Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods
Abstract
1. Introduction
2. Results and Discussion
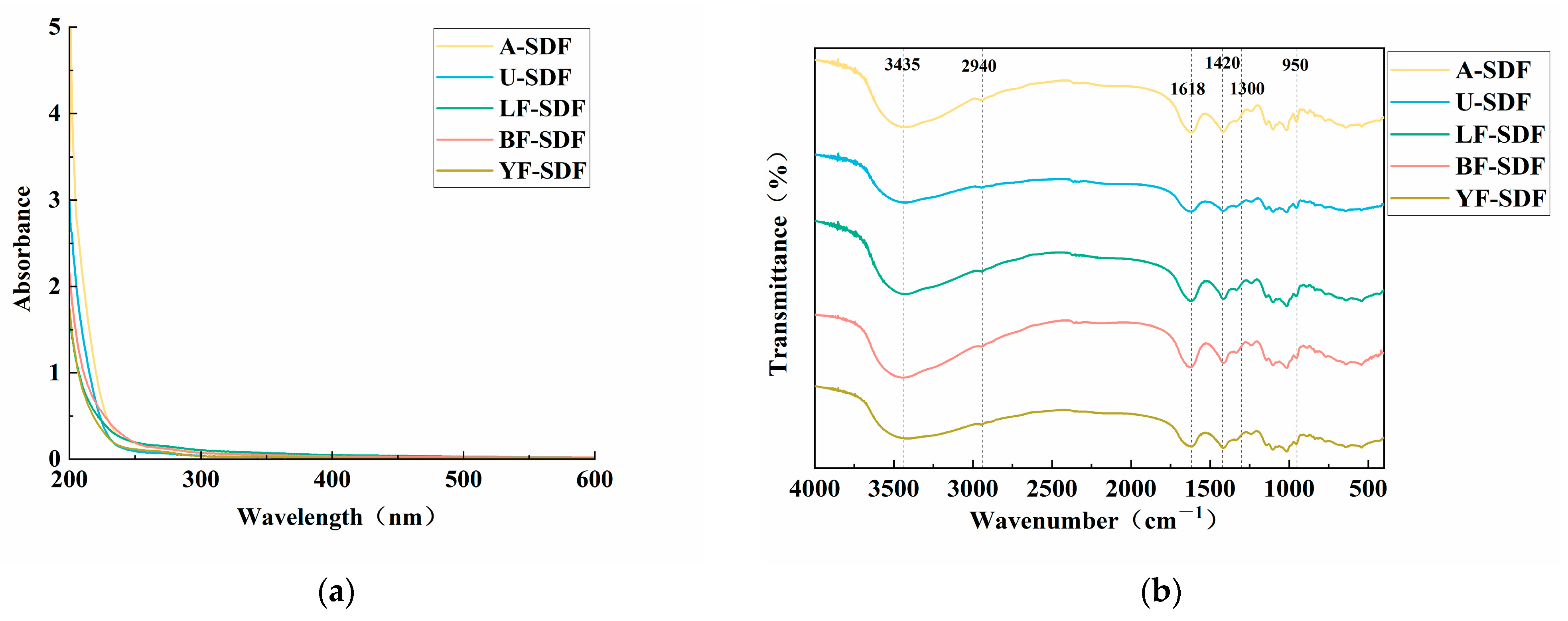
2.1. Spectral Analysis of SDF
2.2. Monosaccharide Composition of the SDF
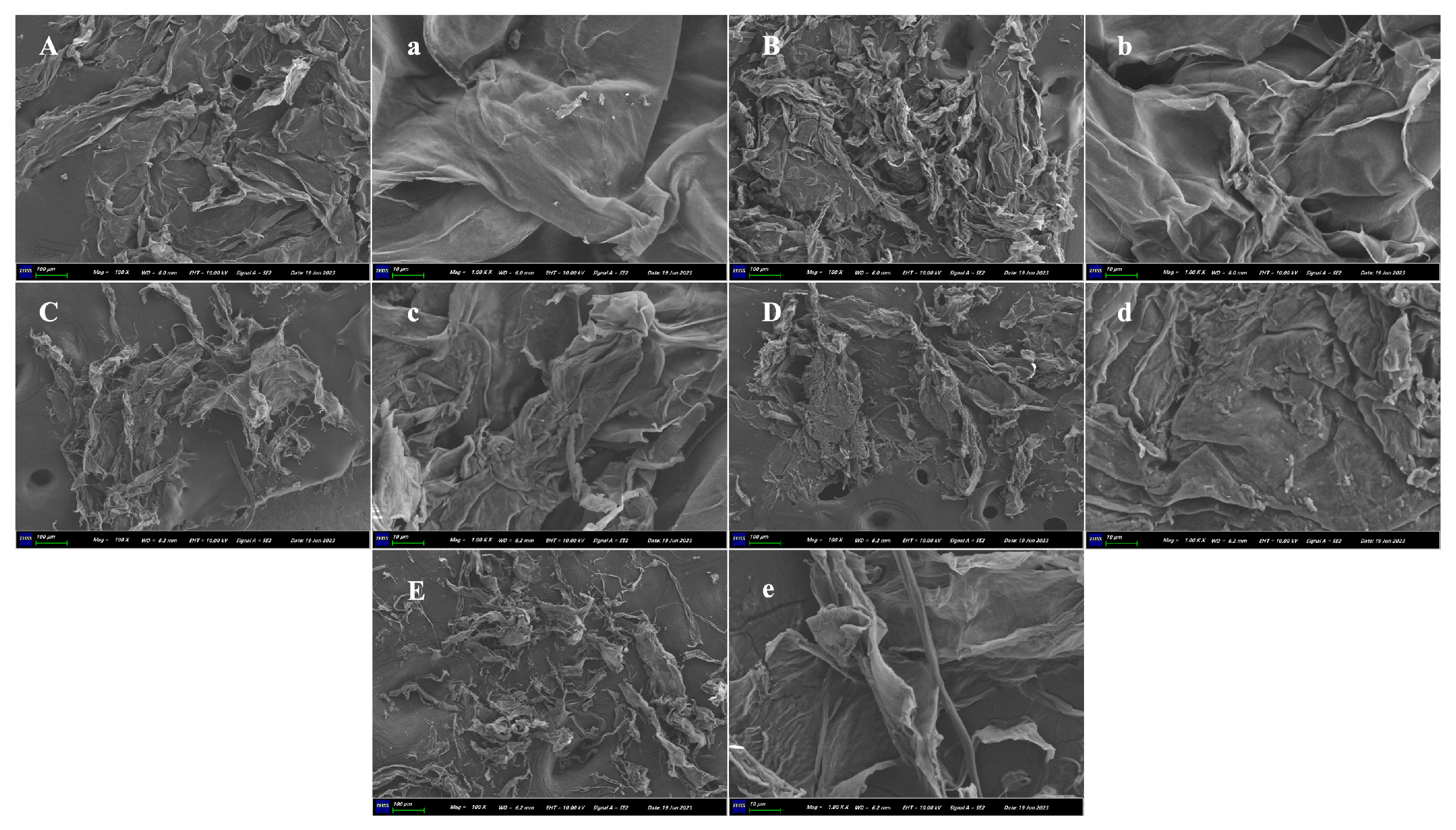
2.3. Particle Size and Surface Morphology
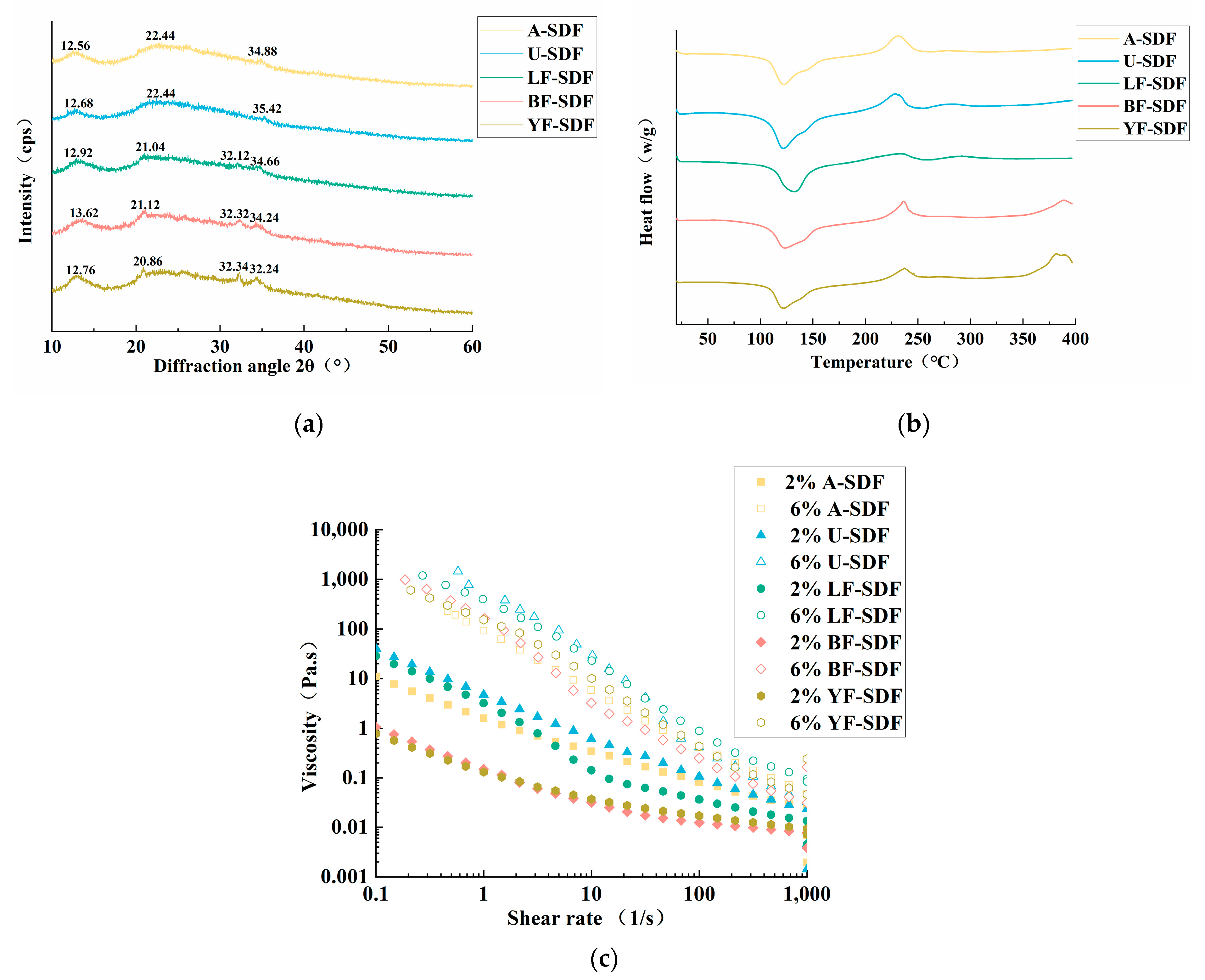
2.4. X-ray Diffraction (XRD) Analysis
2.5. Differential Scanning Calorimetry (DSC) Analysis
2.6. Rheological Properties
2.7. Functional Properties of SDF
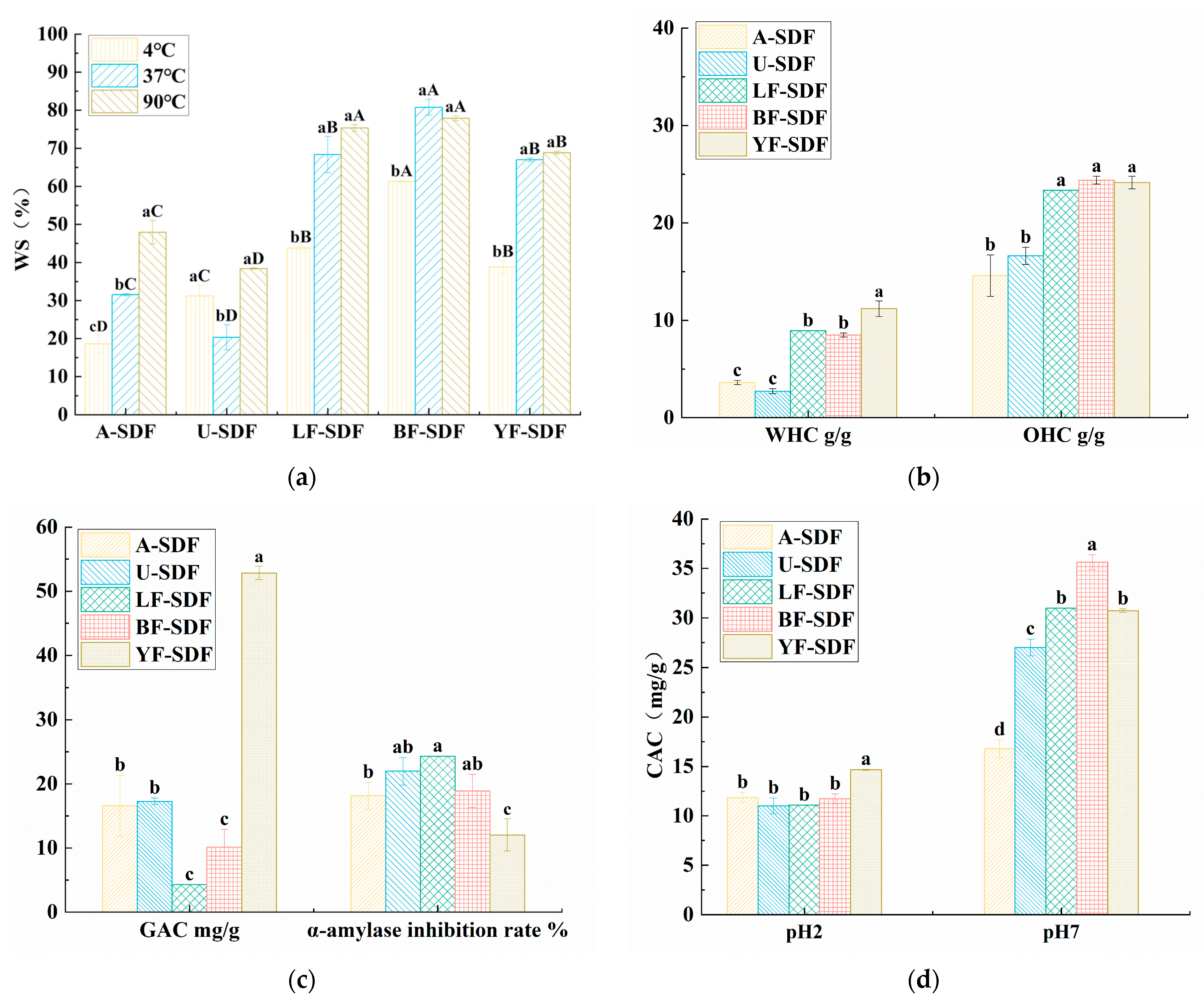
2.7.1. Water Solubility (WS), Water Holding Capacity (WHC), and Oil Holding Capacity (OHC)
2.7.2. Glucose Absorption Capacity (GAC) and the Inhibition Capacities of α-Amylase
2.7.3. Cholesterol Absorption Capacity (CAC)
3. Materials and Methods
3.1. Materials
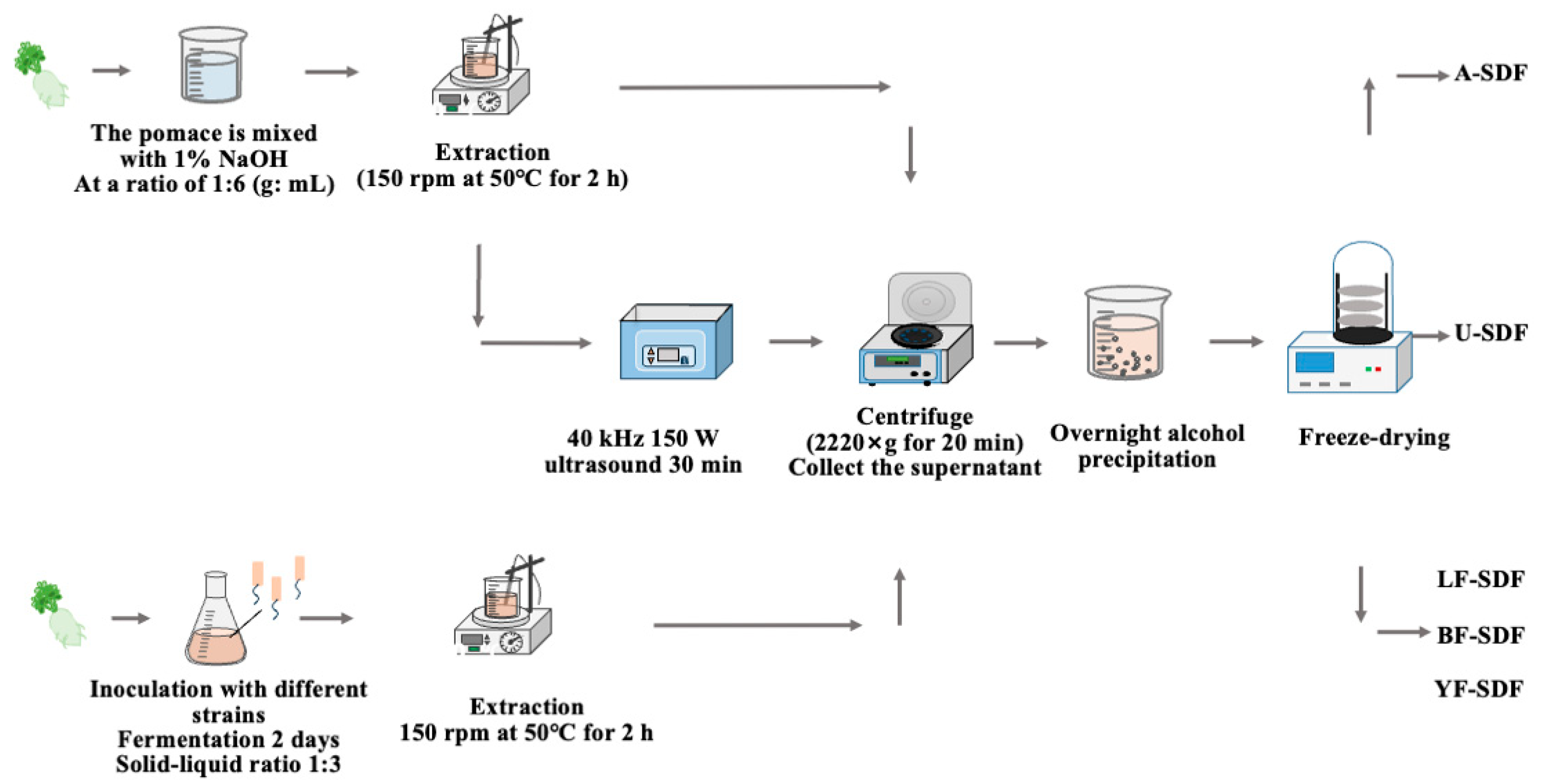
3.2. SDF Extraction from Radish Pomace
3.2.1. Alkali Extraction Method
3.2.2. Ultrasound-Assisted Extraction Method
3.2.3. Fermentation-Assisted Extraction Method
3.3. Spectral Analysis
3.4. Monosaccharide Composition Analysis
3.5. Particle Size and Scanning Electron Microscopy (SEM) Analysis
3.6. X-ray Diffraction(XRD) Analysis
3.7. Differential Scanning Calorimeter (DSC) Analysis
3.8. Rheological Property Analysis
3.9. Functional Properties
3.9.1. Water Solubility (WS)
3.9.2. Water Holding Capacity (WHC)
3.9.3. Oil Holding Capacity (OHC)
3.9.4. Glucose Absorption Capacity (GAC)
3.9.5. Inhibition Capacities of α-Amylase
3.9.6. Cholesterol Absorption Capacity (CAC)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of Dietary Fiber on Human Health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Cao, X.; Wang, J. Preparation and Modification of High Dietary Fiber Flour: A Review. Food Res. Int. 2018, 113, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, B.; Li, L.; Zhao, W. Anti-Obesity Effects of Dietary Fibers Extracted from Flaxseed Cake in Diet-Induced Obese Mice. Nutrients 2023, 15, 1718. [Google Scholar] [CrossRef] [PubMed]
