Comparative Analysis of Phytochemical Profiles and Selected Biological Activities of Various Morphological Parts of Ligustrum vulgare
Abstract
1. Introduction
2. Results and Discussion
2.1. Metabolomic Analysis of L. vulgare Extracts
2.2. Comparative Analyses of Phytochemicals in L. vulgare Extracts
2.3. Quantitative Analysis of Selected Phytochemicals in L. vulgare Extracts
2.4. Antioxidant Activity of L. vulgare Extracts
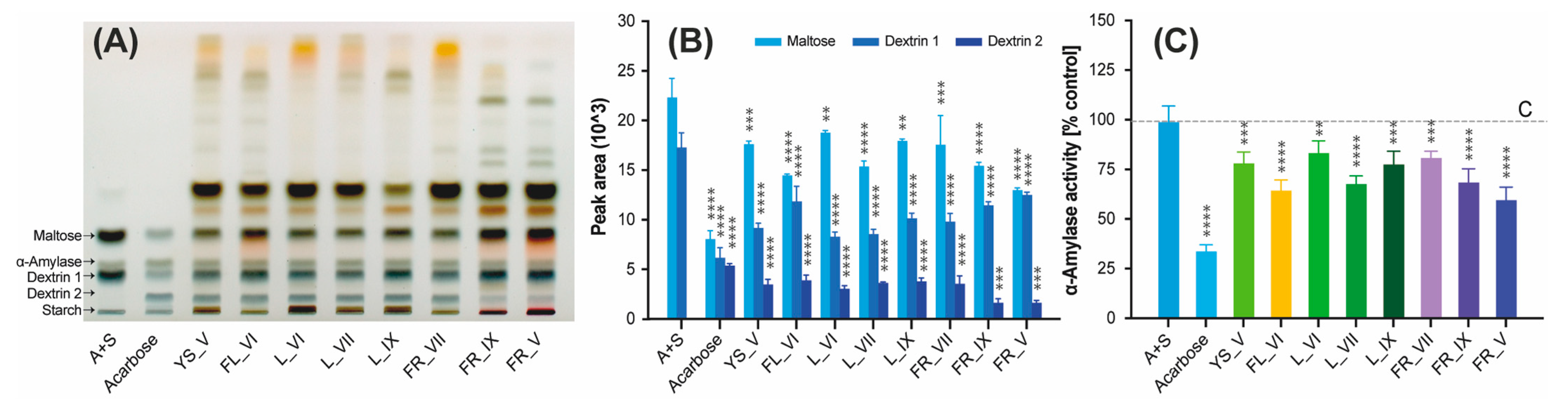
2.5. α-Amylase Inhibitory Activity of L. vulgare Extracts
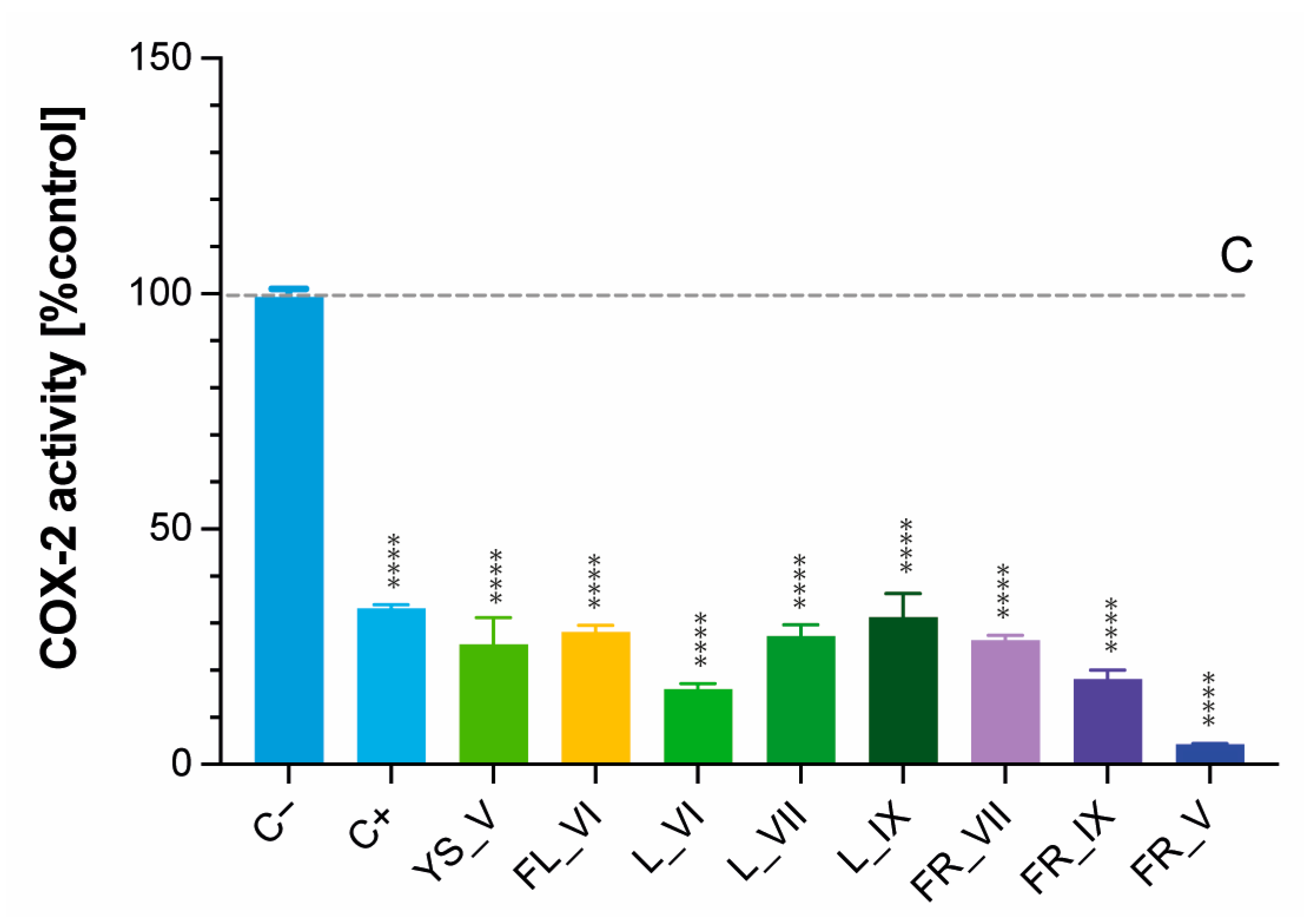
2.6. Cyclooxygenase-2 (COX-2) Inhibitory Activity of L. vulgare Extracts
2.7. Antiproliferative Activity of L. vulgare Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Plant Extract Preparation
3.4. Cell Culture
3.5. Metabolomic Analysis with LC-Q-Orbitrap HRMS
3.6. Quantitative Analysis of Selected Phytochemicals by HPLC-DAD
3.7. Determination of Antioxidant Activity
3.8. Determination of α-Amylase Inhibitory Activity
3.9. Determination of Cyclooxygenase-2 Inhibitory Activity
3.10. Determination of Cytotoxicity by MTT Test
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.L.; Oppong, M.B.; Guo, Y.; Wang, L.Z.; Fang, S.M.; Deng, Y.R.; Gao, X.M. The Oleaceae Family: A Source of Secoiridoids with Multiple Biological Activities. Fitoterapia 2019, 136, 104155. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.R.; Franzyk, H.; Wallander, E. Chemotaxonomy of the Oleaceae: Iridoids as Taxonomic Markers. Phytochemistry 2002, 60, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea Europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; De Kok, R.; Krestovskaja, T.; Morales, R.; et al. Flowering Plants Dicotyledons: Lamiales (except Acanthaceae Including Avicenniaceae). In The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Chemical Composition, Bioactivity and Safety Aspects of Kuding Tea—From Beverage to Herbal Extract. Nutrients 2020, 12, 2796. [Google Scholar] [CrossRef]
- Gao, B.B.; She, G.M.; She, D.M. Chemical Constituents and Biological Activities of Plants from the Genus Ligustrum. Chem. Biodivers. 2013, 10, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, C.; Wang, Z.; Liu, X.; You, Y.; Wei, H.; Guo, T. Ligustri Lucidi Fructus as a Traditional Chinese Medicine: A Review of Its Phytochemistry and Pharmacology. Nat. Prod. Res. 2015, 29, 493–510. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Alamo, C.; García-García, P. “The Herbs That Have the Property of Healing…,”: The Phytotherapy in Don Quixote. J. Ethnopharmacol. 2006, 106, 429–441. [Google Scholar] [CrossRef]
- Pieroni, A.; Pachaly, P. An Ethnopharmacological Study on Common Privet (Ligustrum Vulgare) and Phillyrea (Phillyrea Latifolia). Fitoterapia 2000, 71, S89–S94. [Google Scholar] [CrossRef]
- Pieroni, A. Medicinal Plants and Food Medicines in the Folk Traditions of the Upper Lucca Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef]
- Nash, R.J. Toxic Plants: Dangerous to Humans and Animals By Jean Bruneton (Translated by Caroline K. Hatton). X+545 Pages. Andover, UK: Intercept Ltd, 1999. ISBN 1 898298 62 9. New Phytol. 2000, 148, 57–58. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Vincieri, F.F.; Gravano, E.; Tattini, M. HPLC Analysis of Flavonoids and Secoiridoids in Leaves of Ligustrum vulgare L. (Oleaceae). J. Agric. Food Chem. 2000, 48, 4091–4096. [Google Scholar] [CrossRef] [PubMed]
- Mučaji, P.; Záhradníková, A.; Bezáková, L.; Cupáková, M.; Rauová, D.; Nagy, M. HPLC Determination of Antilipoxygenase Activity of a Water Infusion of Ligustrum vulgare L. Leaves and Some of Its Constituents. Molecules 2011, 16, 8198–8208. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Senkardes, I.; Bibi Sadeer, N.; Fawzi Mahomoodally, M.; Lucini, L. Metabolomics-Based Profiling with Chemometric Approach to Delineate the Bio-Pharmaceutical Properties of Fruit Extracts from Ligustrum vulgare L. Ind. Crops Prod. 2019, 140, 111635. [Google Scholar] [CrossRef]
- Fu, B.; Feng, S.; Song, X.; Guo, T.; Shi, Y.; Zhang, Y.; Liu, Q. The Oleoside-Type Secoiridoid Glycosides: Potential Secoiridoids with Multiple Pharmacological Activities. J. Mol. Struct. 2023, 1283, 135286. [Google Scholar] [CrossRef]
