Fructan Concentrations in Cooked Cereal Grains as a Nutritional Consideration for Low-FODMAP Diet
Abstract
1. Introduction
2. Results
2.1. Water Absorption of Groats
2.2. Color Parameters in Freshly Cooked Groats
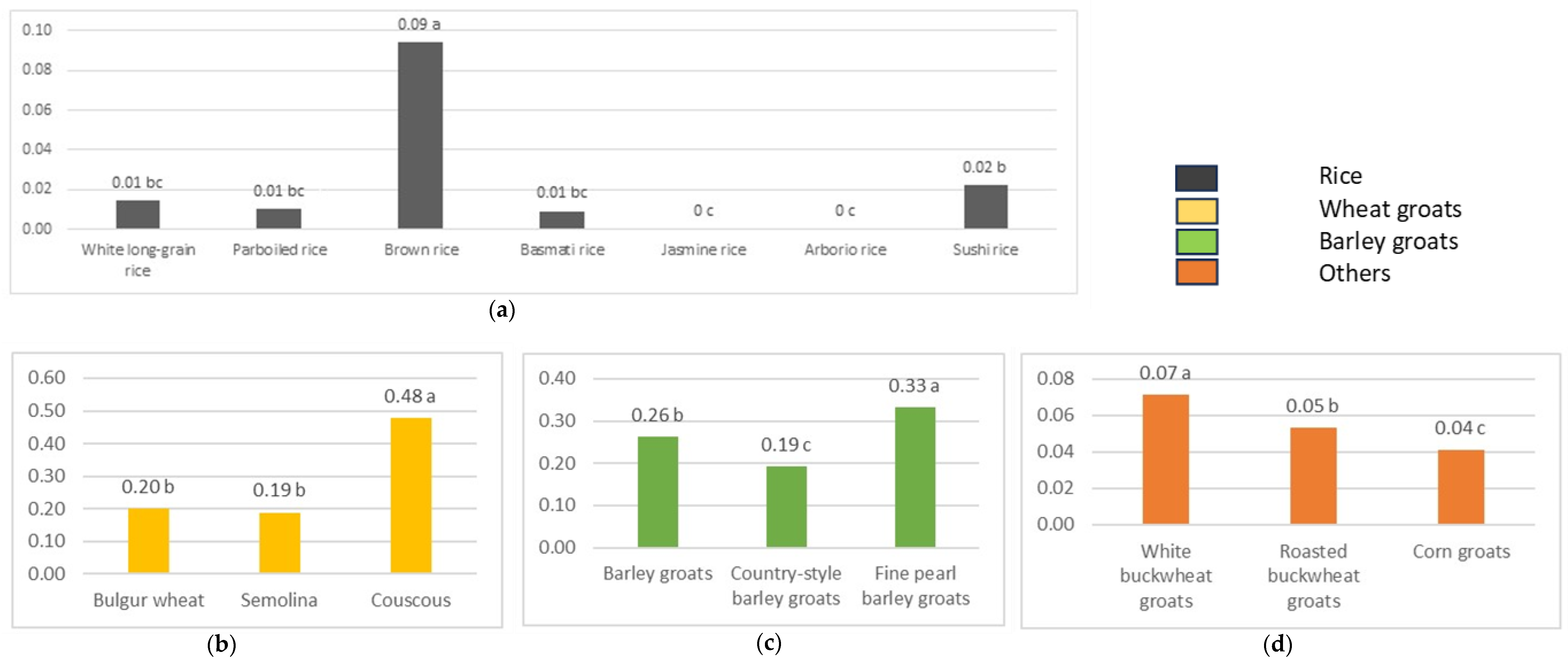
2.3. Fructan Content in Cooked Cereal Grains
2.4. Correlation between Fructan Content and Color Parameters of Groats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Water Absorption of Groats
4.4. Cooked Grains Color
4.5. Fructans Content
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cummings, J.; Stephens, A. Carbohydrates terminology and classification. Eur. J. Clin. Nutr. 2007, 61, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.; Shepherd, S.; Gibson, P.; Muir, J. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Probert, H.; Van Loo, J.; Rastall, R.; Roberfroid, M. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; McCartney, A.; Rastall, R. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005, 93, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef] [PubMed]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.; Mearin, F.; Chang, L.; Chey, W.; Lembo, A.; Simren, M.; Spiller, R. Bowel Disorders. J. Gastroenterol. 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.; Ford, A. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef]
- Adrych, K. Zespół jelita drażliwego w świetle najnowszych wytycznych. Forum. Med. Rodz. 2018, 12, 224–233. [Google Scholar]
- Gąsiorowska, J.; Czerwionka-Szaflarska, M. Zespół przerostu flory bakteryjnej jelita cienkiego a zespół jelita nadwrażliwego. Prz. Gastroenterol. I 2011, 8, 165–171. [Google Scholar]
- Sachdeva, S.; Rawat, A.K.; Reddy, R.S.; Puri, A.S. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: Frecquency and predictors. J. Gastroenterol. Hepatol. 2011, 26, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Hozyasz, K. Nieceliakalna nadwrażliwość na gluten (NCNG)—Choroba ponownie odkryta. Fam. Med. Prim. 2016, 18, 79–83. [Google Scholar]
- Jarocka-Cyrta, E.; Przybyłowicz, K.; Nosek, H. Rola FODMAP w zaburzeniach czynnościowych przewodu pokarmowego. Część 1. Nietolerancja FODMAP. Patomechanizmy i obraz kliniczny. Stand. Med. Pediatr. 2015, 12, 80–86. [Google Scholar]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.; Muir, J. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Zannini, E.; Arendt, E. Characterization of the FODMAP-profile in cereal-product ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. J. Gastroenterol. 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Haskå, L.; Nyman, M.; Andersson, R. Distribution and characterisation of fructan in wheat milling fractions. J. Cereal Sci. 2008, 48, 768–774. [Google Scholar] [CrossRef]