- Meneguelli, T.S.; Kolba, N.; Misra, A.; Dionísio, A.P.; Kravchychyn, A.C.P.; Silva, B.P.D.; Martino, H.S.D.; Hermsdorff, H.H.M.; Tako, E. Intra-Amniotic Administration of Cashew Nut (Anacardium Occidentale L.) Soluble Extract Improved Gut Functionality and Morphology in vivo (Gallus Gallus). Nutrients 2023, 15, 2378. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hong, T.; Li, N.; Zang, B.; Wu, X. Soluble Dietary Fiber Improves Energy Homeostasis in Obese Mice by Remodeling the Gut Microbiota. Biochem. Biophys. Res. Commun. 2018, 498, 146–151. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, B.; Zhang, Z.; Yang, K.; Cai, M.; Hu, W.; Guo, Y.; Xia, Q.; Wu, W. Positive Effects of Dietary Fiber from Sweet Potato [Ipomoea Batatas (L.) Lam.] Peels by Different Extraction Methods on Human Fecal Microbiota in Vitro Fermentation. Front. Nutr. 2022, 9, 986667. [Google Scholar] [CrossRef]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and Phytochemical Characterization of Radish (Raphanus Sativus): A Systematic Review. Trends Food Sci. Tech. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Paul, S.; Geng, C.; Yang, T.; Yang, Y.; Chen, J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica Rapa). J. Food Sci. 2019, 84, 19–30. [Google Scholar] [CrossRef]
- Sani, I.K.; Masoudpour-Behabadi, M.; Sani, M.A.; Motalebinejad, H.; Juma, A.S.M.; Asdagh, A.; Eghbaljoo, H.; Khodaei, S.M.; Rhim, J.-W.; Mohammadi, F. Value-Added Utilization of Fruit and Vegetable Processing by-Products for the Manufacture of Biodegradable Food Packaging Films. Food Chem. 2023, 405, 134964. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of Fruits and Vegetable By-Products for Isolation of Dietary Fibres and Its Potential Application as Functional Ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Xie, J.; Peng, G.; Hu, X.; Xie, J.; Chen, Y.; Dong, R.; Si, J.; Yang, C.; Yu, Q. Physicochemical Characteristics of Soluble Dietary Fiber Obtained from Grapefruit Peel Insoluble Dietary Fiber and Its Effects on Blueberry Jam. Foods 2022, 11, 3735. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Li, L.; Liu, H. Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Z.; Zhang, Y.; Gao, B.; Niu, Y.; Yu, L.L. The Structural and Functional Characteristics of Soluble Dietary Fibers Modified from Tomato Pomace with Increased Content of Lycopene. Food Chem. 2022, 382, 132333. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gui, R.; Li, N.; Wu, Y.; Chen, J.; Wu, X.; Qin, Z.; Yang, S.-T.; Li, X. Production of Soluble Dietary Fibers and Red Pigments from Potato Pomace in Submerged Fermentation by Monascus Purpureus. Process Biochem. 2021, 111, 159–166. [Google Scholar] [CrossRef]
- Jia, M.; Chen, J.; Liu, X.; Xie, M.; Nie, S.; Chen, Y.; Xie, J.; Yu, Q. Structural Characteristics and Functional Properties of Soluble Dietary Fiber from Defatted Rice Bran Obtained through Trichoderma Viride Fermentation. Food Hydrocolloid. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Ameer, K. The Influence of Different Extraction Methods on the Structure, Rheological, Thermal and Functional Properties of Soluble Dietary Fiber from Sanchi (Panax Notoginseng) Flower. Foods 2022, 11, 1995. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Fu, X.; Huang, Q.; Chen, Q. Physicochemical, Functional and Biological Properties of Soluble Dietary Fibers Obtained from Rosa Roxburghii Tratt Pomace Using Different Extraction Methods. Process Biochem. 2023, 128, 40–48. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wang, J.-D.; Cai, Z.-H.; Huang, H.; Zhang, S.; Fu, L.-N.; Zhao, P.-Q.; Yan, X.-Y.; Fu, Y.-J. Improved Physicochemical and Functional Properties of Dietary Fiber from Rosa Roxburghii Pomace Fermented by Bacillus Natto. Food Biosci. 2022, 50, 102030. [Google Scholar] [CrossRef]