- Litewski, S.; Mróz, M.; Bartoszek, A.; Kusznierewicz, B. Post-Chromatographic Derivatization Coupled with Mass Spectrometry as a Method of Profiling and Identification of Antioxidants; Ligustrum vulgare Phytocomplex as an Example. Molecules 2023, 28, 8000. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, W.; Liu, Q.; Xu, J.; Bao, B.; Shan, M.; Cao, Y.; Cheng, F.; Ding, A.; Zhang, L. Application of UHPLC-ESI-Q-TOF-MS to Identify Multiple Constituents in Processed Products of the Herbal Medicine Ligustri Lucidi Fructus. Molecules 2017, 22, 689. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.D.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS Based Metabolic Profiling of the Potential Olea Europaea by-Product “Wood” and Its Comparison with Leaf Counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef]
- Tótha, G.; Barabás, C.; Tóth, A.; Kéry, Á.; Béni, S.; Boldizsár, I.; Varga, E.; Noszál, B. Characterization of Antioxidant Phenolics in Syringa vulgaris L. Flowers and Fruits by HPLC-DAD-ESI-MS. Biomed. Chromatogr. 2016, 30, 923–932. [Google Scholar] [CrossRef]
- Wozniak, M.; Michalak, B.; Wyszomierska, J.; Dudek, M.K.; Kiss, A.K. Effects of Phytochemically Characterized Extracts from Syringa Vulgaris and Isolated Secoiridoids on Mediators of Inflammation in a Human Neutrophil Model. Front. Pharmacol. 2018, 9, 349. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic Profiling of Olives and Olive Oil Process-Derived Matrices Using UPLC-DAD-ESI-QTOF-HRMS Analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef]
- Tóth, G.; Alberti, Á.; Sólyomváry, A.; Barabás, C.; Boldizsár, I.; Noszál, B. Phenolic Profiling of Various Olive Bark-Types and Leaves: HPLC-ESI/MS Study. Ind. Crops Prod. 2015, 67, 432–438. [Google Scholar] [CrossRef]
- Kuhnert, N.; Jaiswal, R.; Matei, M.F.; Sovdat, T.; Deshpande, S. How to Distinguish between Feruloyl Quinic Acids and Isoferuloyl Quinic Acids by Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2010, 24, 1575–1582. [Google Scholar] [CrossRef]
- Liao, X.; Hong, Y.; Chen, Z. Identification and Quantification of the Bioactive Components in Osmanthus Fragrans Roots by HPLC-MS/MS. J. Pharm. Anal. 2021, 11, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Boakye-Yiadom, M.; Liu, E.; Zhang, Y.; Li, W.; Song, X.; Fu, F.; Gao, X. Structural Characterisation and Identification of Phenylethanoid Glycosides from Cistanches Deserticola Y.C. Ma by UHPLC/ESI-QTOF-MS/MS. Phytochem. Anal. 2012, 23, 668–676. [Google Scholar] [CrossRef]
- Romero, C.; Garcia, P.; Brenes, M.; García, A.; Garrido, A. Phenolic Compounds in Natural Black Spanish Olive Varieties. Eur. Food Res. Technol. 2002, 215, 489–496. [Google Scholar] [CrossRef]
- Sanz, M.; De Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrella, I.; Pinto, E. LC-DAD/ESI-MS/MS Study of Phenolic Compounds in Ash (Fraxinus excelsior L. and F. Americana L.) Heartwood. Effect of Toasting Intensity at Cooperage. J. Mass. Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Bryś, M.; Urbańska, K.; Olas, B. Novel Findings Regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Gąsińska, E.; Leśniak, A.; Krawczyk, P.; Kiss, A.K.; Naruszewicz, M.; Bujalska-Zadrożny, M. Inhibitory Effect of Ligustrum vulgare Leaf Extract on the Development of Neuropathic Pain in a Streptozotocin-Induced Rat Model of Diabetes. Phytomedicine 2018, 49, 75–82. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and Biotransformations of Phenol-Conjugated Oleosidic Secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef]
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and Secoiridoids in Raw Material (Olea europaea L. Leaves) and Commercial Food Supplements. Eur. Food Res. Technol. 2017, 243, 429–435. [Google Scholar] [CrossRef]
- Ranalli, A.; Marchegiani, D.; Contento, S.; Girardi, F.; Nicolosi, M.P.; Brullo, M.D. Variations of Iridoid Oleuropein in Italian Olive Varieties during Growth and Maturation. Eur. J. Lipid Sci. Technol. 2009, 111, 678–687. [Google Scholar] [CrossRef]
