fermented by Single and Mixed Strains Using Untargeted Metabolomics. Foods 2022, 11, 966. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, T.; Zhang, Y.; Song, B.; Pang, X.; Lv, J. Effects of Monascus on Proteolysis, Lipolysis, and Volatile Compounds of Camembert-Type Cheese during Ripening. Foods 2022, 11, 1662. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Liu, D.; Zhang, W.; Ma, L.; Wang, S. Physicochemical properties of a new structural lipid from the enzymatical incorporation of flaxseed oil into mutton tallow. Heliyon 2022, 8, e09615. [Google Scholar] [CrossRef]
- Martín-Garcia, A.; Comas-Basté, O.; Riu-Aumatell, M.; Latorre-Moratalla, M.; López-Tamames, E. Changes in the Volatile Profile of Wheat Sourdough Produced with the Addition of Cava Lees. Molecules 2022, 27, 3588. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Wang, X.; Wang, R.; Ren, F.; Zhang, Q.; Shan, Y.; Ding, S. Characterization of Volatile Component Changes in Jujube Fruits during Cold Storage by Using Headspace-Gas Chromatography-Ion Mobility Spectrometry. Molecules 2019, 24, 3904. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Pan, Q.; Wang, X.; Li, P.; Cai, K.; Chen, C. Effect of salt mixture on flavor of reduced-sodium restructured bacon with ultrasound treatment. Food Sci. Nutr. 2020, 8, 3857–3871. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Lu, H.; Liu, J.; Song, C.; Xu, Z.; Yang, H.; Shang, X.; Feng, T. Comparative Aroma Profile Analysis and Development of a Sensory Aroma Lexicon of Seven Different Varieties of Flammulina velutipes. Front. Nutr. 2022, 9, 827825. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tian, T.; Liu, Z.; Yu, H.; Yuan, H.; Tian, H. Comparative Study on Volatile Flavor of Chinese Acid-curd Cheese Using Sensory Evaluation. Food Sci. 2023, 44, 228–236. [Google Scholar] [CrossRef]
- Feng, T.; Shui, M.; Song, S.; Zhuang, H.; Sun, M.; Yao, L. Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach. Molecules 2019, 24, 3305. [Google Scholar] [CrossRef] [PubMed]
- Sakin-Yilmazer, M.; Dirim, S.; Di, P.; Kaymak-Ertekin, F. Yoghurt with candied chestnut: Freeze drying, physical, and rheological behaviour. J. Food Sci. Technol. 2014, 51, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, P.; Zhou, J.; He, J.; Cai, J. The Improvement of Sensory and Bioactive Properties of Yogurt with the Introduction of Tartary Buckwheat. Foods 2022, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, Z.; Lin, L.; Zhang, C.; Wei, C. Analysis of the Effect of Mixed Fermentation on the Quality of Distilled Jujube Liquor by Gas Chromatography-Ion Mobility Spectrometry and Flavor Sensory Description. Foods 2023, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Hu, H.; Cui, A.; Tang, H.; Liu, Y. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT 2021, 149, 111963. [Google Scholar] [CrossRef]
- Cao, L.; Jia, P.; Liu, H.; Kang, S.; Jiang, S.; Pang, M. Effects of High-Canolol Phenolic Extracts on Fragrant Rapeseed Oil Quality and Flavor Compounds during Frying. Foods 2023, 12, 827. [Google Scholar] [CrossRef] [PubMed]



| Milk Fat Content | Hardness/1/N | Cohesiveness | Springiness/mm | Gumminess/N | Chewiness/mj |
|---|---|---|---|---|---|