- Li, Y.; Niu, L.; Guo, Q.; Shi, L.; Deng, X.; Liu, X.; Xiao, C. Effects of Fermentation with Lactic Bacteria on the Structural Characteristics and Physicochemical and Functional Properties of Soluble Dietary Fiber from Prosomillet Bran. LWT 2022, 154, 112609. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Chang, N.; Sun, L.; Bello, A.; Deng, L.; Zhao, L.; Egbeagu, U.U.; Wang, B.; Zhao, Y.; et al. Exploration of β-Glucosidase-Producing Microorganisms Community Structure and Key Communities Driving Cellulose Degradation during Composting of Pure Corn Straw by Multi-Interaction Analysis. J. Environ. Manag. 2023, 325, 116694. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Omedi, J.O.; Huang, W.; Zheng, J.; Zeng, Y.; Huang, J.; Zhang, B.; Zhou, L.; Li, N.; Gao, T.; et al. Antioxidant, Flavor Profile and Quality of Wheat Dough Bread Incorporated with Kiwifruit Fermented by β-Glucosidase Producing Lactic Acid Bacteria Strains. Food Biosci. 2022, 46, 101450. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Chen, Y.; Xie, J.; Song, Y.; Chang, X.; Liu, S.; Wang, Z.; Hu, X.; Yu, Q. Effects of Fermentation on the Structural Characteristics and in Vitro Binding Capacity of Soluble Dietary Fiber from Tea Residues. LWT 2020, 131, 109818. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C.; Li, J.; Hussain, S.; Yan, S.; Wang, Q. Effects of Extrusion on Structural and Physicochemical Properties of Soluble Dietary Fiber from Nodes of Lotus Root. LWT 2018, 93, 204–211. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural Characteristics and Functional Properties of Rice Bran Dietary Fiber Modified by Enzymatic and Enzyme-Micronization Treatments. LWT-Food Sci. Technol. 