Catalytic Hydroconversion of Model Compounds over Ni/NiO@NC Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Catalyst Characterizations
2.2. Catalyst Screening
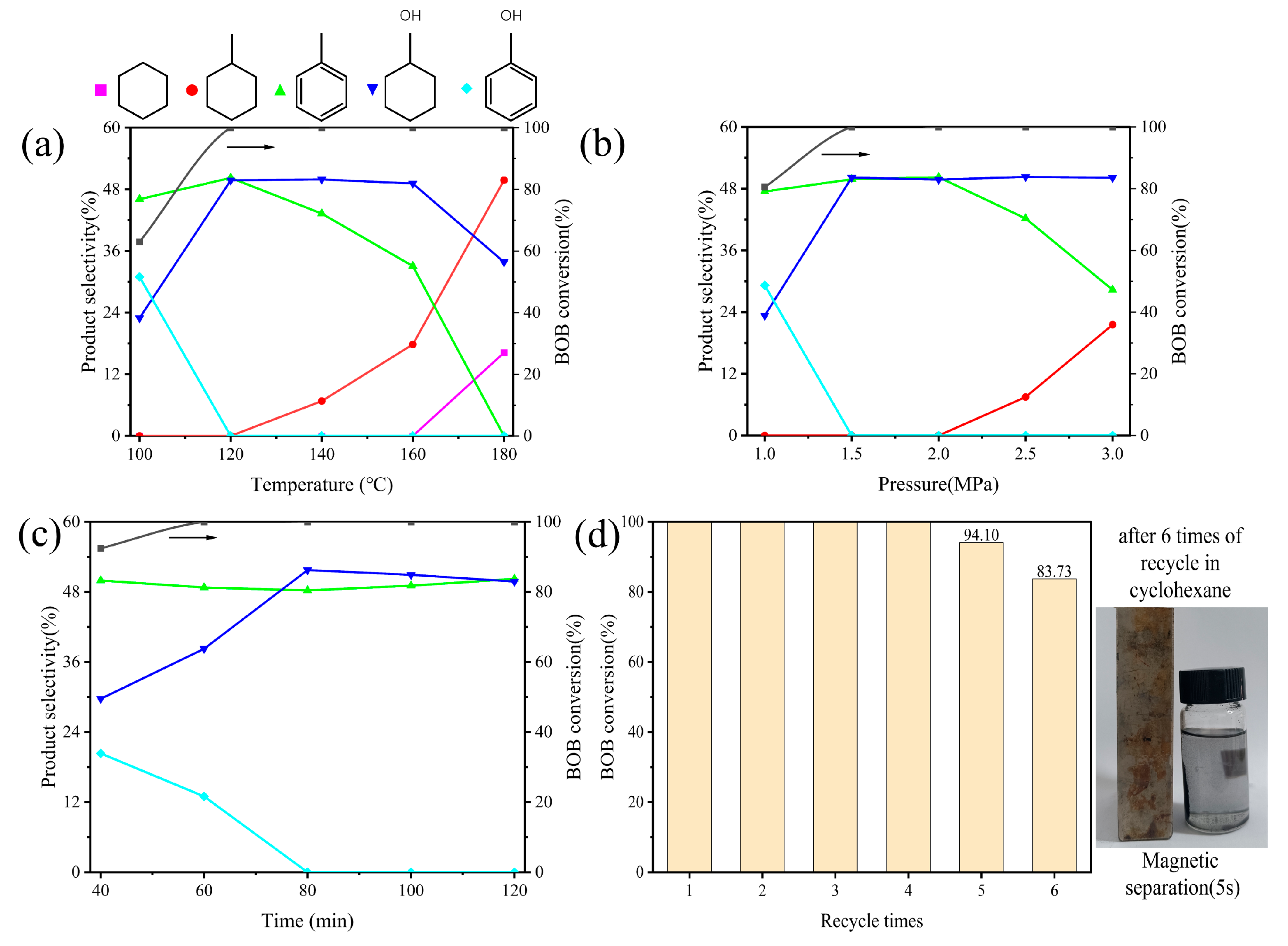
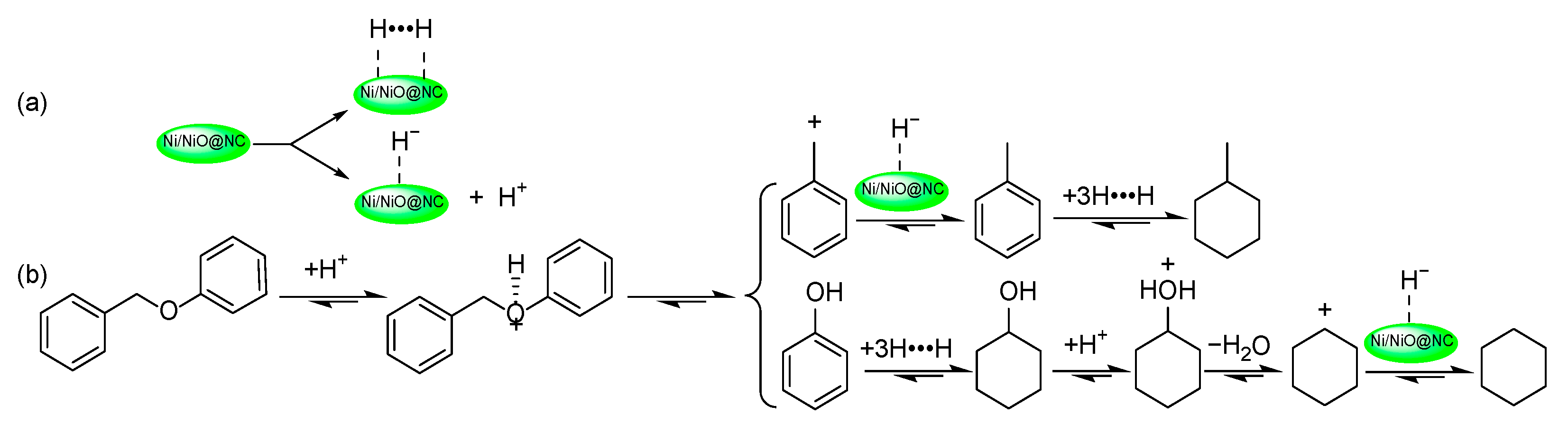
2.3. CHC of BOB under Different Reaction Conditions
2.4. Cyclic Stability of Ni/NiO@NC10,000-10-350
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Hydrogenation and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.P.; Li, G.Y.; Guo, R.; Li, A.Q.; Liang, Y.H. Theoretical and Experimental Insight into Coal Structure: Establishing a Chemical Model for Yuzhou Lignite. Energy Fuels 2017, 31, 124–132. [Google Scholar] [CrossRef]
- Li, S.; Du, F.F.; Ma, Z.H.; Zhang, L.H.; Liu, Z.Q.; Zhao, T.S.; Wei, X.Y.; Xu, M.L.; Cong, X.S. Insight into the Compositional Features of Organic Matter in Xilinguole Lignite through Two Mass Spectrometers. ACS Omega 2022, 7, 46384–46390. [Google Scholar] [CrossRef]
- Liu, M.X.; Lei, Z.P.; Cao, X.Z.; Yan, J.C.; Shui, H.F.; Wang, Z.C.; Hu, J.B.; Hong, M.Q. Construction of Macromolecules of Depolymerized Lignite. ACS Omega 2023, 8, 22820–22826. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Zhang, X.Q.; Gao, J.; Wei, X.Y.; Xue, C.X.; Li, Y.J.; Gao, Y.; Liu, G.H.; Bai, J.J.; Ma, X.R.; et al. Deep hydroconversion of ethanol-soluble portion from the ethanolysis of Hecaogou subbituminous coal to ultra-clean liquid fuel over hierarchical porous zeolite Y supported Ni–Co nanoparticles. Energy Inst. 2021, 99, 88–96. [Google Scholar] [CrossRef]
- Kang, Y.H.; Wei, X.Y.; Liu, G.H.; Ma, X.R.; Gao, Y.; Li, X.; Li, Y.J.; Ma, F.Y.; Yan, L.; Zong, Z.M. Catalytic Hydroconversion of Ethanol-Soluble Portion from the Ethanolysis of Hecaogou Subbituminous Coal Extraction Residue to Clean Liquid Fuel over a Zeolite Y/ZSM-5 Composite Zeolite-Supported Nickel Catalyst. Energy Fuels 2019, 34, 4799–4807. [Google Scholar] [CrossRef]
