Gluten-Free Sweet Potato Flour: Effect of Drying Method and Variety on the Quality and Bioactivity
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of the Raw Material
2.2. CIELab Colour
2.3. Proximate Composition and Mineral Content
2.4. Bioactive Composition
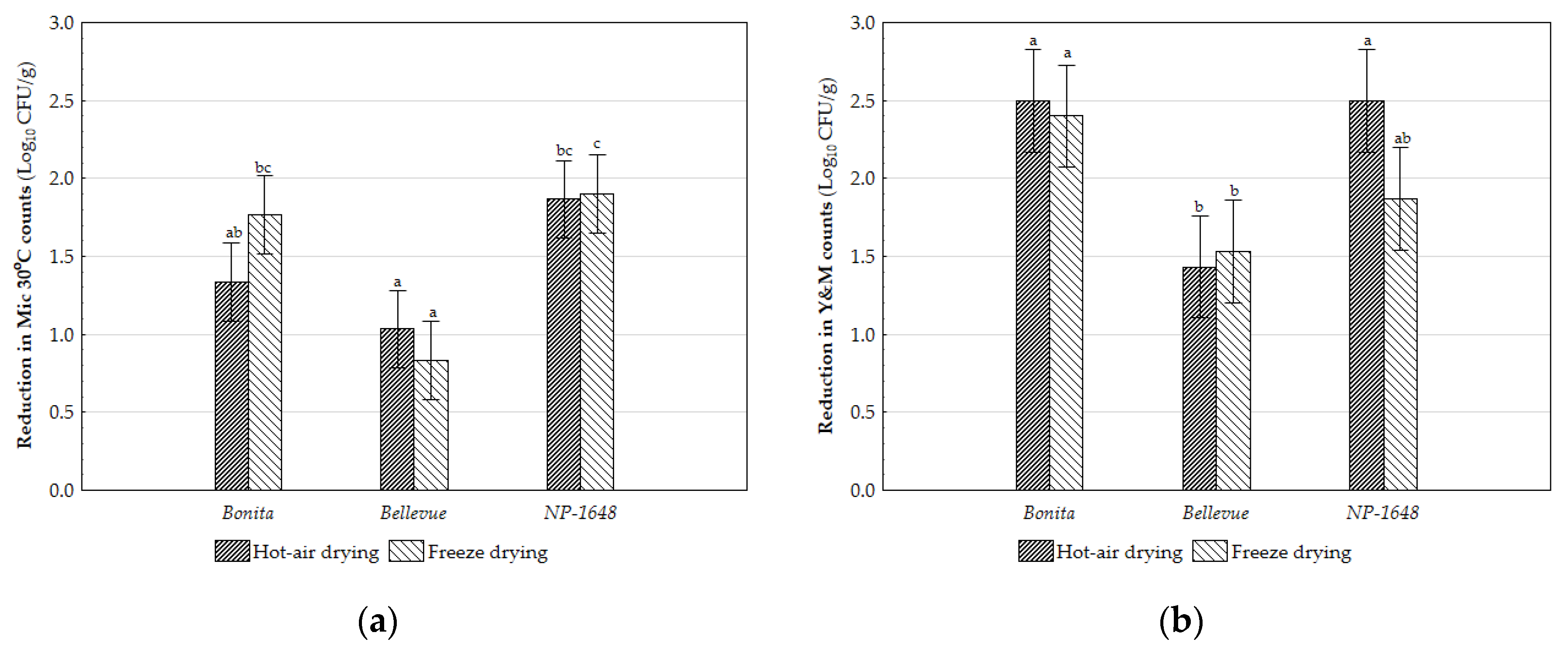
2.5. Microbial Counts
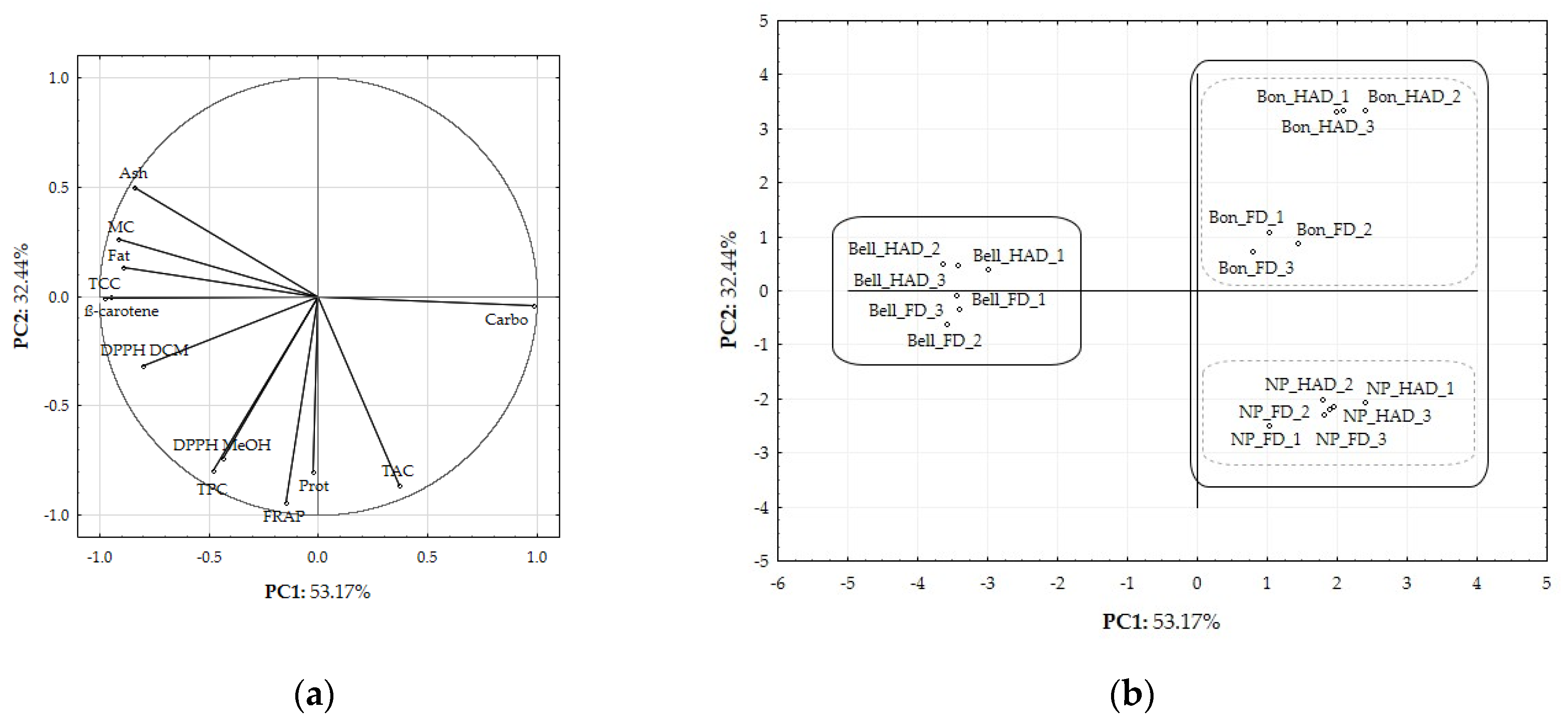
2.6. PCA Modelling
3. Materials and Methods
3.1. Plant Material and Sweet Potato Flour Preparation
3.2. Analytical Procedures
3.2.1. Starch Content
3.2.2. Proximate Composition and Mineral Content
3.2.3. CIELab Colour Measurements
3.2.4. Extract Preparation for TPC and AOx Determinations
3.2.5. Total Phenolic Content
3.2.6. Antioxidant Activity (DPPH and FRAP Methods)
3.2.7. Total Carotenoid Content
3.2.8. Total Anthocyanin Content
3.2.9. β-Carotene Content
3.3. Microbial Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cartabiano-Leite, C.E.; Porcu, O.M.; de Casas, A.F. Sweet potato (Ipomoea batatas L. Lam) nutritional potential and social relevance: A review. Int. J. Eng. Res. Appl. 2020, 10, 23–40. [Google Scholar] [CrossRef]
- FAOSTAT Database. Agriculture Holdings Cultivated for the Production of Crops. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 14 October 2024).