- Czerwińska, M.; Warowny, M.; Kiss, A. HPLC-DAD-MS Analysis of Extracts from Flowers, Leaves, Fruits and Branches of Ligustrum vulgare and Their Effect on Cytokines Secretion by Human Neutrophils. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The Relationship between Standard Reduction Potentials of Catechins and Biological Activities Involved in Redox Control. Redox Biol. 2018, 17, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Bartoszek, A. Relationship between Chemical Structure and Biological Activity Evaluated In Vitro for Six Anthocyanidins Most Commonly Occurring in Edible Plants. Molecules 2023, 28, 6156. [Google Scholar] [CrossRef]
- Jakubek, P.; Suliborska, K.; Kuczyńska, M.; Asaduzzaman, M.; Parchem, K.; Koss-Mikołajczyk, I.; Kusznierewicz, B.; Chrzanowski, W.; Namieśnik, J.; Bartoszek, A. The Comparison of Antioxidant Properties and Nutrigenomic Redox-Related Activities of Vitamin C, C-Vitamers, and Other Common Ascorbic Acid Derivatives. Free Radic. Biol. Med. 2023, 209, 239–251. [Google Scholar] [CrossRef]
- Mróz, M.; Kusznierewicz, B. Phytochemical Screening and Biological Evaluation of Greek Sage (Salvia Fruticosa Mill.) Extracts. Sci. Rep. 2023, 13, 22309. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Mróz, M.; Koss-Mikołajczyk, I.; Namieśnik, J. Comparative Evaluation of Different Methods for Determining Phytochemicals and Antioxidant Activity in Products Containing Betalains–Verification of Beetroot Samples. Food Chem. 2021, 362, 130132. [Google Scholar] [CrossRef]
- Peddio, S.; Padiglia, A.; Cannea, F.B.; Crnjar, R.; Zam, W.; Sharifi-Rad, J.; Rescigno, A.; Zucca, P. Common Bean (Phaseolus Vulgaris L.) α-Amylase Inhibitors as Safe Nutraceutical Strategy against Diabetes and Obesity: An Update Review. Phytother. Res. 2022, 36, 2803–2823. [Google Scholar] [CrossRef]
- Macková, A.; Mučaji, P.; Widowitz, U.; Bauer, R. In Vitro Anti-Inflammatory Activity of Ligustrum vulgare Extracts and Their Analytical Characterization. Nat. Prod. Commun. 2013, 8, 1509–1512. [Google Scholar] [CrossRef]
- Ćurčić, M.G.; Stanković, M.S.; Mrkalić, E.M.; Matović, Z.D.; Banković, D.D.; Cvetković, D.M.; Dačić, D.S.; Marković, S.D. Antiproliferative and Proapoptotic Activities of Methanolic Extracts from Ligustrum vulgare L. as an Individual Treatment and in Combination with Palladium Complex. Int. J. Mol. Sci. 2012, 13, 2521–2534. [Google Scholar] [CrossRef]
- Zarić, M.; Popović, S.; Baskić, D.; Jovanović, D.; Djurdjević, P.; Zarić, R.Ž.; Čanović, P.; Zelen, I. Ligustrum vulgare Leaves and Fruit Extracts Induce Apoptosis of Human Leukemia Cells. Period. Biol. 2021, 123, 71–77. [Google Scholar] [CrossRef]
- Liu, Z. Preparation of Botanical Samples for Biomedical Research. Endocr. Metab. Immune Disord.-Drug Targets 2008, 8, 112–121. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. Juices from Non-Typical Edible Fruits as Health-Promoting Acidity Regulators for Food Industry. LWT 2015, 64, 845–852. [Google Scholar] [CrossRef]
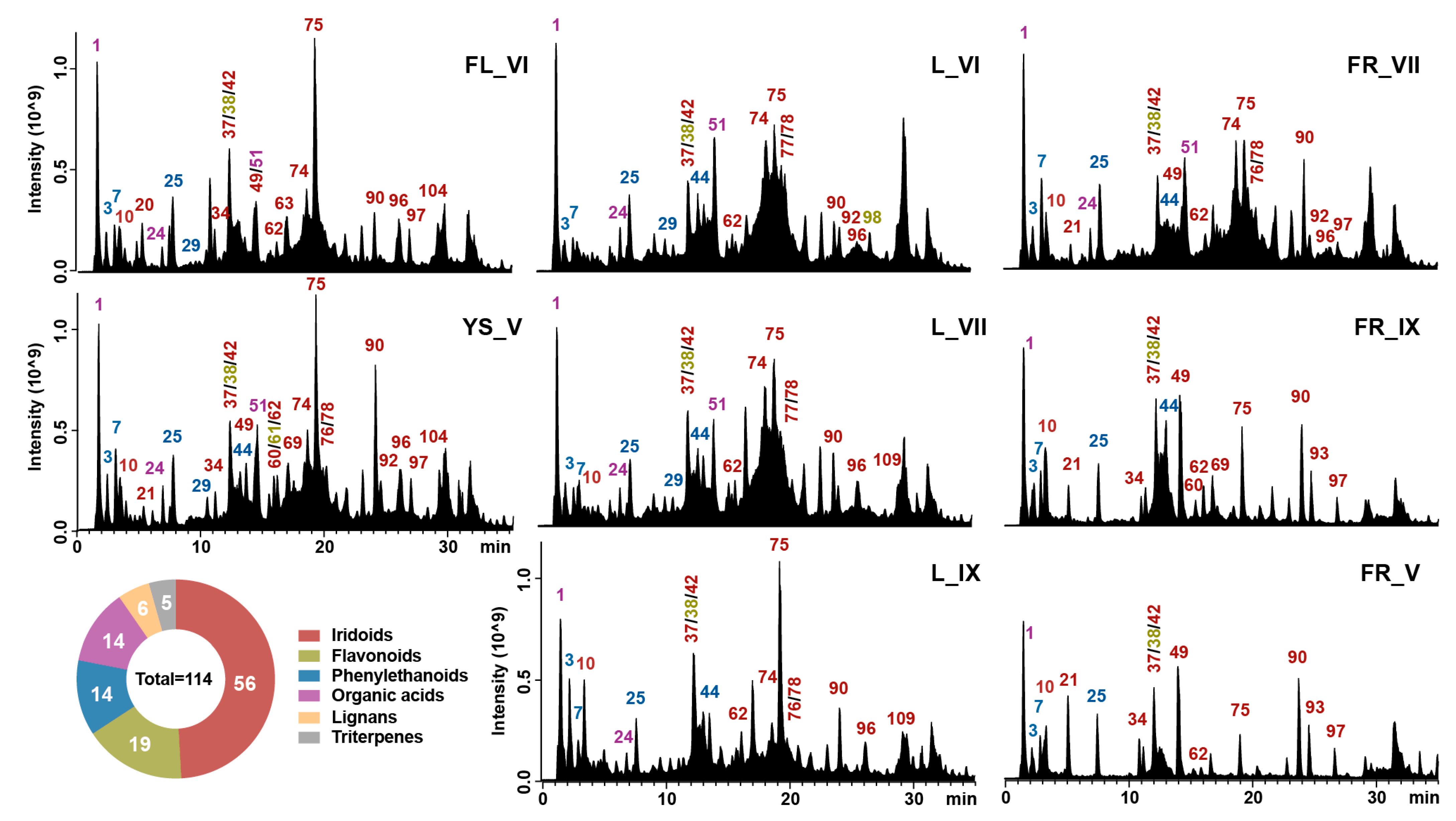
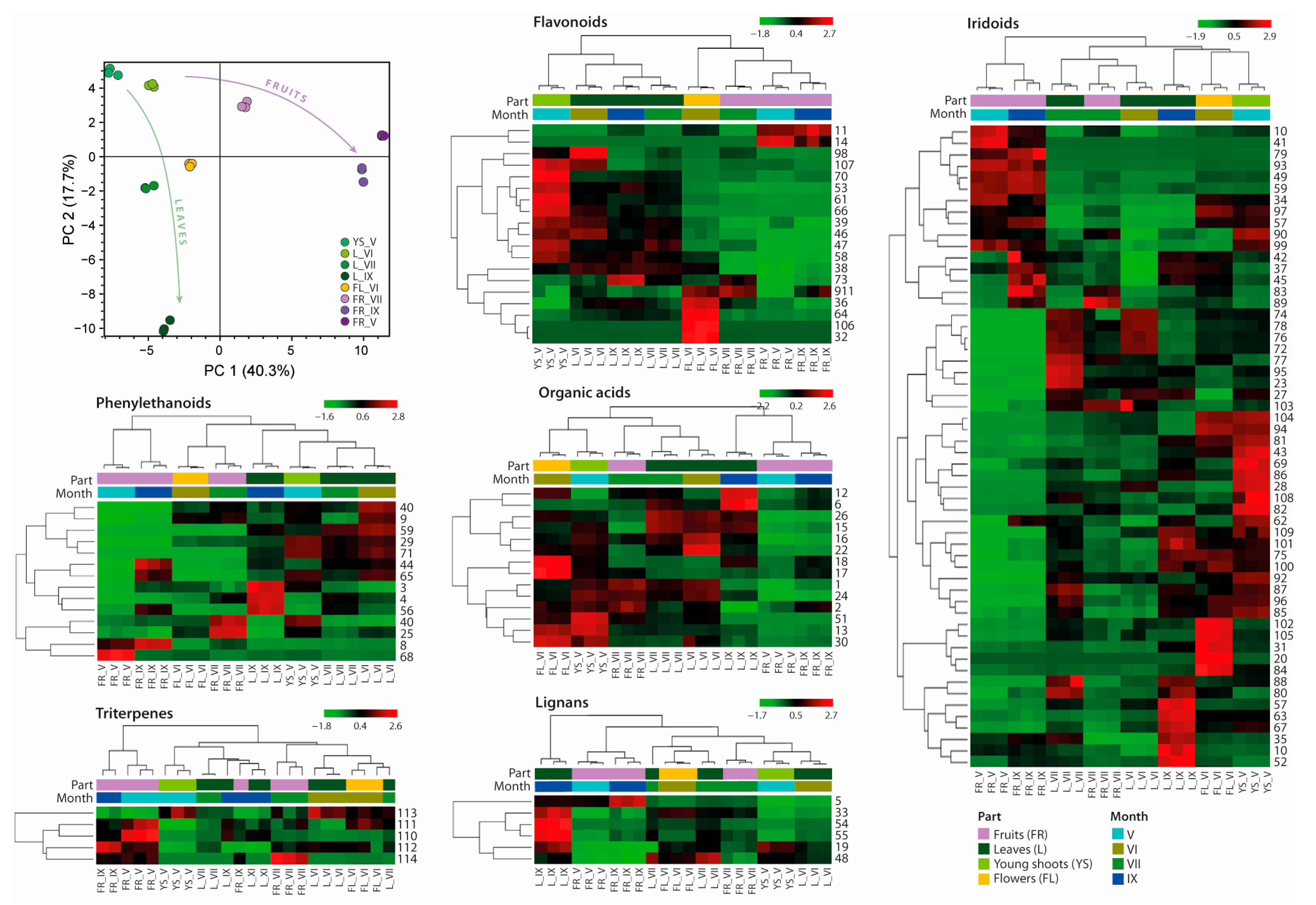


| No | Rt [min] | Compound | Formula | Theoretical [M − H]− | Experimental [M − H]− | Δm [ppm] | MS/MS | Ref. |
|---|---|---|---|---|---|---|---|---|
| Organic acids and derivatives | ||||||||
| 1 | 1.58 | D-(-)-Quinic acid | C7H12O6 | 191.05557 | 191.05513 | 2.28 | 85.028; 93.033; 59.01238; 71.012; 109.028 | [18,19] |
| 2 | 1.74 | Citric acid | C6H8O7 | 191.01918 | 191.01891 | 1.44 | 87.007; 85.028; 111.007 | [19] |
| 6 | 2.91 | Caffeoylquinic acid 1 | C16H18O9 | 353.08726 | 353.08771 | −1.27 | 191.055; 135.044; 179.034; 136.047; 161.023; 180.037; 85.028; 173.044; 111.044 | [19] |
| 12 | 3.66 | Syringin * | C17H24O9 | 417.13969 | 417.14023 | −1.29 | 209.081; 194.058; 176.047; 328.599; 268.111; 318.322; 259.172; 356.034; 117.326 | [20] |
| 13 | 3.75 | p-Coumaroylhexaric acid | C15H16O10 | 355.06653 | 355.06705 | −1.47 | 85.028; 57.033; 209.030; 191.019; 59.012; 129.018; 133.013; 86.031; 111.007 | [21] |
| 15 | 4.11 | p-Coumaroyl acid hexoside 1 | C15H18O8 | 325.09235 | 325.09287 | −1.60 | 119.049; 163.039; 120.052; 91.513; 174.794; 236.837; 183.034; 232.073; 167.113 | [22] |
| 16 | 4.48 | Caffeoylquinic acid 2 | C16H18O9 | 353.08726 | 353.08783 | −1.61 | 135.044; 173.044; 191.055; 179.034; 93.033; 137.023; 136.047; 174.048; 85.028 | [19] |