- Li, W.T.; Wei, X.Y.; Liu, X.X.; Guo, L.L.; Qi, S.C.; Li, Z.K.; Zhang, D.D.; Zong, Z.M. Catalytic hydroconversion of methanol-soluble portion from Xiaolongtan lignite over difunctional Ni/Z5A. Fuel Process. Technol. 2016, 148, 146–154. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wei, X.Y.; Wu, H.H.; Li, W.T.; Zhang, Y.Y.; Zong, Z.M.; Ma, F.Y.; Liu, J.M. Difunctional nickel/microfiber attapulgite modified with an acidic ionic liquid for catalytic hydroconversion of lignite-related model compounds. Fuel 2017, 204, 236–242. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, W.X.; Bai, J.J.; Zhu, Q.Y.; Li, X.L.; Liu, G.H.; Wei, X.Y.; Kang, Y.H.; Li, Y.J.; Ma, X.R.; et al. Investigation on the catalytic performance of magnetic copper ferrite nanoparticles in the catalytic hydroconversion of Hanglaiwan long flame coal. Fuel 2023, 353, 129173. [Google Scholar] [CrossRef]
- Liu, F.J.; Guo, J.P.; Liu, G.H.; Bie, L.L.; Zhao, Y.P.; Huang, Z.X.; Wei, X.Y. Effect of temperature on catalytic hydrocracking of Xiaolongtan lignite over a mesoporous silica-coated Fe3O4 supported magnetic solid base for producing aromatics. J. Energy Inst. 2020, 94, 352–359. [Google Scholar] [CrossRef]
- Trautmann, M.; Lang, S.; Traa, Y. Direct liquefaction of lower-rank coals and biocoals with magnetically separable catalysts as a sustainable route to fuels. Fuel 2015, 151, 102–109. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wei, X.Y.; Zhou, X.; Xiao, Y.C.; Li, Z.K.; Guo, X.H.; Wang, S.K.; Zhang, Y.Y.; Zong, Z.M.; Ma, F.Y.; et al. An acidic ionic liquid modified microfiber attapulgite-supported nickel for catalytic hydroconversion of A,Ω-diarylalkanes. Fuel Process. Technol. 2017, 161, 85–94. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.Q.; Bai, J.; Li, X. Transformation and roles of inherent mineral matter in direct coal liquefaction: A mini-review. Fuel 2017, 197, 209–216. [Google Scholar] [CrossRef]
- Jin, L.J.; Han, K.M.; Wang, J.Y.; Hu, H.Q. Direct liquefaction behaviors of Bulianta coal and its macerals. Fuel Process. Technol. 2014, 128, 232–237. [Google Scholar] [CrossRef]
- Niu, B.; Jin, L.J.; Li, Y.; Shi, Z.W.; Li, Y.T.; Hu, H.Q. Mechanism of hydrogen transfer and role of solvent during heating-up stage of direct coal liquefaction. Fuel Process. Technol. 2017, 160, 130–135. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Ma, F.Y.; Liu, X.J.; Wu, H.H.; Niu, C.G.; Su, X.T.; Liu, J.M. Large-scale synthesis and catalysis of oleic acid-coated Fe2O3 for co-liquefaction of coal and petroleum vacuum residues. Fuel Process. Technol. 2015, 139, 173–177. [Google Scholar] [CrossRef]