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Amagloh, F.C.; Kaaya, A.N.; Yada, B.; Chelangat, D.M.; Katungisa, A.; Amagloh, F.K.; Tumuhimbise, G.A. Bioactive compounds and antioxidant activities in peeled and unpeeled sweet potato roots of different varieties and clones in Uganda. Futur. Foods 2022, 6, 100183. [Google Scholar] [CrossRef]
- Bach, D.; Bedin, A.C.; Lacerda, L.G.; Nogueira, A.; Demiate, I.M. Sweet Potato (Ipomoea batatas L.): A Versatile Raw Material for the Food Industry. Braz. Arch. Biol. Technol. 2021, 64, 1–14. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef]
- Dereje, B.; Girma, A.; Mamo, D.; Chalchisa, T. Functional properties of sweet potato flour and its role in product development: A review. Int. J. Food Prop. 2020, 23, 1639–1662. [Google Scholar] [CrossRef]
- Radojčin, M.; Pavkov, I.; Kovačević, D.B.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of selected drying methods and emerging drying intensification technologies on the quality of dried fruit: A review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pereira, N.; Silva, M.; Alvarenga, N.; Ramos, A.C.; Alegria, C.; Abreu, M. Influence of Air-Drying Conditions on Quality, Bioactive Composition and Sensorial Attributes of Sweet Potato Chips. Foods 2023, 12, 1198. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Prieto, M.A.; Venskutonis, P.R.; Daglia, M.; Devkota, H.P.; Baldi, A.; Ezzat, S.M.; Gómez-Gómez, L.; Salama, M.M.; et al. Effects of different drying techniques on the quality and bioactive compounds of plant-based products: A critical review on current trends. Dry. Technol. 2022, 40, 1539–1561. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Deng, B.; Ru, W.; Tong, C.; Bao, J. Physicochemical, Nutritional, and Antioxidant Properties in Seven Sweet Potato Flours. Front. Nutr. 2022, 9, 923257. [Google Scholar] [CrossRef]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, S.Y.; Yang, J.W.; Lee, J.S.; Oh, S.D.; Oh, S.; Lee, S.M.; Lim, M.H.; Park, S.K.; Jang, J.-S.; et al. Comparative analysis of phytochemicals and polar metabolites from colored sweet potato (Ipomoea batatas L.) tubers. Food Sci. Biotechnol. 2016, 25, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R. Interpretação de Resultados de Ensaios Microbiológicos em Alimentos Prontos Para Consumo e em Superfícies do Ambiente de Preparação e Distribuição Alimentar: Valores-guia. Lisboa, 2019. Available online: http://repositorio.insa.pt//handle/10400.18/5610 (accessed on 14 October 2024).
- Ahmed, M.; Sorifa, A.M.; Eun, J.B. Effect of pre-treatments and drying temperatures on sweet potato flour. Int. J. Food Sci. Technol. 2010, 45, 726–732. [Google Scholar] [CrossRef]
- Dhara, J.; Saha, S.K.; Saha, M.; Chakraborty, R. Study on drying kinetics, antioxidant activity, total bioactive compounds, physicochemical properties and microstructural characteristics of dehydrated star fruits (Averrhoa carambola) by different drying methods. Sustain. Food Technol. 2023, 1, 590–602. [Google Scholar] [CrossRef]
- Desale, F.H.; Sasanatayart, R. Effect of Drying Temperature on Functional and Digestive Properties of Sweet Potato Flour. In Proceedings of the 63rd the IRES International Conference, Bangkok, Thailand, 5–6 March 2017. [Google Scholar]
- CXS 152-1985; Standard for Wheat Flour. FAO: Roma, Italy, 2021.
