The Promising Role of Polyphenols in Skin Disorders
Abstract
1. Introduction
Source of the Data
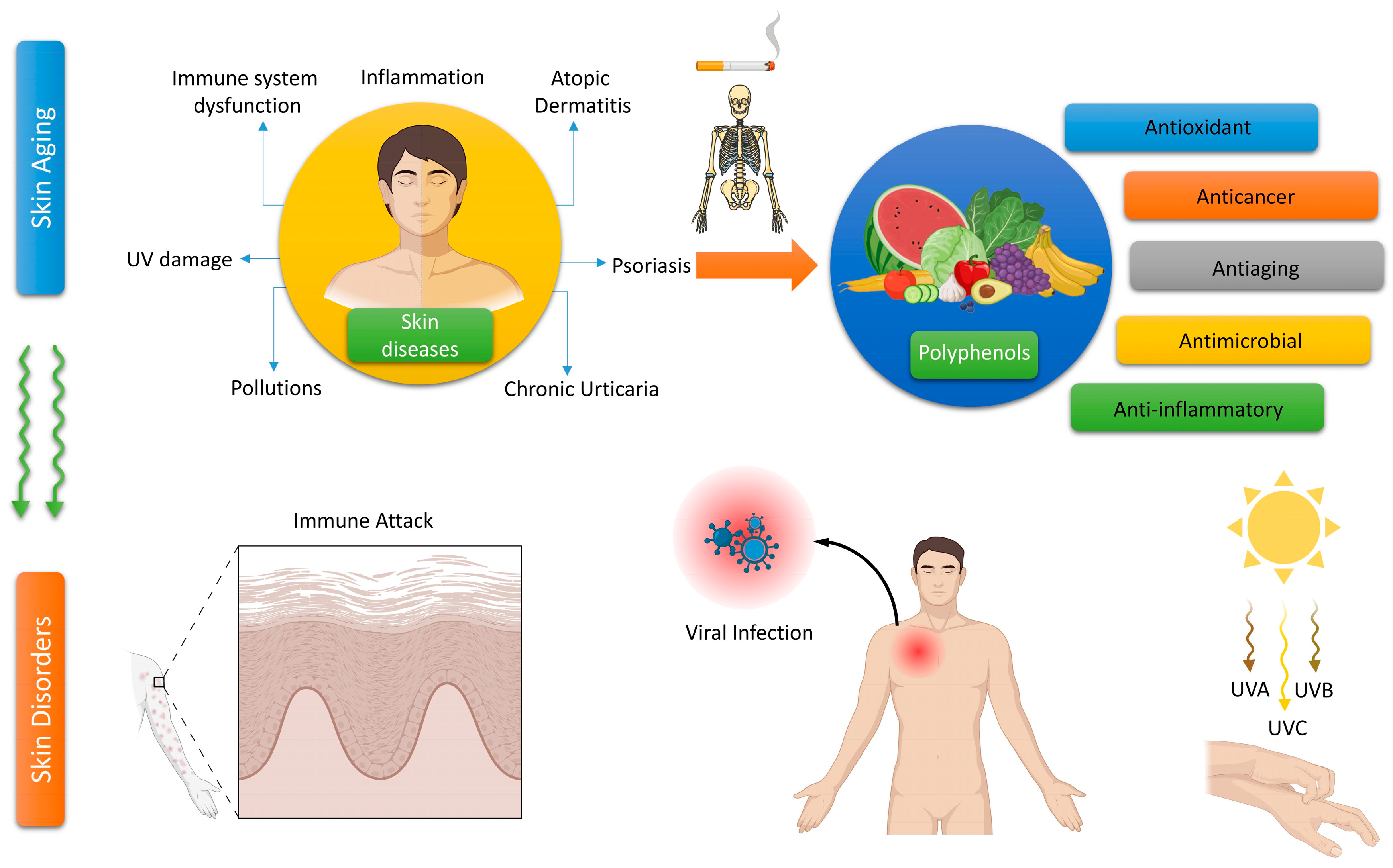
2. Polyphenols and Their Importance to Skin Health
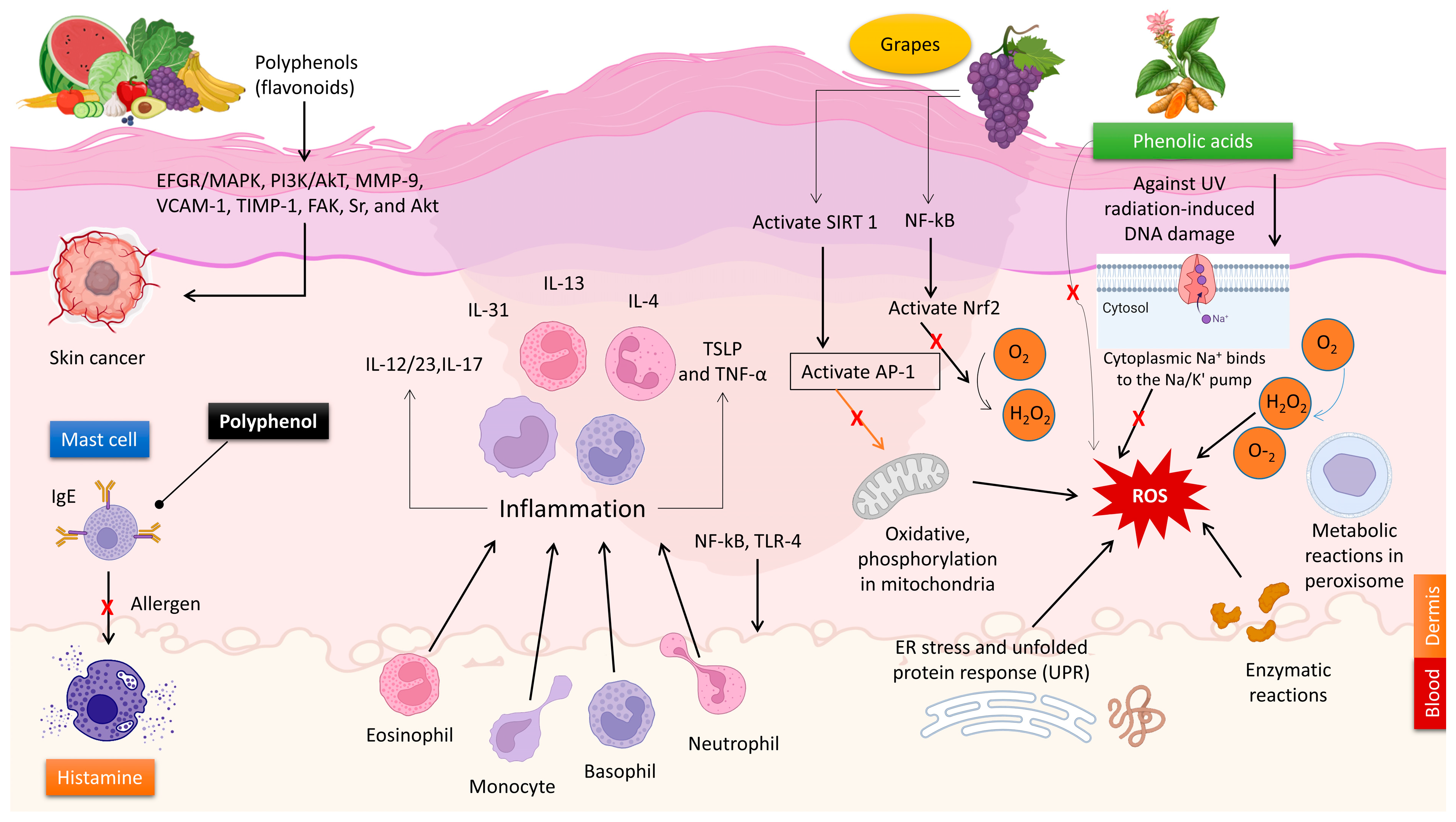
2.1. The Antioxidant Characteristics of Polyphenols
2.2. The Anti-Inflammatory Characteristics of Polyphenols
2.3. The Antimicrobial Characteristics of Polyphenols
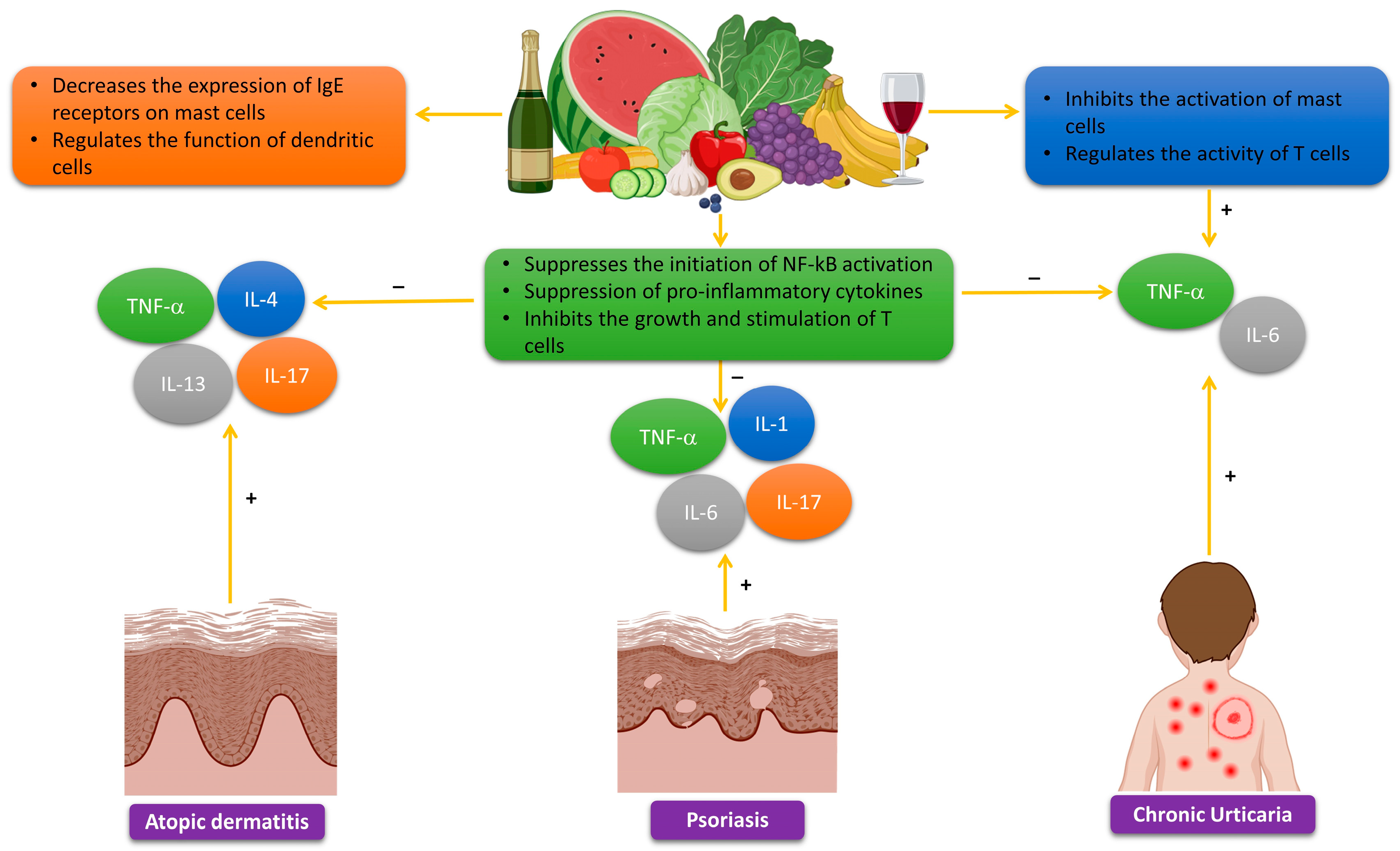
2.4. The Effects of Polyphenols on Allergic Reactions
2.5. Skin Cancer Prevention through Polyphenols
2.6. The Application of Polyphenols in UV Skin Protection
2.7. The Use of Polyphenols in Anti-Aging Cosmetics
3. Polyphenols and Their Significance in Skin Disease Therapy
3.1. Polyphenols for the Treatment of Vitiligo
3.2. Polyphenols for the Treatment of Atopic Dermatitis
3.3. Polyphenols for the Treatment of Acne Vulgaris
3.4. Polyphenols in the Treatment of Psoriasis
3.5. Polyphenols in the Treatment of Chronic Urticaria
4. Assessment of Plant Polyphenols for Skin Disease Therapy
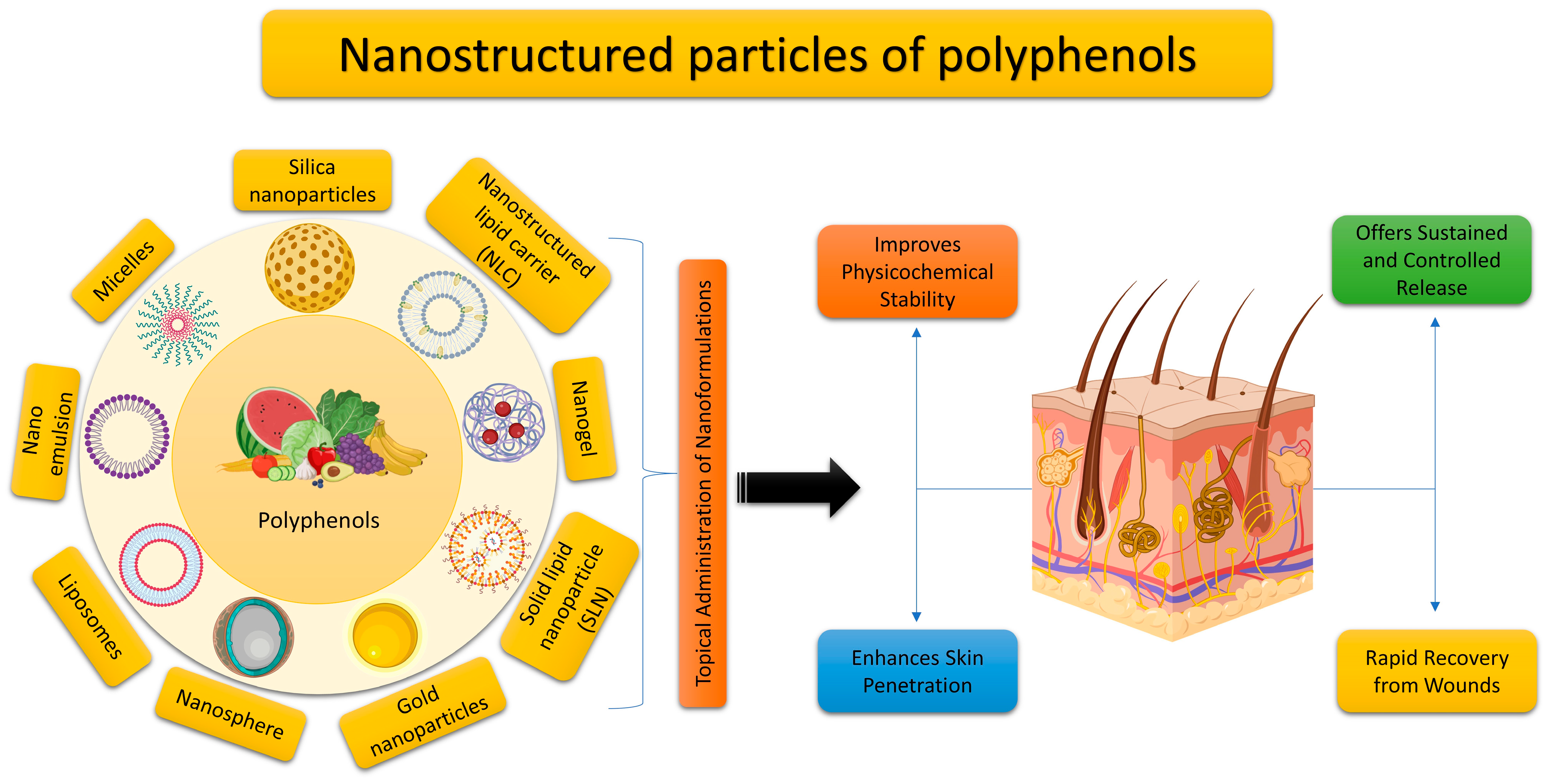
5. Utilizing Nano Delivery Systems for the Topical Application of Plant Polyphenols
6. Conclusions and Prospects for the Future
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.A.; Karimi, J.; Talebi, S.S.; Piri, H. The Association of COVID-19 and Reactive Oxygen Species Modulator 1 (ROMO1) with Oxidative Stress. Chonnam Med. J. 2022, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zarbafian, M.; Dayan, S.; Fabi, S.G. Teachings from COVID-19 and aging-An oxidative process. J. Cosmet. Dermatol. 2020, 19, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Krutmann, J. Air pollution (particulate matter and nitrogen dioxide) and skin aging. Hautarzt 2019, 70, 158–162. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxid. Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Yu, X.M.; Shoaib, M.; Cheng, X.R.; Cui, Y.L.; Hussain, S.; Yan, J.; Zhou, J.; Chen, Q.; Gu, Y.F.; Zou, L.K.; et al. Role of rhizobia in promoting non-enzymatic antioxidants to mitigate nitrogen-deficiency and nickel stresses in Pongamia pinnata. Ecotoxicol. Environ. Saf. 2022, 241, 113789. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Nevinsky, G.A. Essential Protective Role of Catalytically Active Antibodies (Abzymes) with Redox Antioxidant Functions in Animals and Humans. Int. J. Mol. Sci. 2022, 23, 3898. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.-K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local Melatoninergic System as the Protector of Skin Integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Boehm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczynski, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Izykowska, I.; Piotrowska, A.; Podhorska-Okolow, M.; Cegielski, M.; Zabel, M.; Dziegiel, P. The protective role of melatonin in the course of UV exposure. Postep. Hig. I Med. Dosw. 2008, 62, 23–27. [Google Scholar]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Xian, D.; Guo, M.; Xu, J.; Yang, Y.; Zhao, Y.; Zhong, J. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021, 26, 134–146. [Google Scholar] [CrossRef]
- Rubio, C.P.; Cerón, J.J. Spectrophotometric assays for evaluation of Reactive Oxygen Species (ROS) in serum: General concepts and applications in dogs and humans. BMC Vet. Res. 2021, 17, 226. [Google Scholar] [CrossRef]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef]
- Tsuchida, K.; Kobayashi, M. Oxidative stress in human facial skin observed by ultraweak photon emission imaging and its correlation with biophysical properties of skin. Sci. Rep. 2020, 10, 9626. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Tran, J.T.; Diaz, M.J.; Rodriguez, D.; Kleinberg, G.; Aflatooni, S.; Palreddy, S.; Abdi, P.; Taneja, K.; Batchu, S.; Forouzandeh, M. Evidence-Based Utility of Adjunct Antioxidant Supplementation for the Prevention and Treatment of Dermatologic Diseases: A Comprehensive Systematic Review. Antioxidants 2023, 12, 1503. [Google Scholar] [CrossRef]
- Wölfle, U.; Bauer, G.; Meinke, M.C.; Lademann, J.; Schempp, C.M. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol. Physiol. 2014, 27, 316–332. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovener. 2012, 21, 33–36. [Google Scholar]
- Pai, V.V.; Shukla, P.; Kikke, N.N. Antioxidants in dermatology. Indian Dermatol. Online J. 2014, 5, 210–214. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Rhie, G.; Shin, M.H.; Seo, J.Y.; Choi, W.W.; Cho, K.H.; Kim, K.H.; Park, C.; Eun, H.C.; Chung, J.H. Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Investig. Dermatol. 2001, 117, 1212–1217. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R.G. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Ndiaye, M.A.; Nihal, M.; Wood, G.S.; Ahmad, N. Skin, reactive oxygen species, and circadian clocks. Antioxid. Redox Signal. 2014, 20, 2982–2996. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Asad, M.H.B.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longevity. Oxid. Med. Cell. Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef]
- Sriram, R.; Gopal, V. Aging Skin and Natural Bioactives that Impede Cutaneous Aging: A Narrative Review. Indian J. Dermatol. 2023, 68, 424. [Google Scholar]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Passali, D.; Spinosi, M.C.; Crisanti, A.; Bellussi, L.M. Mometasone furoate nasal spray: A systematic review. Multidiscip. Respir. Med. 2016, 11, 18. [Google Scholar] [CrossRef]
- Michalak-Stoma, A.; Pietrzak, A.; Szepietowski, J.C.; Zalewska-Janowska, A.; Paszkowski, T.; Chodorowska, G. Cytokine network in psoriasis revisited. Eur. Cytokine Netw. 2011, 22, 160–168. [Google Scholar] [CrossRef]
- van de Kerkhof, P.C.; Griffiths, C.E.; Reich, K.; Leonardi, C.L.; Blauvelt, A.; Tsai, T.F.; Gong, Y.; Huang, J.; Papavassilis, C.; Fox, T. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 2016, 75, 83–98.e4. [Google Scholar] [CrossRef]
- Strober, B.; Leonardi, C.; Papp, K.A.; Mrowietz, U.; Ohtsuki, M.; Bissonnette, R.; Ferris, L.K.; Paul, C.; Lebwohl, M.; Braun, D.K.; et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: Etanercept comparisons and integrated data. J. Am. Acad. Dermatol. 2017, 76, 432–440.e17. [Google Scholar] [CrossRef]
- Farahnik, B.; Beroukhim, K.; Abrouk, M.; Nakamura, M.; Zhu, T.H.; Singh, R.; Lee, K.; Bhutani, T.; Koo, J. Brodalumab for the Treatment of Psoriasis: A Review of Phase III Trials. Dermatol. Ther. 2016, 6, 111–124. [Google Scholar] [CrossRef]
- Targan, S.R.; Feagan, B.; Vermeire, S.; Panaccione, R.; Melmed, G.Y.; Landers, C.; Li, D.; Russell, C.; Newmark, R.; Zhang, N.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients with Moderate-to-Severe Crohn’s Disease. Am. J. Gastroenterol. 2016, 111, 1599–1607. [Google Scholar] [CrossRef]
- Bucio-Noble, D.; Kautto, L.; Krisp, C.; Ball, M.S.; Molloy, M.P. Polyphenol extracts from dried sugarcane inhibit inflammatory mediators in an in vitro colon cancer model. J. Proteom. 2018, 177, 1–10. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef]