- Xia, Q.N.; Cuan, Q.; Liu, X.H.; Gong, X.Q.; Lu, G.H.; Wang, Y.Q. Pd/NbOPO4 Multifunctional Catalyst for the Direct Production of Liquid Alkanes from Aldol Adducts of Furans. Angew. Chem. 2015, 126, 9913–9918. [Google Scholar] [CrossRef]
- Gao, D.N.; Xiao, Y.; Varma, A. Guaiacol hydrodeoxygenation over platinum catalyst: Reaction pathways and kinetics. Ind. Eng. Chem. Res. 2015, 54, 10638–10644. [Google Scholar] [CrossRef]
- Luo, Z.C.; Zheng, Z.X.; Wang, Y.C.; Sun, G.; Jiang, H.; Zhao, C. Hydrothermally stable Ru/HZSM-5-catalyzed selective hydrogenolysis of lignin-derived substituted phenols to bio-arenes in water. Green Chem. 2016, 18, 5845–5858. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, C.L.; Wan, H.P.; Lee, H.T.; Liu, C.F. Catalytic Hydrodeoxygenation of Guaiacol on Rh-Based and Sulfided CoMo and NiMo Catalysts. Energy Fuels 2011, 25, 890–896. [Google Scholar] [CrossRef]
- Gao, X.Q.; Zhu, S.H.; Li, Y.W. Selective hydrogenolysis of lignin and model compounds to monophenols over AuPd/CeO2. Mol. Catal. 2019, 462, 69–76. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, Y.Z.; Yang, Y.L.; Zhang, B.S.; Wang, S.; Lin, J.D.; Wan, S.L.; Wang, Y. Selective hydrogenolysis of aryl ether bond over Ru-Fe bimetallic catalyst. Catal. Today 2021, 365, 199–205. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.Y.; Zhang, M.; Zong, Z.M. Catalytic hydroconversion of aryl ethers over a nickel catalyst supported on acid-modified zeolite 5A. Fuel Process. Technol. 2018, 177, 345–352. [Google Scholar] [CrossRef]
- Liu, G.H.; Zong, Z.M.; Liu, F.J.; Meng, X.L.; Zhang, Y.Y.; Wang, S.K.; Li, S.; Zhu, C.; Wei, X.Y.; Ma, F.Y.; et al. Deep hydroconversion of ethanol-soluble portion from the ethanolysis of Dahuangshan lignite to clean liquid fuel over a mordenite supported nickel catalyst. J. Anal. Appl. Pyrolysis 2019, 139, 13–21. [Google Scholar] [CrossRef]