- Hasmadi, M.; Merlynda, M.; Mansoor, A.H.; Salwa, I.; Zainol, M.K.; Jahurul, M.H.A. Comparative studies of the physicochemical and functional properties of sweet potato (Ipomoea batatas l.) flour. Food Res. 2021, 5, 145–152. [Google Scholar] [CrossRef]
- Santi, E.N.; Murdianto, W.; Ahmadi, N.R.; Waryat; Sulistyaningrum, A. Physicochemical Characteristics of Three Local Sweet Potato Flour from East Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2022, 1024, 012037. [Google Scholar] [CrossRef]
- Fadhli, M.K.; Arpi, N.; Noviasari, S. Chemical characteristics of three variations of sweet potato (Ipomoea batatas L.) flour with physical modifications. IOP Conf. Ser. Earth Environ. Sci. 2023, 1183, 012053. [Google Scholar] [CrossRef]
- Castro-Mendoza, M.P.; Navarro-Cortez, R.O.; Hernández-Uribe, J.P.; Bello-Pérez, L.A.; Vargas-Torres, A. Sweet potato color variety and flour production drying method determine bioactive compound content and functional properties of flour. J. Food Process. Preserv. 2022, 46, e16852. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Bian, X.; Guo, K.; Zhou, L.; Wei, C. Characterization and comparative study of starches from seven purple sweet potatoes. Food Hydrocoll. 2018, 80, 168–176. [Google Scholar] [CrossRef]
- Mihály-Langó, B.; Ács, K.; Berényi, A.; Maróti Tóth, K.; Táborosi Ábrahám, Z.; Gáll, T.; Ács, E. Rheological properties and characterisation of some bioactive components in flours made of different coloured sweet potato (Ipomoea batatas L.) genotypes. Acta Aliment. 2023, 52, 570–578. [Google Scholar] [CrossRef]
- Silva, G.V.; Machado, B.A.S.; Oliveira, W.P.; Silva, C.F.G.; Quadros, C.P.; Druzian, J.I.; Ferreira, E.D.S.; Umsza-Guez, M.A. Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”. Molecules 2020, 25, 3701. [Google Scholar] [CrossRef]
- Zubia, C.S.; Babaran, G.M.O.; Duque, S.M.M.; Mopera, L.E.; Flandez, L.E.L.; Castillo-Israel, K.A.T.; Reginio Jr, F.C. Impact of drying on the bioactive compounds and antioxidant properties of bignay [Antidesma bunius (L.) Spreng.] pomace. Food Prod. Process. Nutr. 2023, 5, 1–13. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Chittrakorn, S.; Weerawatanakorn, M.; Dangpium, N. Effect of drying conditions on properties, pigments and antioxidant activity retentions of pretreated orange and purple-fleshed sweet potato flours. J. Food Sci. Technol. 2016, 53, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Chang, Y.-H.; Shao, Y.-Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2006, 98, 529–538. [Google Scholar] [CrossRef]
- Lako, J.; Trenerry, V.C.; Wahlqvist, M.; Wattanapenpaiboon, N.; Sotheeswaran, S.; Premier, R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007, 101, 1727–1741. [Google Scholar] [CrossRef]
- Sasanatayart, R.; Liangpanth, T.; Desale, F.H. Effect of Drying and in vitro Gastrointestinal Digestion on Antioxidant Activities of Orange and Purple Fleshed Sweet Potatoes. Food Appl. Biosci. J. 2019, 7, 90–104. Available online: https://li01.tci-thaijo.org/index.php/fabjournal/article/view/176618 (accessed on 14 October 2024).
