Utilizing Integrated UHPLC-Q-Exactive Orbitrap-MS, Multivariate Analysis, and Bioactive Evaluation to Distinguish between Wild and Cultivated Niudali (Millettia speciosa Champ.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Compounds
2.1.1. Identification of Terpenoids
2.1.2. Identification of Flavonoids
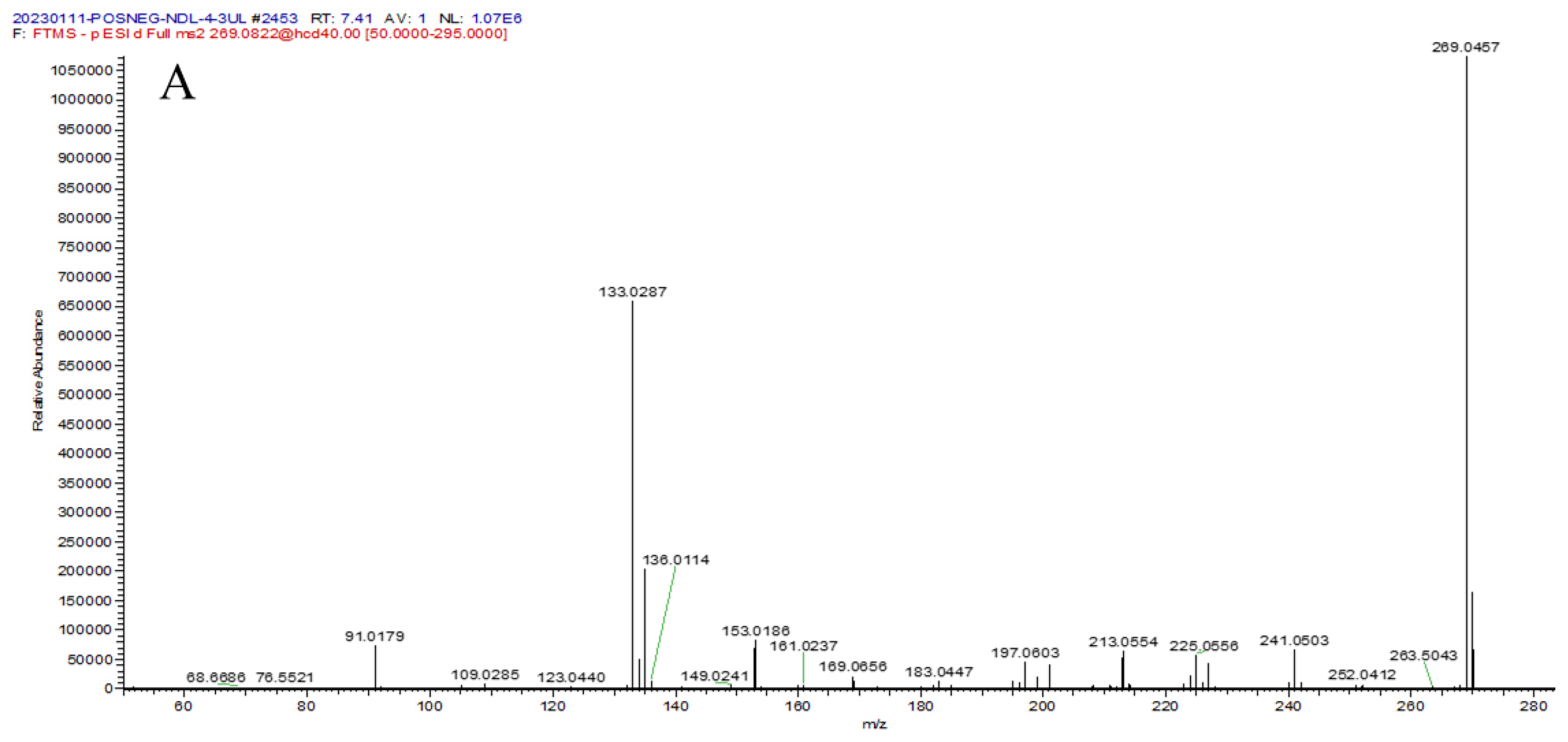
Identification of Flavonols
Identification of Chalcones, Dihydroflavonoids, and Pterocarpans
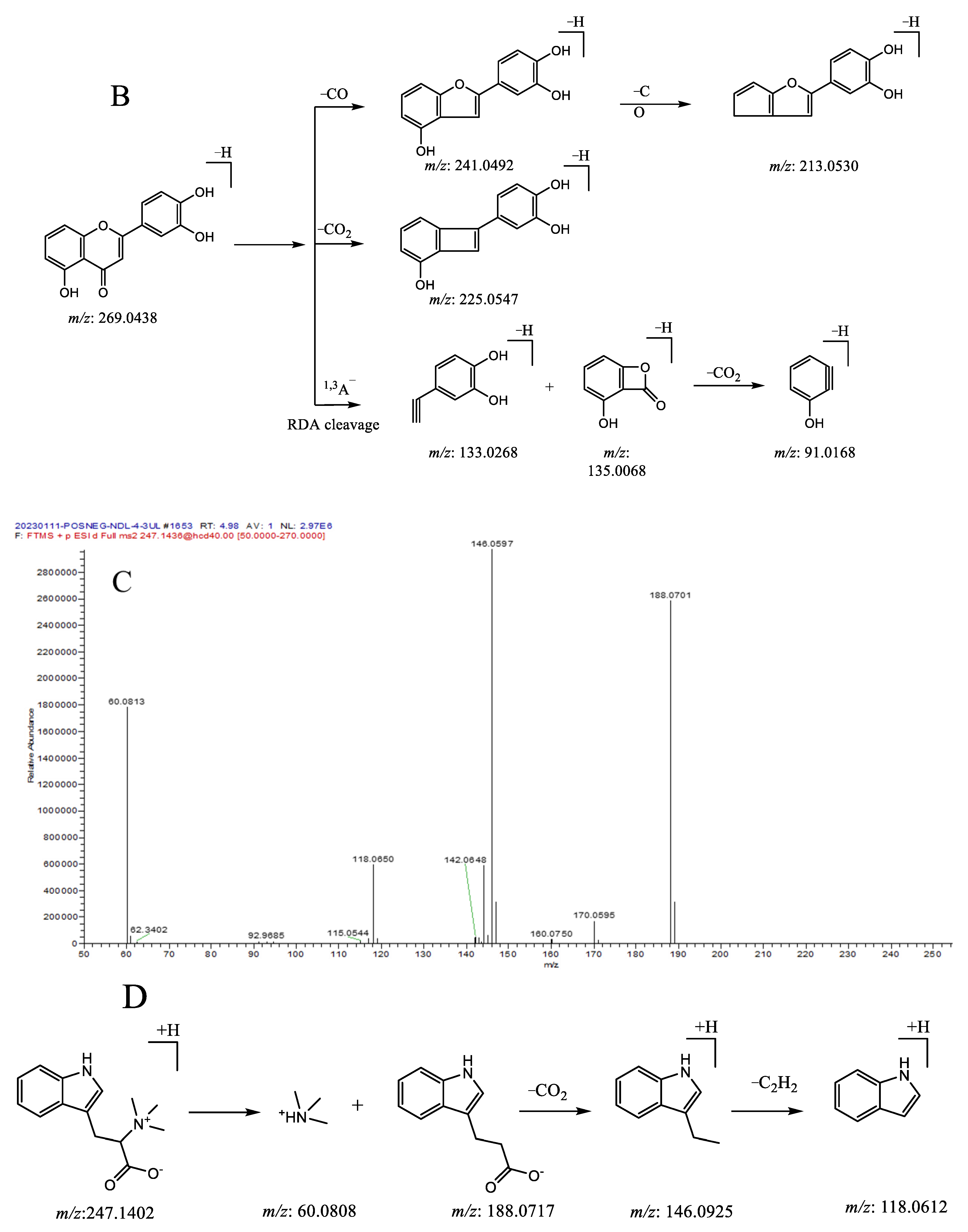
2.1.3. Identification of Alkaloids
2.1.4. Identification of Rotenoids
2.1.5. Identification of Other Compounds
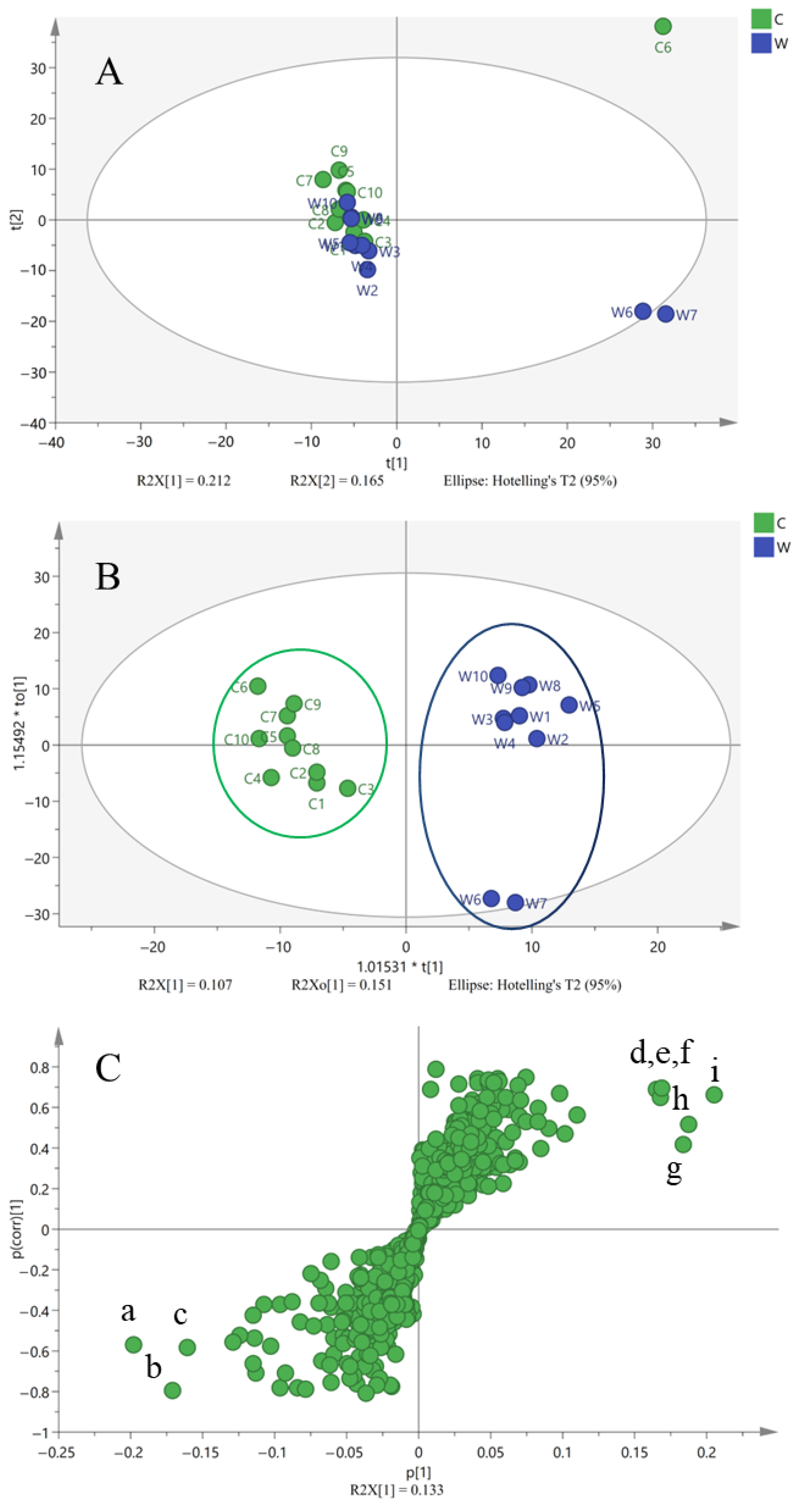
2.2. Principal Components Analysis (PCA)
2.3. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
2.4. Antioxidative Activity Evaluation
2.5. Anti-Fatigue Evaluation
2.6. The Possibility of Cultivation
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Materials
3.3. Preparation of Samples
3.4. Preparation of Standard Solution
3.5. UHPLC-Q-Exactive Orbitrap-MS Conditions
3.6. Antioxidant Activity on ABTS and DPPH
3.6.1. ABTS Radical Scavenging Activity
3.6.2. DPPH Radical Scavenging Activity
3.7. In Vivo Anti-Fatigue Experiment
3.7.1. Animals and Treatments
3.7.2. Forced Swim Test
3.7.3. Biochemical Assays
3.8. Data Acquisition and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.Y.; Liu, P.H.; Ma, S.S.; Wang, S.L.; Li, A.; Liu, J.G.; Wang, M. Botanical characteristics, chemical and nutritional composition and pharmacological and toxicological effects of medicinal and edible plant Millettia speciosa Champ. Food Sci. 2017, 38, 293–306. [Google Scholar]
- Li, R.R.; Chen, Z.K.; Gao, S.; Liang, S.W. Study progress of Millettia speciosa. Asia-Pac. Trad. Med. 2010, 6, 165–167. [Google Scholar]
- Nasiruddin, N.; Yu, Z.X.; Guangying, T.Z.C.; Ji, M.H. Allelopathic and Medicinal plant. 26. Millettia speciosa. Allelopath. J. 2020, 51, 125–146. [Google Scholar] [CrossRef]
- Yu, D.; Liang, X. Characterization and Identification of Isoflavonoids in the Roots of Millettia speciosa Champ. by UPLC-Q-TOF-MS/MS. Current Pharm. Anal. 2019, 15, 580–591. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhang, M.; Yang, Q.; Wang, Q.L.; Ma, B.K.; Li, Z.Y.; Wang, Z.N. Metabolomic profiling of M. speciosa champ at different growth stages. Food Chem. 2021, 376, 131941. [Google Scholar] [CrossRef]
- Zhu, B.S.; Jiang, J.Q.; Long, L.; Liang, Y.L.; Qin, Z.G.; Lai, K.P. A Research Survey of Edible Millettia speciosa Soup. China Condiment. 2020, 45, 133–136. [Google Scholar]
- Zhao, Z.; Huang, Y.Q.; Pan, Y.L.; Wei, L.; Tang, T.; Chen, Z.L. Study on Healthy Herbal Beverage of Millettia speciosa Champ.and Green Tea. J. Guangxi Agric. 2021, 36, 33–42. [Google Scholar]