- Middleton, E., Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Wei, B.L.; Weng, J.R.; Chiu, P.H.; Hung, C.F.; Wang, J.P.; Lin, C.N. Antiinflammatory flavonoids from Artocarpus heterophyllus and Artocarpus communis. J. Agric. Food Chem. 2005, 53, 3867–3871. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef]
- Karasawa, K.; Uzuhashi, Y.; Hirota, M.; Otani, H. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. J. Agric. Food Chem. 2011, 59, 11287–11293. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Zoratti, M. Resveratrol and health: The starting point. Chembiochem 2012, 13, 1256–1259. [Google Scholar] [CrossRef]
- Akyol, S.; Ozturk, G.; Ginis, Z.; Armutcu, F.; Yigitoglu, M.R.; Akyol, O. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): Therapeutic perspectives. Nutr. Cancer 2013, 65, 515–526. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, T. Beyond skin white spots: Vitiligo and associated comorbidities. Front. Med. 2023, 10, 1072837. [Google Scholar] [CrossRef]
- Qaâdan, F.; Nahrstedt, A.; Schmidt, M.; Mansoor, K. Polyphenols from Ginkgo biloba. Sci. Pharm. 2010, 78, 897–907. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, M.; Randy, A.; Nam, E.J.; Nho, C.W. Effects of Hovenia dulcis Thunb. extract and methyl vanillate on atopic dermatitis-like skin lesions and TNF-α/IFN-γ-induced chemokines production in HaCaT cells. J. Pharm. Pharmacol. 2016, 68, 1465–1479. [Google Scholar] [CrossRef]
- Boos, A.C.; Hagl, B.; Schlesinger, A.; Halm, B.E.; Ballenberger, N.; Pinarci, M.; Heinz, V.; Kreilinger, D.; Spielberger, B.D.; Schimke-Marques, L.F.; et al. Atopic dermatitis, STAT3- and DOCK8-hyper-IgE syndromes differ in IgE-based sensitization pattern. Allergy 2014, 69, 943–953. [Google Scholar] [CrossRef]
- Lee, D.Y.; Hwang, C.J.; Choi, J.Y.; Park, M.H.; Song, M.J.; Oh, K.W.; Son, D.J.; Lee, S.H.; Han, S.B.; Hong, J.T. Inhibitory Effect of Carnosol on Phthalic Anhydride-Induced Atopic Dermatitis via Inhibition of STAT3. Biomol. Ther. 2017, 25, 535–544. [Google Scholar] [CrossRef]
- D’Antuono, I.; Carola, A.; Sena, L.M.; Linsalata, V.; Cardinali, A.; Logrieco, A.F.; Colucci, M.G.; Apone, F. Artichoke Polyphenols Produce Skin Anti-Age Effects by Improving Endothelial Cell Integrity and Functionality. Molecules 2018, 23, 2729. [Google Scholar] [CrossRef]
- Carolina Oliveira Dos Santos, L.; Spagnol, C.M.; Guillot, A.J.; Melero, A.; Corrêa, M.A. Caffeic acid skin absorption: Delivery of microparticles to hair follicles. Saudi Pharm. J. 2019, 27, 791–797. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M.; et al. Proanthocyanidins: Target compounds as antibacterial agents. J. Agric. Food Chem. 2008, 56, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Synergistic In Vitro Antimicrobial Activity of Pomegranate Rind Extract and Zinc (II) against Micrococcus luteus under Planktonic and Biofilm Conditions. Pharmaceutics 2021, 13, 851. [Google Scholar] [CrossRef]
- Betts, J.W.; Hornsey, M.; Higgins, P.G.; Lucassen, K.; Wille, J.; Salguero, F.J.; Seifert, H.; la Ragione, R.M. Restoring the activity of the antibiotic aztreonam using the polyphenol epigallocatechin gallate (EGCG) against multidrug-resistant clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2019, 68, 1552–1559. [Google Scholar] [CrossRef]
- Álvarez, N.M.; Ortíz, A.A.; Martínez, O.C. In Vitro antibacterial activity of Curcuma longa (Zingiberaceae) against nosocomial bacteria in Montería, Colombia. Rev. Biol. Trop. 2016, 64, 1201–1208. [Google Scholar]
- Wang, J.; Zhang, X.; Gao, L.; Wang, L.; Song, F.; Zhang, L.; Wan, Y. The synergistic antifungal activity of resveratrol with azoles against Candida albicans. Lett. Appl. Microbiol. 2021, 72, 688–697. [Google Scholar] [CrossRef]
- Stróżek, J.; Samoliński, B.K.; Kłak, A.; Gawińska-Drużba, E.; Izdebski, R.; Krzych-Fałta, E.; Raciborski, F. The indirect costs of allergic diseases. Int. J. Occup. Med. Environ. Health 2019, 32, 281–290. [Google Scholar] [CrossRef]
- Canonica, G.W.; Cox, L.; Pawankar, R.; Baena-Cagnani, C.E.; Blaiss, M.; Bonini, S.; Bousquet, J.; Calderón, M.; Compalati, E.; Durham, S.R.; et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ. J. 2014, 7, 6. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Dębińska, A.; Sozańska, B. Dietary Polyphenols—Natural Bioactive Compounds with Potential for Preventing and Treating Some Allergic Conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef]
- Persia, F.A.; Mariani, M.L.; Fogal, T.H.; Penissi, A.B. Hydroxytyrosol and oleuropein of olive oil inhibit mast cell degranulation induced by immune and non-immune pathways. Phytomedicine 2014, 21, 1400–1405. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yan, G.H. Silibinin attenuates mast cell-mediated anaphylaxis-like reactions. Biol. Pharm. Bull. 2009, 32, 868–875. [Google Scholar] [CrossRef]
- Sato, Y.; Akiyama, H.; Matsuoka, H.; Sakata, K.; Nakamura, R.; Ishikawa, S.; Inakuma, T.; Totsuka, M.; Sugita-Konishi, Y.; Ebisawa, M.; et al. Dietary carotenoids inhibit oral sensitization and the development of food allergy. J. Agric. Food Chem. 2010, 58, 7180–7186. [Google Scholar] [CrossRef]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Sunami, E.; Takahashi, K.; Nagawa, H. Catechin inhibits adhesion and migration of peripheral blood B cells by blocking CD11b. Immunopharmacol. Immunotoxicol. 2011, 33, 391–397. [Google Scholar] [CrossRef]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef]
- Isacescu, E.; Chiroi, P.; Zanoaga, O.; Nutu, A.; Budisan, L.; Pirlog, R.; Atanasov, A.G.; Berindan-Neagoe, I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants 2023, 12, 407. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Tyciakova, S.; Valova, V.; Svitkova, B.; Matuskova, M. Overexpression of TNFα Induces Senescence, Autophagy and Mitochondrial Dysfunctions in Melanoma Cells. BMC Cancer 2021, 21, 507. [Google Scholar] [CrossRef]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate Fruit Extract Modulates UV-B-Mediated Phosphorylation of Mitogen-Activated Protein Kinases and Activation of Nuclear Factor Kappa B in Normal Human Epidermal Keratinocytes Paragraph Sign. Photochem. Photobiol. 2005, 81, 38–45. [Google Scholar]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Katiyar, S.K.; Ballestas, M.E.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin Inhibits Human Melanoma Cell Invasion through Promotion of Mesenchymal to Epithelial Transition and by Targeting MAPK and NFκB Signaling Pathways. PLoS ONE 2014, 9, e86338. [Google Scholar] [CrossRef]
- Xu, A.; Lee, J.; Zhao, Y.; Wang, Y.; Li, X.; Xu, P. Potential Effect of EGCG on the Anti-Tumor Efficacy of Metformin in Melanoma Cells. J. Zhejiang Univ. Sci. B 2021, 22, 548–562. [Google Scholar] [CrossRef]
- Xiao, P.; Yang, J.; Sun, J.; Guo, L. Anticancer Effects of Kaempferol in A375 Human Malignant Melanoma Cells Are Mediated via Induction of Apoptosis, Cell Cycle Arrest, Inhibition of Cell Migration and Downregula-Tion of m-TOR/PI3K/AKT Pathway. J. Balk. Union Oncol. 2018, 23, 218–223. [Google Scholar]