- Liu, G.H.; Zhang, Z.W.; Li, X.L.; Kang, Y.H.; Lu, C.Y.; Chen, J.Z.; Liu, F.J.; Bai, H.C.; Wei, X.Y. Catalytic Degradation and Directional Upgrading of Zhunnan Lignite: Double Constraint of Active Hydrogen and Effective Acquisition of Derived Arenes over Nickel Ferrite. Energy Fuels 2021, 35, 19943–19952. [Google Scholar] [CrossRef]
- Hu, Y.H.; Jiang, G.C.; Xu, G.Q.; Mu, X.D. Hydrogenolysis of lignin model compounds into aromatics with bimetallic Ru-Ni supported onto nitrogen-doped activated carbon catalyst. Mol. Catal. 2018, 445, 316–326. [Google Scholar] [CrossRef]
- Gao, W.R.; Lin, Z.X.; Chen, H.R.; Yan, S.S.; Zhu, H.N.; Zhang, H.; Sun, H.Q.; Zhang, S.; ZHang, S.J.; Wu, Y.L. Roles of graphitization degree and surface functional groups of N-doped activated biochar for phenol adsorption. J. Anal. Appl. Pyrolysis 2022, 167, 105700. [Google Scholar] [CrossRef]
- Tian, J.Q.; Li, H.C.; Zeng, X.; Wang, Z.C.; Huang, J.; Zhao, C. Facile immobilization of Ni nanoparticles into mesoporous MCM-41 channels for efficient methane dry reforming. Chin. J. Catal. 2019, 40, 1395–1404. [Google Scholar] [CrossRef]
- Pandurangan, A.; Pichaikaran, S. Rh/Ni wet-impregnated Ia3d mesostructured aluminosilicate and r-GO catalyst for hydrodeoxygenation of phenoxy benzene. N. J. Chem. 2017, 41, 7893–7907. [Google Scholar]
- Fang, S.Q.; Cui, Z.B.; Zhu, Y.T.; Wang, C.G.; Bai, J.; Zhang, X.H.; Xu, Y.; Liu, Q.Y.; Chen, L.G.; Zhang, Q.; et al. In situ synthesis of biomass-derived Ni/C catalyst by self-reduction for the hydrogenation of levulinic acid to γ-valerolactone. J. Energy Chem. 2019, 37, 204–214. [Google Scholar] [CrossRef]
- Xie, J.X.; Cao, J.P.; Jiang, W.; Zhao, X.Y.; Zhao, L.; Zhang, C.; Bai, H.C. Nickel loaded on biochar prepared from different carbon sources for selective hydrogenolysis of diphenyl ether. Fuel Process. Technol. 2022, 231, 107219. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.P.; Wang, L.L.; Liang, J.Z.; Wei, X.J. Selective hydrogenolysis of aryl ethers over a nitrogen-doped porous carbon supported Ni–CeO2 catalyst at low temperature. Catal. Sci. Technol. 2021, 11, 3241–3250. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, L.J.; Xu, G.Y.; Jia, W.D.; Ma, Y.F.; Zhang, Y. Selective hydrodeoxygenation of lignin-derived phenols to cyclohexanols or cyclohexanes over magnetic CoNx@NC catalysts under mild conditions. ACS Catal. 2016, 6, 7611–7620. [Google Scholar] [CrossRef]