| 17 | 4.50 | Vanilin | C8H8O3 | 151.03952 | 151.03893 | 3.94 | 123.044; 93.033; 108.020; 121.028; 95.049; 105.033; 67.017; 77.038; 137.023 | [19,23] |
| 18 | 4.68 | p-Coumaroyl acid hexoside 2 | C15H18O8 | 325.09235 | 325.09293 | −1.79 | 145.028; 117.033; 146.032; 59.012; 119.049; 89.023; 163.039; 101.023; 161.060 | [22] |
| 22 | 6.17 | Esculetin | C9H6O4 | 177.01879 | 177.01837 | 2.34 | 89.038; 105.033; 133.028; 93.033; 81.033; 95.049; 121.028; 149.023; 177.018 | [19,23] |
| 24 | 6.70 | p-Cumaroylquinic acid | C16H18O8 | 337.09235 | 337.09283 | −1.45 | 173.044; 93.033; 119.049; 163.039; 111.044; 137.023; 174.048; 155.034; 67.017 | [22] |
| 26 | 7.58 | Feruloylquinic acid | C17H20O9 | 367.10291 | 367.10327 | −0.98 | 173.044; 93.033; 134.036; 193.050; 111.044; 174.048; 137.023; 155.034; 67.017 | [24] |
| 30 | 10.26 | p-Coumaric acid | C9H8O3 | 163.03952 | 163.03900 | 3.18 | 119.049; 93.033; 120.052; 117.033; 91.054; 65.038; 94.036; 104.0253; 103.124 | [22] |
| 51 | 14.35 | Cinnamoylquinic acid | C16H18O7 | 321.09743 | 321.09778 | −1.08 | 173.044; 93.033; 147.044; 111.044; 137.023; 174.048; 71.012; 155.034; 59.012 | - |
| Phenylethanoids | ||||||||
| 3 | 2.26 | Hydroxytyrosol glucoside | C14H20O8 | 315.10800 | 315.10852 | −1.67 | 123.044; 153.054; 124.047; 154.058; 59.012; 71.012; 135.044; 89.023; 101.023; 108.020 | [22] |
| 4 | 2.38 | Bioside | C20H30O12 | 461.16591 | 461.16644 | −1.17 | 113.023; 135.044; 71.012; 89.023; 461.166; 59.012; 101.023; 315.108; 85.028; 161.044 | [25] |
| 7 | 2.92 | Salidroside (tyrosol glucoside) | C14H20O7 | 299.11308 | 299.11346 | −1.29 | 59.012; 71.012; 119.049; 89.023; 101.023; 85.028; 113.023; 95.012; 58.005; 137.059 | [18] |
| 8 | 2.94 | Osmanthuside H | C19H28O11 | 431.15534 | 431.15558 | −0.55 | 89.023; 59.012; 101.023; 71.012; 119.034; 119.049; 149.044; 113.023; 131.034; 191.055 | [18] |
| 9 | 3.08 | Hydroxytyrosol | C8H10O3 | 153.05517 | 153.05458 | 3.85 | 123.044; 122.036; 95.049; 93.033; 108.020; 81.033; 67.017; 121.028; 95.012; 124.047 | [18] |
| 25 | 7.50 | Echinacoside 1 | C35H46O20 | 785.25043 | 785.25067 | −0.31 | 785.251; 623.219; 161.023; 179.034; 786.249; 91.514; 623.141; 434.588; 463.301; 58.550 | ST |
| 29 | 10.26 | Echinacoside 2 | C35H46O20 | 785.25043 | 785.25110 | −0.86 | 785.250; 786.254; 623.219; 161.023; 624.221; 162.027; 366.595; 384.669; 106.970; 363.523 | [18] |
| 40 | 12.31 | Hydroxytyrosol acetate | C10H12O4 | 195.06574 | 195.06544 | 1.49 | 59.012; 60.016; 166.294; 142.985; 177.482; 210.096; 99.362; 159.217; 73.254; 58.937 | [22] |
| 44 | 12.92 | Verbascoside 1 | C29H36O15 | 623.19760 | 623.19794 | −0.54 | 161.023; 623.198; 461.166; 135.044; 179.034; 113.023; 315.111; 133.027; 71.012; 305.068 | [18,22] |
| 56 | 14.85 | Verbascoside 2 | C29H36O15 | 623.19760 | 623.19812 | −0.83 | 623.198; 161.023; 624.201; 461.166; 462.170; 162.027; 135.044; 113.023; 251.056; 179.034 | [18,22] |
| 59 | 15.22 | Verbascoside 3 | C29H36O15 | 623.19760 | 623.19788 | −0.44 | 161.023; 623.198; 461.166; 624.202; 162.027; 462.170; 135.044; 179.034; 113.023; 315.108 | [18,22] |
| 65 | 16.44 | Isosyringalide rhamnoside 1 | C29H36O14 | 607.20269 | 607.20331 | −1.03 | 145.028; 461.166; 607.203; 163.039; 462.170; 146.0317; 608.206; 113.023; 135.044; 315.109 | [7] |
| 68 | 16.79 | Syringalide A | C23H26O10 | 461.14478 | 461.14523 | −0.99 | 161.023; 461.145; 162.0267; 462.149; 179.034; 135.044; 133.028; 89.023; 101.023; 221.045 | [26] |
| 71 | 17.57 | Isosyringalide rhamnoside 2 | C29H36O14 | 607.20269 | 607.20325 | −0.93 | 145.028; 461.166; 607.203; 462.170; 163.039; 608.208; 146.032; 113.023; 135.044; 153.055 | [7] |
| Flavonoids | ||||||||
| 11 | 3.65 | Taxifolin-3-O-glucoside | C21H22O12 | 465.10331 | 465.10364 | −0.72 | 285.040; 125.023; 275.056; 178.998; 177.018; 181.013; 153.018; 151.002; 303.051 | [23] |
| 14 | 4.00 | Cyanidin-3-O-rutinoside | C27H30O15 | 593.15065 | 593.15125 | −1.00 | 285.040; 284.032; 593.151; 594.155; 286.044; 299.056; 125.023; 149.044; 89.023 | [27] |
| 32 | 10.69 | Luteolin-4’,7-O-diglucoside | C27H30O16 | 609.14557 | 609.14600 | −0.71 | 284.032; 285.040; 609.146; 610.149; 286.043; 429.081; 178.998; 283.024; 257.044 | [22] |