- Kim, H.; Park, J.; Tak, K.H.; Bu, S.Y.; Kim, E. Chemopreventive Effects of Curcumin on Chemically Induced Mouse Skin Carcinogenesis in BK5.Insulin-like Growth Factor-1 Transgenic Mice. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 883–892. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic Acid Inhibits Proliferation and Migration, Promotes Apoptosis and Enhances Cisplatin Sensitivity of Melanoma Cells through Inhibiting ADAM17/EGFR/AKT/GSK3β Axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Lim, J.H.; Lim, B.O. Apigenin Attenuates Melanoma Cell Migration by Inducing Anoikis through Integrin and Focal Adhesion Kinase Inhibition. Molecules 2015, 20, 21157–21166. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, J.; Wu, Q.; Qian, J.; Yang, C.; Bo, P. Genistein Inhibits the Growth and Regulates the Migration and Invasion Abilities of Melanoma Cells via the FAK/Paxillin and MAPK Pathways. Oncotarget 2017, 8, 21674–21691. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chang, L.; Qu, Y.; Liang, J.; Jin, W.; Xia, X. Tea Polyphenols Inhibit the Proliferation, Migration, and Invasion of Melanoma Cells through the down-Regulation of TLR4. Int. J. Immunopathol. Pharmacol. 2018, 32, 0394632017739531. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C. Interferons α and β in Cancer: Therapeutic Opportunities from New Insights. Nat. Rev. Drug Discov. 2019, 18, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, L.; Sun, Y.; Sun, L.; Yin, Q.; Deng, S.; Niu, L.; Lou, F.; Wang, Z.; Xu, Z.; et al. Melanoma Suppression by Quercein Is Correlated with RIG-I and Type I Interferon Signaling. Biomed. Pharmacother. 2020, 125, 109984. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.R.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix Metalloproteinase-9 (MMP-9) and Its Inhibitors in Cancer: A Minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Zhang, X.M.; Huang, S.P.; Xu, Q. Quercetin Inhibits the Invasion of Murine Melanoma B16-BL6 Cells by Decreasing pro-MMP-9 via the PKC Pathway. Cancer Chemother. Pharmacol. 2004, 53, 82–88. [Google Scholar] [CrossRef]
- Cao, H.H.; Tse, A.K.W.; Kwan, H.Y.; Yu, H.; Cheng, C.Y.; Su, T.; Fong, W.F.; Yu, Z.L. Quercetin Exerts Anti-Melanoma Activities and Inhibits STAT3 Signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin Inhibits Proliferation and Induces Apoptosis of Human Melanoma Cells In Vivo and In Vitro by Suppressing MMP-2 and MMP-9 through the PI3K/AKT Pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Choi, E.O.; Cho, E.J.; Jeong, J.W.; Park, C.; Hong, S.H.; Hwang, H.J.; Moon, S.K.; Son, C.G.; Kim, W.J.; Choi, Y.H. Baicalein Inhibits the Migration and Invasion of B16F10 Mouse Melanoma Cells through Inactivation of the PI3K/Akt Signaling Pathway. Biomol. Ther. 2017, 25, 213–221. [Google Scholar] [CrossRef]
- Trapp, V.; Parmakhtiar, B.; Papazian, V.; Willmott, L.; Fruehauf, J.P. Anti-Angiogenic Effects of Resveratrol Mediated by Decreased VEGF and Increased TSP1 Expression in Melanoma-Endothelial Cell Co-Culture. Angiogenesis 2010, 13, 305–315. [Google Scholar] [CrossRef]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic Acid Exerts Anti-Angiogenic and Anti-Tumor Activity by Targeting Fibroblast Growth Factor Receptor 1-Mediated Angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef]
- Lee, S.H.W.; Koo, B.S.E.; Park, S.Y.I.; Kim, Y.M.I. Anti-Angiogenic Effects of Resveratrol in Combination with 5-Fluorouracil on B16 Murine Melanoma Cells. Mol. Med. Rep. 2015, 12, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Byun, S.; Lee, E.J.; Kim, J.E.; Bode, A.M.; Dong, Z.; Lee, H.J. Myricetin Inhibits UVB-Induced Angiogenesis by Regulating PI-3 Kinase In Vivo. Carcinogenesis 2010, 31, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Peng, B.; Nayak, Y.; Wang, C.; Si, F.; Liu, X.; Dou, J.; Xu, H.; Peng, G. Baicalein and Baicalin Promote Melanoma Apoptosis and Senescence via Metabolic Inhibition. Front. Cell Dev. Biol. 2020, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.H.; Jang, H.; Park, S.Y.; Seol, J.W. Naringenin Exerts Anticancer Effects by Inducing Tumor Cell Death and Inhibiting Angiogenesis in Malignant Melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Agilan, B.; Rajendra Prasad, N.; Kanimozhi, G.; Karthikeyan, R.; Ganesan, M.; Mohana, S.; Velmurugan, D.; Ananthakrishnan, D. Caffeic Acid Inhibits Chronic UVB-Induced Cellular Proliferation Through JAK-STAT3 Signaling in Mouse Skin. Photochem. Photobiol. 2016, 92, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Keravis, T.; Favot, L.; Abusnina, A.A.; Anton, A.; Justiniano, H.; Soleti, R.; Alibrahim, E.A.; Simard, G.; Andriantsitohaina, R.; Lugnier, C. Delphinidin Inhibits Tumor Growth by Acting on VEGF Signalling in Endothelial Cells. PLoS ONE 2015, 10, e0145291. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Chu, J.H.; Kwan, H.Y.; Su, T.; Yu, H.; Cheng, C.Y.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; et al. Inhibition of the STAT3 Signaling Pathway Contributes to Apigenin-Mediated Anti-Metastatic Effect in Melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, P.; Chou, H.; Li, J.; Wang, K.; Li, H. Herbacetin Suppressed MMP9 Mediated Angiogenesis of Malignant Melanoma through Blocking EGFR-ERK/AKT Signaling Pathway. Biochimie 2019, 162, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Kora´c, R.; Khambholja, K. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phyther. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Wach, A.; Pyrzyńska, K.; Biesaga, M. Quercetin content in some food and herbal samples. Food Chem. 2007, 100, 699–704. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J.V. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B Biol. 2006, 84, 21–27. [Google Scholar] [CrossRef]
- Ozkur, M.K.; Bozkurt, M.S.; Balabanli, B.; Aricioglu, A.; Ilter, N.; Gurer, M.A.; Inaloz, H.S. The effect of EGb 761 on lipid peroxide levels and superoxide dismutase activity in sunburn. Photodermatol. Photoimmunol. Photomed. 2002, 18, 117–120. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Mantena, S.K.; Meeran, S.M. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS ONE 2011, 6, e21410. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.O.; Wang, Y.; Stebbins, W.G.; Gao, D.; Zhou, X.; Phelps, R.; Lebwohl, M.; Wei, H. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis 2006, 27, 1627–1635. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wu, W.; Chen, H.C.; Fang, H. Genistein protects against UVB-induced senescence-like characteristics in human dermal fibroblast by p66Shc down-regulation. J. Dermatol. Sci. 2010, 58, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef] [PubMed]
- Widyarini, S. Protective effect of the isoflavone equol against DNA damage induced by ultraviolet radiation to hairless mouse skin. J. Vet. Sci. 2006, 7, 217–223. [Google Scholar] [CrossRef]
- Choi, H.S.; Park, E.D.; Park, Y.; Han, S.H.; Hong, K.B.; Suh, H.J. Topical application of spent coffee ground extracts protects skin from ultraviolet B-induced photoaging in hairless mice. Photochem. Photobiol. Sci. 2016, 15, 779–790. [Google Scholar] [CrossRef]