- Belkacemi, L. Blanching effect on physicochemical and functional properties of flours processed from peeled and unpeeled white-Fleshed sweet potato Algerian cultivar. Food Sci. Technol. 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Oke, M.O.; Workneh, T.S. A review on sweet potato post-harvest processing and preservation technology. Afr. J. Agric. Res. 2013, 8, 4990–5003. [Google Scholar] [CrossRef]
- Akcicek, A.; Avci, E.; Tekin-Cakmak, Z.H.; Kasapoglu, M.Z.; Sagdic, O.; Karasu, S. Influence of Different Drying Techniques on the Drying Kinetics, Total Bioactive Compounds, Anthocyanin Profile, Color, and Microstructural Properties of Blueberry Fruit. ACS Omega 2023, 8, 41603–41611. [Google Scholar] [CrossRef]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT Food Sci. Technol. 2019, 118, 108830. [Google Scholar] [CrossRef]
- Ozay-Arancioglu, I.; Bekiroglu, H.; Karadag, A.; Saroglu, O.; Tekin-çakmak, Z.H.; Karasu, S. Effect of different drying methods on the bioactive, microstructural, and in-vitro bioaccessibility of bioactive compounds of the pomegranate arils. Food Sci. Technol. 2022, 42, 1–9. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of drying methods on total phenolic contents and antioxidant capacity of the pomelo (Citrus grandis (L.) Osbeck) peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Vimala, B.; Nambisan, B.; Hariprakash, B. Retention of carotenoids in orange-fleshed sweet potato during processing. J. Food Sci. Technol. 2011, 48, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Effects of different drying methods on the bioactive compounds and antioxidant properties of edible Centaurea (Centaurea cyanus) petals. Braz. J. Food Technol. 2018, 21, e2017211. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Chang, K.C. Sulfite and Starch Affect Color and Carotenoids of Dehydrated Carrots (Daucus carota) during Storage. J. Food Sci. 1995, 60, 324–326. [Google Scholar] [CrossRef]
- Bechoff, A. Investigating Carotenoid Loss After Drying and Storage of Orange-Fleshed Sweet Potato. Ph.D. Thesis, University of Greenwich, London, UK, 2010. Available online: http://gala.gre.ac.uk/4031/ (accessed on 20 October 2024).
- Çoklar, H.; Akbulut, M. Effect of Sun, Oven and Freeze-Drying on Anthocyanins, Phenolic Compounds and Antioxidant Activity of Black Grape (Ekşikara) (Vitis vinifera L.). S. Afr. J. Enol. Vitic. 2017, 38, 264–272. [Google Scholar] [CrossRef]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of Philippine sweet potato (Ipomoea batatas) varieties. Food Chem. 2009, 113, 1133–1138. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Brandão, T.R.S.; Silva, C.L.M. Influence of Drying Processes and Pretreatments on Nutritional and Bioactive Characteristics of Dried Vegetables: A Review. Food Eng. Rev. 2015, 134–163. [Google Scholar] [CrossRef]
- National Institute of Health. Vitamin A and Carotenoids. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/#en24 (accessed on 21 October 2024).
- Zavareze, E.D.R.; Halal, S.L.M.; Pereira, J.M.; Radünz, A.L.; Elias, M.C.; Dias, A.R.G. Caracterização química e rendimento de extração de amido de arroz com diferentes teores de amilose. Braz. J. Food Technol. 2009. Available online: https://bjft.ital.sp.gov.br/especiais/especial_2009/v11_edesp_06.pdf (accessed on 20 October 2024).
- NP-875:1994; Food for Animals: Determination of Moisture Content. Instituto Português da Qualidade: Lisbon, Portugal, 1994.
- AOAC. Ash of Flour (Direct Method): Method 923.03. In Official Methods of Analysis, 18th ed.; AOAC International Publisher: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Leitão, I.; Sales, J.; Martins, L.L.; Mourato, M.P. Response to stress induced by different potentially toxic elements (As, Cd, Cu and Na) in rapeseed leaves. Plant Physiol. Rep. 2021, 26, 478–490. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. J. Sci. Food Agri. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Talcott, S.T.; Howard, L.R. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999, 47, 2109–2115. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- ISO 4833-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2008.