| 36 | 11.71 | Eriodyctiol glucoside | C21H22O11 | 449.10839 | 449.10898 | −1.31 | 151.002; 287.056; 135.044; 288.060; 175.003; 152.006; 125.023; 193.014; 68.994 | [23] |
| 38 | 12.14 | Quercetin-3-O-rutinoside | C27H30O16 | 609.14557 | 609.14575 | −0.31 | 609.146; 301.035; 300.027; 610.149; 302.039; 178.998; 343.046; 302.006; 179.195 | ST |
| 39 | 12.28 | Luteolin-7-O-rutinoside | C27H30O15 | 593.15065 | 593.15106 | −0.69 | 285.040; 593.151; 594.154; 286.044; 273.076; 274.079; 307.082; 361.094; 327.052 | [22] |
| 46 | 13.40 | Kaemferol-7-O-glucoside | C21H20O11 | 447.09274 | 447.09311 | −0.83 | 285.040; 284.032; 447.093; 448.097; 327.051; 284.000; 269.046; 297.039 | [18] |
| 47 | 13.43 | Kaemferol-3-O-rutinoside | C27H30O15 | 593.15065 | 593.15094 | −0.49 | 593.151; 594.154; 285.040; 284.032; 447.093; 286.045; 448.097; 91.513; 594.254; 327.049 | [18] |
| 53 | 14.44 | Ligustroflavone | C33H40O19 | 723.21365 | 723.21362 | 0.03 | 723.214; 724.218; 269.045; 270.049; 577.159; 559.147; 268.036; 428.833; 159.412; 103.969 | [7] |
| 58 | 15.22 | Luteolin-7-O-rhamnoside | C27H30O15 | 593.15065 | 593.15094 | −0.49 | 285.040; 593.151; 284.032; 594.154; 286.044; 327.051; 151.002; 257.047; 444.174; 99.526 | [22] |
| 61 | 15.65 | Apigenin-7-O-rutinoside 1 | C27H30O14 | 577.15574 | 577.15607 | −0.58 | 269.045; 270.048; 577.156; 311.056; 65.002; 578.155; 67.388; 63.022; 64.999; 91.507 | [18] |
| 64 | 16.12 | Naringenin hexoside | C21H22O10 | 433.11348 | 433.11392 | −1.03 | 271.061; 151.002; 272.065; 119.049; 112.984; 93.033; 68.994; 177.018; 152.006; 175.002 | [23] |
| 66 | 16.68 | Apigenin-7-O-rutinoside 2 | C27H30O14 | 577.15574 | 577.15607 | −0.58 | 269.045; 577.156; 578.160; 270.049; 268.037; 413.088; 311.056; 431.100; 457.112; 101.023 | [18] |
| 70 | 17.22 | Apigenin-7-O-glucoside | C21H20O10 | 431.09783 | 431.09821 | −0.88 | 268.038; 269.044; 431.098; 432.101; 270.049; 311.056; 283.060; 281.046; 341.068; 151.002 | [18,22] |
| 73 | 18.03 | Chrysoeriol-7-O-glucoside | C22H22O11 | 461.10839 | 461.10870 | −0.68 | 446.085; 461.109; 283.024; 298.048; 299.054; 447.089; 462.112; 284.030; 313.035; 297.040 | [19] |
| 91 | 24.17 | Eriodictyol | C15O12O6 | 287.05557 | 287.05603 | −1.62 | 135.044; 151.002; 107.012; 65.002; 136.047; 83.012; 63.022; 109.028; 152.006; 108.016 | [23] |
| 98 | 26.74 | Luteolin | C15H10O6 | 285.03992 | 285.04041 | −1.72 | 133.028; 285.040; 151.002; 175.039; 107.012; 149.023; 199.039; 286.044; 217.050; 134.031 | [18,23] |
| 106 | 29.31 | Naringenin | C15H12O15 | 271.06065 | 271.06134 | −2.55 | 119.049; 107.012; 151.003; 65.002; 83.012; 63.023; 187.039; 120.052; 93.033; 161.060 | [20,23] |
| 107 | 29.40 | Apigenin | C15H10O5 | 269.04500 | 269.04541 | −1.52 | 117.033; 151.002; 149.023; 269.045; 107.012; 65.002; 225.055; 118.036; 121.028; 159.044 | [18,22] |
| Iridoids | ||||||||
| 10 | 3.39 | Oleoside | C16H22O16 | 389.10839 | 389.10898 | −1.51 | 69.033; 59.012; 121.064; 89.023; 71.012; 95.049; 101.023; 113.023; 165.054; 119.034 | [18] |
| 20 | 5.09 | Genipin 1-gentiobioside | C23H34O15 | 549.18195 | 549.18243 | −0.88 | 101.023; 163.060; 205.071; 59.012; 103.039; 89.023; 143.034; 73.028; 119.034; 71.012 | - |
| 21 | 5.16 | Oleoside 11-methyl ester 1 | C17H24O11 | 403.12404 | 403.12439 | −0.87 | 59.012; 89.023; 71.012; 101.023; 113.023; 69.033; 119.033; 121.028; 85.028; 127.038 | [18] |
| 23 | 6.29 | Oleoside 11-methyl ester 2 | C17H24O11 | 403.12404 | 403.12448 | −1.09 | 59.012; 89.023; 71.012; 101.023; 113.023; 119.034; 197.081; 85.028; 68.997; 165.055 | [18] |
| 27 | 8.79 | Elenolic acid | C11H14O6 | 241.07122 | 241.07141 | −0.81 | 67.017; 68.997; 111.007; 95.049; 127.039; 139.002; 101.023; 121.028; 69.033; 123.043 | [18] |
| 28 | 10.01 | Genipin | C11H14O5 | 225.07630 | 225.07649 | −0.85 | 68.997; 101.023; 67.017; 127.039; 106.041; 123.044; 108.020; 70.000; 95.049; 125.023 | - |
| 31 | 10.29 | 7-Hydroxyoleuropein | C25H32O14 | 555.17139 | 555.17145 | −0.11 | 151.039; 89.023; 223.060; 152.042; 101.023; 119.034; 59.012; 71.012; 149.023; 113.023 | [18] |
| 34 | 10.92 | Neonuzhenide 1 | C31H42O18 | 701.22930 | 701.22986 | −0.80 | 315.108; 469.135; 437.148; 701.230; 316.111; 539.176; 470.138; 702.231; 357.119; 507.151 | [18] |