- Kano, M.; Kubota, N.; Masuoka, N.; Hori, T.; Miyazaki, K.; Ishikawa, F. Oral administration of fermented soymilk products protects the skin of hairless mice against ultraviolet damage. Nutrients 2016, 8, 514. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the use of botanicals in anti-aging cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Wu, Y.; Wei, H.-C.; Xu, Y.-Y.; Jia, L.-L.; Chen, J.; Yang, X.-S.; Dong, G.-H.; Gao, X.-H.; Chen, H.-D. Protective effects of green tea extracts on photoaging and photommunosuppression. Ski. Res. Technol. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Jung, E.Y.; Shin, K.-S.; Yu, K.-W.; Chang, U.J.; Suh, H.J. Tannase-converted green tea catechins and their anti-wrinkle activity in humans. J. Cosmet. Dermatol. 2013, 12, 137–143. [Google Scholar] [CrossRef]
- Chuarienthong, P.; Lourith, N.; Leelapornpisid, P. Clinical efficacy comparison of anti-wrinkle cosmetics containing herbal flavonoids. Int. J. Cosmet. Sci. 2010, 32, 99–106. [Google Scholar] [CrossRef]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, L.-L.; Zheng, Y.-N.; Xu, X.-G.; Luo, Y.-J.; Wang, B.; Chen, J.Z.S.; Gao, X.-H.; Chen, H.-D.; Matsui, M.; et al. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 345–350. [Google Scholar] [CrossRef]
- Woon, C.G.; Jin, J.H.; Kyung, S.J.; Hwoon, B.J.; Mi, K.Y.; Chool, B.Y. Skin Anti-aging Effects of a Cream Containing Resveratryl Triacetate (RTA). J. Soc. Cosmet. Sci. Korea 2018, 44, 161–170. [Google Scholar]
- Brinke, A.S.; Janssens-Böcker, C.; Kerscher, M. Skin Anti-Aging Benefits of a 2% Resveratrol Emulsion. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 155–168. [Google Scholar] [CrossRef]
- Moyano-Mendez, J.R.; Fabbrocini, G.; De Stefano, D.; Mazzella, C.; Mayol, L.; Scognamiglio, I.; Carnuccio, R.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Enhanced antioxidant effect of trans-resveratrol: Potential of binary systems with polyethylene glycol and cyclodextrin. Drug Dev. Ind. Pharm. 2014, 40, 1300–1307. [Google Scholar] [CrossRef]
- Cornacchione, S.; Sadick, N.S.; Neveu, M.; Talbourdet, S.; Lazou, K.; Viron, C.; Renimel, I.; de Quéral, D.; Kurfurst, R.; Schnebert, S.; et al. In vivo skin antioxidant effect of a new combination based on a specific Vitis vinifera shoot extract and a biotechnological extract. J. Drugs Dermatol. 2007, 6, s8–s13. [Google Scholar]
- Sharif, A.; Akhtar, N.; Khan, M.S.; Menaa, A.; Menaa, B.; Khan, B.A.; Menaa, F. Formulation and evaluation on human skin of a water-in-oil emulsion containing Muscat hamburg black grape seed extract. Int. J. Cosmet. Sci. 2015, 37, 253–258. [Google Scholar] [CrossRef]
- Seneschal, J.; Boniface, K.; D’Arino, A.; Picardo, M. An Update on Vitiligo Pathogenesis. Pigment. Cell Melanoma Res. 2021, 34, 236–243. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, S.; He, Y.; Nian, Q.; Lei, J.; Yao, Y.; Guo, J.; Zeng, J. Plant Derived Compounds as Promising Therapeutics for Vitiligo. Front. Pharmacol. 2021, 12, 685116. [Google Scholar] [CrossRef]
- Dańczak-Pazdrowska, A.; Gornowicz-Porowska, J.; Polańska, A.; Krajka-Kuźniak, V.; Stawny, M.; Gostyńska, A.; Rubiś, B.; Nourredine, S.; Ashiqueali, S.; Schneider, A.; et al. Cellular senescence in skin-related research: Targeted signaling pathways and naturally occurring therapeutic agents. Aging Cell 2023, 22, e13845. [Google Scholar] [CrossRef] [PubMed]
- Shivasaraun, U.V.; Sureshkumar, R.; Karthika, C.; Puttappa, N. Flavonoids as adjuvant in psoralen based photochemotherapy in the management of vitiligo/leucoderma. Med. Hypotheses 2018, 121, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Gianfaldoni, S.; Tchernev, G.; Lotti, J.; Wollina, U.; Satolli, F.; Rovesti, M.; França, K.; Lotti, T. Unconventional Treatments for Vitiligo: Are They (Un) Satisfactory? Open Access Maced. J. Med. Sci. 2018, 6, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, M.; Song, Y. Molecular mechanism of vitiligo treatment by bailing tablet based on network pharmacology and molecular docking. Medicine 2022, 101, e29661. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Yoshiyuki Osamura, R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar] [PubMed]
- Wang, J.Y.; Chen, H.; Wang, Y.Y.; Wang, X.Q.; Chen, H.Y.; Zhang, M.; Tang, Y.; Zhang, B. Network pharmacological mechanisms of Vernonia anthelmintica (L.) in the treatment of vitiligo: Isorhamnetin induction of melanogenesis via up-regulation of melanin-biosynthetic genes. BMC Syst. Biol. 2017, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Ferran, M.; Santamaria-Babi, L.F. Pathological Mechanisms of Skin Homing T Cells in Atopic Dermatitis. World Allergy Organ. J. 2010, 3, 44–47. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Lutter, R.; Jakasa, I.; Koster, E.S.; Saunders, S.; Caspers, P.; Kemperman, P.M.J.H.; Puppels, G.J.; Sandilands, A.; et al. Filaggrin Loss-of-Function Mutations Are Associated with Enhanced Expression of IL-1 Cytokines in the Stratum Corneum of Patients with Atopic Dermatitis and in a Murine Model of Filaggrin Deficiency. J. Allergy Clin. Immunol. 2012, 129, 1031–1039.e1. [Google Scholar] [CrossRef]
- Atopic Dermatitis: Pathogenetic Mechanisms|Clinical and Experimental Dermatology|Oxford Academic. Available online: https://academic.oup.com/ced/article-abstract/25/7/530/6627852 (accessed on 7 February 2024).
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y.M. Cytokine Modulation of Atopic Dermatitis Filaggrin Skin Expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Carrascosa, J.M.; Morgado-Carrasco, D.; Narda, M.; Trullas, C.; Granger, C.; Fabbrocini, G. The Role of Photoprotection in Optimizing the Treatment of Atopic Dermatitis. Dermatol. Ther. 2021, 11, 315–325. [Google Scholar] [CrossRef]
- Spergel, J.M.; Paller, A.S. Atopic Dermatitis and the Atopic March. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Akiyama, H.; Sasai, M.; Taniuchi, S.; Goda, Y.; Toyoda, M.; Kobayashi, Y. Anti-Allergic Effect of Apple Polyphenol on Patients with Atopic Dermatitis: A Pilot Study. Allergol. Int. 2000, 49, 69–73. [Google Scholar] [CrossRef]
- Kundu, J.K.; Shin, Y.K.; Surh, Y.-J. Resveratrol Modulates Phorbol Ester-Induced pro-Inflammatory Signal Transduction Pathways in Mouse Skin in Vivo: NF-ΚB and AP-1 as Prime Targets. Biochem. Pharmacol. 2006, 72, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef] [PubMed]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol Ameliorates 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis-like Lesions through Effects on the Epithelium. PeerJ 2016, 4, e1889. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef]
- Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef]
- Kim, H.K.; Chang, H.K.; Baek, S.Y.; Chung, J.O.; Rha, C.S.; Kim, S.Y.; Kim, B.J.; Kim, M.N. Treatment of Atopic Dermatitis Associated with Malassezia Sympodialis by Green Tea Extracts Bath Therapy: A Pilot Study. Mycobiology 2012, 40, 124–128. [Google Scholar] [CrossRef]
- Cervi, V.F.; Saccol, C.P.; Sari, M.H.M.; Martins, C.C.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.; Luchese, C.; Wilhelm, E.A.; Cruz, L. Pullulan Film Incorporated with Nanocapsules Improves Pomegranate Seed Oil Anti-Inflammatory and Antioxidant Effects in the Treatment of Atopic Dermatitis in Mice. Int. J. Pharm. 2021, 609, 121144. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Nishida, N.; Ito, H. The Inhibitory Effect of a Polyphenol Concentrate from Pomegranate Juice on 2, 4-Dinitrofluorobenzene-Induced Contact Hypersensitivity in Mice. Food Sci. Technol. Res. 2018, 24, 169–175. [Google Scholar] [CrossRef]