- Sahasakul, Y.; Aursalung, A.; Thangsiri, S.; Temviriyanukul, P.; Inthachat, W.; Pongwichian, P.; Sasithorn, K.; Suttisansanee, U. Nutritional Compositions, Phenolic Contents and Antioxidant Activities of Rainfed Rice Grown in Different Degrees of Soil Salinity. Foods 2023, 12, 2870. [Google Scholar] [CrossRef]
- Sharma, G.M.; Pereira, M.; Wang, S.S.; Chirtel, S.J.; Whitaker, T.B.; Wehling, P.; Arlinghaus, M.; Canida, T.; Jackson, L.S.; Williams, K.M. Evaluation of sampling plans for measurement of gluten in oat groats. Food Control 2023, 114, 107241. [Google Scholar] [CrossRef]
- Andersson, A.; Andersson, R.; Piironen, V.; Lampi, A.; Nyström, L.; Boros, D.; Fraś, A.; Gebruers, K.; Courtin, C.; Delcour, J.; et al. Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the healthgrain diversity screen. Food Chem. 2013, 136, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.; Kaiser, I.; Trebstein, A.; Henle, T. Heat-induced degradation of inulin. Eur. Food Res. Technol. 2005, 220, 466–470. [Google Scholar] [CrossRef]
- Bustos, M.; Pérez, G.; León, A. Effect of Four Types of Dietary Fiber on the Technological Quality of Pasta. Food Sci. Technol. Int. 2011, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, C.; Andersson, A.; Andersson, R.; Mangelsen, E.; Sun, C.; Åman, P. Relationship of Grain Fructan Content to Degree of Polymerisation in Different Barleys. Food Sci. Nutr. 2014, 5, 581–589. [Google Scholar] [CrossRef]
- Radoš, K.; Čukelj Mustač, N.; Varga, K.; Drakula, S.; Voučko, B.; Ćurić, D.; Novotni, D. Development of High-Fibre and Low-FODMAP Crackers. Foods 2022, 11, 2577. [Google Scholar] [CrossRef] [PubMed]
- Glibowski, P.; Skrzypek, M.; Ćwiklińska, M.; Drozda, M.; Kowalska, A. Chemical stability of fructans in apple beverages and their influence on chronic constipation. Food Funct. 2020, 11, 3860–3866. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Raczyk, M. FODMAP reduction strategies for nutritionally valuable baking products: Current state and future challenges. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. FODMAP modulation as a dietary therapy for IBS: Scientific and market perspective. Compr. Rev. Food. Sci. Food. Saf. 2022, 21, 1491–1516. [Google Scholar] [CrossRef]