| 35 | 11.33 | Oleuropeinic acid | C25H30O15 | 569.15065 | 569.15088 | −0.40 | 151.039; 209.045; 331.082; 177.018; 123.044; 183.065; 89.023; 165.054; 195.029; 221.008 | [18] |
| 37 | 12.10 | 10-Hydroxyoleuropein 1 | C25O32O14 | 555.17139 | 555.17163 | −0.44 | 273.077; 89.023; 137.023; 119.033; 101.023; 111.044; 59.012; 307.082; 181.050; 275.056 | [18] |
| 41 | 12.55 | Nuzhenide 1 | C31H42O17 | 685.23438 | 685.23474 | −0.53 | 453.140; 299.113; 223.061; 89.023; 101.023; 421.150; 119.034; 179.055; 59.012; 121.028 | [18,22] |
| 42 | 12.61 | 10-Hydroxyoleuropein 2 | C25H32O14 | 555.17139 | 555.17163 | −0.44 | 195.065; 273.077; 239.055; 89.023; 361.093; 307.082; 137.023; 387.093; 119.034; 101.023 | [18,23] |
| 43 | 12.84 | Neonuzhenide 2 | C31H42O18 | 701.22930 | 701.22961 | −0.46 | 315.108; 316.112; 135.044; 59.013; 89.023; 119.033; 179.055; 101.022; 59.009; 153.054 | [18] |
| 45 | 13.13 | 10-Hydroxyoleuropein 3 | C25H32O14 | 555.17139 | 555.17181 | −0.77 | 195.065; 273.077; 239.056; 89.023; 361.093; 307.082; 387.094; 137.023; 119.034; 101.023 | [18] |
| 49 | 14.08 | Nuzhenide 2 | C31H42O17 | 685.23438 | 685.23499 | −0.88 | 453.140; 421.151; 299.113; 101.023; 223.061; 454.144; 89.023; 523.182; 119.034; 422.155 | [18] |
| 50 | 14.22 | Nuzhenide 3 | C31H42O17 | 685.23438 | 685.23505 | −0.97 | 453.140; 421.151; 299.114; 101.023; 223.060; 89.023; 454.143; 523.182; 119.034; 422.154 | [18] |
| 52 | 14.38 | Oleuropein dihexoside | C31H42O18 | 701.22930 | 701.22961 | −0.46 | 539.177; 377.124; 275.092; 307.082; 540.180; 469.135; 179.055; 378.127; 437.146; 223.0604 | [22,23] |
| 57 | 15.14 | Neonuzhenide 3 | C31H42O18 | 701.22930 | 701.22961 | −0.46 | 315.108; 316.111; 179.055; 275.092; 307.082; 135.044; 377.124; 89.023; 701.229; 223.060 | [18] |
| 60 | 15.29 | Nuzhenide 4 | C31H42O17 | 685.23438 | 685.23431 | 0.10 | 299.113; 223.060; 89.023; 623.198; 119.034; 101.023; 179.055; 113.023; 300.117; 71.012 | [18] |
| 62 | 15.91 | 10-Hydroxyligstroside 1 | C25H32O13 | 539.17647 | 539.17706 | −1.10 | 291.087; 101.023; 275.092; 111.044; 89.023; 127.039; 377.124; 59.012; 292.091; 239.056 | [25] |
| 63 | 16.03 | Comselogoside 1 | C25H28O13 | 535.14517 | 535.14581 | −1.20 | 265.071; 205.050; 163.039; 235.061; 183.065; 145.028; 59.012; 121.064; 69.033; 177.055 | [22] |
| 67 | 16.73 | Caffeoyl-6’-secologanoside | C25H28O14 | 551.14009 | 551.14056 | −0.87 | 161.023; 281.066; 507.151; 389.109; 162.027; 179.034; 345.119; 251.056; 59.012; 323.077 | [22] |
| 69 | 16.90 | 10-Hydroxyligstroside 2 | C25H32O13 | 539.17647 | 539.17706 | −1.10 | 101.023; 89.023; 291.087; 221.045; 275.092; 153.055; 59.012; 119.034; 211.061; 71.012 | [25] |
| 72 | 17.91 | Oleacein 1 | C17H20O6 | 319.11817 | 319.11874 | −1.81 | 69.033; 59.012; 95.049; 70.036; 139.075; 123.044; 139.039; 113.023; 96.052; 67.017 | [22] |
| 74 | 18.74 | Oleacein 2 | C17H20O6 | 319.11817 | 319.11853 | −1.14 | 69.033; 59.012; 95.049; 70.036; 139.075; 139.039; 123.044; 96.052; 113.023; 67.017 | [22] |
| 75 | 19.03 | Oleuropein 1 | C25H32O13 | 539.17647 | 539.17676 | −0.53 | 89.023; 275.093; 307.082; 95.049; 149.023; 59.012; 101.023; 139.039; 275.056; 119.034 | ST |
| 76 | 19.40 | Oleacein 3 | C17H20O6 | 319.11817 | 319.11874 | −1.81 | 69.033; 59.012; 95.049; 70.036; 139.075; 139.039; 123.044; 96.052; 113.023; 107.049 | [22] |
| 77 | 19.73 | Oleacein 4 | C17H20O6 | 319.11817 | 319.11868 | −1.62 | 69.033; 59.012; 95.049; 70.036; 139.075; 139.039; 113.023; 123.044; 96.052; 67.017 | [22] |
| 78 | 19.88 | Oleacein 5 | C17H20O6 | 319.11817 | 319.11874 | −1.81 | 69.033; 59.012; 95.049; 107.049; 70.036; 137.059; 139.039; 121.028; 113.023; 139.075 | [22] |
| 79 | 20.06 | G-13/Oleonuezhenide 1 | C48H64O27 | 1071.35568 | 1071.35584 | −0.14 | 1071.355; 771.235; 685.235; 1072.358; 523.182; 772.239; 403.124; 909.303; 686.238; 910.307 | [18] |
| 80 | 20.52 | Oleuropein 2 | C25H32O13 | 539.17647 | 539.17706 | −1.10 | 89.023; 275.093; 149.023; 95.049; 59.012; 307.082; 101.023; 139.039; 119.034; 275.057 | [18] |
| 81 | 20.64 | Comselogoside 2 | C25H28O13 | 535.14517 | 535.14587 | −1.32 | 145.028; 121.064; 163.039; 205.050; 59.0124; 265.071; 69.033; 146.032; 345.119; 165.055 | [22] |