- Chen, M.; Shi, X.M.; Zhou, X.P.; Shi, K.H.; Zhang, Z.F.; Zhang, J.J. HPLC Simultaneous Determination of Three Chemical Components in Millettia Speciosa from Different Habitats. Asia-Pacific Trad. Med. 2022, 18, 66–70. [Google Scholar]
- Zhang, J.G.; Shi, X.M.; Chen, M.; Zhou, X.P.; Meng, X.L. HPLC Fingerprint and Chemical Pattern Recognition of Wild and Cultivated Millettia speciosa Champ. Med. Plant 2020, 11, 82–86. [Google Scholar]
- Xue, S.J.; Wang, L.L.; Chen, S.Q.; Cheng, Y.X. Simultaneous Analysis of Saccharides between Fresh and Processed Radix Rehmanniae by HPLC and UHPLC-LTQ-Orbitrap-MS with Multivariate Statistical Analysis. Molecules 2018, 23, 541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Chen, J.; Yang, J.L.; Shi, Y.P. UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta 2018, 180, 389–395. [Google Scholar] [CrossRef]
- Li, Q.Q.; Liang, X.W.; Zhao, L.; Zhang, Z.Y.; Xue, X.F.; Wang, K.; Wu, L.M. UPLC-Q-Exactive Orbitrap/MS-Based Lipidomics Approach to Characterize Lipid Extracts from Bee Pollen and Their in Vitro Anti-Inflammatory Properties. J. Agric. Food Chem. 2017, 65, 6848–6860. [Google Scholar] [CrossRef]
- Fu, C.X.; Liu, M.S.; Li, Y.S.; Wang, K.H.; Yang, B.; Deng, L.J.; Zheng, G.D. UPLC-Q-Exactive Orbitrap MS Analysis for Identification of Lipophilic Components in Citri Sarcodactylis Fructus from Different Origins in China Using Supercritical CO2 Fluid Extraction Method. ACS Omega 2020, 5, 11013–11023. [Google Scholar] [CrossRef]
- Lan, Z.W.; Zhang, Y.; Sun, Y.; Wang, L.H.; Huang, Y.T.; Cao, H.; Meng, J. Identifying of Anti-Thrombin Active Components from Curcumae Rhizoma by Affinity-Ultrafiltration Coupled With UPLC-Q-Exactive Orbitrap/MS. Front. Pharmacol. 2021, 12, 769021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Wang, J.J.; Yang, L.; Wang, Y.; Jin, W.F.; Li, J.; Zhang, Z.F. Comprehensive Quality Evaluation of Polygonatum cyrtonema and Its Processed Product: Chemical Fingerprinting, Determination and Bioactivity. Molecules 2023, 28, 4341. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Silva, A.S. Natural Antioxidants: Innovative Extraction and Application in Foods. Foods 2021, 10, 937. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, J.J.; Wang, Y.; Chen, M.; Shi, X.M.; Zhou, X.P.; Zhang, Z.F. Phytochemistry and Antioxidant Activities of the Rhizome and Radix of Millettia speciosa Based on UHPLC-Q-Exactive Orbitrap-MS. Molecules 2022, 27, 7398. [Google Scholar] [CrossRef]
- Zhao, X.N.; Liang, J.L.; Chen, H.B.; Liang, Y.E.; Guo, H.Z.; Su, Z.R.; Zhang, X.J. Anti-Fatigue and Antioxidant Activity of the Polysaccharides Isolated from Millettiae speciosae Champ. Leguminosae. Nutrients 2015, 7, 8657–8669. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Furukawa, M.; Isobe, S.; Makino, M.; Akiyama, T.; Koyama, T.; Fujjimoto, Y. New Oleanane-Type Triterpene Saponins from Millettia speciosa. Heterocycles 2003, 60, 655–661. [Google Scholar]