- Min, S.-Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.-R.; Vanarsa, K.; Kim, H.-Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis through Indoleamine-2, 3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef]
- Ahn, S.-C.; Kim, G.-Y.; Kim, J.-H.; Baik, S.-W.; Han, M.-K.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Kang, J.-H.; Kim, B.-H. Epigallocatechin-3-Gallate, Constituent of Green Tea, Suppresses the LPS-Induced Phenotypic and Functional Maturation of Murine Dendritic Cells through Inhibition of Mitogen-Activated Protein Kinases and NF-ΚB. Biochem. Biophys. Res. Commun. 2004, 313, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.F.; Hu, Y.; Wu, W.F.; Du, Q.Q.; Wang, Z.X.; Chen, T.T.; Shen, Q.; Liu, L.; Jiang, C.P.; Li, H.; et al. Explore the Anti-Acne Mechanism of Licorice Flavonoids Based on Metabonomics and Microbiome. Front. Pharmacol. 2022, 13, 832088. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Womens Dermatol. 2017, 4, 56–71. [Google Scholar] [CrossRef]
- Bhate, K.; Williams, H.C. Epidemiology of acne vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Lim, H.J.; Kang, S.H.; Song, Y.J.; Jeon, Y.D.; Jin, J.S. Inhibitory Effect of Quercetin on Propionibacterium acnes-induced Skin Inflammation. Int. Immunopharmacol. 2021, 96, 107557. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.S.; Nasr, M.; Abdel-Aziz, R.T.A.; Moftah, N.H.; El Shaer, A.; Polycarpou, E.; Mamdouh, W.; Sammour, O. Cosm-nutraceutical nanovesicles for acne treatment: Physicochemical characterization and exploratory clinical experimentation. Int. J. Pharm. 2020, 577, 119092. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Kim, I.H.; Seo, J.J. In vitro activity of kaempferol isolated from the Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. J. Microbiol. 2007, 45, 473–477. [Google Scholar]
- Lu, P.H.; Hsu, C.H. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019, 53, 829–840. [Google Scholar] [CrossRef]
- Nair, P.A.; Badri, T. Psoriasis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Guarneri, F.; Bertino, L.; Pioggia, G.; Casciaro, M.; Gangemi, S. Therapies with Antioxidant Potential in Psoriasis, Vitiligo, and Lichen Planus. Antioxidants 2021, 10, 1087. [Google Scholar] [CrossRef]
- Nowak-Perlak, M.; Szpadel, K.; Jabłόnska, I.; Pizon, M.; Wόzniak, M. Promising Strategies in Plant-Derived Treatments of Psoriasis-Update of In Vitro, In Vivo, and Clinical Trials Studies. Molecules 2022, 27, 591. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Gębka, N.; Adamczyk, J.; Gębka-Kępińska, B.; Mizgała-Izworska, E. The role of flavonoids in prevention and treatment of selected skin diseases. J. Pre-Clin. Clin. Res. 2022, 16, 99–107. [Google Scholar] [CrossRef]
- Klisic, A.; Bakic, M.; Karanikolic, V. Comparative Analysis of Redox Homeostasis Biomarkers in Patients with Psoriasis and Atopic Dermatitis. Antioxidants 2023, 12, 1875. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Vasiadi, M.; Therianou, A.; Theoharides, T.C. Luteolin inhibits human keratinocyte activation and decreases NF-κB induction that is increased in psoriatic skin. PLoS ONE 2014, 9, e90739. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, M.; Zang, X.; Liu, Q.; Du, J.; Hu, J.; Zhang, L.; Du, Z.; Xiang, Z. Luteolin attenuates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response. Biomed. Pharmacother. 2020, 131, 110696. [Google Scholar] [CrossRef]
- Pal, H.C.; Chamcheu, J.C.; Adhami, V.M.; Wood, G.S.; Elmets, C.A.; Mukhtar, H.; Afaq, F. Topical application of delphinidin reduces psoriasiform lesions in the flaky skin mouse model by inducing epidermal differentiation and inhibiting inflammation. Br. J. Dermatol. 2015, 172, 354–364. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol. Physiol. 2015, 28, 177–188. [Google Scholar] [CrossRef]
- Hung, C.H.; Wang, C.N.; Cheng, H.H.; Liao, J.W.; Chen, Y.T.; Chao, Y.W.; Jiang, J.L.; Lee, C.C. Baicalin Ameliorates Imiquimod-Induced Psoriasis-Like Inflammation in Mice. Planta Med. 2018, 84, 1110–1117. [Google Scholar] [CrossRef]
- Wang, P.W.; Lin, T.Y.; Yang, P.M.; Fang, J.Y.; Li, W.T.; Pan, T.L. Therapeutic efficacy of Scutellaria baicalensis Georgi against psoriasis-like lesions via regulating the responses of keratinocyte and macrophage. Biomed. Pharmacother. 2022, 155, 113798. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Z.; Cao, Z.; Shi, Y.; Yang, N.; Cao, G.; Zhang, C.; Sun, R.; Zhang, C. Topical astilbin ameliorates imiquimod-induced psoriasis-like skin lesions in SKH-1 mice via suppression dendritic cell-Th17 inflammation axis. J. Cell. Mol. Med. 2022, 26, 1281–1292. [Google Scholar] [CrossRef]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Nettis, E.; Foti, C.; Ambrifi, M.; Baiardini, I.; Bianchi, L.; Borghi, A.; Caminati, M.; Canonica, G.W.; Casciaro, M.; Colli, L.; et al. Urticaria: Recommendations from the Italian Society of Allergology, Asthma and Clinical Immunology and the Italian Society of Allergological, Occupational and Environmental Dermatology. Clin. Mol. Allergy 2020, 18, 8. [Google Scholar] [CrossRef]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of New Oxidative Stress Markers in Chronic Spontaneous Urticaria. Postep. Dermatol. Alergol. 2017, 34, 448–452. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Riso, G.; Di Salvo, E.; Casciaro, M.; Giuffrida, R.; Minciullo, P.L.; Guarneri, F.; Nettis, E.; Gangemi, S. Oxidative Stress Involvement in Urticaria. J. Biol. Regul. Homeost. Agents 2020, 34, 675–678. [Google Scholar]
- Wang, D.; Tang, H.; Shen, Y.; Wang, F.; Lin, J.; Xu, J. Activation of the blood coagulation system in patients with chronic spontaneous urticaria. Clin. Lab. 2015, 61, 1283–1288. [Google Scholar] [CrossRef]
- Greaves, M.W.; Tan, K.T. Chronic Urticaria: Recent Advances. Clin. Rev. Allerg. Immunol. 2007, 33, 134–143. [Google Scholar] [CrossRef]
- Grattan, C.E.H.; Wallington, T.B.; Warin, R.P.; Kennedy, C.T.C.; Lbradfield, J.W. A Serological Mediator in Chronic Idiopathic Urticaria—A Clinical, Immunological and Histological Evaluation. Br. J. Dermatol. 1986, 114, 583–590. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kaplan, A.P. A Role for C5a in Augmenting IgG-Dependent Histamine Release from Basophils in Chronic Urticaria. J. Allergy Clin. Immunol. 2002, 109, 114–118. [Google Scholar] [CrossRef]
- Soundararajan, S.; Kikuchi, Y.; Joseph, K.; Kaplan, A.P. Functional Assessment of Pathogenic IgG Subclasses in Chronic Autoimmune Urticaria. J. Allergy Clin. Immunol. 2005, 115, 815–821. [Google Scholar] [CrossRef]
- Zhou, B.; Li, J.; Liu, R.; Zhu, L.; Peng, C. The role of crosstalk of immune cells in pathogenesis of chronic spontaneous urticaria. Front. Immunol. 2022, 13, 879754. [Google Scholar] [CrossRef]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; Di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef]