- STATISTICA (Data Analysis Software System); Version 8.0; StatSoft Inc.: Tulsa, OK, USA, 2007.
- Jackson, J.E. A User’s Guide to Principal Components; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1991. [Google Scholar] [CrossRef]
- Larrigaudière, C.; Lentheric, I.; Puy, J.; Pintó, E. Biochemical characterisation of core browning and brown heart disorders in pear by multivariate analysis. Postharvest. Biol. Technol. 2004, 31, 29–39. [Google Scholar] [CrossRef]


| Quality Attribute | Variety | ||
|---|---|---|---|
| Bonita | Bellevue | NP1648 | |
| Moisture content (%) | 75.8 a ± 0.3 | 82.6 b ± 1.0 | 77.0 a ± 1.9 |
| aw | 0.97 a ± 0.0 | 0.97 a ± 0.0 | 0.97 a ± 0.0 |
| Starch content (%) | 53.3 c ± 1.5 | 48.9 b ± 1.4 | 45.0 a ± 0.7 |
| Pulp’s CIELab colour | |||
| L* | 86.4 c ± 0.3 | 72.1 b ± 0.5 | 38.7 a ± 1.3 |
| a* | −3.5 a ± 0.2 | 24.6 b ± 1.9 | 24.5 b ± 0.6 |
| b* | 25.2 b ± 0.7 | 43.8 c ± 3.3 | 2.8 a ± 0.4 |
| Croma | 25.5 a ± 2.5 | 50.3 b ± 5.3 | 24.7 a ± 4.2 |
| Hue | 97.9 c ± 1.4 | 69.7 b ± 2.6 | 6.7 a ± 2.9 |
| Total phenolic content (mg gallic acid eq./100 g db) | 76.4 a ± 8.3 | 173.2 b ± 23.2 | 226.9 c ± 5.0 |
| Antioxidant activity | |||
| Hydrophilic DPPH (µmol trolox eq./100 g db) | 12,487.5 a ± 1863.4 | 43,461.0 c ± 1192.7 | 34,359.3 b ± 159.2 |
| Lipidic DPPH (µmol trolox eq./100 g db) | 3330.8 a ± 140.5 | 5172.4 b ± 172.9 | 3106.3 a ± 193.0 |
| FRAP (µmol FeSO4·7H2O/100 g db) | 23.8 a ± 1.0 | 66.9 b ± 4.3 | 103.3 c ± 1.6 |
| Total carotenoid content (mg β-carotene/100 g db) | 0.4 a ± 0.1 | 49.3 b ± 2.0 | 1.7 a ± 0.2 |
| β-carotene content (mg/100 g db) | n.d | 27.2 ± 4.3 | n.d |
| Total anthocyanin content (mg cyanidin-3-glucoside/100 g db) | 7.4 a ± 0.7 | 14.9 b ± 2.4 | 27.3 c ± 0.7 |
| Total microorganisms at 30 °C (log10 CFU/g) | 4.8 a ± 0.1 | 5.0 ab ± 0.1 | 5.3 b ± 0.3 |
| Yeasts and moulds (log10 CFU/g) | 4.3 b ± 0.2 | 3.5 a ± 0.1 | 3.7 a ± 0.1 |
| Processing | Variety | Chroma | °h 1 | WI | ΔE |
|---|---|---|---|---|---|
| Raw material | Bonita | 25.5 a ± 2.5 | 97.9 h ± 1.4 | 71.1 g ± 2.1 | - |
| HAD | 17.5 bc ± 0.5 | 94.6 g ± 0.3 | 80.4 b ± 0.9 | 9.5 b ± 0.7 | |
| FD | 16.0 b ± 0.5 | 101.5 i ± 0.2 | 82.6 b ± 0.8 | 12.0 c ± 1.0 | |