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Lettow, M.; Grabarics, M.; Mucha, E.; Thomas, D.A.; Polewski, Ł.; Freyse, J.; Rademann, J.; Meijer, G.; von Helden, G.; Pagel, K. IR Action Spectroscopy of Glycosaminoglycan Oligosaccharides. Anal. Bioanal. Chem. 2020, 412, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Jiwani, S.I.; Gillis, R.B.; Besong, D.; Almutairi, F.; Erten, T.; Kök, M.S.; Harding, S.E.; Paulsen, B.S.; Adams, G.G. Isolation and Biophysical Characterisation of Bioactive Polysaccharides from Cucurbita Moschata (Butternut Squash). Polymers 2020, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, P.; Tang, Y.; Pang, Y.; Sun, J.; Li, J.; Rao, C.; Wu, C.; He, X.; Li, L.; et al. Evaluation of Bioactive Compounds and Bioactivities in Plum (Prunus Salicina Lindl.) Wine. Front. Nutr. 2021, 8, 766415. [Google Scholar] [CrossRef]
- Liang, X.; Liu, M.; Guo, S.; Zhang, F.; Cui, W.; Zeng, F.; Xu, M.; Qian, D.; Duan, J. Structural Elucidation of a Novel Arabinogalactan LFP-80-W1 from Lycii Fructus with Potential Immunostimulatory Activity. Front. Nutr. 2023, 9, 1067836. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, W.; Dang, Y.; Gao, X. Physical Properties and Functional Characteristics of Broccoli-Soluble Dietary Fiber. Food Biosci. 2023, 56, 103272. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.-N.; Zhou, X.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y. Effect of Chemical and Enzymatic Modifications on the Structural and Physicochemical Properties of Dietary Fiber from Purple Turnip (Brassica Rapa L.). LWT 2021, 145, 111313. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of Modification Methods on Physicochemical and Structural Properties of Soluble Dietary Fiber from Corn Bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Y.; Liao, A.-M.; Huang, J.-H.; Zhang, J.-G.; Thakur, K.; Wei, Z.-J. The Rheological Properties of Differentially Extracted Polysaccharides from Potatoes Peels. Int. J. Biol. Macromol. 2019, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, W.; Yang, Y.; Xing, J.; Lin, Y. Penicillium Sp. Cis16 Improves Soluble Dietary Fiber Content in Citrus Dregs Fermentation. Food Biotechnol. 