| 82 | 20.72 | Nuzhenide aglycone | C25H32O12 | 523.18156 | 523.18219 | −1.21 | 101.023; 89.023; 119.034; 59.012; 121.028; 453.141; 71.012; 113.023; 119.048; 137.060 | [18] |
| 83 | 21.46 | Oleoacteooside | C46H58O25 | 1009.31890 | 1009.31897 | −0.07 | 1009.319; 1010.323; 847.278; 623.199; 848.278; 745.234; 665.209; 815.238; 161.023; 777.227 | [25] |
| 84 | 21.84 | Lucidumoside C | C27H36O14 | 583.20269 | 583.20337 | −1.17 | 151.039; 89.023; 223.061; 119.034; 101.023; 59.012; 179.055; 71.012; 152.042; 113.023 | [18] |
| 85 | 22.23 | Oleuropein aglycone 1 | C19H22O8 | 377.12365 | 377.12421 | −1.49 | 95.049; 111.007; 139.002; 101.023; 139.039; 149.023; 127.039; 69.033; 68.997; 59.0124 | [18] |
| 86 | 22.37 | Comselogoside 3 | C25H28O13 | 535.14517 | 535.14569 | −0.97 | 145.028; 121.064; 163.039; 265.071; 205.050; 69.033; 146.032; 59.012; 345.119; 165.054 | [22] |
| 87 | 22.55 | Oleuropein aglycone 2 | C19H22O8 | 377.12365 | 377.12421 | −1.49 | 95.049; 111.007; 139.002; 139.039; 101.023; 149.023; 127.039; 69.033; 59.012; 68.996 | [18] |
| 88 | 22.83 | 10-Hydroxyligstroside aglycone 1 | C19H22O8 | 377.12365 | 377.12402 | −1.00 | 101.023; 127.039; 111.007; 68.997; 111.044; 139.002; 85.028; 171.029; 93.033; 137.059 | [25] |
| 89 | 22.96 | Excelside B | C31H42O17 | 685.23438 | 685.23468 | −0.44 | 291.087; 361.129; 259.097; 101.023; 113.023; 292.091; 223.061; 161.044; 362.132; 127.039 | [25] |
| 90 | 23.84 | Ligustroside 1 | C25H32O12 | 523.18156 | 523.18201 | −0.86 | 291.087; 101.023; 259.097; 127.039; 292.091; 111.007; 69.033; 139.039; 89.023; 68.997 | [18] |
| 92 | 24.24 | 10-Hydroxyligstroside aglycone 2 | C19H22O8 | 377.12365 | 377.12408 | −1.17 | 101.023; 111.007; 127.039; 95.049; 139.002; 68.997; 111.044; 85.028; 171.029; 139.039 | [25] |
| 93 | 24.62 | GL-3/Oleonezuhine 2 | C48H64O27 | 1071.35568 | 1071.35547 | 0.20 | 685.235; 686.238; 523.182; 771.235; 1071.355; 1072.359; 403.124; 909.303; 772.239; 524.185 | [18] |
| 94 | 25.65 | 6′-O-trans-Cinnamoyl 8-epikingisidic acid | C25H28O12 | 519.15026 | 519.15070 | −0.85 | 121.064; 147.044; 161.060; 59.012; 189.055; 69.033; 183.065; 165.055; 95.049; 122.068 | [18] |
| 95 | 25.66 | Ligustroside 2 | C25H32O12 | 523.18156 | 523.18201 | −0.86 | 101.023; 291.087; 223.060; 89.023; 59.012; 361.129; 127.0387; 259.097; 71.012; 292.091 | [18] |
| 96 | 25.86 | Oleuropein aglycone 3 | C19H22O8 | 377.12365 | 377.12408 | −1.17 | 95.049; 111.007; 139.039; 101.023; 139.002; 149.023; 127.039; 69.033; 68.997; 59.012 | [18] |
| 97 | 26.71 | GL-3/Oleonezuhine 3 | C48H64O27 | 1071.35568 | 1071.35559 | 0.08 | 1071.355; 685.235; 1072.358; 909.303; 523.182; 910.306; 686.238; 771.234; 403.124; 839.261 | [18] |
| 99 | 27.45 | Ligustroside aglycone 1 | C19H22O7 | 361.12873 | 361.12924 | −1.42 | 101.023; 127.039; 111.007; 68.997; 69.033; 139.002; 171.029; 153.018; 137.059; 67.017 | [22] |
| 100 | 28.64 | Oleuropein aglycone 4 | C19H22O8 | 377.12365 | 377.12421 | −1.49 | 95.049; 111.007; 139.002; 101.023; 139.039; 149.023; 127.039; 69.033; 59.012; 121.028 | [22] |
| 101 | 28.83 | Jaspolyoside | C42H54O23 | 925.29777 | 925.29846 | −0.75 | 539.177; 540.180; 377.124; 925.298; 926.301; 275.092; 307.082; 521.166; 378.127; 403.124 | [19] |
| 102 | 28.95 | Oleuropein 3 | C25H32O13 | 539.17647 | 539.17706 | −1.10 | 89.023; 275.093; 307.082; 95.049; 149.023; 101.023; 59.012; 139.039; 275.057; 119.033 | [18] |
| 103 | 29.01 | 10-Hydroxyoleuropein 4 | C25H32O14 | 555.17139 | 555.17169 | −0.55 | 195.065; 273.077; 89.023; 239.055; 361.093; 307.082; 137.023; 387.093; 119.034; 101.023 | [18] |
| 104 | 29.04 | 6′-O-cis-Cinnamoyl-8-epikingisidic acid | C25H28O12 | 519.15026 | 519.15045 | −0.38 | 147.044; 69.033; 121.064; 161.060; 59.012; 165.054; 189.055; 95.049; 121.028; 139.002 | [18] |
| 105 | 29.25 | Oleuropein 4 | C25H32O13 | 539.17647 | 539.17719 | −1.33 | 89.023; 101.023; 275.093; 307.082; 95.049; 59.012; 149.023; 119.034; 139.039; 275.057 | [22] |
| 108 | 29.43 | Ligustroside aglycone 2 | C19H22O7 | 361.12873 | 361.12927 | −1.50 | 101.023; 127.039; 111.007; 68.997; 69.033; 139.002; 171.029; 153.018; 67.017; 139.039 | [22] |
| 109 | 29.49 | Oleuropein aglycone 5 | C19H22O8 | 377.12365 | 377.12408 | −1.17 | 95.049; 111.007; 139.002; 101.023; 139.039; 149.023; 127.039; 69.033; 68.997; 59.012 | [18] |