| Raw material | Bellevue | 50.3 f ± 5.3 | 60.7 e ± 2.6 | 42.5 a ± 5.4 | - |
| HAD | 37.6 e ± 1.7 | 75.1 f ± 0.6 | 56.7 e ± 0.8 | 18.2 a ± 0.6 | |
| FD | 32.2 d ± 0.6 | 47.7 d ± 0.3 | 61.8 f ± 1.1 | 21.5 e ± 0.8 | |
| Raw material | NP1648 | 24.7 a ± 4.2 | 6.7 b ± 2.9 | 33.8 c ± 4.2 | - |
| HAD | 19.0 c ± 0.7 | 5.0 a ± 0.3 | 50.1 d ± 1.1 | 16.8 d ± 1.0 | |
| FD | 26.3 a ± 0.6 | 20.3 c ± 0.3 | 45.1 a ± 1.5 | 17.8 a ± 1.4 |
| Drying Method | Variety | Moisture Content (%) | Fat (%) | Protein (%) | Carbohydrates (%) | Ash (%) |
|---|---|---|---|---|---|---|
| HAD | Bonita | 3.0 a ± 0.1 | 0.5 a ± 0.1 | 4.9 a ± 0.0 | 88.0 a ± 0.2 | 3.7 b ± 0.0 |
| FD | 3.7 c ± 0.3 | 0.3 a ± 0.1 | 4.8 a ± 0.1 | 87.6 a ± 0.1 | 3.6 b ± 0.1 | |
| HAD | Bellevue | 5.2 e ± 0.1 | 0.7 b ± 0.1 | 5.6 b ± 0.0 | 84.5 b ± 0.2 | 4.0 c ± 0.1 |
| FD | 4.6 d ± 0.2 | 0.9 b ± 0.1 | 5.4 b ± 0.1 | 85.1 b ± 0.3 | 4.0 c ± 0.1 | |
| HAD | NP1648 | 2.2 b ± 0.2 | 0.4 a ± 0.1 | 6.2 c ± 0.0 | 87.9 a ± 0.3 | 3.3 a ± 0.1 |
| FD | 2.8 a ± 0.1 | 0.3 a ± 0.1 | 6.0 c ± 0.0 | 87.9 a ± 0.1 | 3.3 a ± 0.0 |
| Drying Method | Variety | Na | K | Ca | Mg | P | S |
|---|---|---|---|---|---|---|---|
| HAD | Bonita | 16.5 a ± 1.7 | 220.6 a ± 20.0 | 16.6 a ± 1.2 | 15.8 c ± 1.2 | 30.4 b ± 2.5 | 14.4 a ± 1.0 |
| FD | 16.5 a ± 0.9 | 221.2 a ± 10.6 | 13.1 a ± 0.8 | 12.7 bc ± 0.6 | 28.6 b ± 1.2 | 13.7 a ± 0.6 | |
| HAD | Bellevue | 7.1 b ± 0.1 | 263.9 c ± 4.6 | 15.5 a ± 0.3 | 10.5 a ± 0.2 | 22.8 b ± 0.4 | 14.2 a ± 0.3 |
| FD | 7.7 b ± 0.1 | 253.5 c ± 6.7 | 13.9 a ± 0.2 | 10.1 a ± 0.2 | 23.7 b ± 0.7 | 14.0 a ± 0.2 | |
| HAD | NP1648 | 18.1 a ± 1.2 | 168.8 b ± 10.3 | 28.6 b ± 1.6 | 13.4 bc ± 0.7 | 34.1 a ± 2.1 | 16.6 a ± 1.0 |
| FD | 18.2 a ± 2.2 | 150.7 b± 16.0 | 26.7 b ± 3.0 | 12.1 bc ± 1.3 | 33.7 a ± 4.0 | 15.1 a ± 1.8 |
| Processing | Variety | TPC (mg GAE/100 g db) | TCC (mg β-Carotene/100 g db) | TAC (mg Cyd-3-glu/100 g db) | β-Carotene (mg/100 g db) | FRAP (mmol FeSO4(7 H2O)/100 g db) | DPPH MeOH (µmol TE/100 g db) | DPPH DCM (µmol TE/100 g db) |
|---|---|---|---|---|---|---|---|---|
| Raw material | Bonita | 76.4 a ± 8.3 | 0.4 a ± 0.1 | 7.4 a ± 0.7 | n.d | 23.8 a ± 1.0 | 12,487.5 c ± 1863.4 | 3330.8 d ± 140.5 |
