Effect of Novel Processing Techniques on the Carotenoid Release during the Production of Red Guava Juice
Abstract
1. Introduction
2. Results and Discussion
2.1. Juice Yield
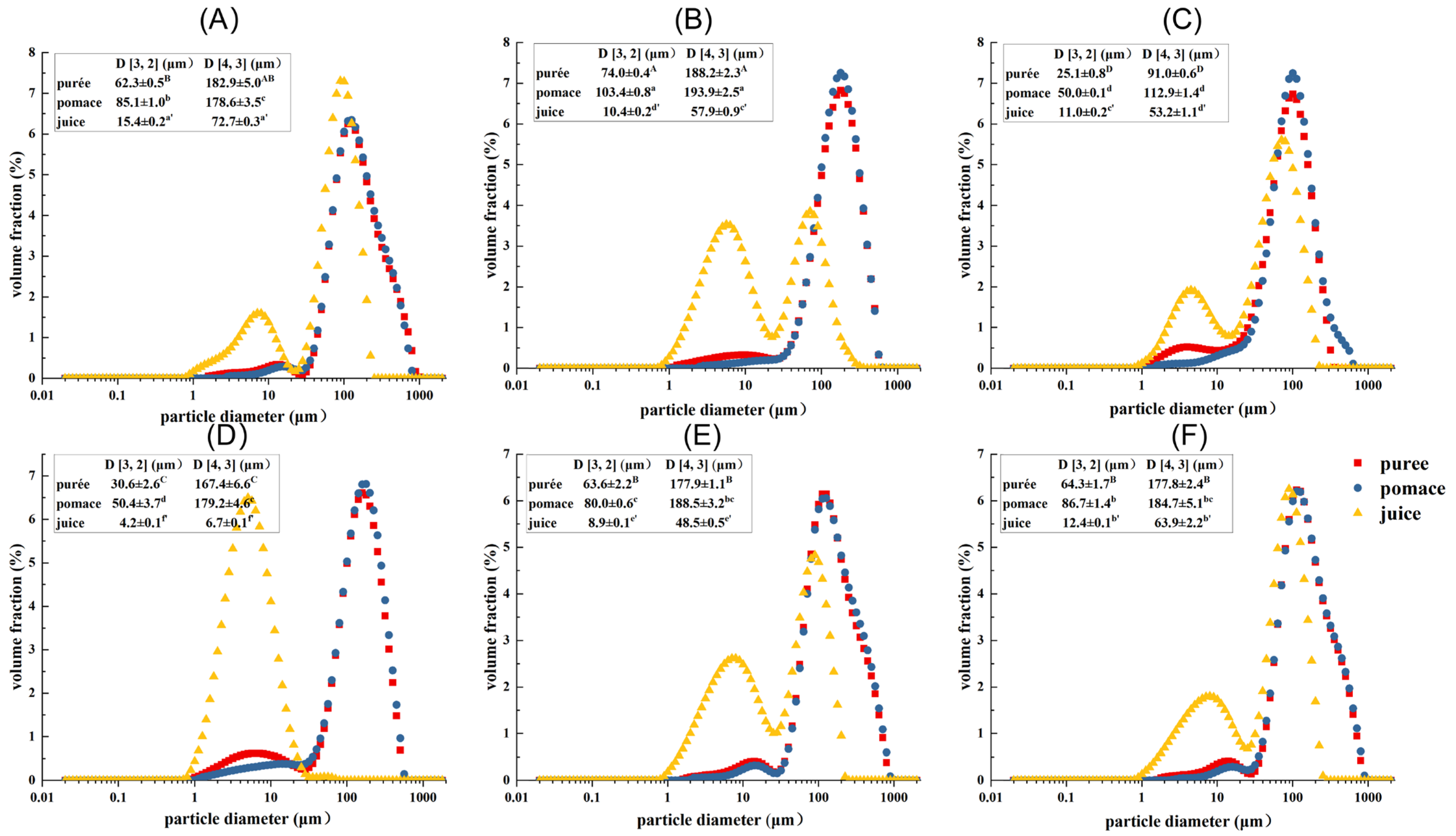
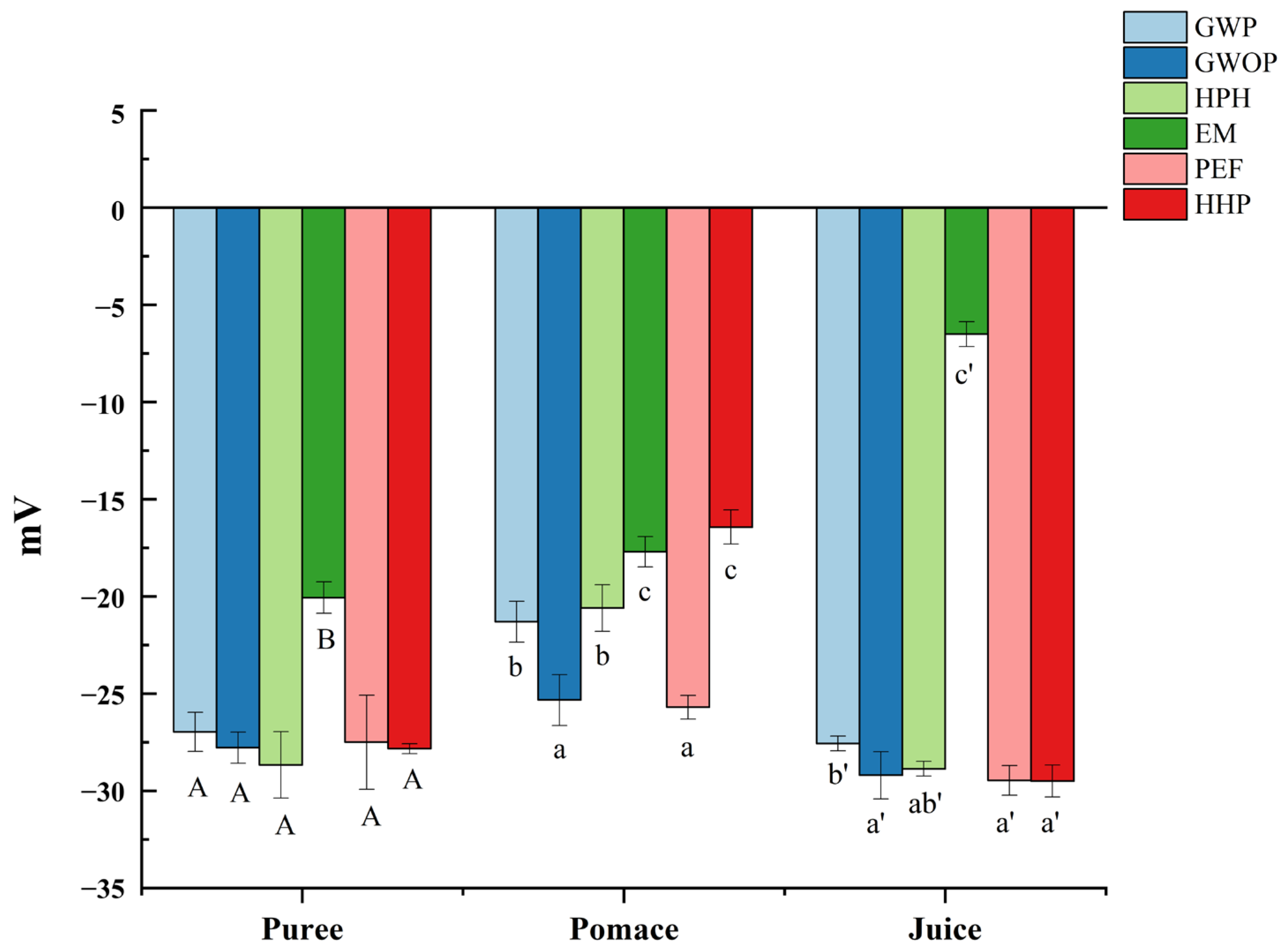
2.2. Particle Size Distribution and Zeta Potential
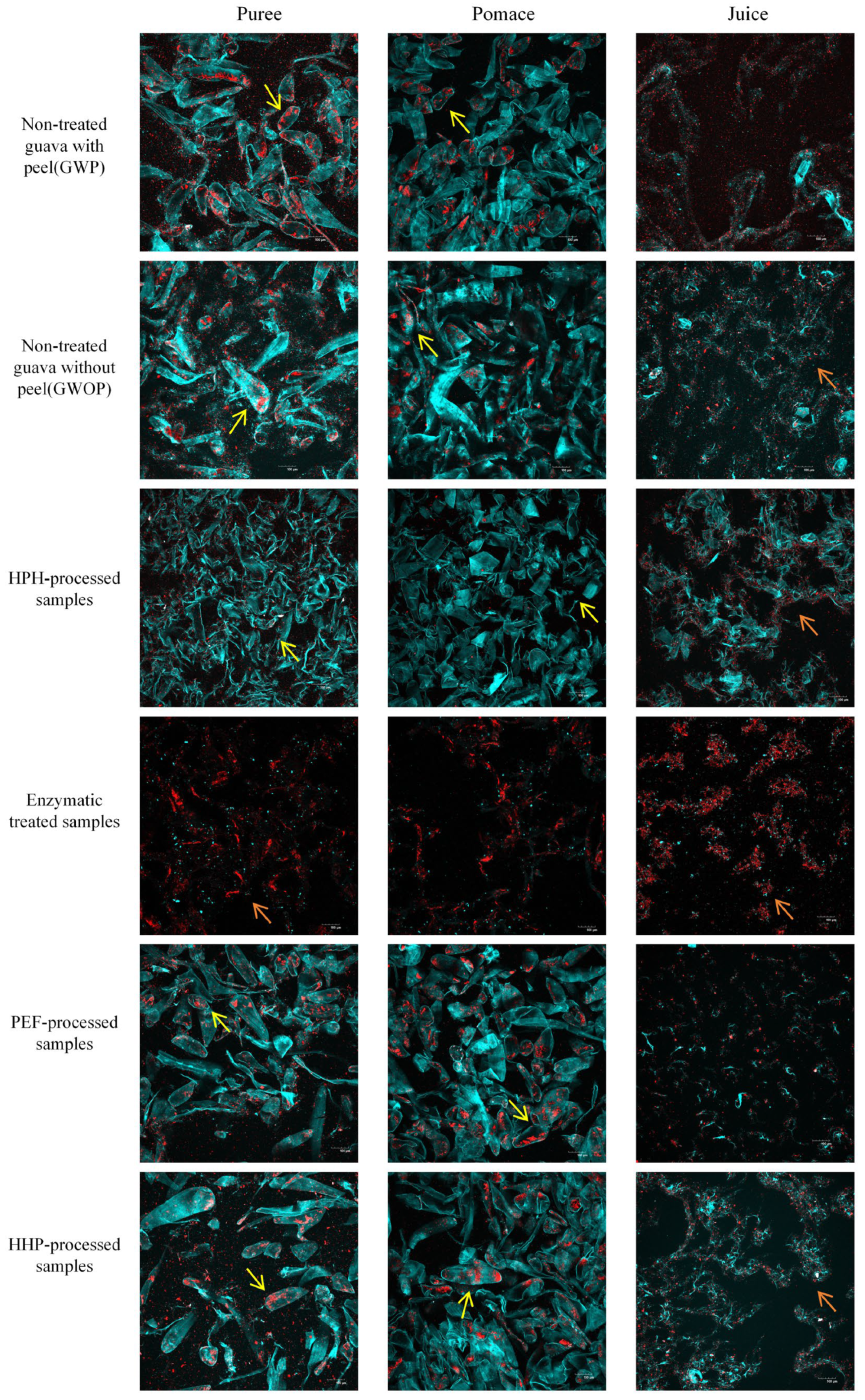
2.3. Microstructural Characterization
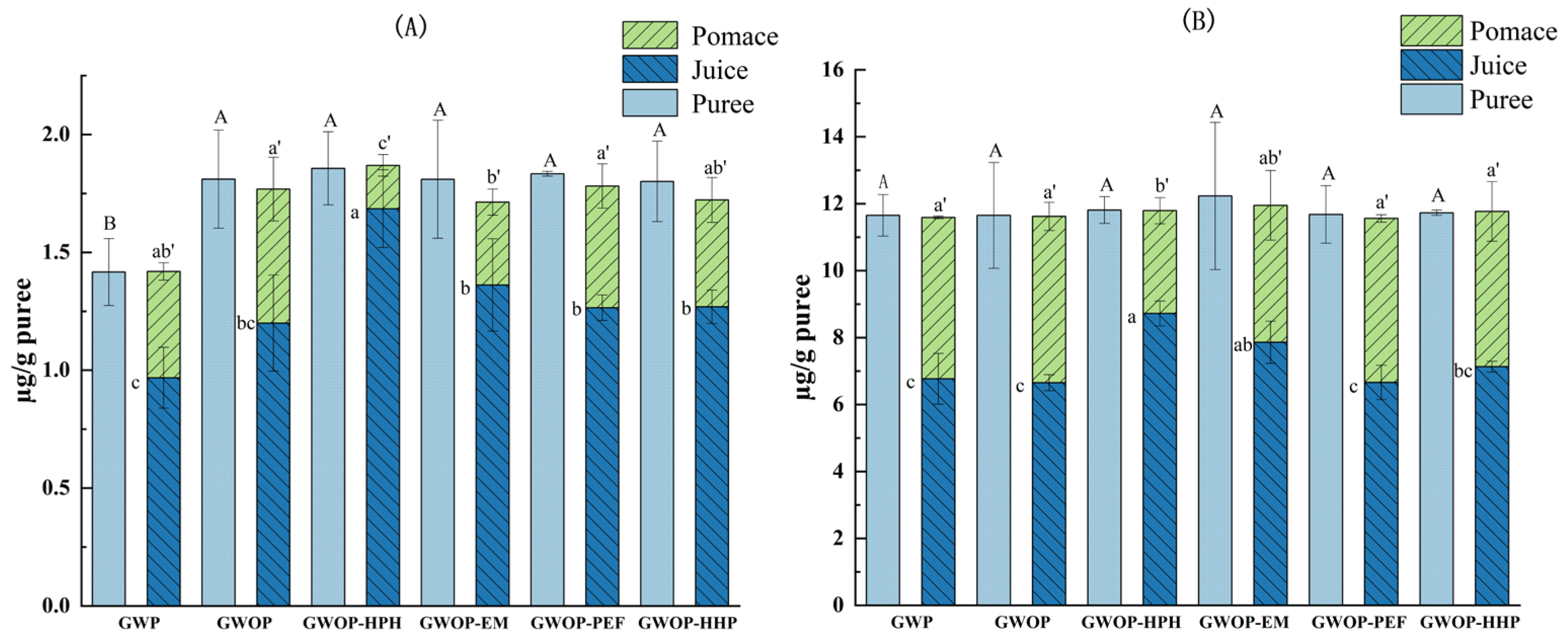
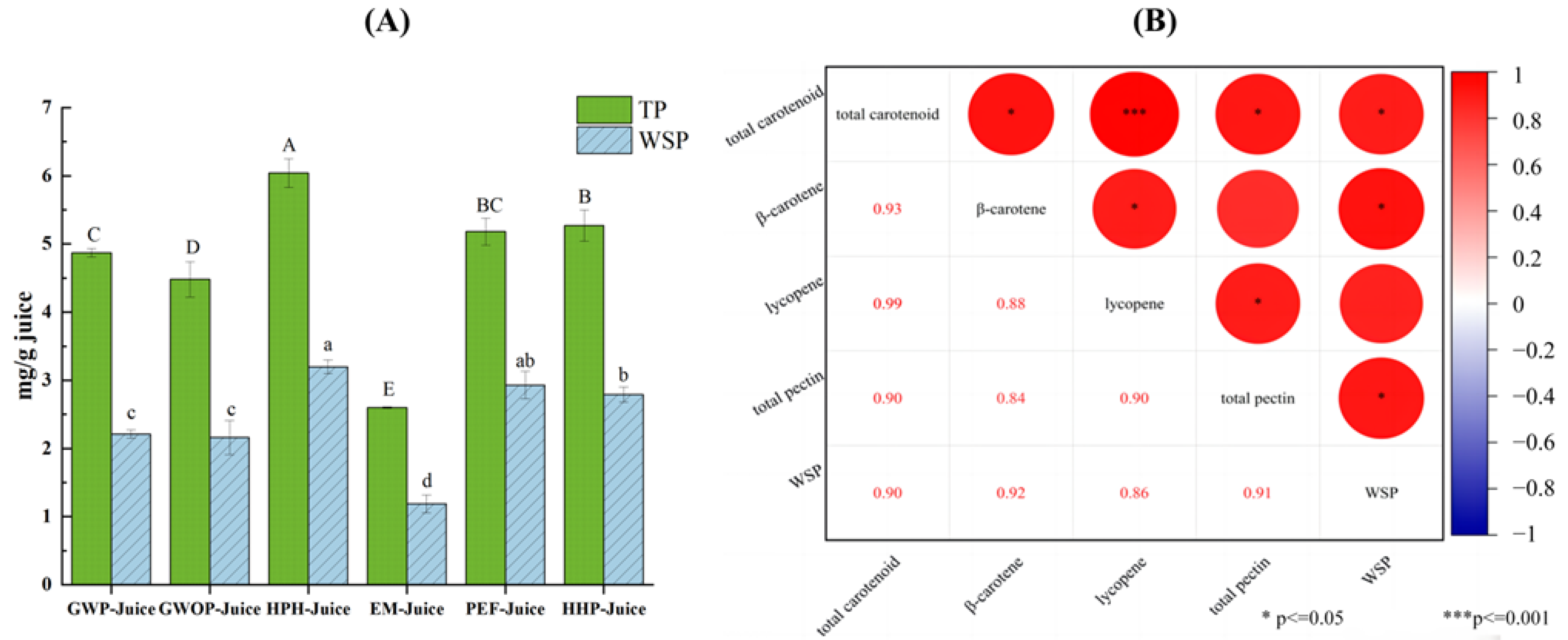
2.4. Effect of Processing Treatments on Carotenoid Contents of Puree, Pomace, and Juice
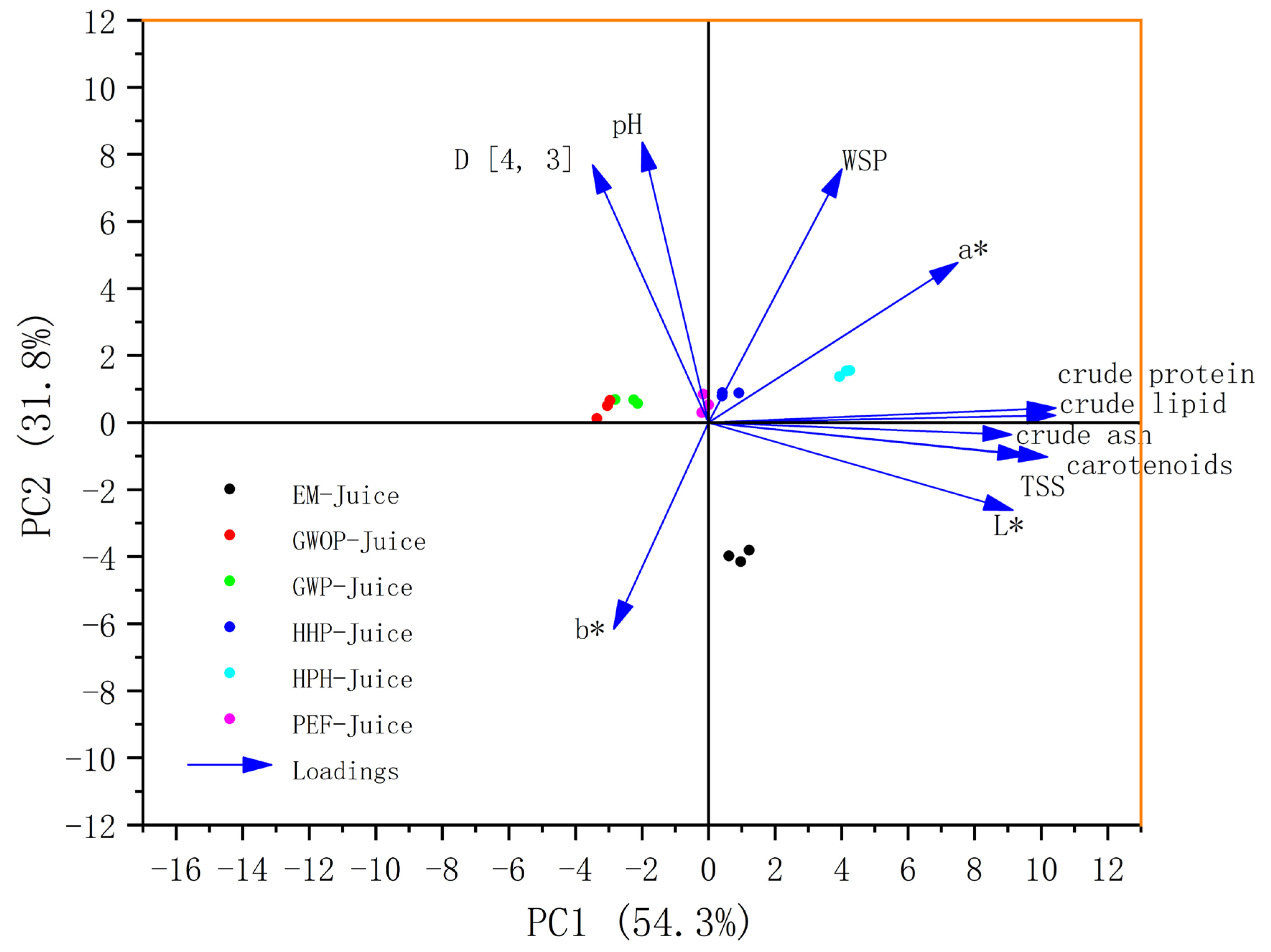
2.5. Compositional Analyses and PCA of Samples
3. Materials and Methods
3.1. Materials
3.2. Preparation of Guava Purees, Pomace, and Juices
3.2.1. Preparation of Guava Purees
3.2.2. High-Pressure Homogenization
3.2.3. Enzymatic Treatment
3.2.4. Pulsed Electric Field Treatment
3.2.5. High Hydrostatic Pressure
3.3. Mass Yield and Microstructural Analyses
3.3.1. Mass Yield of Juice and Pomace Samples
3.3.2. Confocal Laser Scanning Microscopy (CLSM)
3.3.3. Particle Size Distribution (PSD)
3.3.4. Zeta Potential
3.4. Carotenoid Extraction and HPLC Analysis
3.4.1. Carotenoid Extraction
3.4.2. Determination of Carotenoids by High Performance Liquid Chromatography (HPLC)
3.5. Compositional Analyses and Quality Attributes of Samples
3.5.1. Determination of the Dry Matter, Total Lipid, Total Protein, and Ash Content
3.5.2. Determination of pH and Total Soluble Sugars (TSS)
3.5.3. Determination of Color
3.5.4. Determination of Total Pectin and Water-Soluble Pectin Content (WSP)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ninga, K.A.; Carly Desobgo, Z.S.; De, S.; Nso, E.J. Pectinase hydrolysis of guava pulp: Effect on the physicochemical characteristics of its juice. Heliyon 2021, 7, e08141. [Google Scholar] [CrossRef]