| Grains | Moisture Content [%] |
|---|---|
| Rice | |
| White long-grain rice | 70.66 ± 0.34 b |
| Parboiled rice | 72.91 ± 0.09 a |
| Brown rice | 63.15 ± 0.14 d |
| Basmati rice | 62.67 ± 0.33 de |
| Jasmine rice | 65.52 ± 0.31 c |
| Arborio rice | 61.71 ± 0.29 e |
| Sushi rice | 59.83 ± 0.17 f |
| Wheat groats | |
| Bulgur wheat | 59.53 ± 0.47 b |
| Semolina | 77.16 ± 0.16 a |
| Couscous | 76.57 ± 0.36 a |
| Barley groats | |
| Barley groats | 55.17 ± 0.17 c |
| Country-style barley groats | 75.82 ± 0.18 b |
| Fine pearl barley groats | 76.93 ± 0.08 a |
| Others | |
| White buckwheat groats | 71.29 ± 0.28 b |
| Roasted buckwheat groats | 62.33 ± 0.33 c |
| Corn groats | 88.97 ± 0.04 a |
| Group | Grains | L* | a* | b* | Visualization |
|---|---|---|---|---|---|
| Rice | White long-grain rice | 77.85 b | −3.85 d | 12.05 f |  |
| Parboiled rice | 75.25 c | −2.95 b | 14.70 d |  | |
| Brown rice | 67.10 d | −0.55 a | 18.80 a |  | |
| Basmati rice | 78.05 b | −3.05 b | 13.95 e |  | |
| Jasmine rice | 79.90 a | −4.25 e | 14.15 e |  | |
| Arborio rice | 77.55 b | −3.55 c | 15.55 b |  | |
| Sushi rice | 79.70 a | −4.45 f | 15.15 c |  | |
| Wheat groats | Bulgur wheat | 66.95 c | −1.95 a | 28.70 b |  |
| Semolina | 78.70 a | −6.50 c | 17.90 c |  | |
| Couscous | 74.75 b | −3.05 b | 29.80 a |  | |
| Barley groats | Barley groats | 57.85 c | 1.85 a | 18.95 a |  |
| Country-style barley groats | 62.15 b | 0.45 a | 17.75 b |  | |
| Fine pearl barley groats | 63.10 a | 0.90 a | 18.05 b |  | |
| Others | White buckwheat groats | 54.90 b | 2.30 b | 13.55 b |  |
| Roasted buckwheat groats | 46.50 c | 5.05 a | 13.95 b |  | |
| Corn groats | 75.80 a | −3.90 c | 45.70 a |  |
| Grains | Fructan Content [g/100 g] |
|---|---|
| Rice | |
| White long-grain rice | 0.05 ± 0.04 b |
| Parboiled rice | 0.04 ± 0.01 b |
| Brown rice | 0.26 ± 0.01 a |
| Basmati rice | 0.03 ± 0.01 b |
| Jasmine rice | 0.00 ± 0.00 b |
| Arborio rice | 0.00 ± 0.00 b |
| Sushi rice | 0.06 ± 0.01 b |
| Wheat groats | |
| Bulgur wheat | 0.50 ± 0.01 c |
| Semolina | 0.83 ± 0.03 b |
| Couscous | 2.04 ± 0.01 a |
| Barley groats | |
| Barley groats | 0.59 ± 0.02 c |
| Country-style barley groats | 0.80 ± 0.01 b |
| Fine pearl barley groats | 1.44 ± 0.01 a |
| Others | |
| White buckwheat groats | 0.19 ± 0.01 b |
| Roasted buckwheat groats | 0.19 ± 0.00 b |
| Corn groats | 0.37 ± 0.01 a |
| MC | L* | a* | b* | FDM | FFC | |