- Wang, C.H.; Wang, Y.; Wang, G.C.; Zha, Q.; Zhang, X.Q.; Ye, C.W. Chemical constituents from roots of Millettia speciosa. Chin. Trad. Herb. Drugs 2008, 39, 972–975. [Google Scholar]
- Chen, X.; Zhang, X.T.; Mou, L.T.; Ren, H.X.; Zhang, Y.; Wang, L.H.; Sun, C.H. Characterization of chemical constituents and identification of absorbed prototypes components in rat serum of Scutellaria baicalensis by UHPLC-Q-Orbitrap-MS. Chin. Trad. Herb. Drugs 2023, 54, 2722–2732. [Google Scholar]
- Zhang, S.Y.; Cheng, J.; Chen, W.J.; Ling, X.M.; Zhao, Y.Y.; Feng, J.; Liang, H. Interactions between thrombin and natural products of Millettia nitita var. hirsutissima using capillary zone electrophoresis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 4107–4114. [Google Scholar] [CrossRef]
- Ding, P.; Qiu, J.Y.; Ying, G.; Dai, L. Chemical Constituents of Millettia speciosa. Chin. Herb. Med. 2014, 6, 332–334. [Google Scholar] [CrossRef]
- Zong, X.K.; Lai, F.L.; Wang, Z.N.; Wang, J.R. Studies on Chemical Constituents of Root of Millettia speciosa. J. Chin. Med. Mater. 2009, 32, 520–521. [Google Scholar]
- Fu, M.Q.; Xiao, G.S.; Xu, Y.J.; Wu, J.J.; Chen, Y.L.; Qiu, S. Chemical Constituents from Roots of Millettia speciosa. Chin. Herb. Med. 2016, 8, 385–389. [Google Scholar] [CrossRef]
- Zhang, H.W.; Ding, G.; Li, R.T.; Wei, J.H.; Zhou, Z.M. Isolation, identification and quantitative analysis of hypaphorine in the root of Millettia speciosa Champ. Chin. J. Pharm. Anal. 2011, 31, 1024–1026. [Google Scholar]
- Chen, D.L.; Liu, Y.Y.; Ma, G.X.; Zhu, N.L.; Wu, H.F.; Wang, D.L.; Xu, X.D. Two new rotenoids from the roots of Millettia speciosa. Phytochem. Lett. 2015, 12, 196–199. [Google Scholar] [CrossRef]
- Yin, T.; Tu, G.; Zhang, Q.; Wang, B.; Zhao, Y. Three new phenolic glycosides from the caulis of Millettia speciosa. Magn. Reason. Chem. 2008, 46, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.P.; He, L.L.; Mo, C.M.; Zhang, Z.F.; Long, H.R.; Gu, X.Y.; Wei, Y. Rapid Evaluation of Chemical Consistency of Artificially Induced and Natural Resina Draconis Using Ultra-Performance Liquid Chromatography Quadrupole-Time-of-Flight Mass Spectrometry-Based Chemical Profiling. Molecules 2018, 23, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; He, L.L.; Wang, J.J.; Yang, L.; Chen, M.; Liang, Z.C.; Zhang, Z.F. Chemical change and mechanism of Millettia speciosa with sulfur fumigation by UHPLC–QTOF–MS/MS. Arab. J. Chem. 2024, 17, 105509. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Li, L.; Wang, J.J.; Jin, W.F.; Wang, Y.; Zhang, Z.F. A strategy for antioxidant quality evaluation of Aster yunnanensis based on fingerprint-activity relationship modeling and chemometric analysis. Arab. J. Chem. 2023, 16, 104755. [Google Scholar] [CrossRef]