| 5% | 0.034 ± 0.006 d | 0.644 ± 0.032 ab | 9.564 ± 0.030 d | 0.023 ± 0.003 d | 0.264 ± 0.096 c |
| 10% | 0.049 ± 0.004 c | 0.621 ± 0.014 b | 11.249 ± 0.200 c | 0.030 ± 0.002 c | 0.371 ± 0.063 c |
| 15% | 0.060 ± 0.016 bc | 0.636 ± 0.007 ab | 13.794 ± 0.255 b | 0.043 ± 0.003 b | 0.645 ± 0.009 b |
| 20% | 0.062 ± 0.006 bc | 0.664 ± 0.04 a | 14.067 ± 1.680 ab | 0.045 ± 0.004 b | 0.689 ± 0.069 b |
| 25% | 0.070 ± 0.004 b | 0.650 ± 0.098 a | 14.995 ± 0.230 ab | 0.045 ± 0.003 b | 0.716 ± 0.212 b |
| 30% | 0.103 ± 0.023 a | 0.664 ± 0.005 a | 15.432 ± 0.608 a | 0.065 ± 0.004 a | 1.033 ± 0.047 a |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 491.74 | 14 | 35.12 | 19.71 | <0.0001 | *** |
| A—Content of cultures | 10.08 | 1 | 10.08 | 5.66 | 0.0322 | * |
| B—Content of fats | 12.00 | 1 | 12.00 | 6.73 | 0.0212 | * |
| C—Fermentation time | 4.08 | 1 | 4.08 | 2.29 | 0.1524 | |
| D—Fermentation temperature | 33.33 | 1 | 33.33 | 18.70 | 0.0007 | *** |
| AB | 4.00 | 1 | 4.00 | 2.24 | 0.1563 | |
| AC | 2.25 | 1 | 2.25 | 1.26 | 0.2801 | |
| AD | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | |
| BC | 0.2500 | 1 | 0.2500 | 0.1403 | 0.7136 | |
| BD | 30.25 | 1 | 30.25 | 16.97 | 0.0010 | *** |
| CD | 2.25 | 1 | 2.25 | 1.26 | 0.2801 | |
| A2 | 2.96 | 1 | 2.96 | 1.66 | 0.2187 | |
| B2 | 182.21 | 1 | 182.21 | 102.24 | <0.0001 | *** |
| C2 | 173.71 | 1 | 173.71 | 97.47 | <0.0001 | *** |
| D2 | 165.42 | 1 | 165.42 | 92.82 | <0.0001 | *** |
| Residual | 24.95 | 14 | 1.78 | |||
| Lack of Fit | 21.75 | 10 | 2.17 | 2.72 | 0.1737 | |
| Pure Error | 3.20 | 4 | 0.8000 | |||
| Cor Total | 516.69 | 28 | ||||
| R2 | 0.9517 | Std. Dev. | 1.33 | |||
| Adjusted R2 | 0.9034 | Mean | 78.90 | |||
| Predicted R2 | 0.7479 | C.V. % | 1.69 | |||
| Adeq Precision | 16.4218 |
| Count | Compound | CAS# | Formula | MW | RI a | Rt [sec] b | Dt c [RIPrel] | Comment |
|---|---|---|---|---|---|---|---|---|
| V1 | 2-Nonanone | C821556 | C9H18O | 142.2 | 1091.6 | 481.454 | 1.40809 | |
| V2 | 3-Octanone | C106683 | C8H16O | 128.2 | 985.5 | 335.61 | 1.30991 | |
| V3 | Benzaldehyde | C100527 | C7H6O | 106.1 | 954.8 | 309.657 | 1.14748 | |
| V4 | 2-Heptanone | C110430 | C7H14O | 114.2 | 888.3 | 254.655 | 1.26113 | monomer |
| V5 | 2-Heptanone | C110430 | C7H14O | 114.2 | 888.3 | 254.655 | 1.63297 | dimer |
| V6 | Ethyl lactate | C97643 | C5H10O3 | 118.1 | 832.7 | 225.752 | 1.15009 | |
| V7 | Cyclopentanone | C120923 | C5H8O | 84.1 | 792.5 | 204.866 | 1.1021 | |
| V8 | 2,3-Butanediol | C513859 | C4H10O2 | 90.1 | 790.5 | 203.795 | 1.38336 | |
| V9 | Methylpyrazine | C109080 | C5H6N2 | 94.1 | 791.5 | 204.331 | 1.07755 | |
| V10 | 3-Hydroxy-2-butanone | C513860 | C4H8O2 | 88.1 | 703.4 | 167.648 | 1.06192 | monomer |
| V11 | 3-Hydroxy-2-butanone | C513860 | C4H8O2 | 88.1 | 703.4 | 167.648 | 1.32867 | dimer |
| V12 | 2-Methylpropanoic acid | C79312 | C4H8O2 | 88.1 | 766.5 | 193.353 | 1.1646 | |
| V13 | 4-Methyl-3-penten-2-one | C141797 | C6H10O | 98.1 | 789.4 | 203.26 | 1.43582 | |
| V14 | 2-Pentanone | C107879 | C5H10O | 86.1 | 664.8 | 155.331 | 1.11773 | monomer |
| V15 | 2-Pentanone | C107879 | C5H10O | 86.1 | 665.8 | 155.598 | 1.36885 | dimer |