- Kaur, H.; Ghosh, M. Probiotic fermentation enhances bioaccessibility of lycopene, polyphenols and antioxidant capacity of guava fruit (Psidium guajava L). J. Agric. Food Res. 2023, 14, 100704. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, Q.; Wu, H.; Ma, X.; Xu, W.; Li, L.; Liang, Q.; Wang, S. Metabolomics and transcriptomics analyses reveal the potential molecular mechanisms of flavonoids and carotenoids in guava pulp with different colors. Sci. Hortic. 2022, 305, 111384. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Culver, C.A. Alternatives to those artificial FD&C food colorants. Ann. Rev. Food Sci. Technol. 2012, 3, 59–77. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Krinsky, N.I. Effects of carotenoids in cellular and animal systems. Am. J. Clin. Nutr. 1991, 53, 238S–246S. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Mannozzi, C.; Fauster, T.; Haas, K.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Role of thermal and electric field effects during the pre-treatment of fruit and vegetable mash by pulsed electric fields (PEF) and ohmic heating (OH). Innov. Food Sci. Emerg. Technol. 2018, 48, 131–137. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Hu, K.; Chen, D.; Chen, M.; Xiang, A.; Xie, B.; Sun, Z. Effect of high pressure processing on gastrointestinal fate of carotenoids in mango juice: Insights obtained from macroscopic to microscopic scales. Innov. Food Sci. Emerg. Technol. 2023, 85, 103325. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Chandel, A.; Smitha, P.M.; Semwal, A.D. High pressure homogenization and retention of bioactive compounds in fruits and vegetables products. Food Hum. 2023, 1, 1559–1569. [Google Scholar] [CrossRef]
- La, H.J.; Choi, G.G.; Cho, C.; Seo, S.H.; Srivastava, A.; Jo, B.H.; Lee, J.Y.; Jin, Y.S.; Oh, H.M. Increased lipid productivity of Acutodesmus dimorphus using optimized pulsed electric field. J. Appl. Phycol. 2016, 28, 931–938. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Boulet, C.; Yildiz, S.; Sablani, S.; Tang, J.; Barbosa-Cánovas, G.V. Effect of high hydrostatic pressure on microbial inactivation and quality changes in carrot-orange juice blends at varying pH. LWT 2022, 159, 113219. [Google Scholar] [CrossRef]