- Gangemi, S.; Minciullo, P.L.; Magliacane, D.; Saitta, S.; Loffredo, S.; Saija, A.; Cristani, M.; Marone, G.; Triggiani, M. Oxidative Stress Markers Are Increased in Patients with Mastocytosis. Allergy 2015, 70, 436–442. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hu, S.; Ge, S.; Jia, M.; Wang, N. Resveratrol Inhibits MRGPRX2-Mediated Mast Cell Activation via Nrf2 Pathway. Int. Immunopharmacol. 2021, 93, 107426. [Google Scholar] [CrossRef]
- Bunselmeyer, B.; Laubach, H.J.; Schiller, M.; Stanke, M.; Luger, T.A.; Brehler, R. Incremental Build-up Food Challenge—A New Diagnostic Approach to Evaluate Pseudoallergic Reactions in Chronic Urticaria: A Pilot Study. Clin. Exp. Allergy 2009, 39, 116–126. [Google Scholar] [CrossRef]
- Shaik, Y.; Caraffa, A.; Ronconi, G.; Lessiani, G.; Conti, P. Impact of Polyphenols on Mast Cells with Special Emphasis on the Effect of Quercetin and Luteolin. Cent. Eur. J. Immunol. 2018, 43, 476–481. [Google Scholar] [CrossRef]
- dos Anjos Oliveira Ferreira, L.; de Paula Barros de Melo, C.; Saito, P.; Iwanaga, C.C.; Nakamura, C.V.; Casagrande, R.; da Conceição Torrado Truiti, M. Nectandra Cuspidata Fraction and the Isolated Polyphenols Protect Fibroblasts and Hairless Mice Skin from UVB-Induced Inflammation and Oxidative Stress. J. Photochem. Photobiol. B Biol. 2020, 205, 111824. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Fattori, V.; Bussmann, A.J.C.; Bottura, C.; Fonseca, M.J.V.; Vignoli, J.A.; Baracat, M.M.; et al. Trans-Chalcone, a Flavonoid Precursor, Inhibits UV-Induced Skin Inflammation and Oxidative Stress in Mice by Targeting NADPH Oxidase and Cytokine Production. Photochem. Photobiol. Sci. 2017, 16, 1162–1173. [Google Scholar] [CrossRef]
- Hossain, S.J.; Tsujiyama, I.; Takasugi, M.; Islam, M.A.; Biswas, R.S.; Aoshima, H. Total Phenolic Content, Antioxidative, Anti-Amylase, Anti-Glucosidase, and Antihistamine Release Activities of Bangladeshi Fruits. Food Sci. Technol. Res. 2008, 14, 261–268. [Google Scholar] [CrossRef]
- Cuffaro, D.; Digiacomo, M.; Macchia, M. Dietary Bioactive Compounds: Implications for Oxidative Stress and Inflammation. Nutrients 2023, 15, 4966. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef]
- Lee, T.H.; Do, M.H.; Oh, Y.L.; Cho, D.W.; Kim, S.H.; Kim, S.Y. Dietary Fermented Soybean Suppresses UVB-Induced Skin Inflammation in Hairless Mice via Regulation of the MAPK Signaling Pathway. J. Agric. Food Chem. 2014, 62, 8962–8972. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phyther. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Sobiepanek, A.; Milner-Krawczyk, M.; Bobecka-Wesołowska, K.; Kobiela, T. The effect of delphinidin on the mechanical properties of keratinocytes exposed to UVB radiation. J. Photochem. Photobiol. B 2016, 164, 264–270. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn polyphenol extract inhibits UV-B-induced skin photoaging by regulating MMP expression and type I procollagen production in mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Q.; Zou, J.; Zhao, Y.; Chang, X. Protective effects of hawthorn (Crataegus pinnatifida) polyphenol extract against UV-B-induced skin damage by modulating the p53 mitochondrial pathway in vitro and in vivo. J. Food Biochem. 2019, 43, e12708. [Google Scholar]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UV-B-induced photo-aging by polyphenolic-rich Spatholobus Suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, B.; Zhuang, Y. Effects of rambutan (Nephelium lappaceum) peel phenolics and Leu-Ser-Gly-Tyr-Gly-Pro on hairless mice skin photoaging induced by ultraviolet irradiation. Food Chem. Toxicol. 2019, 129, 30–37. [Google Scholar] [CrossRef]
- Lee, H.J.; Im, A.R.; Kim, S.M.; Kang, H.S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (mmp)-9 expression via mitogen activated protein kinase (mapk)-dependent signaling pathways. BMC Complement. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef]
- Kim, H.I.; Jeong, Y.U.; Kim, J.H.; Park, Y.J. 3, 5, 6, 7, 8, 3′, 4′-Heptamethoxyflavone, a citrus flavonoid, inhibits collagenase activity and induces type I procollagen synthesis in HDFn cells. Int. J. Mol. Sci. 2018, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a Flavonoid-Based Compound of Leopard Lily Rhizome, Attenuates UV-B-Induced Apoptosis and Collagen Degradation by Inhibiting Oxidative Stress in Human Keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.R.; Gewandter, J.S.; Bautista, J.; Heckler, C.E.; Strasser, J.; Dyk, P.; Anderson, T.; Gross, H.; Speer, T.; Dolohanty, L.; et al. Utility of topical agents for radiation dermatitis and pain: A randomized clinical trial. Support. Care Cancer 2020, 28, 3303–3311. [Google Scholar] [CrossRef]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric tonic as a treatment in scalp psoriasis: A randomized placebo-control clinical trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Datta, R.N.; Bhattacharyya, B.; Bandopadhyay, S.K. Emollient and antipruritic effect of Itch cream in dermatological disorders: A randomized controlled trial. Indian J. Pharmacol. 2005, 37, 253–254. [Google Scholar] [CrossRef]
- Kasai, K.; Yoshimura, M.; Koga, T.; Arii, M.; Kawasaki, S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006, 52, 383–388. [Google Scholar] [CrossRef]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-blinded, placebo-controllecl trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005, 31, 855–859. [Google Scholar] [CrossRef]
- Granger, C.; Aladren, S.; Delgado, J.; Garre, A.; Trullas, C.; Gilaberte, Y. Prospective evaluation of the efficacy of a food supplement in increasing photoprotection and improving selective markers related to skin photo-ageing. Dermatol. Ther. 2020, 10, 163–178. [Google Scholar] [CrossRef]
- Charoenchon, N.; Rhodes, L.E.; Nicolaou, A.; Williamson, G.; Watson, R.E.B.; Farrar, M.D. Ultraviolet radiation-induced degradation of dermal extracellular matrix and protection by green tea catechins: A randomized controlled trial. Clin. Exp. Dermatol. 2022, 47, 1314–1323. [Google Scholar] [CrossRef]
- Megow, I.; Darvin, M.E.; Meinke, M.C.; Lademann, J. A randomized controlled trial of green tea beverages on the in vivo radical scavenging activity in human skin. Skin Pharmacol. Physiol. 2017, 30, 225–233. [Google Scholar] [CrossRef]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Ohtake, Y.; Kanda, T. Administration of Apple Polyphenol Supplements for Skin Conditions in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, D.K.; Yee, N.J.; Kaur Ambar Jeet Singh, B.J.; Panneerselvam, J.; Madheswaran, T.; Chellian, J.; Satija, S.; Mehta, M.; Gulati, M.; Gupta, G.; et al. Formulation and characterization of glibenclamide and quercetin-loaded chitosan nanogels targeting skin permeation. Ther. Deliv. 2019, 10, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Du, Y.; Takhistov, P.; Michniak-Kohn, B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013, 441, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous Silica as Topical Nanocarriers for Quercetin: Characterization and in Vitro Studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, W.; Guo, C.; Zhai, G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int. J. Nanomed. 2011, 6, 1621–1630. [Google Scholar]