2022, 36, 191–208. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, J.; Xie, Q.; Huang, L.; Shen, D.; Li, G. Dietary Fiber from Navel Orange Peel Prepared by Enzymatic and Ultrasound-Assisted Deep Eutectic Solvents: Physicochemical and Prebiotic Properties. Foods 2023, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.S.; Baldelli, A.; Pratap-Singh, A. Characterization of Selected Dietary Fibers Microparticles and Application of the Optimized Formulation as a Fat Replacer in Hazelnut Spreads. Food Res. Int. 2023, 165, 112466. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yang, C.; Si, J.; Chen, Y.; Xie, J.; Tang, J.; Dong, X.; Cheng, Y.; Hu, X.; Yu, Q. Fortified Yogurt with High-Quality Dietary Fiber Prepared from the by-Products of Grapefruit by Superfine Grinding Combined with Fermentation Treatment. LWT 2023, 188, 115396. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Tian, Y.; Song, Z.; Ai, L. Interaction between Barley β-Glucan and Corn Starch and Its Effects on the in Vitro Digestion of Starch. Int. J. Biol. Macromol. 2019, 141, 240–246. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, S.; Li, Y.; Tian, J.; Zhang, C. Structure, Physicochemical Properties and Effects on Nutrients Digestion of Modified Soluble Dietary Fiber Extracted from Sweet Potato Residue. Food Res. Int. 2021, 150, 110761. [Google Scholar] [CrossRef]
- He, T.; Zhang, X.; Zhao, L.; Zou, J.; Qiu, R.; Liu, X.; Hu, Z.; Wang, K. Insoluble Dietary Fiber from Wheat Bran Retards Starch Digestion by Reducing the Activity of Alpha-Amylase. Food Chem. 2023, 426, 136624. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Qin, L.; Wang, Y.; Chen, F.; Qu, C.; Miao, J. Physicochemical Properties of the Soluble Dietary Fiber from Laminaria Japonica and Its Role in the Regulation of Type 2 Diabetes Mice. Nutrients 2022, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yang, C.; Qin, X.; Si, J.; Dong, X.; Hu, X.; Yu, Q. Effects of Cellulose-Degrading Fungus Penicillium Griseofulvum on the Structure Characteristics and Adsorption Properties of Soluble Dietary Fiber from Citrus aurantium L. Food Biosci. 2023, 55, 102999. [Google Scholar] [CrossRef]