- Jun, X. High-Pressure Processing as Emergent Technology for the Extraction of Bioactive Ingredients From Plant Materials. Crit. Rev. Food Sci. Nutr. 2013, 53, 837–852. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K.; Cameron, R.G. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour. Technol. 2007, 98, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Atencio, S.; Verkempinck, S.H.E.; Bernaerts, T.; Reineke, K.; Hendrickx, M.; Van Loey, A. Impact of processing on the production of a carotenoid-rich Cucurbita maxima cv. Hokkaido pumpkin juice. Food Chem. 2022, 380, 132191. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; González-Laredo, R.F.; Olmedilla-Alonso, B. Natural pigments of plant origin: Classification, extraction and application in foods. Food Chem. 2023, 398, 133908. [Google Scholar] [CrossRef]
- Toy, J.Y.H.; Lu, Y.; Huang, D.; Matsumura, K.; Liu, S.-Q. Enzymatic treatment, unfermented and fermented fruit-based products: Current state of knowledge. Crit. Rev. Food Sci. Nutr. 2022, 62, 1890–1911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.P.; Patel, H.; Sugandha. Enzymatic added extraction and clarification of fruit juices—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Esteve, M.J.; Frígola, A. High Pressure Treatment Effect on Physicochemical and Nutritional Properties of Fluid Foods During Storage: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 307–322. [Google Scholar] [CrossRef]
- Torregrosa, F.; Cortés, C.; Esteve, M.J.; Frígola, A. Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. J. Agric. Food Chem. 2005, 53, 9519–9525. [Google Scholar] [CrossRef]
- Bengtsson, H.; Tornberg, E. Physicochemical characterization of fruit and vegetable fiber suspensions. i: Effect of homogenization. J. Food Oral Process. Res. 2011, 42, 268–280. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Jiang, S.-W.; Cao, X.-M.; Jiang, S.-T.; Pan, L.-J. Effect of high pressure homogenization (HPH) on the physical properties of taro (Colocasia esculenta (L). Schott) pulp. J. Food Eng. 2016, 177, 1–8. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, Y.; Wei, L.; Xu, L.; Cao, X.; Liu, H.; Li, J. Effect of particle size on the stability and flavor of cloudy apple juice. Food Chem. 2020, 328, 126967. [Google Scholar] [CrossRef]