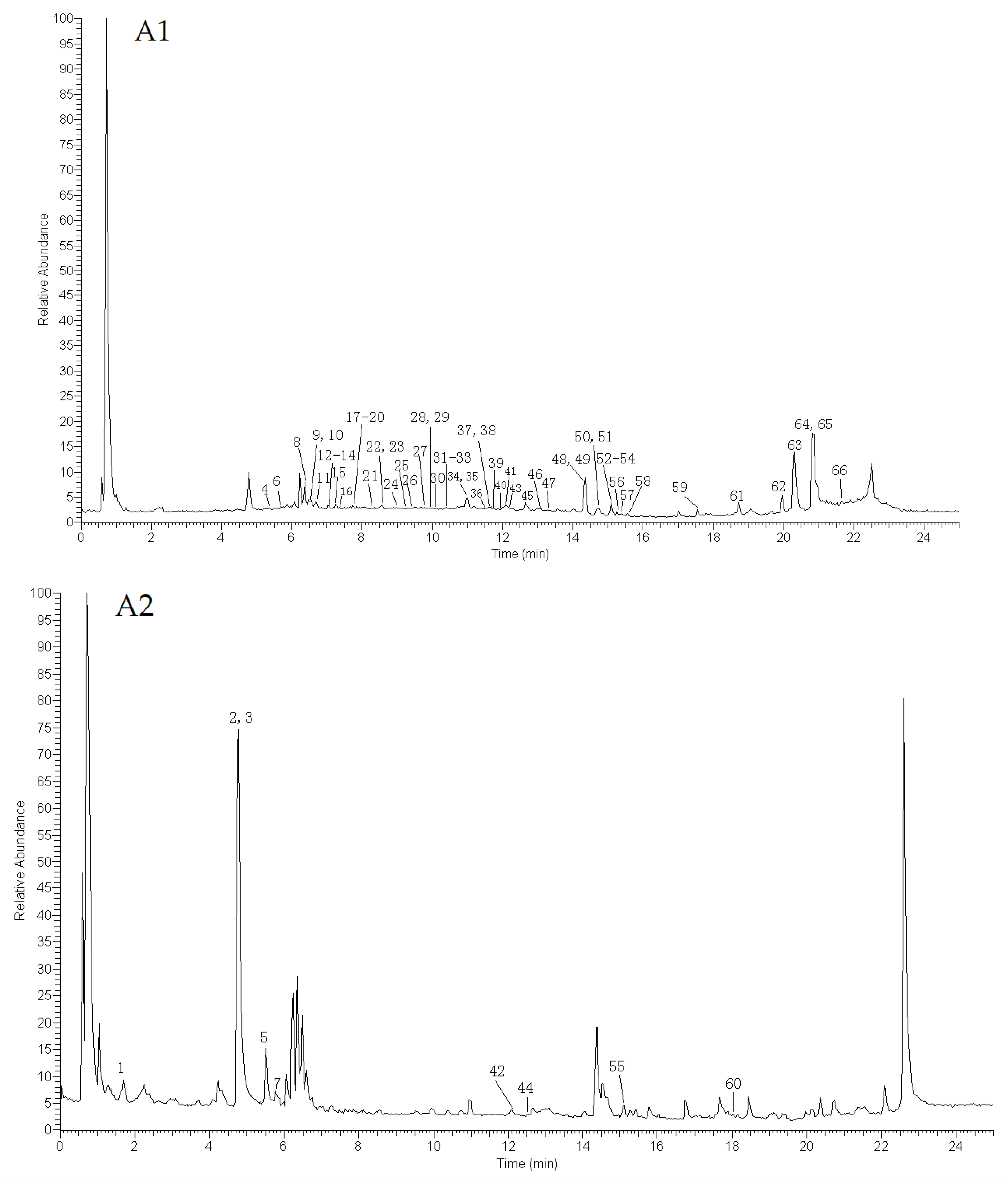
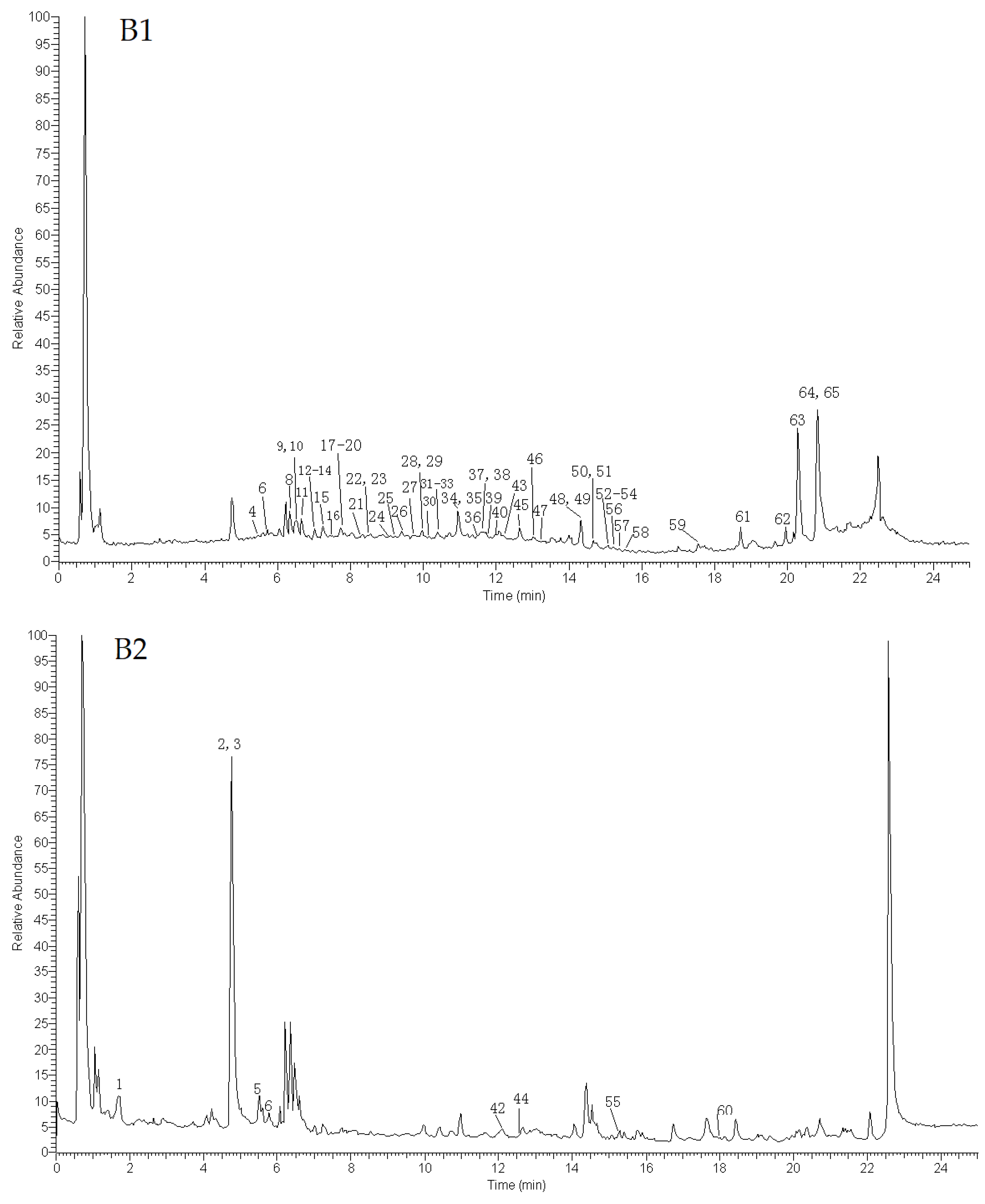

| NO. | Retention Time (min) | ESI-MS (m/z) | Error (ppm) | MS/MS Fragments Ions | Formula | Identification |
|---|---|---|---|---|---|---|
| 1 | 1.72 | 254.1609 [M + H]+ | −0.2 | 196.1158, 195.1123, 167.1175, 125.0707 | C11H19N5O2 | unknown |
| 2 | 4.78 | 188.0703 [M + H]+ | −1.5 | 142.0648, 118.0650, | C11H9O2N | 3-Indoleacrylic acid |
| 3 | 4.78 | 247.1437 [M + H]+ | −1.4 | 188.0701, 146.0594, 118.0650, 60.0183 | C14H18O2N2 | Hypaphorine |
| 4 | 5.45 | 581.1526 [M − H]− | 3.4 | 563.1313, 287.0566, 269.0458, 259.0615, 243.0670, 163.0030, 133.0281, 125.0236 | C26H30O15 | Okanin 4′-alpha-l-arabinofuranosyl-(1→4)-glucoside |
| 5 | 5.51 | 201.1385 [M + H]+ | −2.8 | 186.1147 | C13H16N2 | dehydrostobadine |
| 6 | 5.67 | 583.1678 [M − H]− | 2.6 | 433.1331, 301.0920, 167.0343, 152.0107, 123.0443 | C26H32O15 | seguinoside K |
| 7 | 5.77 | 217.1335 [M + H]+ | −0.3 | 202.10956, 186.1142, 130.0649 | C13H16N2O | Adrenoglomerulotropin |
| 8 | 6.41 | 1089.5494 [M − H]− | −0.2 | 942.8404, 793.4399, 471.3649 | C53H86O23 | Soyasapogrnol B 3-O-α-l-arabinopyranosyl-(1→2)-β-d-galactopyranosyl-(1→2)-glucuronopyranosyl-22-O-β-d-glucopyranoside |
| 9 | 6.55 | 1119.5594 [M − H]− | 1.4 | 911.4915, 793.4419, 630.0288, 452.1937 | C54 H88O24 | 23-hydroxy-pomalic acid 3-O-α-L-rhamnopyranosyl-(1→4)-β-d-glucopyranosyl-(1→6)-β-d-galactopyranosyl-28-O-β-d-glucopyranoside |
| 10 | 6.58 | 1087.5344 [M − H]− | 1.8 | 821.4017, 556.54883, 487.3268, 435.1130 | C53H84O23 | Oleanolic acid 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl-(1→2)-β-d-galactopyranosyl-28-O-β-d-glucopyranoside |
| 11 | 6.69 | 1117.5437 [M − H]− | −1.7 | 1055.5314, 791.4255, 685.3199 | C54H86O24 | 3β,22,24-trihydroxyolean-12-en-29-oic acid 3-O-α-l-rhamnopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranoyl-22-O-β-d-glucopyranoside |
| 12 | 6.95 | 269.0458 [M − H]− | 4.9 | 241.0505, 225.0556, 197.0605 | C15H10O5 | Baicalein |
| 13 | 7.02 | 971.4858 [M + HCOO]− | 1.9 | 629.3689, 471.3462 | C47H74O18 | 3β-olean-12-en-28,29-dioic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside |
| 14 | 7.02 | 1103.5643 [M − H]− | 4.3 | 895.5084, 777.4471, 571.3987, 455.3559 | C54H88O23 | Oleanolic acid 3-O-β-d-glucopyranosyl- (1→6)-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl-28-O-β-d-glucopyranoside |
| 15 | 7.25 | 1103.5631 [M − H]− | −3.2 | 957.5154, 777.4453, 616.3993, 457.3670 | C54H88O23 | Soyasapogrnol B 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranosyl-(1→2)-glucuronopyranosyl-22-O-β-d-glucopyranoside |