- Ma, L.; Wang, R.; Li, Y.H.; Liu, X.F.; Zhang, Q.Q.; Dong, X.Y.; Zang, S.Q. Apically Co-nanoparticles-wrapped nitrogen-doped carbon nanotubes from a single-source MOF for efficient oxygen reduction. J. Mater. Chem. A 2018, 6, 24071–24077. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, W.G.; Zhang, B.W.; Yin, S.M.; Huang, Y.Z.; Kraft, M.; Xu, R. Nickel Nanoparticles Encapsulated in Few-Layer Nitrogen-Doped Graphene Derived from Metal–Organic Frameworks as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Wang, J.L.; Jiang, S.X.; Li, P.Y. Recent Progress in Sol-Gel Method for Designing and Preparing Metallic and Alloy Nanocrystals. Acta Phys.-Chim. Sin. 2019, 35, 1186–1206. [Google Scholar] [CrossRef]
- Song, Q.L.; Zhao, Y.P.; Wu, F.P.; Li, G.S.; Fan, X.; Wang, R.Y.; Cao, J.P.; Wei, X.Y. Selective hydrogenolysis of lignin-derived aryl ethers over Co/C@N catalysts. Renew. Energy 2020, 148, 729–738. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Liu, J.X.; Zhang, J.J.; Li, Z.X.; Fu, Y. Selective reductive cleavage of C-O bond in lignin model compounds over nitrogen-doped carbon-supported iron catalysts. Mol. Catal. 2018, 452, 36–45. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Asiri, A.M. An investigation of the thermal decomposition of nickel citrate as a precursor for Ni-NiO composite nanoparticles. Thermochim. Acta 2017, 649, 54–62. [Google Scholar] [CrossRef]
- Yang, W.W.; He, D.H. Role of Poly (N-vinyl-2-pyrrolidone) in Ni dispersion for Highly-Dispersed Ni/SBA-15 Catalyst and Its Catalytic Performance in Carbon Dioxide Reforming of Methane. Appl. Catal. A Gen. 2016, 524, 94–104. [Google Scholar] [CrossRef]
- Wu, S.P.; Li, K.H.; Shi, W.J.; Cai, J.W. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, J.Q.; Yu, C.H.; Yan, K.; Fu, Y.; Xie, H.Q.; Chen, J.; Chu, P.; Wu, X.L. Efficient electromagnetic wave absorption by SiC/Ni/NiO/C nanocomposites. J. Alloys Compd. 2019, 816, 152519. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Grivani, G.; Izadi, S. Temperature effect on preparation of Ni/NiO nanocomposites by solid-state thermal decomposition of bis(N-(2-hydroxyphenyl)cinnamaldimine)nickel(II). J. Therm. Anal. Calorim. 2016, 126, 1105–1109. [Google Scholar] [CrossRef]