- Croak, S.; Corredig, M. The role of pectin in orange juice stabilization: Effect of pectin methylesterase and pectinase activity on the size of cloud particles. Food Hydrocoll. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Lacroix, N.; Fliss, I.; Makhlouf, J. Inactivation of pectin methylesterase and stabilization of opalescence in orange juice by dynamic high pressure. Food Res. Int. 2005, 38, 569–576. [Google Scholar] [CrossRef]
- Liu, L.; You, Y.; Deng, H.; Guo, Y.; Meng, Y. Promoting hydrolysis of apple pomace by pectinase and cellulase to produce microbial oils using engineered Yarrowia lipolytica. Biomass Bioenergy 2019, 126, 62–69. [Google Scholar] [CrossRef]
- Hu, K.; Chen, D.; Chen, M.; Xiang, A.; Xie, B.; Sun, Z. Mechanistic insights into changes in endogenous water soluble pectin and carotenoid bioaccessibility in mango beverage upon high pressure processing. Food Hydrocoll. 2023, 140, 108623. [Google Scholar] [CrossRef]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavcic, D.; Tarek, M. Cell membrane electroporation—Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef]
- Vázquez-Gutiérrez, J.L.; Plaza, L.; Hernando, I.; Sánchez-Moreno, C.; Cano, M. Changes in the structure and antioxidant properties of onions by high pressure treatment. Food Funct. 2013, 4, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, A.Z.; Steck, A.; Pfander, H. Carotenoids from guava (Psidium guajava L.): Isolation and structure elucidation. J. Agric. Food Chem. 1999, 47, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garbanzo, C.; Gleichenhagen, M.; Heller, A.; Esquivel, P.; Schulze-Kaysers, N.; Schieber, A. Carotenoid Profile, Antioxidant Capacity, and Chromoplasts of Pink Guava (Psidium guajava L. Cv. ‘Criolla’) during Fruit Ripening. J. Agric. Food Chem. 2017, 65, 3737–3747. [Google Scholar] [CrossRef]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.-Y. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015, 8, 61–68. [Google Scholar] [CrossRef]
- Jazaeri, S.; Mohammadi, A.; Kermani, A.M.P.; Paliyath, G.; Kakuda, Y. Characterization of lycopene hydrocolloidal structure induced by tomato processing. Food Chem. 2018, 245, 958–965. [Google Scholar] [CrossRef]