| Lignans | ||||||||
| 5 | 2.40 | Acyclodihydroelenolic acid hexoside | C17H28O11 | 407.15534 | 407.15570 | −0.89 | 59.012; 71.012; 151.075; 101.023; 89.023; 113.023; 85.028; 121.064; 73.028; 99.007 | [19] |
| 19 | 4.86 | Cycloolivil glucoside | C26H34O12 | 537.19721 | 537.19757 | −0.68 | 179.070; 195.065; 375.145; 191.070; 180.074; 376.148; 327.124; 345.134; 196.069; 360.121 | [19] |
| 33 | 10.87 | Lariciresinol-4-O-glucoside | C26H34O11 | 521.20229 | 521.20221 | 0.15 | 329.139; 330.143; 349.150; 175.075; 485.203; 178.062; 169.086; 350.153; 71.012; 101.023 | [25] |
| 48 | 13.83 | Pinoresinol-4-O-glucoside | C26H32O11 | 519.18664 | 519.18695 | −0.60 | 151.039; 357.134; 358.138; 152.042; 342.110; 136.015; 175.075; 311.128; 71.012; 101.023 | [25] |
| 54 | 14.58 | Syringaresinol-O-glucoside | C28H36O13 | 579.20777 | 579.20837 | −1.04 | 417.155; 181.050; 418.159; 402.131; 182.053; 166.026; 403.134; 205.086; 371.150; 387.108 | [28] |
| 55 | 14.79 | Medioresinol-4-O-glucopiranoside | C27H34O12 | 549.19721 | 549.19775 | −1.00 | 387.145; 151.039; 181.050; 149.044; 89.023; 388.148; 101.023; 372.121; 131.034; 191.055 | [25] |
| Triterpenes | ||||||||
| 110 | 30.34 | Tormentic acid | C30H48O5 | 487.34235 | 487.34271 | −0.74 | 469.332; 487.343; 470.335; 488.346; 486.330; 423.325; 440.325; 467.317; 425.341; 424.328 | [18] |
| 111 | 31.36 | Colosic acid | C30H48O4 | 471.34744 | 471.34763 | −0.40 | 471.348; 472.351; 61.987; 91.507; 117.966; 222.480; 102.313; 294.133; 221.898; 326.910 | [18] |
| 112 | 31.51 | 3β-O-cis/trans-p-Coumaroylmaslinic acid | C39H54O6 | 617.38422 | 617.38446 | −0.40 | 617.385; 618.388; 619.391; 145.028; 497.328; 119.049; 146.032; 645.004; 73.177; 105.122 | [18] |
| 113 | 32.57 | Ursolic/oleanolic acid | C30H48O3 | 455.35252 | 455.35294 | −0.91 | 455.353; 456.356; 97.360; 118.599; 50.633 | [18] |
| 114 | 33.65 | Acetyloleanolic/ursolic acid | C32H50O4 | 497.36309 | 497.36328 | −0.39 | 497.363; 498.367; 437.340; 429.303; 124.469; 91.513; 61.987; 323.325; 339.940; 111.674 | [18] |
| No. | Compound | Content (mg/g d.w.) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| YS_V | FL_VI | L_VI | L_VII | L_IX | FR_VII | FR_IX | FR_V | ||
| 25 | Echinacoside | 17.13 ± 0.32 (a) | 14.31 ± 0.21 (b) | 14.12 ± 0.41 (b) | 12.62 ± 0.37 (c) | 9.51 ± 0.36 (d) | 28.88 ± 0.21 (e) | 7.44 ± 0.88 (f) | 9.32 ± 0.17 (d) |
| 38 | Quercetin-3-O-rutinoside | 9.54 ± 0.26 (a) | 10.95 ± 0.42 (ab) | 9.78 ± 0.32 (a) | 11.29 ± 0.59 (b) | 13.75 ± 0.96 (c) | 4.92 ± 0.27 (de) | 6.08 ± 0.58 (d) | 3.89 ± 0.16 (e) |
| 75 | Oleuropein | 26.05 ± 1.07 (a) | 33.43 ± 2.48 (b) | 18.85 ± 3.05 (c) | 15.30 ± 0.83 (cd) | 33.20 ± 1.83 (b) | 10.29 ± 2.40 (d) | 3.15 ± 0.52 (e) | 0.67 ± 0.23 (e) |
| Sample Abbreviation | Morphological Part | Month of Harvest |
|---|---|---|
| YS_V | Young shoots | May |
| FL_VI | Flowers | June |
| L_VI | Leaves | June |
| L_VII | Leaves | July |
| L_IX | Leaves | September |
| FR_V | Fruits (from last year) | May |
| FR_VII | Fruits (green) | July |
| FR_IX | Fruits (black) | September |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litewski, S.; Koss-Mikołajczyk, I.; Kusznierewicz, B. Comparative Analysis of Phytochemical Profiles and Selected Biological Activities of Various Morphological Parts of Ligustrum vulgare. Molecules 2024, 29, 399. https://doi.org/10.3390/molecules29020399
Litewski S, Koss-Mikołajczyk I, Kusznierewicz B. Comparative Analysis of Phytochemical Profiles and Selected Biological Activities of Various Morphological Parts of Ligustrum vulgare. Molecules. 2024; 29(2):399. https://doi.org/10.3390/molecules29020399
Chicago/Turabian StyleLitewski, Szymon, Izabela Koss-Mikołajczyk, and Barbara Kusznierewicz. 2024. "Comparative Analysis of Phytochemical Profiles and Selected Biological Activities of Various Morphological Parts of Ligustrum vulgare" Molecules 29, no. 2: 399. https://doi.org/10.3390/molecules29020399
APA StyleLitewski, S., Koss-Mikołajczyk, I., & Kusznierewicz, B. (2024). Comparative Analysis of Phytochemical Profiles and Selected Biological Activities of Various Morphological Parts of Ligustrum vulgare. Molecules, 29(2), 399. https://doi.org/10.3390/molecules29020399