- Diao, Z.J.; Huang, L.Q.; Chen, B.; Gao, T.; Cao, Z.Z.; Ren, X.D.; Zhao, S.J.; Li, S. Amorphous Ni-Ru bimetallic phosphide composites as efficient catalysts for the hydrogenolysis of diphenyl ether and lignin. Fuel 2022, 324, 124489. [Google Scholar] [CrossRef]
- Zhu, M.L.; Wu, J.; Li, S.Q.; Luo, X.J.; Zhang, B.B.; Jiang, M.J.; Xu, X.B.; Sheng, W.Q.; Xu, J.M.; Song, K.X. Flower-like Ni/NiO microspheres decorated by sericin-derived carbon for high-rate lithium-sulfur batteries. Ionics 2021, 27, 5137–5145. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Mi, H.T.; Chang, Y.Q.; Zhou, D.X.; Zhang, L.D.; Yang, M. Interfacial Engineering and Electrocatalytic Hydrogen Evolution Performance of Ni/TiO2-VO Nanowires Self-supporting Thin Films. Chem. J. Chin. Univ. 2023, 44, 8. [Google Scholar]
- Xie, K.H.; Guo, P.J.; Xiong, Z.Y.; Sun, S.F.; Wang, H.J.; Gao, Y.J. Ni/NiO hybrid nanostructure supported on biomass carbon for visible-light photocatalytic hydrogen evolution. J. Mater. Sci. 2021, 56, 12775–12788. [Google Scholar] [CrossRef]
- Zhukova, A.; Fionov, Y.; Chuklina, S.; Mikhalenko, I.; Fionov, A.V.; Isaikina, O.; Zhukov, D.Y.; Lima, A.M. CO2 Reforming of Ethanol over Ni/Al2O3-(Zr–Yb)O2 Catalysts: The Effect of Zr:Al Ratio on Nickel Activity and Carbon Formation. Energy Fuels 2024, 38, 482–498. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.D.; Han, G.Q.; Liu, X.; Zhang, X.Y.; Sun, Y.F.; Znang, M.; Cao, Z.; Sun, Y.J. Enhanced Electrocatalytic Hydrogen Oxidation on Ni/NiO/C Derived from a Nickel-Based Metal–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 10644–10649. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Rai, A.; Yadav, R.; Sinha, A.K. Glucose hydrogenation to sorbitol over unsupported mesoporous Ni/NiO catalyst. J. Mol. Catal. A Chem. 2018, 451, 186–191. [Google Scholar] [CrossRef]