- Zulueta, A.; Barba, F.J.; Esteve, M.J.; Frígola, A. Effects on the carotenoid pattern and vitamin A of a pulsed electric field-treated orange juice–milk beverage and behavior during storage. Eur. Food Res. Technol. 2010, 231, 525–534. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Ancos, B.A.D.; Plaza, L.; Elez-Martínez, P.; Cano, M.P. Nutritional Approaches and Health-Related Properties of Plant Foods Processed by High Pressure and Pulsed Electric Fields. Crit. Rev. Food Sci. Nutr. 2009, 49, 552–576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, D.; Sheng, Y.; Tao, J.; Yang, Y. Carotenoids in Fruits of Different Persimmon Cultivars. Molecules 2011, 16, 624–636. [Google Scholar] [CrossRef]
- Gupta, R.; Kopec, R.E.; Schwartz, S.J.; Balasubramaniam, V.M. Combined pressure-temperature effects on carotenoid retention and bioaccessibility in tomato juice. J. Agric. Food Chem. 2011, 59, 7808–7817. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Stability of avocado paste carotenoids as affected by high hydrostatic pressure processing and storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 121–128. [Google Scholar] [CrossRef]
- Krebbers, B.; Matser, A.M.; Hoogerwerf, S.W.; Moezelaar, R.; Tomassen, M.M.M.; van den Berg, R.W. Combined high-pressure and thermal treatments for processing of tomato puree: Evaluation of microbial inactivation and quality parameters. Innov. Food Sci. Emerg. Technol. 2003, 4, 377–385. [Google Scholar] [CrossRef]
- Kormin, F.; Sakinah, R.A.; Iwansyah, A.C.; Hesan, A. The effect of enzyme concentration on physcical characteristics of pumpkin (Cucurbita moschata) puree and its dried extract. IOP Conf. Ser. Earth Environ. Sci. 2021, 736, 012031. [Google Scholar] [CrossRef]
- Gonnet, J.-F. Colour effects of co-pigmentation of anthocyanins revisited—1. A colorimetric definition using the CIELAB scale. Food Chem. 1998, 63, 409–415. [Google Scholar] [CrossRef]
- Gliemmo, M.F.; Latorre, M.E.; Gerschenson, L.N.; Campos, C.A. Color stability of pumpkin (Cucurbita moschata, Duchesne ex Poiret) puree during storage at room temperature: Effect of pH, potassium sorbate, ascorbic acid and packaging material. LWT—Food Sci. Technol. 2009, 42, 196–201. [Google Scholar] [CrossRef]