| 16 | 7.40 | 269.0457 [M − H]− | 4.9 | 241.0503, 225.0556, 213.0554, 135.0073, 133.0287, 91.0179 | C15H10O5 | 5,3′,4′-trihydroxy-flavone |
| 17 | 7.73 | 283.0615 [M − H]− | 4.4 | 268.0380, 240.0424, 211.0396 | C16H12O5 | Isoprunetin |
| 18 | 7.76 | 255.0664 [M − H]− | 4.8 | 153.0186, 135.0079, 119.0493 | C15H12O4 | Isoliquiritigenin |
| 19 | 7.78 | 1131.5227 [M − H]− | −2.2 | 1090.9832, 805.4061, 536.4517 | C54H84O25 | 3β-olean-12-en-28,29-dioic acid 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranosyl-(1→2)-glucuronopyranosyl-28-O-β-d-glucopyranoside |
| 20 | 7.81 | 1073.5536 [M − H]− | −2.9 | 1056.5305, 777.4403, 457.3699 | C52H84O20 | Oleanolic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-galactopyranoside |
| 21 | 8.26 | 1071.5377 [M − H]− | 1.7 | 1053.5327, 775.4200,435.3287 | C53H84O22 | Betulinic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside |
| 22 | 8.54 | 269.0820 [M − H]− | 4.3 | 254.05722, 225.0553, 119.0494 | C16H14O4 | 4,4′-dihydroxy-2′-methoxychalcone |
| 23 | 8.56 | 793.4055 [M − H]− | 1.8 | 599.3633, 437.3063 | C42H66O14 | Calenduloside F |
| 24 | 9.08 | 271.0614 [M − H]− | 4.7 | 151.0029, 119.0493 | C15H12O5 | Naringenin |
| 25 | 9.32 | 1071.5386 [M − H]− | 2.2 | 775.4217, 435.3235 | C53H84O22 | Oleanolic acid 3-O-α l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d glucuronopyranosyl-28-O-β-d-glucopyranoside |
| 26 | 9.42 | 269.0822 [M − H]− | 3.2 | 254.0581, 175.0394, 161.0237, 133.0287, 117.0339 | C16H14O4 | Medicarpin |
| 27 | 9.81 | 823.4122 [M − H]− | 1.6 | 643.3477, 485.3258 | C42H64O16 | 3β-olean-12-en-28,29-dioic acid 3-O-β-d-glucopyranosyl-(1→2)-glucuronopyranoside |
| 28 | 9.92 | 299.0564 [M − H]− | 1.2 | 284.0328, 271.0611, 253.0505, 161.0240, 137.0236 | C16H12O6 | Tectorigenin |
| 29 | 9.98 | 969.4701 [M − H]− | −0.9 | 780.9517, 643.3474, 485.3314, 205.0711, 163.0606 | C48H74O20 | millettiasaponin B |
| 30 | 10.09 | 283.0613 [M − H]− | 4.7 | 268.0379, 240.0429, 148.0157, 135.0073 | C16H12O5 | calycosin |
| 31 | 10.38 | 971.4856 [M + HCOO]− | −1.4 | 809.4363, 629.3690, 471.3469 | C47H74O18 | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside |
| 32 | 10.42 | 983.4859 [M − H]− | 0.8 | 733.4136, 645.3657, 487.3443 | C49H76O20 | 22β-acetyloxy-3β,24-dihydroxyolean-12-en-29-oic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-β-d-galactopyranoside |
| 33 | 10.43 | 299.0565 [M − H]− | 1.3 | 284.0323, 176.0109, 151.0070, 148.0156, 135.0073 | C16H12O6 | Pratensein |
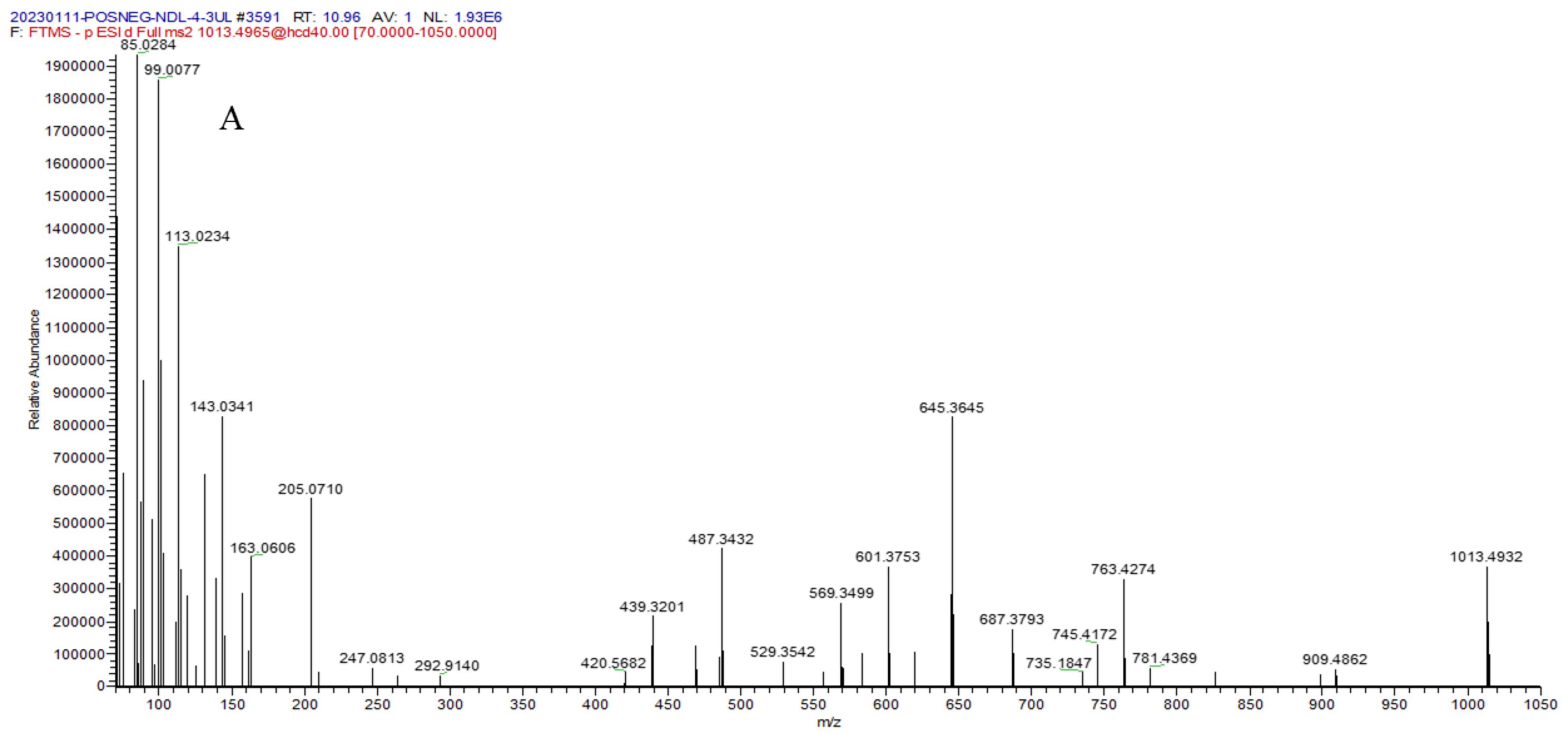
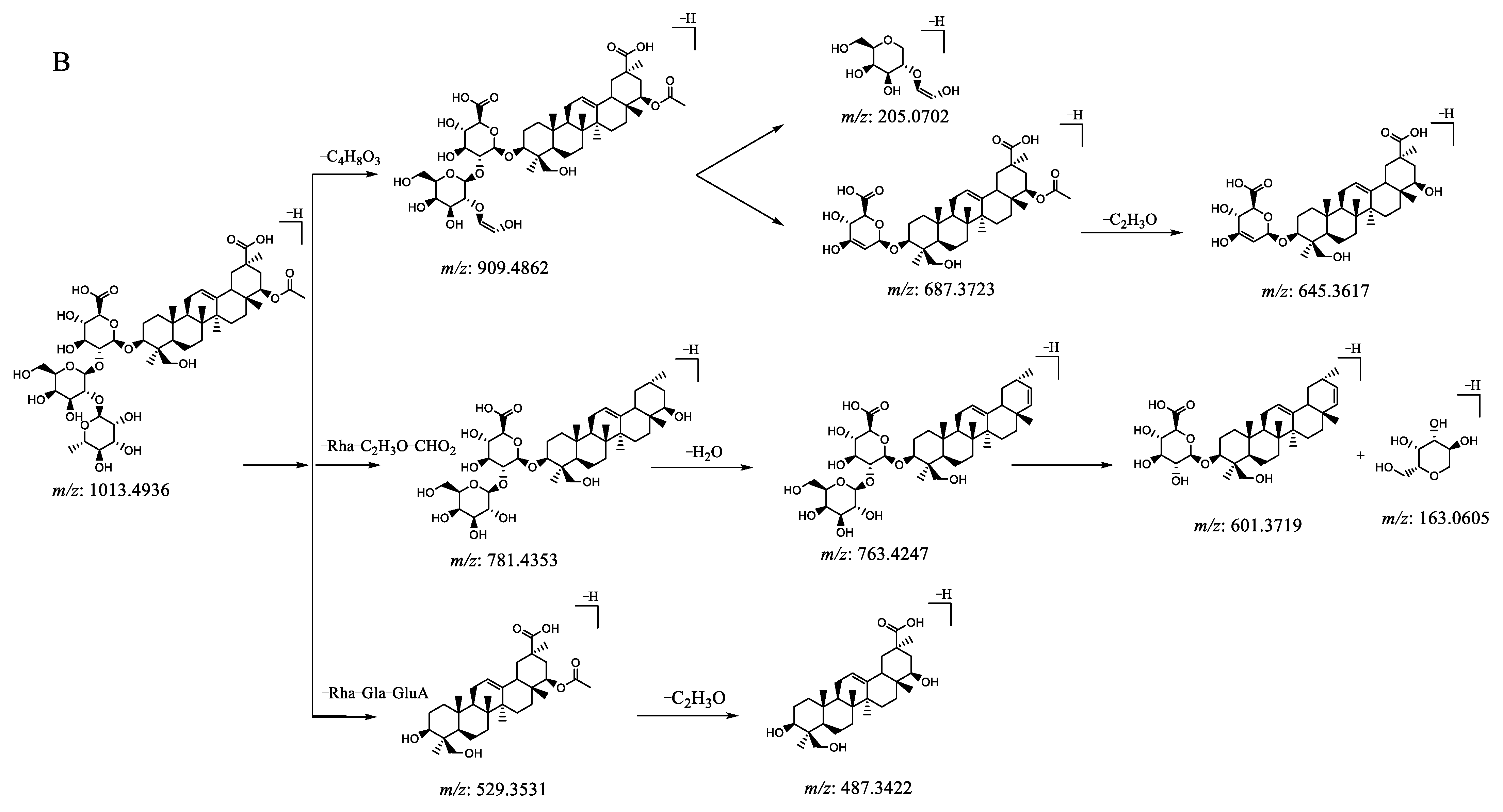