- Greenwald, M.B.Y.; Frusic-Zlotkin, M.; Soroka, Y.; Ben Sasson, S.; Bitton, R.; Bianco-Peled, H.; Kohen, R. Curcumin Protects Skin against UVB-Induced Cytotoxicity via the Keap1-Nrf2 Pathway: The Use of a Microemulsion Delivery System. Oxid. Med. Cell. Longev. 2017, 2017, 5205471. [Google Scholar]
- Gupta, N.K.; Dixit, V.K. Development and evaluation of vesicular system for curcumin delivery. Arch. Dermatol. Res. 2011, 303, 89–101. [Google Scholar] [CrossRef]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanic, Z.; Skalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nacher, A.; Valenti, D.; Fernandez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Yang, C.; Li, F.; Qiu, B.; Ding, W. Enhanced transdermal efficiency of curcumin-loaded peptide-modified liposomes for highly effective antipsoriatic therapy. J. Mater. Chem. B 2021, 9, 4846–4856. [Google Scholar] [CrossRef] [PubMed]
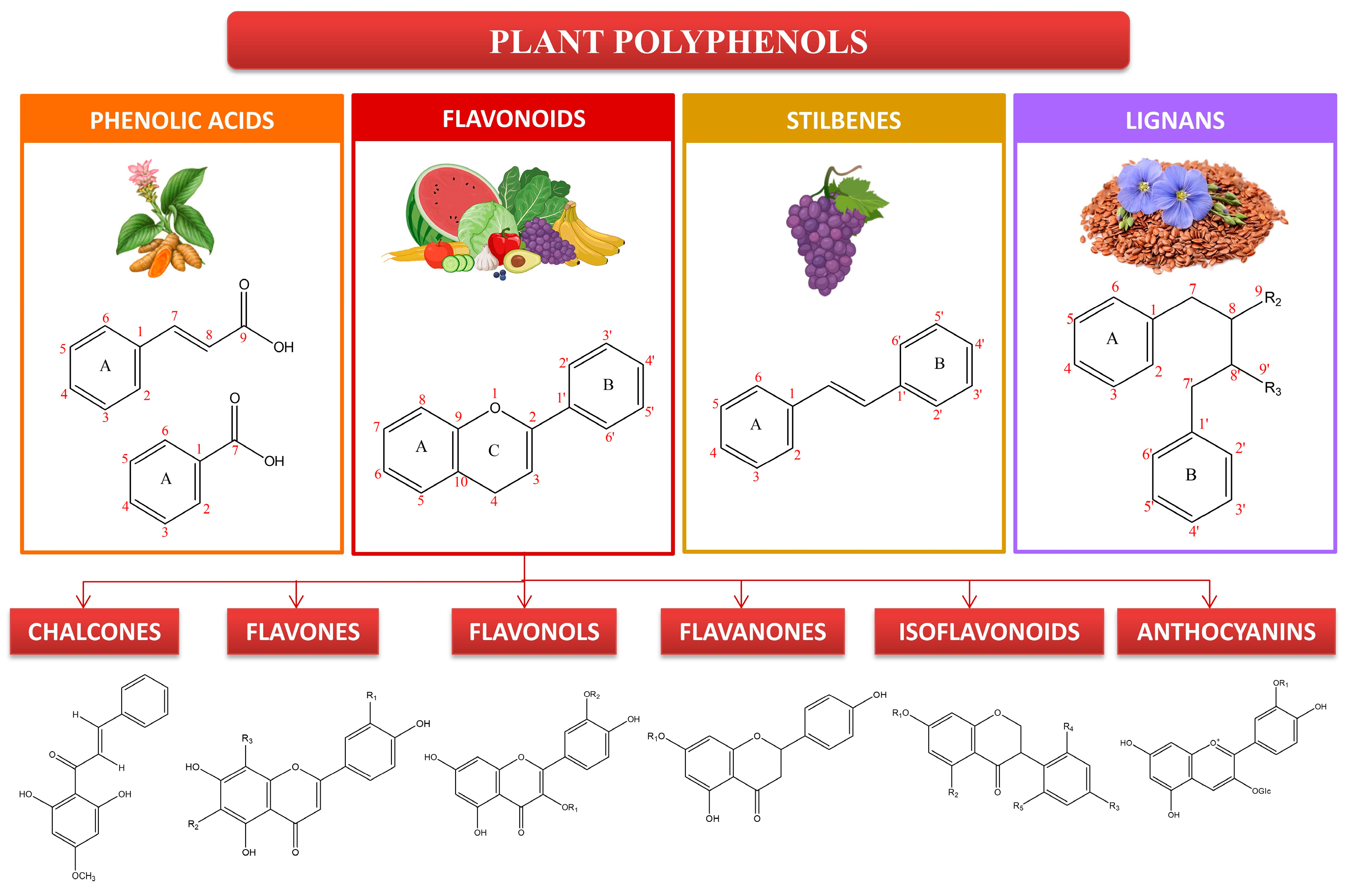
| Polyphenol | Study Type | Study Model | Effects | Targets | Ref. |
|---|---|---|---|---|---|
| EGCG + metformin | In vitro | B16F10 cells | Inhibition of cell growth and STAT3/NF-κb pathway | STAT3 and NF-κb p65 ↓ | [81] |
| Curcumin | In vivo | BK5.IGF-1 transgenic (Tg) mice | Inhibition of tumor growth | IGF-1 ↓ | [83] |
| Genistein | In vitro | B16F10 melanoma cells | Inhibition of cell proliferation, migration, and metastasis | p-p38, p-ERK, and p-JNK ↓ | [86] |
| Quercetin | In vitro | B16 and A375 cells | Suppressed proliferation | RIG-I ↑ IFN-I ↑ STAT1 ↑ | [89] |
| EGCG | In vivo | C57BL/6 mice | Inhibition of tumor growth | STAT1 ↓ | [90] |
| Rosmarinic acid | In vitro | A375 | Inhibits proliferation and migration | ADAM17/EGFR/AKT/GSK3β ↓ | [84] |
| Luteolin | In vitro | A375 | Inhibited the proliferation, migration, and invasion | MMP-2 and MMP-9 ↓ TIMP-1 and TIMP-2 ↑ | [94] |
| Apigenin | In vivo | WT mice TKO mice | Inhibition of UV-induced cutaneous angiogenesis | TSP-1 ↑ | [106] |
| Caffeic acid | In vivo | Male Swiss albino mice | Inhibition of angiogenesis and proliferation | TSP-1 ↑ | [102] |
| Resveratrol | In vitro | Co-culture | Antiangiogenic effects | VEGF ↓ TSP-1 ↑ | [96] |
| EGCG | In vitro | 1205Lu, HS294T, and A375 cells | Inhibition of cell proliferation | STAT1 ↓ | [90] |
| Herbacetin | In vitro | A375 and Hs294T cells | Suppressed angiogenesis | MMP9 ↓ | [105] |
| Polyphenol | Type of Skin Cell | Assay | Effect | References |
|---|---|---|---|---|
| Apigenin | Fibroblasts derived from human foreskin | The cells were co-treated with bleomycin for 24 h at a concentration of either 10 or 20 μM | Downregulated IL-6, IL-8, and IL-1β mRNA expression; Suppressed NF-κB activity | [212] |
| Kaempferol | Fibroblasts derived from human foreskin | The cells were co-treated with bleomycin for 24 h at a concentration of either 10 or 20 μM | Downregulated expression of IL-6, IL-8, and IL-1β mRNA | [212] |
| Quercetin | Fibroblasts derived from human foreskin | The cells were co-treated with bleomycin for 24 h at a concentration of either 10 or 20 μM | Downregulated IL-6, IL-8, and IL-1β mRNA expression; decreased SA-β-Gal activity | [212] |
| Genistein | Co-culture of NHDF with keratinocytes | Applied a concentration of 10 millimolar for a duration of 72 h following exposure to UV radiation | Decreased the production of IL-6; suppressed the phosphorylation of p38, ERK, and JNK | [213] |
| Gallic acid | NDHF, HaCaT | Applied a concentration ranging from 0.1 to 10 millimolar for a duration of 24 h following UV exposure | Reduced levels of IL-6 and MMP-1; reduced generation of ROS; inhibited phosphorylation of AP-1 | [214] |
| Delphinidin | HaCaT | Used either 5 or 10 micromolar concentration before or after exposure to UV | Restored elastic characteristics | [215] |
| Fisetin | Senescent mouse embryonic fibroblasts (MEFs) lacking the Ercc1 gene and human IMR-90 fibroblasts | 48 h duration of treatment with a concentration range of 1 to 15 micromolar | Decrease in the proportion of cells that are positive for SA-ß-Gal | [216] |
| Curcumin/ luteolin | MEFs derived from Ercc1 knockout animals | Treatment for 48 h with a concentration of 5 micromolar | Decrease in the proportion of cells that are positive for SA-ß-Gal | [216] |
| Hawthorn polyphenol extract (HPE) | Human dermal fibroblasts (HDFs) and HaCaT cells were used in conjunction with mice that were 5–6 weeks old. | Cell treatment: HPE (0, 5, 10 µg/mL) for 24 h; mice were administered HPE at doses of 0, 100, and 300 mg/kg.bw.day for a duration of 12 weeks | 1. HPE therapy can enhance cell growth, augment intracellular collagen levels, and decrease MMP–1 synthesis 2. Oral HPE mitigates the harmful effects of UV radiation on the skin by removing ROS, decreasing DNA damage, and suppressing the production of p53 | [217,218] |
| Products containing high levels of polyphenols, such as NutroxsunTM, derived from rosemary and citrus | Adult female | For a prolonged period of time, used NutroxsunTM at a dosage of 250 mg per day for two weeks. For a shorter duration, used NutroxsunTM at a dosage of 100 or 250 mg per day for 24 or 48 h | NutroxsunTM, when consumed as part of a diet, decreases the negative effects of UV radiation on the skin, such as wrinkles and loss of suppleness | [219] |
| Polyphenolic rich extract (SSE and SSW)/Spatholobus Suberectus stem | HaCaT/Human skin | Tg1: The concentration of SSE used was 0, 3, 10, 30, and 300 µg/mL. Tg2: The concentration of SSW used was 0, 3, 10, 30, and 300 µg/mL for a duration of 24 h | 1. The presence of SSE and SSW suppressed the generation of ROS and prevented cellular harm. 2. SSE restores skin by increasing the production of enzymes and proteins in cells, preventing the activation of MAPKs phosphorylation caused by UV radiation, and inhibiting the activity of its downstream transcription factor | [220] |
| Rambutan peel phenolics (RPP)/Nephelium lappaceum; Leu-Ser-Gly-Tyr-Gly-Pro (LSGYGP)/synthetic | Male BALB/c nude mice weighing between 20 and 22 g. | Single group: RPP (100 mg/kg.bw.d), SGYGP (100 mg/kg.bw.d); Composite group: (50 RPP+ 50 LSGYGP) mg/kg.bw.d, (100 RPP + 100 LSGYGP)mg/kg.bw.d/10 weeks | 1. The use of RPP and LSGYGP enhanced skin biochemical markers, tissue structure, and collagen levels. 2. RPP improved the control of oxidative stress and the levels of inflammatory factors. 3. The presence of LSGYGP had a substantial impact on the levels of collagen and hyaluronic acid in the skin | [221] |
| Polyphenols/Flavonoid hesperidin | Hairless male mice that were 6 weeks old. | Water was administered to the control group, while the treatment group received UV radiation along with hesperidin at doses of 0 and 100 mg/kg.bw.d for a duration of 12 weeks | 1. Hesperidin, when taken orally, prevented the thickening of the skin and the production of wrinkles caused by UV- radiation. 2. Hesperidin suppressed the UV-induced activation of MMP-9 and cytokines and prevented the degradation of collagen fibers | [222] |