- Xu, M.; Qin, X.T.; Xu, Y.; Zhang, X.C.; Zheng, L.R.; Liu, J.X.; Wang, M.; Liu, X.; Ma, D. Boosting CO hydrogenation towards C2+ hydrocarbons over interfacial TiO2−x/Ni catalysts. Nat. Commun. 2022, 13, 6720. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, S.Q.; Huang, H.L.; Liu, D.H.; Zhuang, Z.B.; Zhong, C.L. ZIF-67 as Continuous Self-Sacrifice Template Derived NiCo2O4/Co,N-CNTs Nanocages as Efficient Bifunctional Electrocatalysts for Rechargeable Zn–Air Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10021–10029. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Zhang, L.; Huang, J.W.; Peng, L.S.; Deng, M.M.; Li, L.; Li, J.; Chen, H.M.; Wei, Z.D. Boosting Hydrogen Evolution Reaction of Nickel Sulfides by Introducing Nonmetallic Dopants. J. Phys. Chem. C 2020, 124, 24223–24231. [Google Scholar] [CrossRef]
- Qiu, H.G.; Ito, Y.; Cong, W.T.; Tan, Y.W.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M.W. Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production. Angew. Chem. 2015, 127, 14237–14241. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Sui, J.H.; Yin, G.P.; Gao, Y.Z. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2008, 79, 89–99. [Google Scholar] [CrossRef]
- Yang, W.; Fellinger, T.P.; Antonietti, M. Efficient Metal-Free Oxygen Reduction in Alkaline Medium on High-Surface-Area Mesoporous Nitrogen-Doped Carbons Made from Ionic Liquids and Nucleobases. J. Am. Chem. Soc. 2011, 133, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Cao, J.P.; Zhao, X.Y.; Jiang, W.; Zhao, L.; Zhao, M.; Bai, H.C. Selective Cleavage of the Diphenyl Ether C-O Bond over a Ni Catalyst Supported on AC with Different Pore Structures and Hydrophilicities. Energy Fuels 2021, 35, 9599–9608. [Google Scholar] [CrossRef]
- Galindo-Ortega, Y.I.; Infantes-Molina, A.; Huirache-Acuña, R.; Barroso-Martín, I.; Rodríguez-Castellón, E.; Fuentes, S.; Alonso-Nuñez, G.; Zepeda, T.A. Active ruthenium phosphide as selective sulfur removal catalyst of gasoline model compounds. Fuel Process. Technol. 2020, 208, 106507. [Google Scholar] [CrossRef]
- Li, W.T.; Wei, X.Y.; Li, X.K.; Liu, X.X.; Li, Z.K.; Zong, Z.M. Catalytic hydroconversion of lignite-related model compounds over difunctional Ni-Mg2Si/γ-Al2O3. Fuel 2017, 200, 208–217. [Google Scholar] [CrossRef]
- Wei, X.Y.; Liu, F.J.; Wang, B.J.; Wang, B.J.; Zong, Z.M. Temperature-controlled hydrogenation of anthracene over nickel nanoparticles supported on attapulgite powder. Fuel 2018, 223, 222–229. [Google Scholar]