- Kubo, M.T.K.; Augusto, P.E.D.; Cristianini, M. Effect of high pressure homogenization (HPH) on the physical stability of tomato juice. Food Res. Int. 2013, 51, 170–179. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Wu, J.; Yu, Y.; Zou, B.; Li, L. Effects of Pectinase Pre-Treatment on the Physicochemical Properties, Bioactive Compounds, and Volatile Components of Juices from Different Cultivars of Guava. Foods 2023, 12, 330. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Bi, J.; Zhang, B.; Yi, J.; Peng, J. Effects of different crushing and refining treatments on release of carotenoids in carrot juice. Trans. Chin. Soc. Agric. Eng. 2017, 33, 307–314. [Google Scholar] [CrossRef]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.; Van Loey, A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Yanu, P.; Jakmunee, J. Down scaled Kjeldahl digestion and flow injection conductometric system for determination of protein content in some traditional northern Thai foods. Food Chem. 2017, 230, 572–577. [Google Scholar] [CrossRef] [PubMed]
- McCready, R.M.; McComb, E.A. Extraction and Determination of Total Pectic Materials in Fruits. Anal. Chem. 1952, 24, 1986–1988. [Google Scholar] [CrossRef]
| Treatments | Puree Weight/g | Pomace Weight/g | Juice Weight/g | Juice Yield (%) |
|---|---|---|---|---|
| GWP | 200.00 | 50.07 ± 1.42 | 150.86 ± 1.42 | 75.08 ± 0.71 cd |
| GWOP | 200.00 | 51.59 ± 2.96 | 148.54 ± 2.96 | 74.09 ± 1.48 d |
| GWOP-HPH | 200.00 | 39.38 ± 0.91 | 160.62 ± 0.91 | 80.17 ± 0.46 b |
| GWOP-EM | 200.00 | 28.19 ± 0.37 | 171.81 ± 0.37 | 85.58 ± 0.19 a |
| GWOP-PEF | 200.00 | 49.57 ± 1.68 | 150.64 ± 1.68 | 75.24 ± 0.84 cd |
| GWOP-HHP | 200.00 | 48.40 ± 1.04 | 151.76 ± 1.04 | 75.82 ± 0.52 c |
| Blue Signal/AU | Red Signal/AU | |
|---|---|---|
| Puree | ||
| GWP | 73.00 ± 3.57 A | 29.49 ± 3.57 a |
| GWOP | 64.30 ± 4.17 B | 31.95 ± 3.42 a |
| GWOP-HPH | 61.39 ± 3.76 B | 28.50 ± 1.26 a |
| GWOP-EM | 25.18 ± 0.68 C | 21.63 ± 2.41 b |
| GWOP-PEF | 64.99 ± 3.12 B | 32.32 ± 2.82 a |
| GWOP-HHP | 59.12 ± 4.10 B | 28.97 ± 2.03 a |
| Pomace | ||