| 34 | 10.96 | 1013.4932 [M − H]− | −1.2 | 909.4862, 781.4369, 763.4274, 687.3793, 645.3645, 601.3753, 529.3542, 487.3432, 205.0710, 163.0606 | C50H78O21 | millettiasaponin A |
| 35 | 10.96 | 329.2335 [M − H]− | 2.5 | 229.1443, 211.1336 | C18H34O5 | 9,12,13-trihydroxyoctadeca-10(E)-dienoic acid |
| 36 | 11.55 | 281.0457 [M − H]− | 4.6 | 251.0350, 225.0555, 135.0079, 117.0339 | C16H10O5 | 7,4′-dimethoxyisoflavone |
| 37 | 11.63 | 255.0663 [M − H]− | 4.8 | 135.0080, 119.0494 | C15H12O4 | Liquiritigenin |
| 38 | 11.66 | 837.4275 [M + HCOO]− | 3.6 | 733.4119, 645.3645, 487.3445, 439.3220 | C42H64O14 | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranoside |
| 39 | 11.74 | 953.4751 [M − H]− | −2.1 | 627.3541, 537.3569, 469.3323 | C48H74O19 | 3β-olean-12-en-28,29-dioic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranoside |
| 40 | 12.00 | 267.0665 [M − H]− | 4.9 | 252.0429, 223.0397, 135.0080, 132.0287 | C16 H12O4 | Formononetin |
| 41 | 12.08 | 867.4377 [M + HCOO]− | 3.2 | 645.3696, 469.3309 | C42H62O16 | Glycyrrhizic acid |
| 42 | 12.15 | 299.0905 [M + H]+ | 4.5 | 284.0677, 256.0720, 239.0696, 167.0335, 132.0568 | C17H14O5 | Alfalone |
| 43 | 12.23 | 953.4752 [M − H]− | 2.6 | 627.3545, 469.3321 | C48H74O19 | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranosyl-(1→2)-glucuronopyranoside |
| 44 | 12.57 | 299.0906 [M + H]+ | 4.5 | - | C17H14O5 | millettiaosa A |
| 45 | 12.65 | 997.5020 [M − H]− | −0.3 | 933.3743, 747.4297, 629.3691, 585.3782, 539.3754, 471.3483 | C50H78O20 | unknown |
| 46 | 13.06 | 997.5018 [M − H]− | −1.3 | 747.4376, 629.3703, 585.3818, 539.3750, 471.3483, 443.4824 | C50H78O20 | unknown |
| 47 | 13.29 | 971.4857 [M + HCOO]− | −1.4 | 809.4360, 629.3693, 471.3461 | C47H74O18 | Saikogenin G 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-glucuronopyranoside |
| 48 | 14.32 | 911.5012 [M − H]− | 3.5 | 457.3714, 409.3477 | C47H76O17 | Soyasaponin II |
| 49 | 14.33 | 941.5075 [M − H]− | −2.3 | 457.3685, 426.9266 | C48H78O18 | Saikogenin G 3-O-α-l-rhamnopyranosyl-(1→4)-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside |
| 50 | 14.68 | 911.5014 [M − H]− | 2.7 | 472.6508 | C47H76O17 | Saikogenin G 3-O-α-l-arabinopyranosyl- (1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside |
| 51 | 14.68 | 795.4540 [M − H]− | −0.2 | 615.3959, 457.3717 | C42H68O14 | Soyasapogrnol B 3-O-β-d-galactopyranosyl-(1→2)-glucuronopyranoside |
| 52 | 15.02 | 283.0615 [M − H]− | −0.3 | 239.0349, 223.0474, 211.0399, 132.0213 | C16H12O5 | Maackiain |
| 53 | 15.06 | 909.4843 [M − H]− | −1.2 | 455.3547, 407.3309 | C47H74 O17 | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-arabinopyranosyl-(1→2)-β-d-galactopyranosyl-(1→2)-glucuronopyranoside |