|---|---|---|---|---|---|---|
| MC | 1.00 | 0.06 | −0.19 | 0.19 | 0.43 | 0.21 |
| L* | 1.00 | −0.95 | −0.13 | −0.44 | −0.56 | |
| a* | 1.00 | −0.03 | 0.28 | 0.44 | ||
| b* | 1.00 | 0.66 | 0.61 | |||
| FDM | 1.00 | 0.96 | ||||
| FFC | 1.00 |
| Group | Grains | Country of Origin | Manufacturer/Distributor | Cooking Time [min] |
|---|---|---|---|---|
| Rice | White long-grain rice | Myanmar | Sawex Foods, Warsaw, Poland | 14 |
| Parboiled rice | Thailand | Lestello, Cmolas, Poland | 12 | |
| Brown rice | Vietnam | Sawex Foods, Warsaw, Poland | 35 | |
| Basmati rice | India | Lestello, Cmolas, Poland | 15 | |
| Jasmine rice | Thailand | Lidl, Tarnowo Podgórne, Poland | 12 | |
| Arborio rice | Italy | Lidl, Tarnowo Podgórne, Poland | 15 | |
| Sushi rice | Japan | Lidl, Tarnowo Podgórne, Poland | 10 | |
| Wheat groats | Bulgur wheat | Türkiye | Lidl, Tarnowo Podgórne, Poland | 15 |
| Semolina | Poland | Kupiec, Krzymow, Poland | 3 | |
| Couscous | Italy | Sante, Warsaw, Poland | 5 | |
| Barley groats | Barley groats | Poland | Cenos, Września, Poland | 15 |
| Country-style barley groats | Poland | Cenos, Września, Poland | 15 | |
| Fine pearl barley groats | Poland | Kupiec, Krzymow, Poland | 15 | |
| Others | White buckwheat groats | Poland | Sante, Warsaw, Poland | 15 |
| Roasted buckwheat groats | Poland | Sante, Warsaw, Poland | 15 | |
| Corn groats | Poland | Sante, Warsaw, Poland | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pejcz, E.; Wojciechowicz-Budzisz, A.; Spychaj, R.; Gil, Z.; Harasym, J. Fructan Concentrations in Cooked Cereal Grains as a Nutritional Consideration for Low-FODMAP Diet. Molecules 2024, 29, 282. https://doi.org/10.3390/molecules29020282
Pejcz E, Wojciechowicz-Budzisz A, Spychaj R, Gil Z, Harasym J. Fructan Concentrations in Cooked Cereal Grains as a Nutritional Consideration for Low-FODMAP Diet. Molecules. 2024; 29(2):282. https://doi.org/10.3390/molecules29020282
Chicago/Turabian StylePejcz, Ewa, Agata Wojciechowicz-Budzisz, Radosław Spychaj, Zygmunt Gil, and Joanna Harasym. 2024. "Fructan Concentrations in Cooked Cereal Grains as a Nutritional Consideration for Low-FODMAP Diet" Molecules 29, no. 2: 282. https://doi.org/10.3390/molecules29020282
APA StylePejcz, E., Wojciechowicz-Budzisz, A., Spychaj, R., Gil, Z., & Harasym, J. (2024). Fructan Concentrations in Cooked Cereal Grains as a Nutritional Consideration for Low-FODMAP Diet. Molecules, 29(2), 282. https://doi.org/10.3390/molecules29020282