| GWP | 68.88 ± 1.63 A | 23.93 ± 5.39 ab |
| GWOP | 60.05 ± 5.39 B | 23.58 ± 1.04 ab |
| GWOP-HPH | 68.28 ± 0.52 A | 20.03 ± 1.92 c |
| GWOP-EM | 30.39 ± 0.88 C | 21.28 ± 1.56 bc |
| GWOP-PEF | 74.98 ± 3.03 A | 24.96 ± 1.90 a |
| GWOP-HHP | 72.97 ± 3.18 A | 24.44 ± 1.03 a |
| Juice | ||
| GWP | 26.93 ± 0.08 C | 26.57 ± 0.80 b |
| GWOP | 27.96 ± 3.15 C | 25.62 ± 0.37 b |
| GWOP-HPH | 44.97 ± 3.86 A | 31.10 ± 2.58 a |
| GWOP-EM | 17.42 ± 0.82 D | 25.26 ± 3.44 b |
| GWOP-PEF | 18.96 ± 1.46 D | 24.33 ± 3.84 b |
| GWOP-HHP | 33.08 ± 2.33 B | 27.28 ± 1.14 ab |
| Crude Lipid | Crude Protein | Crude Ash | pH (22 °C) | TSS (22 °C) | CIE Color Values | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| Purees | ||||||||
| GWOP | 8.39 ± 0.78 a | 13.68 ± 1.26 b | 7.90 ± 0.13 a | 5.26 ± 0.01 ab | n.a. | 38.62 ± 0.41 bc | 5.42 ± 0.12 c | 0.63 ± 0.04 c |
| GWP | 7.79 ± 0.60 a | 12.97 ± 0.41 b | 7.42 ± 0.12 b | 5.25 ± 0.01 ab | n.a. | 38.43 ± 0.19 c | 5.11 ± 0.05 d | 1.19 ± 0.11 ab |
| GWOP-HPH | 8.63 ± 0.32 a | 14.84 ± 0.67 a | 7.82 ± 0.06 a | 5.28 ± 0.02 a | n.a. | 39.07 ± 0.05 ab | 5.94 ± 0.15 b | 0.76 ± 0.11 c |
| GWOP-EM | 8.48 ± 0.53 a | 13.15 ± 0.05 b | 7.87 ± 0.35 a | 4.17 ± 0.02 c | n.a. | 39.23 ± 0.21 a | 6.18 ± 0.07 a | 1.02 ± 0.11 b |
| GWOP-PEF | 8.50 ± 0.45 a | 13.32 ± 0.14 b | 7.90 ± 0.27 a | 5.24 ± 0.01 b | n.a. | 38.42 ± 0.16 c | 5.84 ± 0.02 b | 1.24 ± 0.11 a |
| GWOP-HHP | 8.46 ± 0.21 a | 13.69 ± 0.09 b | 7.92 ± 0.04 a | 5.26 ± 0.02 ab | n.a. | 38.14 ± 0.44 c | 5.94 ± 0.14 b | 1.25 ± 0.13 a |
| Juices | ||||||||
| GWOP-Juice | 1.40 ± 0.10 d | 4.26 ± 0.75 d | 3.28 ± 0.02 d | 5.31 ± 0.01 a | 0.94 ± 0.02 c | 37.90 ± 0.16 bc | 5.41 ± 0.07 c | 1.53 ± 0.07 b |
| GWP-Juice | 1.10 ± 0.06 d | 4.03 ± 0.02 d | 3.29 ± 0.07 d | 5.33 ± 0.01 a | 0.87 ± 0.01 d | 37.61 ± 0.19 c | 4.48 ± 0.16 e | 0.73 ± 0.19 c |
| HPH-Juice | 6.01 ± 0.17 a | 9.61 ± 0.38 a | 5.65 ± 0.03 a | 5.31 ± 0.01 a | 1.32 ± 0.03 a | 38.79 ± 0.03 a | 6.15 ± 0.08 a | 0.25 ± 0.07 d |
| EM-Juice | 3.54 ± 0.22 b | 6.77 ± 0.13 b | 5.08 ± 0.03 c | 4.22 ± 0.02 b | 1.21 ± 0.05 b | 38.60 ± 0.15 a | 4.81 ± 0.14 d | 2.12 ± 0.13 a |