| 54 | 15.09 | 939.4962 [M − H]− | 2.1 | 613.3740, 455.3528 | C47H72O19 | 3α-hydroxy-11-oxoolean-12-en-30-oic acid 3-O-α-l-rhamnopyranosyl-(1→4)-β-d-glucopyranosyl-(1→6)-β-d-galactopyranoside |
| 55 | 15.19 | 467.1937 [M + Na]+ | 3.4 | 224.1055, 105.0335 | C27H28N2O4 | unknown |
| 56 | 15.24 | 895.5065 [M − H]− | 4.1 | 509.4002, 439.3597 | C47H76O16 | Betulinic acid 3-O-α-l-arabinopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside |
| 57 | 15.32 | 1067.5437 [M − H]− | 3.2 | 921.4910, 583.3988, 457.3726 | C54H84O21 | Soyasaponin VI |
| 58 | 15.56 | 283.0955 [M − H]− | −5.5 | 268.0716, 121.0645 | C17H16O4 | millettiaosa B |
| 59 | 17.55 | 295.2280 [M − H]− | 1.8 | 277.2175, 171.1018 | C18H32O3 | 9-hydroxy-10,12-octadecadienoic acid |
| 60 | 18.02 | 429.3723 [M + Na]+ | −1.3 | 411.3609, 393.3521, 369.3146 | C29H48O2 | 7-Ketositosterol |
| 61 | 18.77 | 455.3533 [M − H]− | −1.5 | - | C30H48O3 | Betulinic acid |
| 62 | 20.07 | 617.385 [M − H]− | 2.4 | 415.2764, 179.0342 | C39H54O6 | pyracrenic acid |
| 63 | 20.45 | 617.3848 [M − H]− | 2.1 | 415.2768, 179.0340 | C39H54O6 | 3-O-Caffeoyloleanolic acid |
| 64 | 20.82 | 603.4056 [M − H]− | 0.5 | 179.0338, 161.0237, 133.0284 | C39H56O5 | erythrodiol-3-caffeate |
| 65 | 20.92 | 603.4058 [M − H]− | 1.0 | 179.0341, 161.0236, 133.0285 | C39H56O5 | Betulin-3-caffeate |
| 66 | 21.67 | 603.4056 [M − H]− | 0.7 | 179.0336, 161.0237, 133.0286 | C39H56O5 | uvaol-caffeate |
| No. | ABTS | DPPH | No. | ABTS | DPPH |
|---|---|---|---|---|---|
| IC50 (mg/mL) | IC50 (mg/mL) | IC50 (mg/mL) | IC50 (mg/mL) | ||
| W1 | 6.18 ± 0.21 | 5.54 ± 0.18 | C1 | 7.18 ± 0.44 | 6.22 ± 0.21 |
| W2 | 5.45 ± 0.10 | 3.23 ± 0.10 | C2 | 7.45 ± 0.18 | 8.32 ± 0.44 |
| W3 | 6.94 ± 0.17 | 4.52 ± 0.22 | C3 | 8.32 ± 0.59 | 7.25 ± 0.28 |
| W4 | 5.55 ± 0.31 | 4.21 ± 0.08 | C4 | 6.62 ± 0.27 | 5.10 ± 0.17 |
| W5 | 4.47 ± 0.24 | 4.33 ± 0.44 | C5 | 8.47 ± 0.34 | 6.45 ± 0.30 |
| W6 | 4.69 ± 0.20 | 3.84 ± 0.12 | C6 | 6.43 ± 0.30 | 5.03 ± 0.15 |
| W7 | 5.54 ± 0.31 | 4.35 ± 0.43 | C7 | 6.74 ± 0.41 | 7.35 ± 0.36 |
| W8 | 4.25 ± 0.08 | 4.26 ± 0.20 | C8 | 7.52 ± 0.16 | 8.27 ± 0.42 |
| W9 | 5.57 ± 0.30 | 5.05 ± 0.24 | C9 | 9.08 ± 0.33 | 7.17 ± 0.16 |
| W10 | 6.02 ± 0.31 | 2.38 ± 0.10 | C10 | 10.02 ± 0.86 | 6.42 ± 0.25 |
| No. | Origin of Sample | Coordinates | Classification | Collection Date |
|---|---|---|---|---|
| W1 | Longmen, Pubei, Guangxi | N 22°09′53.54″ E 109°22′48.06″ | Wild | September 2020 |
| W2 | Xiaojiang, Qinzhou, Guangxi | N 22°15′12.86″ E 109°34′25.93″ | Wild | September 2020 |
| W3 | Naixiao, Nanning, Guangxi | N 22°23′54.99″ E 108°27′43.15″ | Wild | September 2020 |
| W4 | Yanan, Nanning, Guangxi | N 22°30′7.24″ E 108°08′35.83″ | Wild | April 2021 |
| W5 | Nanping, Shangsi, Guangxi | N 22°11′27.65″ E 108°03′41.54″ | Wild | April 2021 |