| V16 | 2-Butanone | C78933 | C4H8O | 72.1 | 556.3 | 126.68 | 1.24385 | |
| V17 | Butanal | C123728 | C4H8O | 72.1 | 597.8 | 137.658 | 1.28961 | |
| V18 | 2-Propanone | C67641 | C3H6O | 58.1 | 475.2 | 105.259 | 1.11326 | |
| V19 | Ethanol | C64175 | C2H6O | 46.1 | 419.4 | 90.532 | 1.04741 | |
| V20 | Propanal | C123386 | C3H6O | 58.1 | 516.7 | 116.237 | 1.04295 | |
| V21 | Hydroxyacetone | C116096 | C3H6O2 | 74.1 | 625.2 | 144.888 | 1.04183 | |
| V22 | 3-Methyl-2-butenal | C107868 | C5H8O | 84.1 | 773.8 | 196.298 | 1.09094 | |
| V23 | Ethyl Acetate | C141786 | C4H8O2 | 88.1 | 598.9 | 137.926 | 1.09429 | |
| V24 | Pentanal | C110623 | C5H10O | 86.1 | 687.1 | 161.221 | 1.41684 | |
| V25 | Hexanal | C66251 | C6H12O | 100.2 | 784.8 | 200.85 | 1.25612 | |
| V26 | Heptanal | C111717 | C7H14O | 114.2 | 898.4 | 261.9 | 1.33202 | |
| V27 | (E)-2-Hexenal | C6728263 | C6H10O | 98.1 | 814.7 | 216.38 | 1.17688 | |
| V28 | Dimethyl sulfide | C75183 | C2H6S | 62.1 | 510.5 | 114.595 | 0.95577 | |
| V29 | 2-Propanol | C67630 | C3H8O | 60.1 | 527.5 | 119.089 | 1.20946 | |
| V30 | 1-Pentanol | C71410 | C5H12O | 88.1 | 756.8 | 189.41 | 1.25209 | |
| V31 | Isobutanol | C78831 | C4H10O | 74.1 | 601.6 | 138.652 | 1.16475 | |
| V32 | 3-Methylbutanal | C590863 | C5H10O | 86.1 | 644.6 | 150.02 | 1.16267 | |
| V33 | 1-Hexanol | C111273 | C6H14O | 102.2 | 868.3 | 244.257 | 1.32146 | |
| V34 | 2-Hexanone | C591786 | C6H12O | 100.2 | 777.4 | 197.77 | 1.18483 | |
| 35 | Nonanal | C124196 | C9H18O | 142.2 | 1109.1 | 506.666 | 1.48711 | |
| 36 | Octanal | C124130 | C8H16O | 128.2 | 1004.2 | 355.979 | 1.41163 | |
| 37 | 1 | unidentified | nd d | nd | 767.2 | 193.62 | 1.33537 | |
| 38 | 2 | unidentified | nd | nd | 775.7 | 197.10 | 1.41238 | |
| 39 | 3 | unidentified | nd | nd | 754.7 | 188.53 | 1.4001 | |
| 40 | 4 | unidentified | nd | nd | 721.8 | 175.15 | 1.40233 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; Tian, Y.; Tao, L.; Xie, J.; Wang, M.; Zhang, F.; Yu, Z.; Sheng, J.; Zhao, C. Exploring the Effect of Milk Fat on Fermented Milk Flavor Based on Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) and Multivariate Statistical Analysis. Molecules 2024, 29, 1099. https://doi.org/10.3390/molecules29051099
Tan C, Tian Y, Tao L, Xie J, Wang M, Zhang F, Yu Z, Sheng J, Zhao C. Exploring the Effect of Milk Fat on Fermented Milk Flavor Based on Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) and Multivariate Statistical Analysis. Molecules. 2024; 29(5):1099. https://doi.org/10.3390/molecules29051099
Chicago/Turabian StyleTan, Chunlei, Yang Tian, Liang Tao, Jing Xie, Mingming Wang, Feng Zhang, Zhijin Yu, Jun Sheng, and Cunchao Zhao. 2024. "Exploring the Effect of Milk Fat on Fermented Milk Flavor Based on Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) and Multivariate Statistical Analysis" Molecules 29, no. 5: 1099. https://doi.org/10.3390/molecules29051099
APA StyleTan, C., Tian, Y., Tao, L., Xie, J., Wang, M., Zhang, F., Yu, Z., Sheng, J., & Zhao, C. (2024). Exploring the Effect of Milk Fat on Fermented Milk Flavor Based on Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) and Multivariate Statistical Analysis. Molecules, 29(5), 1099. https://doi.org/10.3390/molecules29051099