| PEF-Juice | 2.85 ± 0.47 c | 5.81 ± 0.07 c | 5.36 ± 0.02 bc | 5.29 ± 0.01 a | 1.16 ± 0.01 b | 37.87 ± 0.16 bc | 5.45 ± 0.10 bc | 1.43 ± 0.12 b |
| HHP-Juice | 3.03 ± 0.19 c | 6.98 ± 0.03 b | 5.60 ± 0.02 b | 5.31 ± 0.01 a | 1.18 ± 0.02 b | 38.05 ± 0.30 b | 5.65 ± 0.12 b | 1.42 ± 0.23 b |
| Pomace | ||||||||
| GWOP-Pomace | 6.82 ± 0.16 a | 8.87 ± 0.43 a | 4.48 ± 0.06 a | 5.21 ± 0.01 a | n.a. | 41.26 ± 0.15 bc | 10.11 ± 0.17 b | 2.99 ± 0.13 c |
| GWP-Pomace | 6.57 ± 0.78 a | 9.15 ± 0.07 a | 4.50 ± 0.02 a | 5.22 ± 0.02 a | n.a. | 41.73 ± 0.12 bc | 7.56 ± 0.23 d | 1.94 ± 0.02 d |
| HPH-Pomace | 1.56 ± 0.41 c | 4.67 ± 0.14 d | 1.96 ± 0.37 c | 5.21 ± 0.01 a | n.a. | 44.16 ± 0.10 a | 3.79 ± 0.04 e | 2.63 ± 0.24 c |
| EM-Pomace | 4.93 ± 0.09 b | 6.50 ± 0.29 bc | 2.36 ± 0.03 b | 4.15 ± 0.02 b | n.a. | 44.13 ± 0.21 a | 14.74 ± 0.24 a | 5.00 ± 0.26 a |
| PEF-Pomace | 5.24 ± 0.83 b | 6.79 ± 0.15 b | 2.14 ± 0.01 bc | 5.19 ± 0.01 a | n.a. | 42.16 ± 0.27 b | 9.95 ± 0.31 bc | 3.66 ± 0.08 b |
| HHP-Pomace | 5.08 ± 0.52 b | 6.26 ± 0.12 c | 2.25 ± 0.02 b | 5.22 ± 0.02 a | n.a. | 40.95 ± 1.26 c | 9.39 ± 0.76 c | 3.51 ± 0.60 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Chen, Z.; Guo, Z.; Chen, M.; Xie, B.; Sun, Z.; Hu, K. Effect of Novel Processing Techniques on the Carotenoid Release during the Production of Red Guava Juice. Molecules 2024, 29, 487. https://doi.org/10.3390/molecules29020487
Zheng X, Chen Z, Guo Z, Chen M, Xie B, Sun Z, Hu K. Effect of Novel Processing Techniques on the Carotenoid Release during the Production of Red Guava Juice. Molecules. 2024; 29(2):487. https://doi.org/10.3390/molecules29020487
Chicago/Turabian StyleZheng, Xiaoxue, Ziting Chen, Ziming Guo, Mengting Chen, Bijun Xie, Zhida Sun, and Kai Hu. 2024. "Effect of Novel Processing Techniques on the Carotenoid Release during the Production of Red Guava Juice" Molecules 29, no. 2: 487. https://doi.org/10.3390/molecules29020487
APA StyleZheng, X., Chen, Z., Guo, Z., Chen, M., Xie, B., Sun, Z., & Hu, K. (2024). Effect of Novel Processing Techniques on the Carotenoid Release during the Production of Red Guava Juice. Molecules, 29(2), 487. https://doi.org/10.3390/molecules29020487