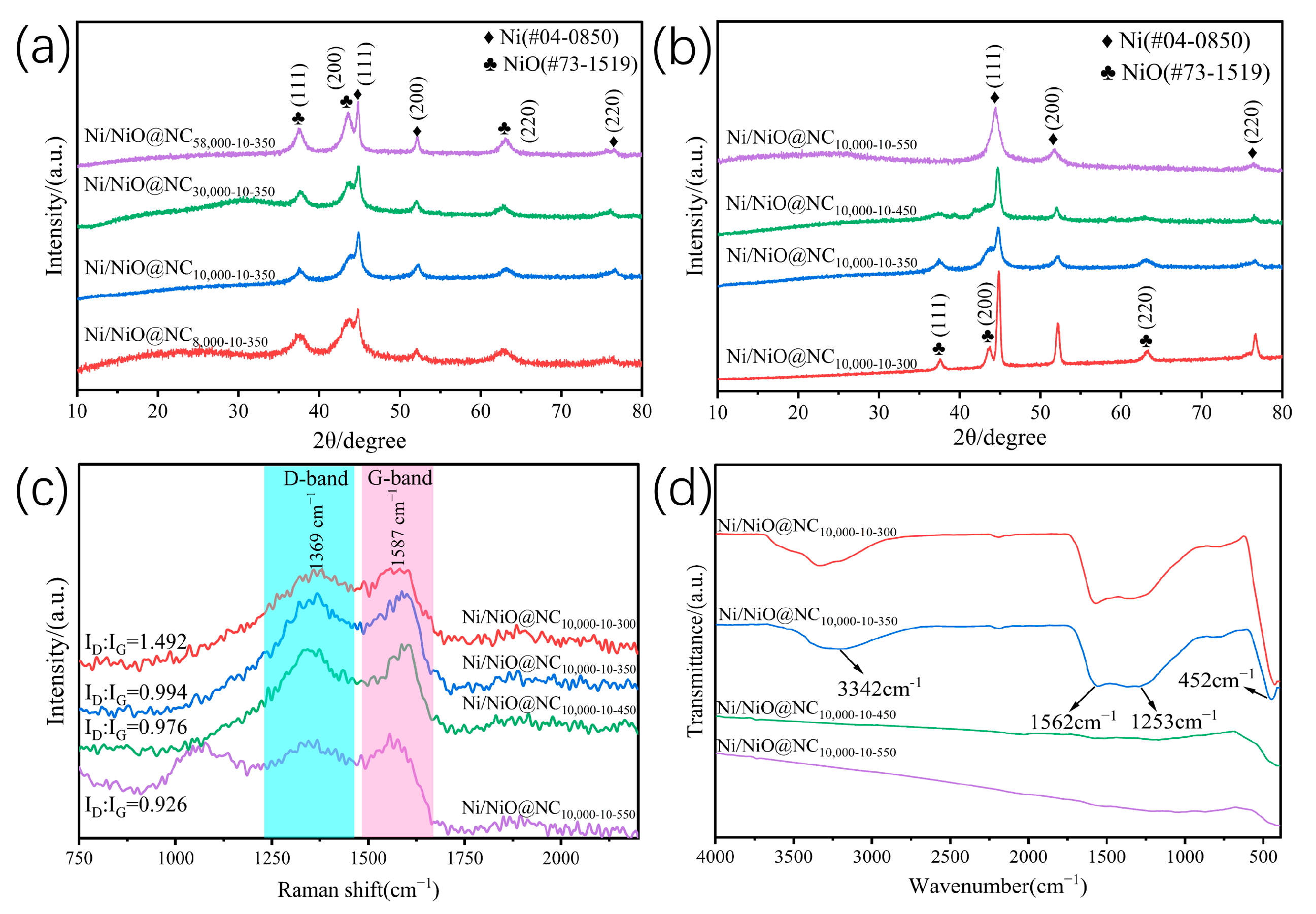


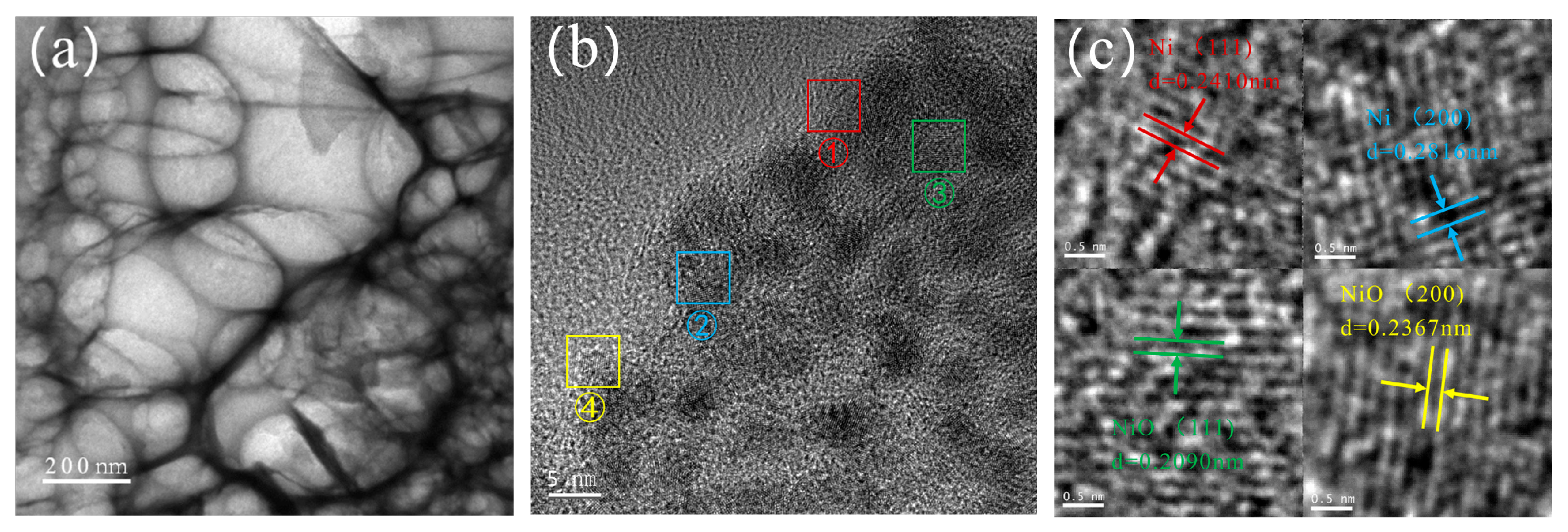
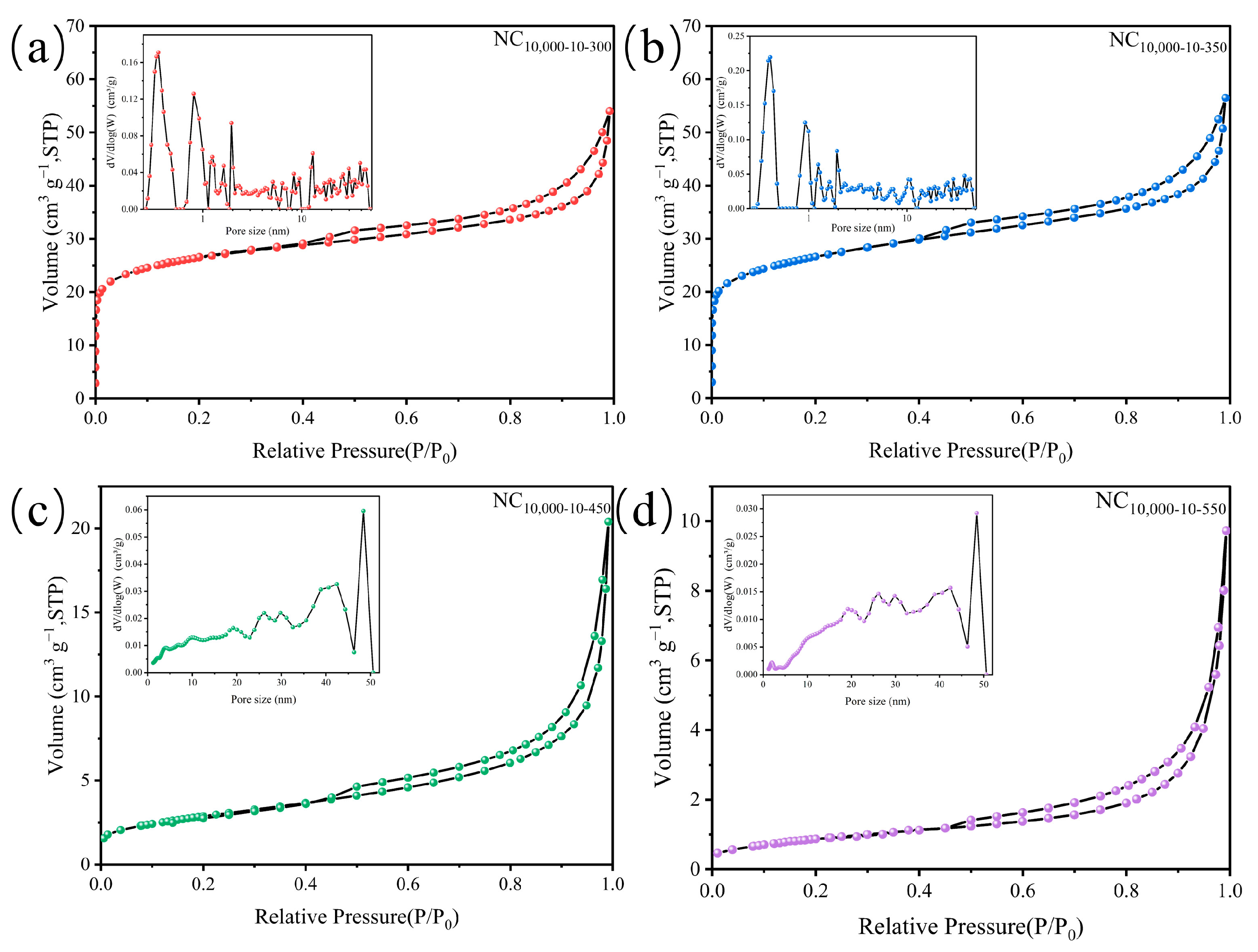
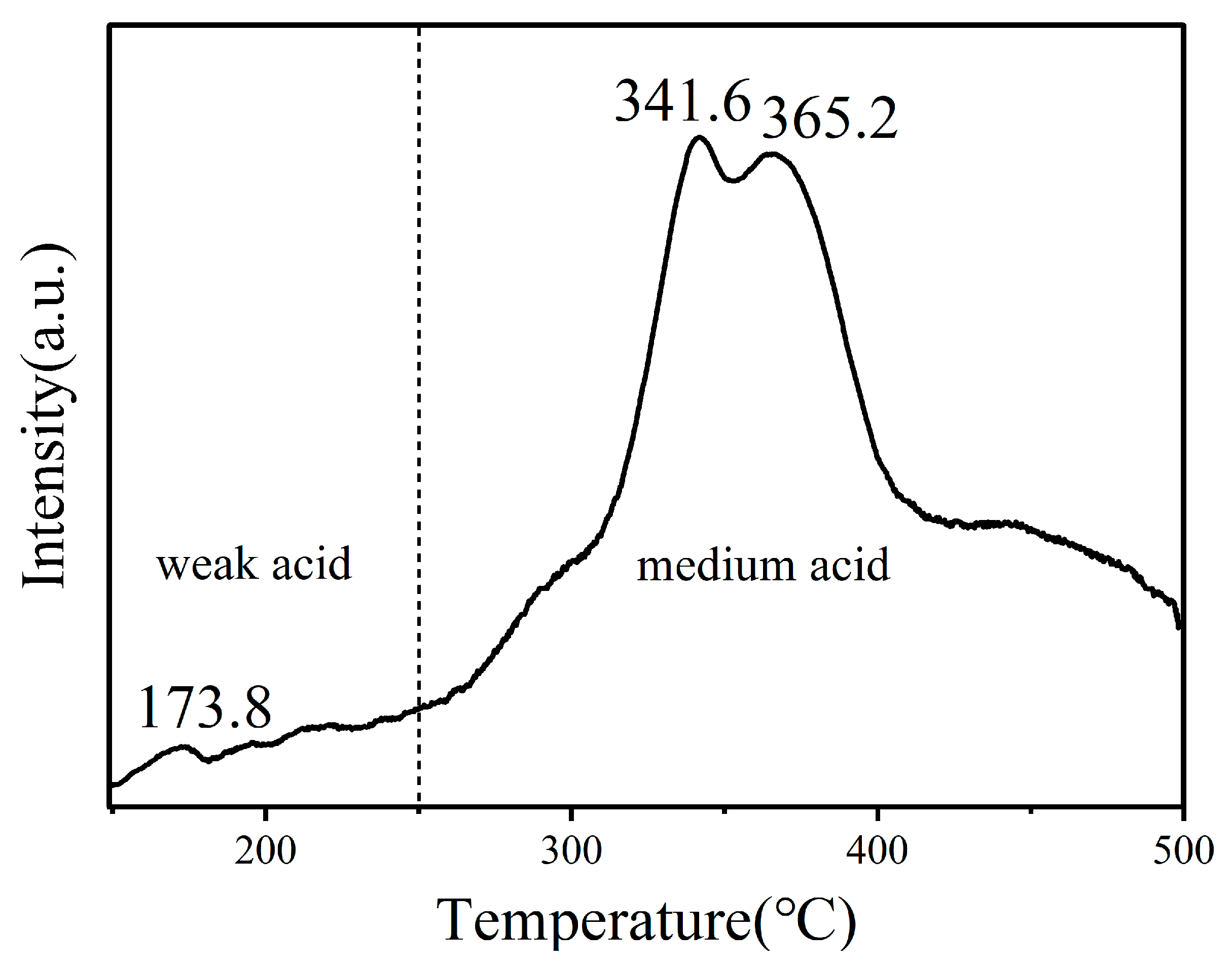
| Entry | Catalysts | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
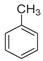 | 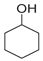 |  | |||
| 1 | None | 0 | - | - | - |
| 2 | Ni/NiO@C10,000-10-350 | 15.74 | 52.37 | - | 47.63 |
| 3 | Ni/NiO@NC8,000-10-350 | 29.25 | 50.53 | - | 49.47 |
| 4 | Ni/NiO@NC10,000-10-350 | 100 | 47.65 | 52.35 | - |
| 5 | Ni/NiO@NC30,000-10-350 | 68.56 | 42.17 | 23.95 | 33.88 |
| 6 | Ni/NiO@NC58,000-10-350 | 22.4 | 52.4 | - | 47.6 |
| 7 | Ni/NiO@NC10,000-5-350 | 91.26 | 47.52 | 38.51 | 13.97 |
| 8 b | Ni/NiO@NC10,000-10-350 | 62.93 | 48.82 | 16.1 | 37.08 |
| 9 b | Ni/NiO@NC10,000-15-350 | 24.42 | 51.91 | - | 48.09 |
| 10 | Ni/NiO@NC10,000-15-350 | 100 | 48.9 | 51.1 | - |
| 11 | Ni/NiO@NC10,000-20-350 | 81.54 | 47.26 | 29.53 | 23.21 |
| 12 | Ni/NiO@NC10,000-10-300 | 43.51 | 47.95 | - | 52.05 |
| 13 | Ni/NiO@NC10,000-10-450 | 37.35 | 49.59 | - | 50.41 |
| 14 | Ni/NiO@NC10,000-10-550 | 0 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Ma, Y.; Tang, Y.; Zhang, Y.; Liu, J.; Zhou, X.; Li, X.; Liu, L. Catalytic Hydroconversion of Model Compounds over Ni/NiO@NC Nanoparticles. Molecules 2024, 29, 755. https://doi.org/10.3390/molecules29040755
Liu T, Ma Y, Tang Y, Zhang Y, Liu J, Zhou X, Li X, Liu L. Catalytic Hydroconversion of Model Compounds over Ni/NiO@NC Nanoparticles. Molecules. 2024; 29(4):755. https://doi.org/10.3390/molecules29040755
Chicago/Turabian StyleLiu, Ting, Yanxi Ma, Yakun Tang, Yue Zhang, Jingmei Liu, Xiaodong Zhou, Xiaohui Li, and Lang Liu. 2024. "Catalytic Hydroconversion of Model Compounds over Ni/NiO@NC Nanoparticles" Molecules 29, no. 4: 755. https://doi.org/10.3390/molecules29040755
APA StyleLiu, T., Ma, Y., Tang, Y., Zhang, Y., Liu, J., Zhou, X., Li, X., & Liu, L. (2024). Catalytic Hydroconversion of Model Compounds over Ni/NiO@NC Nanoparticles. Molecules, 29(4), 755. https://doi.org/10.3390/molecules29040755