- Ma, W.; Liang, Y.; Lin, H.; Chen, Y.; Xie, J.; Ai, F.; Yan, Z.; Hu, X.; Yu, Q. Fermentation of Grapefruit Peel by an Efficient Cellulose-Degrading Strain, (Penicillium YZ-1): Modification, Structure and Functional Properties of Soluble Dietary Fiber. Food Chem. 2023, 420, 136123. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yao, J.; Zhang, T.; Yang, K.; Pan, S. Mixed Fermentation of Navel Orange Peel by Trichoderma Viride and Aspergillus Niger: Effects on the Structural and Functional Properties of Soluble Dietary Fiber. Food Biosci. 2024, 57, 103545. [Google Scholar] [CrossRef]
- Yang, D.; Lin, F.; Huang, Y.; Ye, J.; Xiao, M. Separation, Purification, Structural Analysis and Immune-Enhancing Activity of Sulfated Polysaccharide Isolated from Sea Cucumber Viscera. Int. J. Biol. Macromol. 2020, 155, 1003–1018. [Google Scholar] [CrossRef]
- Wei, C.; He, P.; He, L.; Ye, X.; Cheng, J.; Wang, Y.; Li, W.; Liu, Y. Structure Characterization and Biological Activities of a Pectic Polysaccharide from Cupule of Castanea Henryi. Int. J. Biol. Macromol. 2018, 109, 65–75. [Google Scholar] [CrossRef]
- Li, J.; Niu, D.; Zhang, Y.; Zeng, X.-A. Physicochemical Properties, Antioxidant and Antiproliferative Activities of Polysaccharides from Morinda Citrifolia L. (Noni) Based on Different Extraction Methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef]
- Ouyang, H.; Guo, B.; Hu, Y.; Li, L.; Jiang, Z.; Li, Q.; Ni, H.; Li, Z.; Zheng, M. Effect of Ultra-High Pressure Treatment on Structural and Functional Properties of Dietary Fiber from Pomelo Fruitlets. Food Biosci. 2023, 52, 102436. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of Extraction Methods on the Structural Characteristics and Functional Properties of Dietary Fiber Extracted from Kiwifruit (Actinidia Deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Niu, G.; You, G.; Zhou, X.; Fan, H.; Liu, X. Physicochemical Properties and in vitro Hypoglycemic Activities of Hsian-Tsao Polysaccharide Fractions by Gradient Ethanol Precipitation Method. Int. J. Biol. Macromol. 2023, 231, 123274. [Google Scholar] [CrossRef]
| Monosaccharides (%) | A-SDF | U-SDF | LF-SDF | BF-SDF | YF-SDF |
|---|---|---|---|---|---|
| Mannose | 0.47 | 0.66 | 0.34 | 0.55 | 4.49 |
| Ribose | 0.19 | 0.24 | 0.25 | 0.32 | 0.38 |
| Rhamnose | 2.28 | 3.39 | 4.28 | 5.31 | 4.73 |
| Glucuronic acid | 0.15 | 0.38 | 0.19 | 0.27 | 0.24 |
| Galacturonic acid | 10.21 | 17.84 | 8.37 | 12.99 | 10.30 |
| Glucose | 2.13 | 3.64 | 2.01 | 3.09 | 2.57 |
| Galactose | 2.25 | 3.84 | 2.41 | 2.89 | 2.33 |
| Xylose | 0.13 | 0.22 | 0.07 | 0.11 | 0.15 |
| Arabinose | 3.30 | 5.61 | 2.77 | 3.58 | 3.09 |
| Fucose | 0.37 | 0.53 | 0.44 | 0.48 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Cheng, X.; Ma, B.; Cui, F.; Wang, D.; Shen, R.; Li, X.; Li, J. Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods. Molecules 2024, 29, 500. https://doi.org/10.3390/molecules29020500
Tan X, Cheng X, Ma B, Cui F, Wang D, Shen R, Li X, Li J. Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods. Molecules. 2024; 29(2):500. https://doi.org/10.3390/molecules29020500
Chicago/Turabian StyleTan, Xiqian, Xiaoxiao Cheng, Bingyu Ma, Fangchao Cui, Dangfeng Wang, Ronghu Shen, Xuepeng Li, and Jianrong Li. 2024. "Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods" Molecules 29, no. 2: 500. https://doi.org/10.3390/molecules29020500
APA StyleTan, X., Cheng, X., Ma, B., Cui, F., Wang, D., Shen, R., Li, X., & Li, J. (2024). Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods. Molecules, 29(2), 500. https://doi.org/10.3390/molecules29020500