| Polyphenols/3,5,6,7,8,3,4-heptam-ethoxy flavone (HMF)/C.unshiu peels | HDFn cells (human dermal fibroblast cells) | The samples were treated with different concentrations of HMF (0, 50, 100, 200 µg/mL) for a duration of 24 h | 1. HMF shielded HDFn cells from damage caused by UV radiation by suppressing the production of MMP-1 through phosphorylated MAPK signals 2. HMF modulated the expression of Smad3 and Smad7 proteins in a manner that is dependent on the dosage | [223] |
| Polyphenols/ Tectorigenin/ Belamcanda chinensis L. | Human HaCaT cells | Tg refers to the compound Tectorigenin at concentrations of 0, 0.1, 1, and 10 µM. Cg refers to the control group treated with VC at a concentration of 200 µM for a duration of 24 h | 1. Tectorigenin reduces ROS levels by enhancing the activity of intracellular antioxidant enzymes. 2. Tectorigenin decreases the expression of mmp–1 and hinders the breakdown of collagen. 3. Tectorigenin exerts an inhibitory effect on apoptosis via modulating the expression of caspase–3 and bcl–2 associated proteins | [224] |
| Curcumin gel | The skin of breast cancer patients receiving radiation therapy with a range of 36 to 81 years. | Applied the medication topically containing 4% curcumin three times daily for a duration of one week | Topical use of curcumin as a preventive therapy may effectively manage radiation-induced skin inflammation and alleviate associated pain | [225] |
| Turmeric supplements | Patients between the ages of 18 and 75 with mild scalp psoriasis. | Administered topical cream having turmeric 9% twice a day for a duration of nine weeks | The Dermatology Life Quality Index (DLQI) questionnaire and Psoriasis Area and Severity Index (PASI) scores were evaluated, revealing that the application of turmeric tonic resulted in a considerable reduction in redness, flaking of the scalp and skin abnormalities. | [226] |
| An anti-itch cream containing a combination of several herbs, with a 16% concentration of turmeric extract and a 0.1% concentration of turmeric oil. | Children aged 2 to 12 with atopic dermatitis | Applied a topical cream containing 16% turmeric twice a day | Both the treatment group, which used an anti-itching cream, and the control group, which used Moisturex, showed significant improvements in all measured aspects, including subjective itching severity, clinical evaluation, and overall health | [227] |
| Tablets containing pomegranate extract with a high concentration of ellagic acid | Individuals with skin that has undergone aging due to exposure to UV radiation, typically between the ages of 20 and 40. | Administered at a high dose of 200 mg/d ellagic acid or a low dose of 100 mg/d ellagic acid once a day for a duration of four weeks | The questionnaire results indicated that the decrease in skin luminance values was reduced by 1.35% in the low-dose group and by 1.73% in the high-dose group compared to the initial measurement. Furthermore, there was a noticeable upward trajectory in the quality of certain aspects, such as “facial brightness” and “spots and freckles” | [228] |
| Oral green tea supplement with topical green tea cream | Women having a moderate level of photoaging | Application of a cream containing 10% green tea and taking 300 mg of green tea orally twice a day for a duration of eight weeks | Participants who received a combined treatment of topical and oral green tea demonstrated histological enhancements in elastin levels; however, no noticeable clinical alterations were seen | [229] |
| Multicomponent polyphenol supplement | Moderate photoaging between the ages of 40 and 65 | VitAoX ultra® formula 50 mg Camellia sinensis L, two capsules daily for twelve weeks | Increased antioxidant capability and anti-aging metrics | [230] |
| Taking a vitamin C and green tea supplement orally | Healthy subjects between the ages of 18 and 65 | Green tea extract 450 mg per gelatin capsule daily for 12 weeks | UV protection to fibulin-5 | [231] |
| Freshly prepared green tea beverages | Healthy subjects between the ages of 20 and 55 | Consumption of 600 mL of freshly brewed green tea beverages per day for a duration of 2 weeks | Enhanced Skin radical scavenging activity | [232] |
| Tablets containing polyphenols found in apple | Healthy women between the ages of 20 and 39 | Tablets of 300 or 600 mg daily for 12 weeks | Prevented UV-induced skin pigmentation | [233] |
| Polyphenol | Delivery Systems | Skin Model | Main Results | Reference |
|---|---|---|---|---|
| Quercetin | NLC gel | Strat-M membrane and excised rat skin membrane in Franz diffusion cell | There was a notable increase in the ability of the skin to absorb substances when using this gel, as compared to the traditional gel | [234] |
| Nanogels | Franz diffusion cell apparatus using Strat-M transdermal diffusion membrane | Chitosan-based nanogel has a lower but controlled skin permeation rate | [235] | |
| SLN | Vertical Franz diffusion cells, using full-thickness human skin | The quercetin-containing solid lipid-based nanosystems exhibited greater quercetin retention in the skin compared to the control formulation | [236] | |
| Mesoporous silica | Porcine skin in vertical Franz diffusion cells | Quercetin-loaded mesoporous nanoparticles exhibited enhanced skin accumulation compared to free quercetin | [237] | |
| Nanoparticles | Franz diffusion cells and mice skin | Lecithin-chitosan nanoparticles enhanced the penetration of quercetin and augmented its retention in the epidermis | [238] | |
| Curcumin | Microemulsion | HaCaT cells; Human skin | Substantial amounts of curcumin were detected in the dermis, and the application of curcumin microemulsion reduced the harmful effects of UV radiation on the outer layer of the skin | [239] |
| Phytovesicles | Mice | The phytovesicles demonstrated superior efficacy in comparison to all other preparations and ordinary curcumin in delivering heightened antioxidant and anti-aging benefits | [240] | |
| Liposome (DLs) nanocarriers | Isolated human skin | Consistently infiltrated the skin and improved its biological characteristics | [241] | |
| Transparent plastid nanovesicles | Human keratinocytes | In vitro, it provided protection to human keratinocytes against damage caused by oxidative stress, mitigated inflammation and injury induced by 12-0-tetracyl-chlorowave, decreased edema development, and enhanced the biocompatibility and safety of the components | [242] | |
| Peptide-modified curcumin-loaded liposome (CRC-TD-Lip) | Mice | It demonstrated exceptional stability and a remarkable capacity to encapsulate curcumin, resulting in an expedited transdermal distribution of curcumin and an increased suppression of psoriasis | [243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. https://doi.org/10.3390/molecules29040865
Farhan M. The Promising Role of Polyphenols in Skin Disorders. Molecules. 2024; 29(4):865. https://doi.org/10.3390/molecules29040865
Chicago/Turabian StyleFarhan, Mohd. 2024. "The Promising Role of Polyphenols in Skin Disorders" Molecules 29, no. 4: 865. https://doi.org/10.3390/molecules29040865
APA StyleFarhan, M. (2024). The Promising Role of Polyphenols in Skin Disorders. Molecules, 29(4), 865. https://doi.org/10.3390/molecules29040865