| HAD | 23.3 b ± 3.8 | 0.3 a ± 0.1 | 5.0 a ± 0.6 | n.d | 9.7 c ± 0.4 | 3415.3 b ± 162.0 | 1147.6 a ± 72.0 | |
| FD | 55.6 a ± 2.7 | 0.3 a ± 0.1 | 6.6 a ± 0.7 | n.d | 22.8 a ± 0.7 | 8184.2 a ± 68.5 | 1648.2 ab ± 161.6 | |
| Raw material | Bellevue | 173.2 c ± 23.2 | 49.3 d ± 2.0 | 14.9 c ± 2.3 | 27.2 d ± 4.3 | 66.9 e ± 4.2 | 43,461.0 e ± 1192.7 | 5172.4 e ± 171.9 |
| HAD | 61.6 a ± 1.8 | 33.5 b ± 1.0 | 4.9 a ± 1.4 | 12.7 b ± 0.2 | 24.9 a ± 0.9 | 8414.1 a ± 47.9 | 3149.2 cd ± 308.1 | |
| FD | 74.8 a ± 7.7 | 43.6 c ± 1.6 | 11.2 b ± 0.9 | 20.0 c ± 2.7 | 30.7 b ± 0.4 | 8123.6 a ± 227.0 | 2617.5 bcd ± 233.4 | |
| Raw material | NP1648 | 226.9 d ± 5.0 | 1.7 a ± 0.2 | 27.3 f ± 0.7 | n.d | 103.3 f ± 1.5 | 34,359.3 d ± 159.2 | 3106.3 cd ± 193.0 |
| HAD | 57.5 a ± 3.0 | 0.8 a ± 0.1 | 21.9 e ± 1.0 | n.d | 30.1 b ± 0.5 | 8164.3 a ± 22.7 | 1751.8 ab ± 128.3 | |
| FD | 74.3 a ± 1.7 | 1.1 a ± 0.0 | 18.5 d ± 0.3 | n.d | 40.3 d ± 0.3 | 7853.1 a ± 88.0 | 2122.2 abc ± 158.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, N.; Ramos, A.C.; Alves, M.; Alves, V.D.; Roseiro, C.; Vida, M.; Moldão, M.; Abreu, M. Gluten-Free Sweet Potato Flour: Effect of Drying Method and Variety on the Quality and Bioactivity. Molecules 2024, 29, 5771. https://doi.org/10.3390/molecules29235771
Pereira N, Ramos AC, Alves M, Alves VD, Roseiro C, Vida M, Moldão M, Abreu M. Gluten-Free Sweet Potato Flour: Effect of Drying Method and Variety on the Quality and Bioactivity. Molecules. 2024; 29(23):5771. https://doi.org/10.3390/molecules29235771
Chicago/Turabian StylePereira, Nelson, Ana Cristina Ramos, Marco Alves, Vítor D. Alves, Cristina Roseiro, Manuela Vida, Margarida Moldão, and Marta Abreu. 2024. "Gluten-Free Sweet Potato Flour: Effect of Drying Method and Variety on the Quality and Bioactivity" Molecules 29, no. 23: 5771. https://doi.org/10.3390/molecules29235771
APA StylePereira, N., Ramos, A. C., Alves, M., Alves, V. D., Roseiro, C., Vida, M., Moldão, M., & Abreu, M. (2024). Gluten-Free Sweet Potato Flour: Effect of Drying Method and Variety on the Quality and Bioactivity. Molecules, 29(23), 5771. https://doi.org/10.3390/molecules29235771