| W6 | Naqin, Shangsi, Guangxi | N 22°08′6.14″ E 108°04′17.37″ | Wild | June 2021 |
| W7 | Zhangwang, Pubei, Guangxi | N 22°0′33.95″ E 109°28′47.60″″ | Wild | June 2021 |
| W8 | Guandong, Pubei, Guangxi | N 22°26′35.47″ E 109°41′19.30″ | Wild | October 2021 |
| W9 | Duruan, Jiangmen, Guangdong, | N 22°34′30.73″ E 113°02′45.58″ | Wild | October 2021 |
| W10 | Yayao, Heshan, Guangdong | N 22°42′35.37″ E 113°0′16.14″ | Wild | November 2021 |
| C1 | Dacheng, Qinzhou, Guangxi | N 22°19′45.46″ E 109°25′20.76″ | cultivated | August 2020 |
| C2 | Quanshui, Qinzhou, Guangxi | N 21°56′30.57″ E 109°26′53.65″ | cultivated | August 2020 |
| C3 | Fuwang, Qinzhou, Guangxi | N 22°25′4.50″ E 109°35′18.91″ | cultivated | March 2021 |
| C4 | Gongzheng, Shangsi, Guangxi | N 22°09′43.45″ E 108°08′35.93″ | cultivated | March 2021 |
| C5 | Siyang, Shangsi, Guangxi | N 22°07′30.69″ E 108°06′54.38″ | cultivated | March 2021 |
| C6 | Yanan, Nanning, Guangxi | N 22°22′54.18″ E 108°25′57.85 | cultivated | May 2021 |
| C7 | Nayang, Hengzhou, Guangxi | N 22°41′57.56″ E 109°19′38.74 | cultivated | May 2021 |
| C8 | Yayao, Heshan, Guangdong | N 22°42′27.50″ E 112°59′32.86″ | cultivated | May 2021 |
| C9 | Yayao, Heshan, Guangdong | N 22°42′35.37″ E 113°0′16.14″ | cultivated | August 2021 |
| C10 | Duruan, Jiangmen, Guangdong | N 22°34′43.13″ E 113°02′41.90″ | cultivated | August 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Yang, Q.; Huang, B.; Chen, M.; Liang, Z.; Zhang, Z.; Zhang, J. Utilizing Integrated UHPLC-Q-Exactive Orbitrap-MS, Multivariate Analysis, and Bioactive Evaluation to Distinguish between Wild and Cultivated Niudali (Millettia speciosa Champ.). Molecules 2024, 29, 806. https://doi.org/10.3390/molecules29040806
Zeng Y, Yang Q, Huang B, Chen M, Liang Z, Zhang Z, Zhang J. Utilizing Integrated UHPLC-Q-Exactive Orbitrap-MS, Multivariate Analysis, and Bioactive Evaluation to Distinguish between Wild and Cultivated Niudali (Millettia speciosa Champ.). Molecules. 2024; 29(4):806. https://doi.org/10.3390/molecules29040806
Chicago/Turabian StyleZeng, Yuwei, Qing Yang, Binbin Huang, Ming Chen, Zichang Liang, Zhifeng Zhang, and Jianguang Zhang. 2024. "Utilizing Integrated UHPLC-Q-Exactive Orbitrap-MS, Multivariate Analysis, and Bioactive Evaluation to Distinguish between Wild and Cultivated Niudali (Millettia speciosa Champ.)" Molecules 29, no. 4: 806. https://doi.org/10.3390/molecules29040806
APA StyleZeng, Y., Yang, Q., Huang, B., Chen, M., Liang, Z., Zhang, Z., & Zhang, J. (2024). Utilizing Integrated UHPLC-Q-Exactive Orbitrap-MS, Multivariate Analysis, and Bioactive Evaluation to Distinguish between Wild and Cultivated Niudali (Millettia speciosa Champ.). Molecules, 29(4), 806. https://doi.org/10.3390/molecules29040806






